Abstract
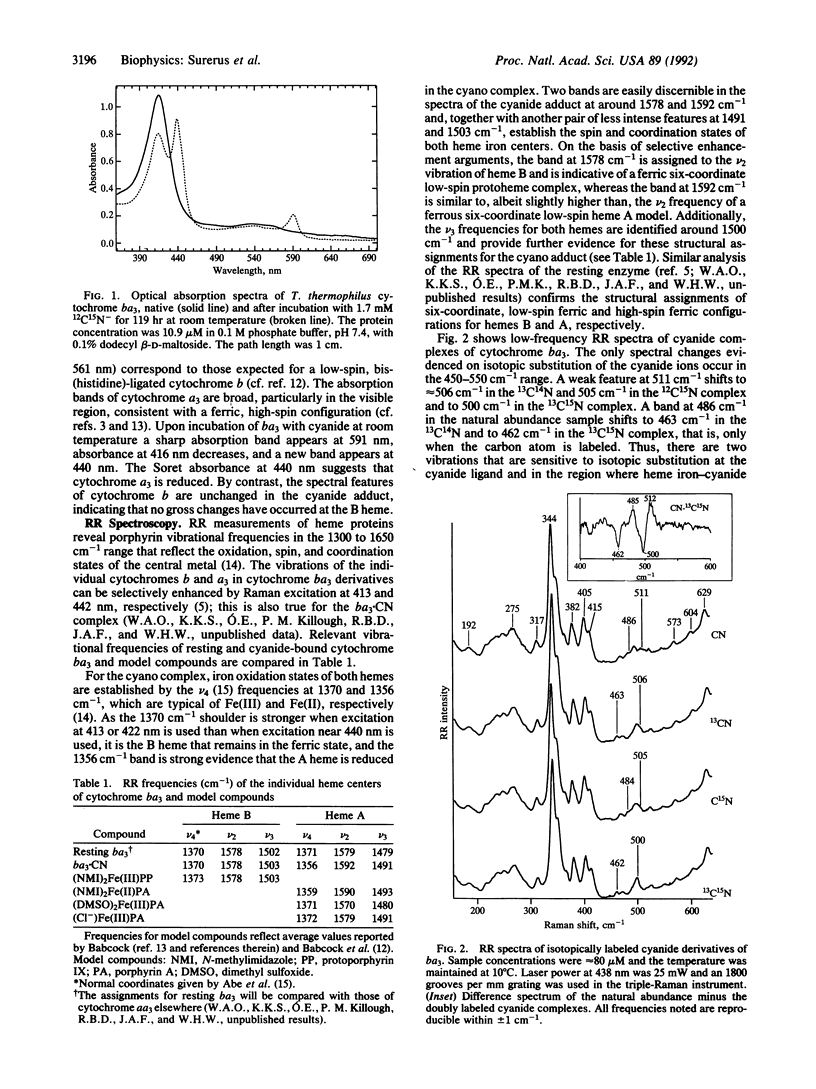
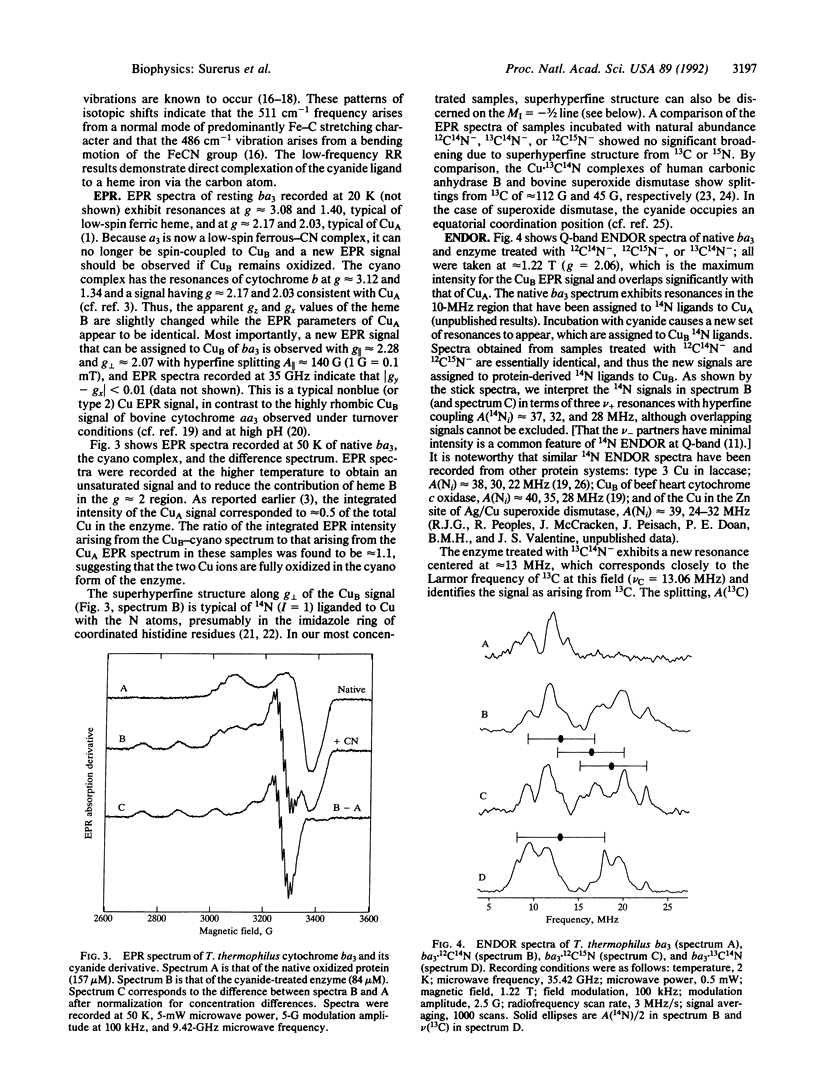
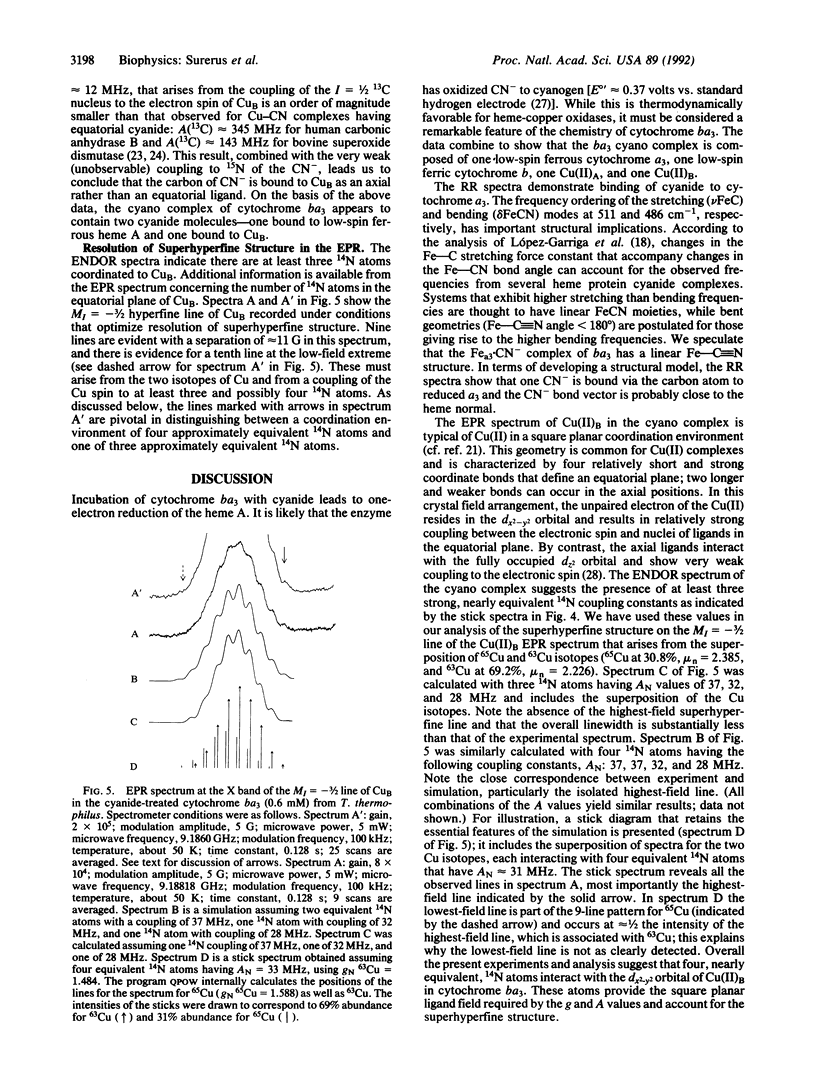
Cytochrome ba3 from Thermus thermophilus reacts slowly with excess HCN at pH 7.4 to create a form of the enzyme in which CuA, cytochrome b, and CuB remain oxidized, while cytochrome a3 is reduced by one electron, presumably with the formation of cyanogen. We have examined this form of the enzyme by UV-visible, resonance Raman, EPR, and electron nuclear double resonance spectroscopies in conjunction with permutations of 13C- and 15N-labeled cyanide. The results support a model in which one CN- binds through the carbon atom to ferrous a3, supporting a low-spin (S = 0) configuration on the Fe; bridging by this cyanide to the CuB is weak or absent. Four 14N atoms, presumably donated by histidine residues of the protein, provide a strong equatorial ligand field about CuB; a second CN- is coordinated through the carbon atom to CuB in an axial position.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Widger W. R., Cramer W. A., Oertling W. A., Metz J. G. Axial ligands of chloroplast cytochrome b-559: identification and requirement for a heme-cross-linked polypeptide structure. Biochemistry. 1985 Jul 2;24(14):3638–3645. doi: 10.1021/bi00335a036. [DOI] [PubMed] [Google Scholar]
- Bott M., Bolliger M., Hennecke H. Genetic analysis of the cytochrome c-aa3 branch of the Bradyrhizobium japonicum respiratory chain. Mol Microbiol. 1990 Dec;4(12):2147–2157. doi: 10.1111/j.1365-2958.1990.tb00576.x. [DOI] [PubMed] [Google Scholar]
- Carithers R. P., Palmer G. Characterization of the potentiometric behavior of soluble cytochrome oxidase by magnetic circular dichroism. Evidence in support of heme-heme interaction. J Biol Chem. 1981 Aug 10;256(15):7967–7976. [PubMed] [Google Scholar]
- Chan S. I., Li P. M. Cytochrome c oxidase: understanding nature's design of a proton pump. Biochemistry. 1990 Jan 9;29(1):1–12. doi: 10.1021/bi00453a001. [DOI] [PubMed] [Google Scholar]
- Cline J., Reinhammar B., Jensen P., Venters R., Hoffman B. M. Coordination environment for the type 3 copper center of tree laccase and CuB of cytochrome c oxidase as determined by electron nuclear double resonance. J Biol Chem. 1983 Apr 25;258(8):5124–5128. [PubMed] [Google Scholar]
- Einarsdóttir O., Killough P. M., Fee J. A., Woodruff W. H. An infrared study of the binding and photodissociation of carbon monoxide in cytochrome ba3 from Thermus thermophilus. J Biol Chem. 1989 Feb 15;264(5):2405–2408. [PubMed] [Google Scholar]
- Haffner P. H., Coleman J. E. Cu (II)-carbon bonding in cyanide complexes of copper enzymes. 13C splitting of the Cu(II) electron spin resonance. J Biol Chem. 1973 Oct 10;248(19):6626–6629. [PubMed] [Google Scholar]
- Han S. H., Madden J. F., Siegel L. M., Spiro T. G. Resonance Raman studies of Escherichia coli sulfite reductase hemoprotein. 3. Bound ligand vibrational modes. Biochemistry. 1989 Jun 27;28(13):5477–5485. doi: 10.1021/bi00439a024. [DOI] [PubMed] [Google Scholar]
- Kent T. A., Münck E., Dunham W. R., Filter W. F., Findling K. L., Yoshida T., Fee J. A. Mössbauer study of a bacterial cytochrome oxidase: cytochrome c1aa3 from Thermus thermophilus. J Biol Chem. 1982 Nov 10;257(21):12489–12492. [PubMed] [Google Scholar]
- Lieberman R. A., Sands R. H., Fee J. A. A study of the electron paramagnetic resonance properties of single monoclinic crystals of bovine superoxide dismutase. J Biol Chem. 1982 Jan 10;257(1):336–344. [PubMed] [Google Scholar]
- López-Garriga J. J., Oertling W. A., Kean R. T., Hoogland H., Wever R., Babcock G. T. Metal-ligand vibrations of cyanoferric myeloperoxidase and cyanoferric horseradish peroxidase: evidence for a constrained heme pocket in myeloperoxidase. Biochemistry. 1990 Oct 9;29(40):9387–9395. doi: 10.1021/bi00492a012. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Morpurgo L., Giovagnoli C., Calabrese L., Mondovì B. Studies of the metal sites of copper proteins. Symmetry of copper in bovine superoxide dismutase and its functional significance. Biochemistry. 1972 May 23;11(11):2187–2192. doi: 10.1021/bi00761a028. [DOI] [PubMed] [Google Scholar]
- Saraste M. Structural features of cytochrome oxidase. Q Rev Biophys. 1990 Nov;23(4):331–366. doi: 10.1017/s0033583500005588. [DOI] [PubMed] [Google Scholar]
- Thomson A. J., Johnson M. K., Greenwood C., Gooding P. E. A study of the magnetic properties of haem a3 in cytochrome c oxidase by using magnetic-circular-dichroism spectroscopy. Biochem J. 1981 Mar 1;193(3):687–697. doi: 10.1042/bj1930687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S., Caughey W. S. Infrared evidence of cyanide binding to iron and copper sites in bovine heart cytochrome c oxidase. Implications regarding oxygen reduction. J Biol Chem. 1990 May 15;265(14):7945–7958. [PubMed] [Google Scholar]
- Yu N. T., Benko B., Kerr E. A., Gersonde K. Iron-carbon bond lengths in carbonmonoxy and cyanomet complexes of the monomeric hemoglobin III from Chironomus thummi thummi: a critical comparison between resonance Raman and x-ray diffraction studies. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5106–5110. doi: 10.1073/pnas.81.16.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann B. H., Nitsche C. I., Fee J. A., Rusnak F., Münck E. Properties of a copper-containing cytochrome ba3: a second terminal oxidase from the extreme thermophile Thermus thermophilus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5779–5783. doi: 10.1073/pnas.85.16.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]



