Abstract
Objective
Acute respiratory tract infections caused by Streptococcus pneumoniae are a leading cause of morbidity and mortality in young children and the elderly. In 2002, Alberta introduced a pneumococcal universal immunization program for children, using Pfizer’s Prevnar 7, a 7-valent pneumococcal conjugate vaccine (PCV7). In this study, we explored the impact of the immunization program on the burden of disease and related health care costs in Alberta, in the context of serotype replacement.
Methods
Using surveillance data from Alberta, we examined the change in costs averted as a result of a decline in invasive pneumococcal disease (IPD) cases caused by PCV7 serotypes, as well as the increase in costs due to serotype replacement. We also calculated the magnitude of positive externalities (indirect effects) in terms of costs averted.
Results
We found that following the introduction of PCV7 (2003–2008), the number of cases of IPD caused by vaccine serotypes declined significantly across all ages. Non-PCV7 IPD cases, on the other hand, increased. Net costs were averted as a result of the implementation of PCV7 universal vaccination in Alberta, after accounting for serotype replacement.
Conclusion
On the basis of the analysis of serotype-specific pneumococcal data, the impact of the Prevnar public immunization program on direct health costs averted in Alberta as a result of reducing IPD cases caused by PCV7 strains amounted to $5.5 million (in 2008 Canadian dollars). However, the unintended effects of serotype replacement resulted in costs incurred of nearly $1.9 million. As a result, on net, the total cost savings for Alberta amounted to about $3.6 million. Irrespective of serotype replacement, the PCV7 immunization program has had a positive impact in terms of health benefits, which translates into health service costs averted.
Key Points
| Decision makers need to know the distribution of serotypes causing pneumococcal disease in their regions. Such information is critical to immunization program planning and evaluation. |
| The economic benefits of public programs, post-implementation, are under-studied and can provide valuable information about the intended and unintended program effects. |
Introduction
Acute respiratory tract infections caused by Streptococcus pneumoniae are a leading cause of morbidity and mortality in young children and the elderly. The burden of disease attributable to S. pneumoniae in the form of invasive pneumococcal disease (IPD) is high. Clinical presentations of IPD infection include invasive pneumonia, meningitis, or bacteremia; sequelae can include death. S. pneumoniae has proven to be fatal in up to 40 % of cases in industrialized countries [1]. It is estimated that before 2002, the Canadian societal costs of pneumococcal-related disease were between $155 million and $295 million annually [2].
There are more than 90 known S. pneumoniae serotypes, each of which varies in frequency. Since 2002, a 7-valent pneumococcal conjugate vaccine (PCV7), targeting seven serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F), has been adopted in the childhood vaccination program in Alberta, Canada. Since its introduction, it has been shown to be effective in reducing the number of cases of pneumococcal disease via direct and indirect protection [3–5]. In particular, there is evidence that invasive disease caused by any one of the seven S. pneumoniae serotypes has dropped by up to 100 % in areas with universal childhood immunization programs [6–8]. However, the number of cases reduced is dependent upon the distribution of serotypes causing disease. Frequencies of serotypes vary by geographic location and time [9, 10], and changes in the distribution of S. pneumoniae caused by non-Prevnar serotypes have been observed following the introduction of the vaccine [3, 7]. This phenomenon, termed serotype replacement, reflects an emergence or increase in the frequency of cases caused by non-vaccine serotypes following the introduction of PCV7. What has not been quantified is the net economic impact of the public immunization program, especially related to the decrease in IPD cases caused by PCV7 serotypes, and the increase in IPD cases caused by non-PCV7 serotypes, or serotype replacement.
Objective of the Study
The objective of this study was to estimate the changes in the incidence of IPD and the resulting impact on health care costs following the introduction of Prevnar 7 in Alberta, accounting for serotype replacement, using observational data.
Methods
IPD Surveillance Data in Alberta
The change in the incidence of pneumococcal disease as a result of Prevnar 7 was estimated using data from the Provincial Public Health Authorities in Alberta. IPD is a notifiable health condition in the province. Isolates matching the Canadian national case definition for IPD are forwarded to the Provincial Public Health Laboratory in Edmonton, Alberta, for serotype analysis [11].1 Details regarding the program have been published elsewhere [11].
Data consisting of reported IPD cases by age, between 2000 and 2008, were obtained from the Provincial Public Health Laboratory and used in the present analysis. The age-specific incidence rates of IPD in Alberta, with ascertained cases subdivided into seven age categories (<2, 2–4, 5–9, 10–14, 15–19, 20–64, and 65+ years of age), were estimated using annual estimates of population by age from Statistics Canada CANSIM Table 051-0001 [13]. Age-specific incidence and fatality rates were further subdivided by the presenting diagnosis (pneumonia, bacteremia, meningitis) on the basis of distributions estimated by Morrow et al. [2] (Table 1).
Table 1.
Epidemiology and direct health service costs
| Age group | ||||||||
|---|---|---|---|---|---|---|---|---|
| <2 years | 2–4 years | 5–9 years | 10–14 years | 15–19 years | 20–39 years | 40–64 years | 65+ years | |
| Distribution (%) | ||||||||
| Hospitalized pneumonia | 74.00 | 74.00 | 88.20 | 62.40 | 62.40 | 66.90 | 66.90 | 81.50 |
| Hospitalized bacteremia | 14.30 | 14.30 | 6.70 | 24.80 | 24.80 | 26.30 | 26.30 | 15.00 |
| Non-hospitalized bacteremia | 8.90 | 8.90 | 4.10 | 9.60 | 9.60 | 5.10 | 5.10 | 2.90 |
| Meningitis | 2.50 | 2.50 | 1.00 | 3.20 | 3.20 | 1.70 | 1.70 | 0.60 |
| Mortality (%) | ||||||||
| Hospitalized pneumonia | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 |
| Bacteremia | 2 | 2 | 2 | 2 | 15 | 15 | 15 | 31 |
| Meningitis | 7 | 7 | 7 | 7 | 28 | 28 | 28 | 28 |
| Direct health service costs ($)a | ||||||||
| Meningitis | 35,017 | 35,017 | 32,981 | 32,981 | 32,981 | 14,170 | 14,170 | 11,304 |
| Hospitalized bacteremia | 6553 | 6553 | 5129 | 5129 | 5129 | 11,697 | 11,697 | 11,287 |
| Non-hospitalized bacteremia | 143 | 143 | 143 | 143 | 143 | 143 | 143 | 143 |
| Hospitalized pneumonia | 2686 | 2686 | 4314 | 7165 | 7165 | 7624 | 7624 | 8031 |
The data in this table are drawn from Morrow et al. [2]
aCosts are expressed in 2008 Canadian dollars
Serotype Replacement
Serotype replacement is a phenomenon whereby serotypes not included in the vaccine have been found to emerge or increase in frequency with the introduction of a vaccine [3, 7]. However, the microbiology regarding S. pneumoniae and how PCV7 may have led to the replacement (increased frequency) of serotypes is not well understood. The current body of evidence on the association between PCV7 and serotype replacement has been generated through observational studies. Studies suggest that, historically, there have been “epidemic” serogroups that have exhibited specific epidemic trends between 1928 and 2009 [9, 10, 14]. Serotypes 1, 2, 3, and 5 have been found to be epidemic throughout the last 75 years [9, 10]. If the incidence of a serotype is known to follow a time trend characterized by outbreaks that occur at predictable intervals, then it is unreasonable to attribute the most recent outbreaks to the vaccine PCV7.2
In addition, the majority of reports [3, 9, 15–17] have inferred a causal relationship based on a temporal association between the vaccine and the redistribution of serotypes with respect to the increased relative frequency of their detection of IPD. On the basis of this inference, it is most plausible to infer serotype replacement resulting only from PCV7 for serotypes found to increase in frequency following the initiation of a public health vaccination program. Conversely, it seems plausible to exclude serotypes that decrease or remain constant in frequency following vaccine introduction. In other words, these serotypes do not appear to be influenced by vaccination at the population level, and changes in these serotypes are therefore not attributed to the vaccine.
The above evidence concerning the relationship between Prevnar and the redistribution of serotypes supports the differentiation of non-Prevnar serotypes into three groups: (1) epidemic serotypes; (2) all increasing serotypes following vaccine introduction (which are referred to as serotype replacement throughout the remainder of this paper); and (3) other serotypes (not related to serotype replacement).
Costing Model
Using the IPD surveillance data, an economic model was developed to compare the incidence rates of IPD disease and associated health service resource use before (2000–2001) and after (2003–2008) the introduction of PCV7 in 2002, by serotypes contained in the vaccine, and by serotype replacement.
Costs
The costs included in the model were direct medical costs associated with direct and indirect protection against IPD for both PCV7 and replaced serotypes. Three direct health service cost measures were calculated: PCV7 costs averted as a result of vaccine protection; non-PCV7 costs incurred as a result of PCV7-related serotype replacement (referred to as PCV7SR); and resulting net costs.3 This analysis was conducted in Microsoft Excel 2012.
Direct health service costs were applied to the expected numbers of age-specific IPD cases (by disease diagnosis) and the numbers of deaths from PCV7 serotypes and PCV7SR. The cost associated with a case fatality was assumed to be $39,000 for any age [18]. This estimate was taken from a study on end-of-life care, and the value reflects the average cost of end-of-life care for patients suffering from infection. All costs are adjusted to reflect $2008 Canadian dollars, using the Canadian Consumer Price Index.
Method for Calculation of Direct Medical Costs Averted as a Result of PCV7
The costs averted were estimated by first calculating the difference between the average annual incidence pre-PCV7 (2000–2001) and post-PCV7 vaccination program implementation [by diagnosis] (2003–2008); this difference can be interpreted as the frequency of prevented cases, assuming that there were no other causes of the observed decline in frequency from one time period to the next. We excluded the first year of the vaccination program to allow for a wash-out period. Second, this annual incidence difference was then multiplied by the number of Albertans in each age category, using 2008 population data to generate an estimate of the total cases averted in Alberta. Third, to calculate the annual total PCV7 costs averted, we multiplied the estimated total cases averted in Alberta per year by the average cost per case. The average cost per case was previously estimated by Morrow et al. [2] and was used in the present study (Table 1). Fourth, the cost post-vaccine program was then calculated by multiplying the annual total PCV7 costs averted by the number of years post-vaccination program implementation (only 6 years of data were available post-vaccination program implementation).
Method for Calculation of Costs Incurred as a Result of PCV7SR
In this analysis, our base case accounts for the costs associated with serotype replacement by including the costs incurred as a result of all increasing non-PCV7 serotypes less epidemic cases (serotypes 1, 2, 3, and 5). We also excluded non-PCV7 serotypes for which changes in frequency did not appear to be related to PCV7 vaccine introduction (i.e., they exhibited a decreasing trend before vaccine introduction or maintained a constant incidence rate throughout the time period). The non-PCV7 serotypes meeting these criteria are referred to as PCV7SR. The costs incurred as a result of PCV7SR are calculated in the same way as PCV7 costs averted.
Method for Calculation of Total Net Costs
Net costs are the sum of costs incurred and averted from 2003 through 2008 as a result of PCV7: the sum of the medical costs averted because of IPD cases being prevented by PCV7, plus costs incurred as a result of additional IPD cases due to serotype replacement (PCV7SR).
Direct and Indirect Vaccine Protection
In 2002, at the time when universal PCV7 vaccination started in Alberta, only children reaching 2 months of age were targeted. At the time, children older than 2 months were not offered the vaccine unless they belonged to a high-risk group [19]. In this study, the measure of direct vaccine protection against IPD is the change in the number of cases of each diagnostic category for children aged less than 8 years.
The non-vaccinated population is assumed to be indirectly protected as a result of the decreased circulation of the disease, similarly evidenced by a decrease in the annual incidence of IPD following the vaccine introduction. Indirect protection was estimated as the difference between the annual incidence of IPD cases across the 6-year vaccination program (in those aged 10 years and older), by presenting diagnosis, before and after the introduction of PCV7. The youngest age considered for evidence of indirect protection was 10 years, since, in 2008, children with direct protection would have been between the ages of 6 and 7 years (within the age category of 5–9 years).
Scenario Analysis
A scenario analysis was conducted to determine the effects of model assumptions. Specifically, given the uncertainty concerning the causal relationship between PCV7 and the redistribution of S. pneumoniae serotypes causing pneumonia, we examined whether the net costs were robust to different assumptions concerning the causality of PCV7 and serotype replacement. In particular, we varied which groups of serotypes were assumed to be included in PCV7SR. Three additional scenarios were considered:
Serotype replacement was unrelated to PCV7.
Only those serotypes that were increasing following the introduction of the vaccine (and not before) were attributed to the vaccine, including epidemic serotypes (base case plus epidemic cases).
All serotype replacement was caused by PCV7, excluding epidemic serotypes.
Results
Changes in Annual Age-Specific IPD Incidence and Case Mortality
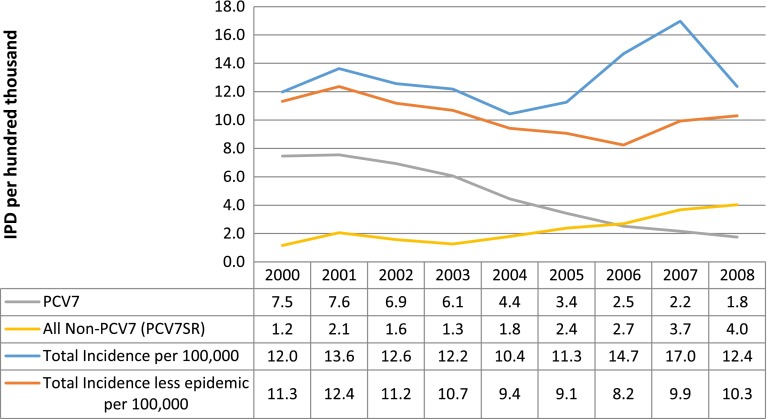
In Alberta, between 2000 and 2008, a total of 3822 IPD cases that were confirmed as positive for S. pneumoniae were identified. Of these, 1348 were caused by the seven serotypes targeted by PCV7 and 2464 were caused by other serotypes (with 503 of these being caused by serotype 5). Ten cases were non-typable and were therefore excluded from the analysis. As can be seen in Fig. 1, the total number of cases reported in 2000, before the vaccine was introduced, was 12.0 per 100,000 people. In 2008, the total IPD annual incidence was 12.4 per 100,000. When epidemic serotypes were excluded, the annual incidence rates in 2000 and 2008 were 11.3 and 10.3 per 100,000, respectively.
Fig. 1.
Invasive pneumococcal disease (IPD) incidence per 100,000 people. PCV7 7-valent pneumococcal conjugate vaccine, PCV7SR 7-valent pneumococcal conjugate vaccine serotype replacement
When serotypes were assessed to identify those that met the study criteria for replacement serotypes, the following serotypes were found to increase in frequency after vaccine introduction but not before: 10A, 11A, 15A, 19A, 23A, 20, 34, 23B, 12F, 15B, 5, 38, and 22F. Figure 1 also depicts the change in the annual IPD incidence by PCV7 serotype and serotype replacement over the 9-year period. These data show an inverse relationship between the incidence of PCV7 and that of PCV7SR; as PCV7 incidence consistently declined over the period, PCV7SR incidence consistently increased. The total annual IPD incidence caused by the seven serotypes contained in PCV7 in 2000 was 7.5 per 100,000, dropping to 1.8 per 100,000 in 2008. However, the total annual incidence of PCV7SR increased from 1.2 to 4.0 per 100,000 over the same period. These findings for PCV7 are consistent with those published elsewhere [3, 6, 7, 11].
Of particular importance is how these changes in IPD incidence were distributed across categories of age, given that the young and old are most susceptible to severe disease outcomes. The distribution of cases caused by PCV7 before and after vaccine introduction indicated direct and indirect protection that may have resulted from PCV7. Evidence of direct protection can be seen in the percentage reduction in the total IPD annual incidence caused by PCV7 serotypes between 2000 and 2008, which was 98 % in infants (Table 2). Other age groups received indirect benefits as a result of infants being immunized, as the percentage reduction in cases caused by PCV7 ranged from 53 to 100 % in age groups of 10 years and over. Every age category showed an increase in PCV7SR, except for the 5- to 19-year age groups. The largest increases were among children aged 2–4 years (527 %), followed by the 20- to 64-year age group (322 %) and seniors (168 %). Overall, PCV7SR serotypes increased between 2000 and 2008 by 246 %. As a result, on net, there was little change in the number of IPD cases caused by all serotypes over the period.
Table 2.
Percentage change in age-specific invasive pneumococcal disease (IPD) incidence (2000 versus 2008)
| PCV7 (%) | PCV7SR (%) | All (%) | All less epidemic (%) | ||
|---|---|---|---|---|---|
| Including PCV7 and epidemic | Excluding PCV7 | ||||
| Age group | |||||
| <2 years | −98 | 119 | −70 | 154 | −71 |
| 2–4 years | −86 | 527 | −57 | 198 | −57 |
| 5–9 years | −42 | 0 | 1 | 154 | 30 |
| 10–14 years | −100 | 0 | 0 | 201 | −33 |
| 15–19 years | −70 | 0 | −55 | −10 | −55 |
| 20–64 years | −53 | 322 | 48 | 134 | 29 |
| 65+ years | −83 | 168 | −7 | 98 | −12 |
| Total | −76 | 246 | 3 | 134 | −9 |
PCV7 7-valent pneumococcal conjugate vaccine, PCV7SR PCV7-related serotype replacement
Along with the overall decline in the incidence of pneumococcal disease following the introduction of PCV7, an average of 4.3 lives were saved annually as a result of protection against serotypes included in PCV7 (Table 3), and 2.5 lives annually were saved once PCV7SR deaths were taken into account. This amounts to 15 lives saved over the 6 years post-vaccine introduction.
Table 3.
Average annual 7-valent pneumococcal conjugate vaccine (PCV7) and PCV7-related serotype replacement (PCV7SR) cases and mortality pre- and post-introduction of the vaccination program in Alberta
| PCV7 cases (N) | PCV7SR cases (N) | Net annual difference in cases (N) | |||||
|---|---|---|---|---|---|---|---|
| Average pre-introduction (2000–2001) | Average post-introduction (2003–2008) | Annual difference post- versus pre-introduction | Average pre-introduction (2000–2001) | Average post-introduction (2003–2008) | Annual difference post- versus pre-introduction | ||
| Hospitalized pneumonia | 177.1 | 79 | 97.9 | 23 | 136 | −113.3 | −15.4 |
| Bacteremia | 40 | 20 | 20.3 | 2 | 3 | −0.9 | 19.3 |
| Meningitis | 4 | 1 | 2.3 | 0 | 0 | −0.1 | 2.2 |
| Non-hospitalized bacteria not getting worse | 14 | 5 | 8.3 | 1 | 1 | −0.4 | 8.0 |
| Non-hospitalized bacteria getting worse (10 %) | 1 | 1 | 0.9 | 0 | 0 | 0.0 | 0.8 |
| Mortality | 9.7 | 5.4 | 4.3 | 4.2 | 6.0 | −1.8 | 2.5 |
Health Ministry Net Costs Resulting from the PCV7 Child Immunization Program
Costs Averted from 2003 to 2008 (Prevnar 7)
The direct health service costs saved by the Health Ministry as a result of the observed declines in PCV7 amounted to $604,000 per year in Alberta (Table 4). The direct health service costs incurred as a result of associated PCV7SR were over $317,000 per year in Alberta. Cost savings were greatest in the vaccinated group (<2-year-olds), with approximately $260,000 in savings annually as a result of direct PCV7 protection, and $250,000 on net ($1.5 million over 6 years). The next most significant savings were in the elderly, with indirect protection resulting in $262,000 in costs averted and $220,000 saved on net annually, or $1.3 million over the 6 years post-vaccination.
Table 4.
Net health service costs of the 7-valent pneumococcal conjugate vaccine (PCV7) vaccination program in Alberta
| PCV7 cost saving post-vaccine ($)a | PCV7SR cost incurred post-vaccine ($)a | Net health service cost post-vaccine ($)a | |
|---|---|---|---|
| Age category | |||
| <2 years | 262,893 | (8914) | 253,980 |
| 2–4 years | 89,861 | (3387) | 86,474 |
| 5–9 years | 5065 | (125) | 49,405 |
| 10–14 years | (1008) | (3626) | (4634) |
| 15–19 years | 15,087 | (4328) | 10,758 |
| 20–64 years | 286,604 | (253,656) | 32,948 |
| 65+ years | 262,768 | (43,175) | 219,592 |
| Annual total | 921,271 | (317,212) | 604,059 |
| 6 years post-introduction total | 5,527,629 | (1,903,273) | 3,624,356 |
Italicized values in parentheses are costs incurred
PCV7SR PCV7-related serotype replacement
aCosts are expressed in 2008 Canadian dollars
Costs Incurred from 2003 to 2008 (Serotype Replacement)
In terms of costs incurred as a result of PCV7SR, the incidence rates and related costs were greatest in the adult age categories. Specifically, those aged 20–64 experienced indirect protection, resulting in $287,000 in costs averted, with $254,000 in costs incurred as a result of PCV7SR. As a result, $33,000 was saved on net annually, or $198,000 over the 6 years post-vaccination.
Total Net Costs Associated with PCV7 Child Immunization Program from 2003 to 2008
Altogether, the direct health costs averted from reducing PCV7 strains amounted to $5.5 million. However, the costs incurred as a result of PCV7SR were nearly $1.9 million. As a result, on net, the total cost savings for Alberta amounted to about $3.6 million.
The reductions in disease incidence and related costs due to indirect effects were a proportion of the net costs calculated above. There was evidence of over $563,000 in costs averted annually as a result of PCV7 across those aged 10 + years in Alberta. However, the costs incurred because of related PCV7SR strains amounted to $305,000. As a result, the net savings as a result of indirect effects were $259,000 annually, or roughly $1.5 million over the 6 years post-vaccination.
Scenario Analysis Results
In assessing the robustness of the estimated direct health service cost impact of PCV7 in Alberta, we considered three alternative scenarios to describe the relationship between PCV7 and serotype replacement. As can be seen in Table 5, there was variation in the total estimated net costs as a result of PCV7, depending upon the assumed serotype replacement. If serotype replacement was unrelated to the vaccine, then the cost savings resulting from PCV7 amounted to $921,000 annually ($5.5 million saved over 6 years). If only those serotypes that were found to have increased following the introduction of PCV7 were considered, including epidemic serotypes, the net annual costs incurred were $188,000 ($1.1 million incurred over 6 years). If the costs associated with all non-PCV7 serotypes were taken into account, excluding epidemic serotypes, the direct medical costs averted were $345,000 annually ($2.0 million over 6 years).
Table 5.
Sensitivity analysis of net health service costs averted (or incurred) annually and over 6 years post-introduction of the vaccination program in Alberta
| Base case: PCV7 related to all increasing non-PCV7 serotypes (excluding epidemic) ($)a | Scenario 1: serotype replacement unrelated to PCV7 ($)a | Scenario 2: PCV7 related to all increasing non-PCV7 serotypes (including epidemic) ($)a | Scenario 3: all non-PCV7 serotypes (excluding epidemic) ($)a | |
|---|---|---|---|---|
| Age group | ||||
| <2 years | 253,980 | 262,893 | 238,978 | 253,205 |
| 2–4 years | 86,474 | 89,861 | 79,674 | 93,151 |
| 5–9 years | 4940 | 5065 | 12,022 | (3170) |
| 10–14 years | (4634) | (1008) | (7063) | (11,981) |
| 15–19 years | 10,758 | 15,087 | 3558 | 11,552 |
| 20–64 years | 32,948 | 286,604 | (700,835) | (161,762) |
| 65+ years | 219,592 | 262,768 | 186,035 | 163,856 |
| Annual total | 604,059 | 921,271 | (187,632) | 344,851 |
| 6 years post-introduction total | 3,624,356 | 5,527,629 | (1,125,792) | 2,069,108 |
Italicized values in parentheses are costs incurred
PCV7 7-valent pneumococcal conjugate vaccine
aCosts are expressed in 2008 Canadian dollars
Discussion
We conducted a retrospective analysis of the cost impact on the health system from introducing PCV7 into Alberta’s childhood vaccination strategy, in the context of serotype replacement. In this analysis, we found that the total number of IPD cases changed little over the period studied: the cases reported in 2000 versus 2008 were 12.0 versus 12.4 per 100,000. When epidemic serotypes were excluded, the annual incidence rates were 11.3 and 10.3 per 100,000, respectively. Meanwhile, PCV7 serotypes decreased by 76 % from 7.5 to 1.8 per 100,000, and PCV7SR increased by 246 % from 1.2 to 4.0 per 100,000.
The net direct medical costs attributed to vaccine implementation are sensitive to assumptions related to serotype replacement. It is not yet known which serotypes increased as a result of the vaccine as compared with other reasons such as antibiotic use [20–23]. After identifying those serotypes most likely to have increased as a result of vaccine introduction, we found that there were still positive economic benefits associated with the vaccine. In total, we found that there was a net health service cost saving due to vaccination of roughly $5.5 million for a population of 3.6 million over the 6 years post-program implementation if serotype replacement was unrelated to the introduction of PCV7. If all increasing non-PCV7 serotypes were causally related to PCV7 (excluding epidemic serotypes 1, 2, 3, and 5), then costs of $3.6 million were averted following vaccine introduction. If the costs associated with all non-PCV7 serotypes were taken into account, including epidemic serotypes, then $1.1 million in direct medical costs were incurred. However, if the costs associated with all non-PCV7 serotypes, excluding epidemic serotypes, were considered, then the direct medical costs averted were $2.0 million over 6 years.
Our base case excluded epidemic serotypes because cases found to traditionally follow a time trend characterized by outbreaks that occur at predictable intervals cannot be confidently attributed to the introduction of PCV7. Of the epidemic serotypes, only serotype 5 was problematic in Alberta over the time period studied. Most of the cases were experienced with homeless populations [24] and accounted for more than $4.5 million in costs incurred as a result of all non-PCV7 serotypes. Serotype 19A was found to have increased substantially following the introduction of PCV7 and accounted for more than half of the serotype replacement costs estimated in this analysis (nearly $1 million in costs incurred). A new vaccine, Prevnar 13 (PCV13), has been developed to target the original seven serotypes, as well as serotypes 1, 3, 5, 6A, 7F, and 19A. We expect that the high costs resulting from serotypes 5 and 19A will have been reduced following the introduction of PCV13 in Alberta in 2010.
Only changes in health service costs have been considered in this analysis; the net medical cost savings, however, have not been considered in the context of total program costs. The estimated program cost of a three-dose set of Prevnar 7 in Alberta is nearly $10 million; a three-dose set costs $284 [5] and is administered to 33,200 newborns annually (given there are 40,000 newborns annually in the province, with a coverage rate of 83 % [13]). The program costs exceed the medical service costs averted as a result of the immunization program. However, this is not an unexpected finding, as previous cost-effectiveness studies predicted that PCV7 would be associated with additional costs for improved health outcomes in terms of life-years or quality-adjusted life-years gained [4, 19, 25]. While none of those models took into account serotype replacement, our analysis suggests that the inclusion of serotype replacement would not have changed their overall conclusions because the impact of serotype replacement are small relative to the overall program costs.
This study should be examined in light of the following limitations. First, the study provided an estimate of the cost impact on the health system when accounting for serotype replacement and was not an economic evaluation of the value for money associated with PCV7, which would include a broader assessment of costs and benefits, including a valuation of the health impact (i.e., health-related quality of life). Second, the time horizon reflected in the available data included 2 years pre-vaccine and 6 years post-vaccine introduction. It is uncertain whether this time horizon was sufficient for observing the full change in disease frequency associated with serotype replacement. However, additional data prior to the year 2000 were unavailable. Third, the majority of the reduction in costs was experienced by infants and seniors. Seniors are recommended to receive a 23-valent polysaccharide vaccine (PPV23), and it is unknown how the administration of PPV23 influenced our results in the senior population; there are no vaccine registries in Alberta. Nevertheless, PPV23 was available prior to the introduction of PCV7 in children, and we observed a clear temporal association between the introduction of PCV7 and the decrease in the incidence of S. pneumoniae in seniors. Fourth, noninvasive pneumococcal disease (NIPD) was not included in this study, because of the lack of incidence data on cases of NIPD.
Conclusion
This study highlighted the importance of collecting serotype-specific data for the purpose of program evaluation. Specifically, the distribution of serotypes in Alberta influenced the health gains resulting from the PCV7 immunization program. The direct health costs averted from reducing PCV7 strains amounted to $5.5 million. However, the unintended effects of serotype replacement resulted in costs incurred of $1.9 million. As a result, on net, the total cost savings for Alberta amounted to about $3.6 million. Irrespective of serotype replacement, the PCV7 immunization program has had a positive impact in terms of health benefits, which translates into health service costs averted.
Acknowledgments
The authors would like to acknowledge Drs. Goodman, Tan, and Ohinmaa, as well as Emily Hastings, for their insight and careful revision of versions of this manuscript. The study was funded through an unrestricted Grant from Pfizer Canada Inc.
Conflict of interest
GJT has received funding for pneumococcal research from Pfizer and Merck-Canada.
Footnotes
Canadian case definition of IPD: isolation of a positive culture of S. pneumoniae from normally sterile body fluid, primarily blood or cerebrospinal fluid [12].
It has been documented that serotype 5 caused large outbreaks in people aged 16–64 years in Alberta between 2006 and 2008, and cases were reported as being largely among Alberta’s homeless populations [3, 14, 24]. Kellner et al. [3, 6] reported that the relationship between serotype 5 and the incidence of the infection is not causally associated with PCV7.
Direct health service costs include the cost of hospitalization and outpatient costs. Costs of health care vary across age and disease presentations. The direct costs accounted for in this analysis include health service costs resulting from hospitalization for IPD outpatient care, as well as any subsequent hospitalizations. These costs of treatment and sequelae for survivors of IPD are taken from Morrow et al. [2].
References
- 1.World Health Organization 23-Valent pneumococcal polysaccharide vaccine: WHO position paper. Wkly Epidemiol Rec. 2008;83(42):100–119. [PubMed] [Google Scholar]
- 2.Morrow A, De Wals P, Petit G, Guay M, Erickson LJ. The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine. Can J Infect Dis Med Microbiol. 2007;18(2):121–127. doi: 10.1155/2007/713576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele D. Changing epidemiology of invasive pneumococcal disease in Canada 1998 to 2007: update from the Calgary Area Streptococcus pneumoniae Research (CASPER) study. Can J Infect Dis. 2009;49:205–212. doi: 10.1086/599827. [DOI] [PubMed] [Google Scholar]
- 4.Chuck A, Jacobs P, Nguyen T, Hanrahan A, Loewen J, Mashinter L, et al. Economic analysis of a public program for routine seven valent pneumococcal conjugate vaccine (PCV-7) in infancy, Alberta. Can Commun Dis Rep. 2008;34(10):1–13. [Google Scholar]
- 5.Chuck AW, Jacobs P, Tyrrell G, Kellner JD. Pharmacoeconomic evaluation of 10- and 13-valent pnumococcal conjugate vaccines. Vaccine. 2010;28:5484–5490. doi: 10.1016/j.vaccine.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 6.Kellner J. Update on the success of the pneumococcal conjugate vaccine. Pediatric Child Health. 2011;16(4):233–236. doi: 10.1093/pch/16.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 8.Advisory Committee on Immunization Practices Update on pediatric invasive pneumococcal disease and recommended use of conjugate pneumococcal vaccines. Can Commun Dis Rep. 2010;36:1–30. [Google Scholar]
- 9.Feikin D, Klugman K. Historical changes in pneumococcal serogroup distribution: implications for the era of pneumococcal conjugate vaccines. Clin Infect Dis. 2002;35(5):547–555. doi: 10.1086/341896. [DOI] [PubMed] [Google Scholar]
- 10.Harboe Z, Benfield T, Valentiner-Branth P, Hjuler T, Lambertsen L, Kaltoft M, et al. Temporal trends in invasive pneumococcal disease and pneumococcal serotypes over 7 decades. Clin Infect Dis. 2010;50(3):329–337. doi: 10.1086/649872. [DOI] [PubMed] [Google Scholar]
- 11.Tyrrell GJ, Lovgrena M, Chuia N, Minion J, Garge S, Kellner JD, et al. Serotypes and antimicrobial susceptibilities of invasive Streptococcus pneumoniae pre- and post-seven valent pneumococcal conjugate vaccine introduction in Alberta, Canada, 2000–2006. Vaccine. 2009;27:3553–3560. doi: 10.1016/j.vaccine.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 12.Health Canada. Case definitions for diseases under national surveillance. Can Commun Dis Rep. 2000;26-S3:51. [PubMed]
- 13.Statistics Canada. Table 051-0001: estimates of population, by age group and sex for July 1, Canada, provinces and territories, annual (persons unless otherwise noted). 2013. http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=0510001&paSer=&pattern=&stByVal=1&p1=1&p2=37&tabMode=dataTable&csid=. Accessed 27 April 2015.
- 14.Tyrrell G, Lovgren M, Ibrahim Q, Garg S, Chui L, Boone T, et al. Epidemic of invasive pneumococcal disease, Western Canada, 2005–2009. Emerg Infect Dis. 2012;18(5):733–740. doi: 10.3201/eid1805.110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lexau C, Lynfield R, Danila R, Pilishvili T, Fackman R, Farley M, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2011;294(16):2043–2051. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 16.Weinberger D, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–1973. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelton S. Replacement pneumococcal disease in perspective. Clin Infect Dis. 2008;46:1353–1355. doi: 10.1086/586748. [DOI] [PubMed] [Google Scholar]
- 18.Fassbender K, Fainsinger R, Carson M, Finegan B. Cost trajectories at the end of life: the Canadian experience. J Pain Symptom Manag. 2009;38(1):75–80. doi: 10.1016/j.jpainsymman.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Lebel MH, Kellner JD, Ford-Jones L, Hvidsten K, Wang EC, Ciuryla V, et al. A pharmacoeconomic evaluation of 7-valent pneumococcal conjugate vaccine in Canada. Can J Infect Dis Med Microbiol. 2003;36(1):259–268. doi: 10.1086/345833. [DOI] [PubMed] [Google Scholar]
- 20.Mulholland K, Satzke C. Serotype replacement after pneumococcal vaccination. Lancet. 2012;379(9824):1387. doi: 10.1016/S0140-6736(12)60588-1. [DOI] [PubMed] [Google Scholar]
- 21.DiNubile M. Serotype replacement after vaccination. Lancet. 2012;379(9824):1388. doi: 10.1016/S0140-6736(12)60590-X. [DOI] [PubMed] [Google Scholar]
- 22.Weinberger D, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378(9807):1962–1973. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hausdorff W, Van Dyke M, Effelterre T. Serotype replacement after pneumococcal vaccination. Lancet. 2012;379(9824):1387–1388. doi: 10.1016/S0140-6736(12)60589-3. [DOI] [PubMed] [Google Scholar]
- 24.Vanderkooi O, Church D, MacDonald J, Zucol F, Kellner J. Community based outbreaks in vulnerable populations of invasive infections caused by Streptococcus pnuemoniae serotypes 5 and 8 in Calgary, Canada. PLoS One. 2011;6(12):e28547. doi: 10.1371/journal.pone.0028547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntosh D, Convay P, Willingham J, Llyod A. The cost-burden of paediatric pneumococcal disease in the UK and the potential cost-effectiveness of prevention using 7-valent pneumococcal conjugate vaccine. Vaccine. 2003;21(19):2564–2572. doi: 10.1016/S0264-410X(03)00031-8. [DOI] [PubMed] [Google Scholar]