Abstract
Introduction
This study examined the use of anti-tumor necrosis factor (anti-TNF) monotherapy, adherence with non-biologic disease-modifying anti-rheumatic drugs (nbDMARDs) in patients receiving a combination of anti-TNF therapies and nbDMARDs, and the impact of nbDMARD adherence on anti-TNF persistence among patients with rheumatoid arthritis (RA).
Methods
Patients with RA (aged ≥18 years) from a US commercial health plan with claims for anti-TNFs (2006–2010) were defined as either biologic-naive or -exposed anti-TNF initiators based on previous nbDMARD use. Adherence to nbDMARDs and anti-TNF persistence were estimated. Cox regression estimated the association between nbDMARD adherence and anti-TNF persistence.
Results
Among 9764 patients identified (mean age 50.2 years; 78% female), 55% of biologic-naive patients and 49% of previously exposed patients initiated any combination therapy during follow-up. Among biologic-naive combination therapy patients, 53% adhered to nbDMARD therapy <80% of the time while receiving anti-TNF therapies; 33% had <60% adherence. Compared with the most adherent patients, patients adherent to nbDMARDs 20% to 79% of the time were 30% to 20% more likely to discontinue their anti-TNF therapy in the period >90 days after starting the anti-TNF therapy. This relationship was not observed for patients with nbDMARD adherence of <20% (who were less likely to discontinue their anti-TNF therapy during the first 90 days of treatment).
Conclusion
Almost one-third of patients with RA receiving anti-TNF therapy received it as pure monotherapy. About one-third of combination therapy recipients had <60% adherence to nbDMARDs. Higher nbDMARD adherence may be associated with better anti-TNF persistence after an initial treatment period.
Electronic supplementary material
The online version of this article (doi:10.1007/s40744-015-0015-x) contains supplementary material, which is available to authorized users.
Keywords: Adherence, Anti-TNF therapy, Combination therapy, Discontinuation, DMARD, Persistence, Rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) affects upwards of an estimated 1.3 million persons in the United States over the age of 18 years, with a growing prevalence among those over the age of 60 [1, 2]. The clinical and economic burden of RA is significant [3], whether measured by the impact on quality of life, direct treatment costs, or indirect costs in the form of productivity loss [4, 5]. Successful treatment of RA can improve patients’ quality of life [5] and reduce costs associated with loss of productivity [6].
Guidelines provided by the American College of Rheumatology call for early, aggressive treatment of RA to slow the progression of the disease [7]. Typically, non-biologic disease-modifying anti-rheumatic drugs (nbDMARDs) are the first line of treatment. Biologics, including anti-tumor necrosis factor drugs (anti-TNFs), are often added to the nbDMARD for patients who do not experience improvement after nbDMARD treatment.
Studies have shown a better response to anti-TNFs when used in combination with nbDMARDs than when used as monotherapies [8, 9]. Adherence to nbDMARDs prescribed in combination with anti-TNFs may impact the benefit obtained with the use of these biologic therapies. The objectives of this study were to examine (1) the use of anti-TNF therapies as monotherapy, (2) adherence with concomitant nbDMARDs in patients receiving a combination of anti-TNFs and nbDMARDs, and (3) the impact of concomitant nbDMARD adherence on anti-TNF persistence in a real-world setting among commercially insured patients with RA.
Methods
Study Design
A retrospective claims-based analysis using data from a US managed care plan was conducted. Patients were followed from anti-TNF initiation to discontinuation of that specific anti-TNF or disenrollment from the managed care plan.
Data Sources
Patients were identified from a large US commercial health plan (Optum Research Database) that represents ~14 million members annually. The administrative claims data included medical claims, pharmacy claims, eligibility information, and linked mortality data from the Social Security Administration’s death master files. The individuals covered by this health plan are geographically diverse across the US, with greatest representation in the South and Midwest US census regions. Patients included in the study were insured in plans that provided coverage for professional (e.g., physician), facility (e.g., hospital), and outpatient prescription medication services. Medical (professional, facility) claims included International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes, procedures recorded with ICD-9-CM procedure codes, Current Procedural Terminology, or Healthcare Common Procedure Coding System codes, site of service codes, and revenue codes (for facilities). Outpatient pharmacy claims provided National Drug Codes for dispensed medications, quantity dispensed, drug strength, and days’ supply. This study was conducted in compliance with the Health Insurance Portability and Accountability Act Privacy Rule.
Patient Selection
Patients aged 18 years or older who were identified as having at least one medical claim for RA (ICD-9-CM diagnosis code of 714.xx) were included if they initiated anti-TNF medications during the patient identification period of January 1, 2006 through December 31, 2010. Patients initiating multiple biologics during the study period were eligible for more than one medication-related cohort, and the date of the first claim for each biologic therapy represented the index date for that therapy. Only those with continuous enrollment for 6 months prior to the index date and 12 months after were included. Anti-TNF medications included adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab, while nbDMARDs included auranofin, aurothioglucose, azathioprine, chloroquine, cyclophosphamide, d-penicillamine, gold sodium thiomalate, cyclosporine, hydroxychloroquine, leflunomide, methotrexate (MTX), and sulfasalazine. Patients with more than one biologic on a particular index date or with evidence of prior use of the index medication were excluded from analysis of that medication. Also excluded were patients with conditions other than RA that were labeled indications for the biologic medications.
Study Measures
Patients were classified into several defined groups based on their treatment patterns with nbDMARD and anti-TNF medications, resulting in 3 main classifications. The first designation was based on prior biologic exposure. ‘Biologic-exposed’ patients were those who had filled a different biologic prescription during the 6-month baseline period prior to the index date, whereas ‘biologic-naive’ patients were those who did not.
The second classification placed patients into either combination therapy or monotherapy groups. ‘Combination therapy initiators’ were those who had filled prescriptions or administrations for nbDMARDs within 30 days after their index date, or who had filled a prescription within 30 days prior to the index date with a medication supply that covered the period through at least 30 days after the index date. ‘Monotherapy initiators’ were patients who initiated anti-TNF therapy and who did not meet the definition for combination therapy initiators. Third, we assessed what proportion of patients stayed on monotherapy for the entire duration of their anti-TNF treatment. ‘Pure monotherapy’ referred to treatment with anti-TNF monotherapy that never included an nbDMARD during anti-TNF follow-up, whereas patients with nbDMARD therapy at any time during anti-TNF follow-up were referred to as the ‘any combination therapy’ group.
The primary study outcomes included adherence with nbDMARDs and persistence with anti-TNF medications. Adherence to nbDMARDs was estimated from the proportion of days covered. Specifically, it represents the percentage of days that patients received any nbDMARD while they were receiving the anti-TNF therapy. Perfect (100%) adherence indicates that patients possessed some nbDMARD 100% of the time during their anti-TNF follow-up based on filled prescriptions or administrations. In addition to adherence with any nbDMARDs, adherence to MTX was assessed separately. Anti-TNF persistence represented the time from the start of use until discontinuation of that anti-TNF therapy (a gap in supply of 60 days or a switch to a different biologic) or censoring due to disenrollment or the end of the study. For medications identified in pharmacy claims, the last date of supply was identified based on the days’ supply on all fills for that medication; for medications administered in a physician office or inpatient setting, the days’ supply was imputed for the last administration based on dosing intervals described on medication labels.
Patient characteristics included age, sex, and geographic location. Clinical characteristics were established during the 6-month baseline period before the index date.
Statistical Analysis
All study variables, including baseline and outcome measures, were analyzed descriptively. Numbers and percentages were calculated for dichotomous and polychotomous variables. Means, standard deviations, and percentiles were calculated for continuous variables. Descriptive analyses are presented separately for biologic-naive and previously exposed patients for each index anti-TNF. When comparing groups, the appropriate statistical test (e.g., t test, Mann–Whitney U test, χ 2 test) was used based on the distribution of the variable(s) of interest. Bivariate comparisons of baseline characteristics and outcome measures are presented. Multivariate Cox proportional hazards models were used to assess the association between nbDMARD adherence and anti-TNF persistence (i.e., time to discontinuation) for each patient’s first observed anti-TNF therapy, among patients who initiated their anti-TNF therapy as combination therapy with an nbDMARD. The Cox models included nbDMARD adherence as a time-dependent factor by measuring adherence at each instance of anti-TNF discontinuation and treating the non-discontinuing patients at that time point as controls. Adherence was modeled as an ordinal variable in 20% increments, and the reference group consisted of patients with >80% adherence. In addition, based on initial review of the results, the model included interaction terms for the nbDMARD adherence with the time from initiation of the anti-TNF therapy, as the initial descriptive results suggested that the effect of nbDMARD adherence on anti-TNF discontinuation varied by time. Time from anti-TNF initiation was dichotomized as ≤90 days and >90 days from initiation; the hazard ratios for the effect of nbDMARD adherence are presented separately for each of these time periods. These models were also adjusted for line of therapy, specific anti-TNF medication, age, sex, Charlson comorbidity score, and baseline use of injectable or oral corticosteroids. SAS version 9.2 (SAS Institute Inc, Cary, NC) was used for constructing the analytic data set and for statistical analyses.
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
Sample Selection and Baseline Characteristics
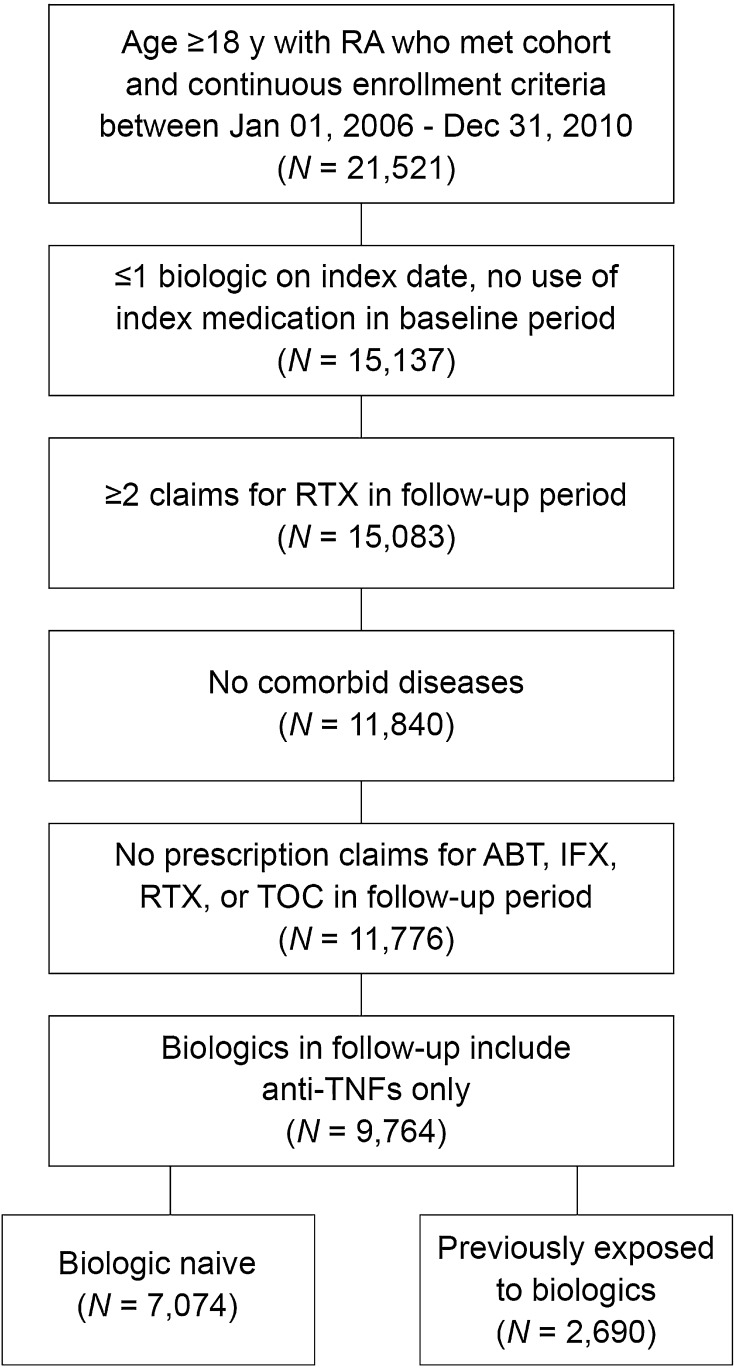
After applying all of the inclusion and exclusion criteria, the final study population included a total of 9764 patients, with 7074 patients classified as biologic naive and 2690 classified as previously biologic exposed (Fig. 1). The mean age of the study population was ~50 years and study patients were predominately female (Table 1). More than half of all patients were located geographically in the South. Corticosteroids were used by 62% of patients with first-line monotherapy and 70% of patients with first-line combination therapy, although the proportion of days covered was low (17% and 22% of days for monotherapy and combination therapy, respectively).
Fig. 1.
Patient selection. For each qualifying line subsequent to the first line, patients can appear multiple times in this data set (i.e., multiple records per patient). ABT abatacept, anti-TNFs anti-tumor necrosis factor drugs, IFX infliximab, RTX rituximab, TOC tocilizumab
Table 1.
Baseline demographics and clinical characteristics of the study population
| Total | Pure monotherapy | Any combination therapy | |
|---|---|---|---|
| Biologic-naive patients, n | 7074 | 1896 | 5178 |
| Length of follow-up, days (SD) | 471.4 (416.4) | 332.6 (362.4) | 522.3 (423.3) |
| Age, years (SD) | 50.2 (11.9) | 48.8 (13.0) | 50.8 (11.5) |
| Male, n (%) | 1610 (22.8) | 425 (22.4) | 1185 (22.9) |
| Baseline (6-month) non-biologic DMARD, n (%) | 5434 (76.8) | 804 (42.4) | 4630 (89.4) |
| Patients previously exposed to biologics,a n | 2690 | 844 | 1846 |
| Length of follow-up, days (SD) | 380.8 (348.1) | 282.6 (294.4) | 425.8 (361.3) |
| Age, years (SD) | 50.2 (11.5) | 48.6 (12.2) | 50.9 (11.1) |
| Male, n (%) | 506 (18.8) | 162 (19.2) | 344 (18.6) |
| Baseline (6-month) non-biologic DMARD, n (%) | 1.912 (71.1) | 269 (31.9) | 1643 (89.0) |
DMARD disease-modifying anti-rheumatic drug, SD standard deviation
aPatients with ≥1 anti-tumor necrosis factor in baseline period. Data are mean (SD) unless otherwise indicated
Outcomes
Monotherapy Initiators vs. Combination Therapy Initiators
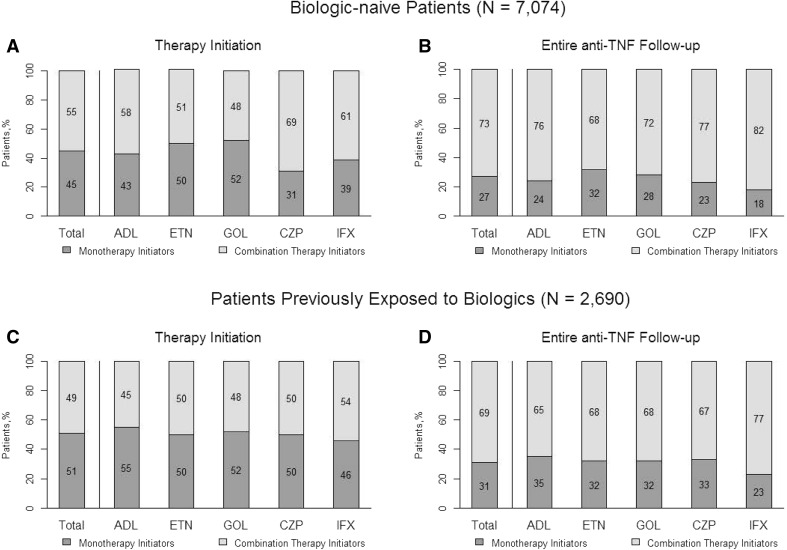
Among all patients initiating anti-TNFs, 45% of biologic-naive patients and 51% of biologic-exposed patients initiated an anti-TNF agent as a monotherapy; the remaining patients initiated anti-TNFs as part of combination therapy (Fig. 2). This was similar among the individual anti-TNF agents: 31% to 52% of biologic-naive patients and 46% to 55% of biologic-exposed patients were monotherapy initiators.
Fig. 2.
Distribution of therapy groups by anti-TNF therapy at a the time of anti-TNF initiation and b during anti-TNF follow-up in the biologic-naive group, and at c the time of anti-TNF initiation and d during anti-TNF follow-up in the biologic-experienced group. ADL adalimumab, anti-TNF anti-tumor necrosis factor, CZP certolizumab pegol, ETN etanercept, GOL golimumab, IFX infliximab
Pure Monotherapy vs. Any Combination Therapy Users
During anti-TNF follow-up, 27% of all patients in the biologic-naive group and 31% of all patients in the biologic-exposed group received anti-TNF therapy as pure monotherapy; the remaining patients received an nbDMARD during follow-up (Fig. 2). When stratified by the individual anti-TNF agents, the percentage of patients identified as pure monotherapy ranged from 18% to 32% for the biologic-naive group and 23% to 35% for those previously exposed to biologics. 42% of biologic-naive patients on pure monotherapy and 89% of biologic-naive patients on combination therapy were administered an nbDMARD during the 6-month baseline period prior to initiating anti-TNF medication (Table 1); for patients previously exposed to biologics, those values were 32% and 89%, respectively.
Adherence to nbDMARDs Among Any Combination Therapy Users
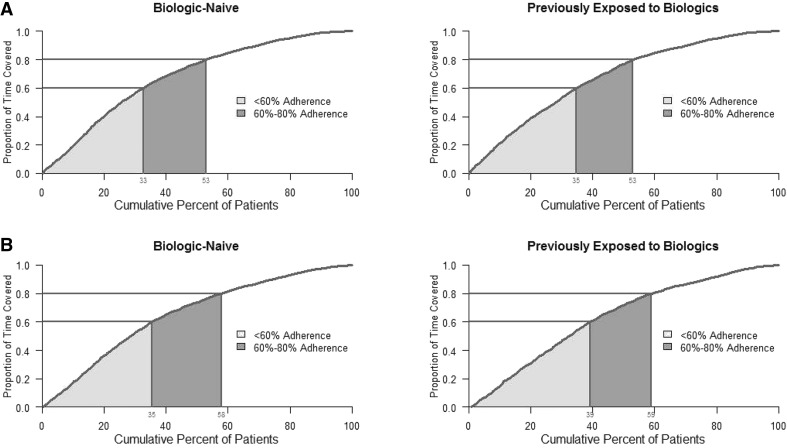
Among biologic-naive combination therapy patients, 53% of patients adhered to nbDMARD therapy <80% of the time while receiving anti-TNFs (proportion of days covered <80%); 33% of the patients had <60% adherence (Fig. 3a). Among biologic-naive patients who received anti-TNF combination therapy with MTX, 58% had <80% adherence to MTX and 35% had <60% adherence to MTX while receiving the anti-TNF (Fig. 3b). Similar results were observed for anti-TNF patients previously exposed to biologics (Fig. 3c, d).
Fig. 3.
a Non-biologic disease-modifying anti-rheumatic drug and b methotrexate adherence in patients receiving any combination
Association Between Concomitant nbDMARD Adherence and Anti-TNF Persistence
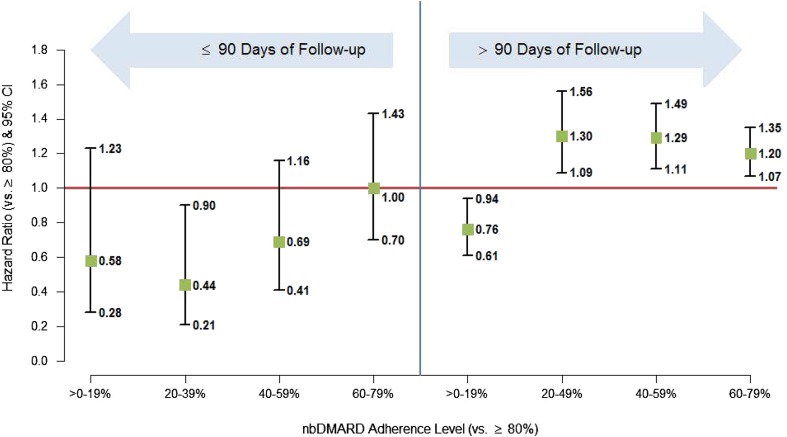
Average persistence with anti-TNF treatment was lower for patients receiving pure monotherapy (333 days for biologic-naive and 283 days for previously exposed patients) than for combination therapy users (522 days for biologic-naive and 426 days for previously exposed patients). Overall, we did not find a significant association between adherence to concomitant nbDMARDs and anti-TNF persistence. It is known that while some biologic users may not respond right from the outset (primary non-response), a proportion of patients may fail to maintain their initial response (secondary non-response). We therefore examined the association within subgroups stratified by duration of follow-up (≤90 days and >90 days) to determine whether nbDMARD adherence would have an impact within those time frames on primary or secondary non-response, respectively (Fig. 4). Within the first 90 days there was no statistically significant difference in anti-TNF persistence for patients with different levels of nbDMARD adherence, with the exception of patients with adherence of 20% to 39%, who were significantly less likely to discontinue than patients with adherence >80% (hazard ratio, 0.437; P < 0.0001; 95% confidence interval, 0.212, 0.901). However, after the first 90 days, the results suggest that for patients taking nbDMARDs at least 20% of the time, there may be a dose–response effect. Compared with the patients most adherent to their nbDMARDs (>80%), the patients with 20% to 39% adherence to their nbDMARD were 1.3 times more likely to discontinue their anti-TNF, whereas patients with 40% to 59% and 60% to 79% nbDMARD adherence were 1.29 and 1.20 times more likely to discontinue their anti-TNF, respectively.
Fig. 4.
Adjusted hazard ratios for discontinuation of index anti-TNF medication at varying levels of nbDMARD adherence during anti-TNF treatment period. Anti-TNF anti-tumor necrosis factor, CI confidence interval, nbDMARD non-biologic anti-rheumatic disease-modifying drug
Discussion
Previous research has demonstrated enhanced efficacy of the anti-TNF therapies infliximab, etanercept, adalimumab, and golimumab when used in combination with MTX, compared with using these therapies as monotherapy [10–15]. We examined the prevalence of anti-TNF monotherapy in a real-world setting and found that about one-third of patients with RA receiving anti-TNF therapy received it as pure monotherapy, which is consistent with previously published data [16, 17]. Furthermore, even among patients in our study cohort receiving combination therapy (anti-TNF + nbDMARD), one-third had an nbDMARD adherence rate of <60%, suggesting that a substantial proportion of this patient population might not be receiving the full benefit offered by using the anti-TNF medications with concomitant nbDMARDs. Previous estimates of DMARD adherence vary substantially across studies. In a recent reviews of the literature [18, 19], the authors found reported adherence rates that ranged from 22% to 107%, although there were a variety of study methods and definitions employed in the studies reviewed. Additionally, adherence can change over time, as some patients are completely non-adherent while others may have periods of adherence. A study from 1999 found nearly one-quarter of patients with RA consistently non-adherent [20], whereas in a more recent study that number was just over 10% [21]. Despite these difficulties, a recent prospective study that utilized electronic monitoring of medications (a method thought to provide some of the most accurate estimates of adherence) reported that 21% of patients with RA had an average adherence to DMARDs of ≥80% [22], which is noticeably lower than the 41% to 47% of patients found in our study.
The reasons for non-adherence with nbDMARDs may be varied. For MTX, non-adherence has been shown to be associated with longer disease duration and low or moderate disease activity [23]. Furthermore, while serious side effects of MTX are rare [24], patient concerns regarding potential adverse effects of RA medication may influence their adherence [25]. A full understanding of the factors associated with adherence in patients with RA continues to be elusive [18]; however, our data do not allow for further investigation as to the reasons for non-adherence. One possibility is that patients use corticosteroids as a replacement for combination therapy or to compensate for low adherence. However, in our study, the proportion of time that patients used corticosteroids was low (17–22% of days), suggesting that corticosteroids may have been used for flare-ups or to treat other conditions.
Persistence is measured as the time from initiation of therapy to discontinuation of therapy, which could be a proxy for clinical effectiveness, tolerability, and patient/provider preferences. Several studies have demonstrated better persistence of anti-TNFs when combined with nbDMARDs [8, 16, 17, 26–29], but the impact of adherence with concomitant nbDMARDs on persistence with the anti-TNF therapy has not been studied to our knowledge. In our study, there was a significant association between nbDMARD adherence and anti-TNF discontinuation in the period after the first 90 days. During that time period, compared with the most adherent patients (>80%), anti-TNF persistence worsened as nbDMARD adherence decreased. It is likely that the lack of an association in the first 90 days after starting the anti-TNF reflected discontinuations due to primary non-response to the anti-TNF therapy, which is typically considered to be in the first 12 weeks [30]. One hypothesis is that after the first 90 days, the difference in persistence may be linked to differences in the maintenance of response achieved by those on combination therapy. MTX co-administration may increase the bioavailability of anti-TNF therapies and reduce the development of anti-drug antibodies, thus prolonging the therapeutic effect of the anti-TNF [31]. Those with concomitant therapy with an nbDMARD typically have lower rates of discontinuation due to adverse events [8, 26, 28]. One hypothesis offered to explain this is that nbDMARDs (MTX specifically) may help reduce or prevent human anti-chimeric antibodies known to be induced by anti-TNFs [8, 26, 32]. This has not been formally tested, and the literature posing this hypothesis acknowledges possible confounding due to unmeasured patient characteristics, such as the ability to tolerate, and the possibility of partially responding to, treatment with nbDMARDs. Although most physicians believe their patients are compliant with combination therapy, our review of claims data demonstrate that this is not occurring in the majority of patients. Given that there are 53% to 59% of patients who are prescribed combination therapy but in fact >80% of the time only use biologic monotherapy, consideration should be given to prescribing a biologic that has demonstrated efficacy when used either as a monotherapy or in combination with nbDMARDs. Biologics and other medications that are effective as monotherapy may expand the range of treatment options for patients with RA and warrant further investigation [33].
No consensus exists regarding the level of medication adherence that is considered optimal for patients with RA in a real-world setting, although a systematic review from 2010 concluded that “most studies show that adherence is inadequate in many patients” [34]. Vermeer et al. reported an adherence of 69% [35] and suggested that perfect adherence for patients with RA is not realistic due to the existence of side effects, comorbidities, and the fact that patients with RA—who frequently assess their disease severity differently than does their physician [36]—may be involved in decisions regarding their treatment. While our study did not suggest a clear threshold for adequate adherence with concomitant nbDMARDs, we did observe an impact on persistence with anti-TNFs after the initial 90-day period. Patients with <80% adherence with their nbDMARD had a 1.2 to 1.3 times higher risk of discontinuing their anti-TNF therapy compared with patients who had >80% nbDMARD adherence. The lowest nbDMARD adherence category (0–19%) was an exception in this dose–response relationship and actually demonstrated greater anti-TNF persistence than the high adherence group. The reasons for this remain unclear, but one possible explanation is that this group represents patients who are more responsive (i.e., respond well to their anti-TNF therapy) and therefore do not require concomitant nbDMARD therapy. This explanation may also drive the finding that in the first 90 days of anti-TNF therapy, patients with relatively low nbDMARD adherence (20–39%) were less likely to discontinue their anti-TNF medication.
Claims database analysis allows for estimation of real-world treatment patterns, and the strength of our analysis derives from the large, geographically diverse population studied. All retrospective database analyses are subject to certain limitations and the results of this study must be interpreted with appropriate consideration of these limitations. Claims data are collected primarily for payment purposes, not research, and are subject to coding errors. Additionally, certain information was not readily available in claims data that could have had an effect on study outcomes, such as certain clinical and disease-specific parameters, which may result in residual confounding due to factors that were not measured; however, the analyses adjusted for comorbidity levels and other characteristics measurable in claims data. Similarly, claims data do not contain the rationale for why patients were prescribed medications, so reasons for patients being treated with monotherapy versus combination therapy were unknown. A limitation of this analysis is that there may be unmeasured characteristics of some patients that could influence both adherence with nbDMARDs and persistence with anti-TNFs. For patients who have a general tendency to be adherent to all of their medications (a ‘healthy behavior’ tendency), for example, the association between adherence and persistence seen in this study may be in part due to that unmeasured factor.
We were unable to identify reasons for non-compliance in claims data. In addition, because the measures of adherence were based on a combination of filled prescriptions and administrations in medical claims, the lowest level is driven in part by the nature of filled prescriptions, which typically are written for a 30-day period; this may partially explain the lack of effect in the first 90 days after anti-TNF initiation. Adherence was calculated based on information about filled prescriptions provided in claims data and may be overestimated because it is not known whether patients consumed the filled prescriptions.
The data used for this study come from a managed care population; therefore, results of this analysis are primarily applicable to patients with RA in managed care settings and may not be applicable to patients who are uninsured or covered through Medicaid.
Conclusions
In conclusion, our results are consistent with other studies in that we found that almost one-third of patients with RA receiving anti-TNF therapy received it as pure monotherapy. Furthermore, one-third of those receiving any combination therapy (anti-TNF + nbDMARDs) had <60% adherence to nbDMARDs. Physicians should take this into consideration when prescribing combination therapy and may want to consider prescribing a biologic that is known to be efficacious when used as a monotherapy or combination with nbDMARDs. We also found that lack of adherence with nbDMARDs resulted in poorer anti-TNF persistence in the period >90 days after starting the anti-TNF therapy, suggesting that concomitant nbDMARD use may play a role in maintenance of response to anti-TNF therapy. Further research is warranted to assess the reasons for, and effects of, the observed lower adherence to concomitant nbDMARDs on clinical outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by Genentech, Inc., a member of the Roche group. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. The authors thank Jennie Best, of Genentech, Inc. for review of the manuscript. Support for third-party writing assistance, furnished by Denise Kenski, PhD, from Health Interactions, was provided by F. Hoffmann-La Roche Ltd. Sponsorship and article processing charges for this study were funded by F. Hoffmann-La Roche Ltd.
Conflict of interest
Sarika Ogale is an employee (and stockholder) of Genentech, Inc. Nicole M. Engel-Nitz is an employee of Optum and stockholder of UnitedHealth Group. Mahesh Kulakodlu was an employee of Optum at the time of this study.
Compliance with ethics guidelines
The study databases satisfy the conditions set forth in Sections 164.514 (a)–(b)1ii of the Health Insurance Portability and Accountability Act of 1996 privacy rule regarding the determination and documentation of statistically de-identified data. Because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, Institutional Review Board approval to conduct this study was not necessary. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Rasch EK, Hirsch R, Paulose-Ram R, Hochberg MC. Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis Rheum. 2003;48:917–926. doi: 10.1002/art.10897. [DOI] [PubMed] [Google Scholar]
- 3.Michaud CM, McKenna MT, Begg S, et al. The burden of disease and injury in the United States 1996. Popul Health Metr. 2006;4:11. doi: 10.1186/1478-7954-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furneri G, Mantovani LG, Belisari A, et al. Systematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritis. Clin Exp Rheumatol. 2012;30(4 Suppl 73):S72–S84. [PubMed] [Google Scholar]
- 5.Strand V, Burmester GR, Ogale S, Devenport J, John A, Emery P. Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24-week randomized controlled RADIATE study. Rheumatol (Oxf) 2012;51:1860–1869. doi: 10.1093/rheumatology/kes131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuya H, Kasama T, Isozaki T, et al. Effect of TNF antagonists on the productivity of daily work of patients with rheumatoid arthritis. J Multidiscip Healthc. 2013;6:25–30. doi: 10.2147/jmdh.s39158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soliman MM, Ashcroft DM, Watson KD, et al. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:583–589. doi: 10.1136/ard.2010.139774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nixon R, Bansback N, Brennan A. The efficacy of inhibiting tumour necrosis factor α and interleukin 1 in patients with rheumatoid arthritis: a meta-analysis and adjusted indirect comparisons. Rheumatol (Oxf) 2007;46:1140–1147. doi: 10.1093/rheumatology/kem072. [DOI] [PubMed] [Google Scholar]
- 10.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 11.Breedveld FC, Keystone E, van der Heijde D, et al. Initial combination therapy with adalimumab plus methotrexate leads to better long-term outcomes than with either monotherapy in patients with early rheumatoid arthritis: 8-year results of an open-label extension of a phase 3 trial [abstract] Arthritis Rheum. 2011;63(Suppl S10):1231. [Google Scholar]
- 12.Buckley F, Finkckh A, Huizinga T, Dejonckheere F, Jansen J. Comparative efficacy of biologics as monotherapy and in combination with methotrexate in rheumatoid arthritis patients with an inadequate response to conventional dmards: a network meta-analysis [abstract]. Arthritis Rheum. 2012;64(Suppl S10):S918.
- 13.Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor α given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD study. Ann Rheum Dis. 2009;68:789–796. doi: 10.1136/ard.2008.099010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijde D, Klareskog L, Rodriguez-Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063–1074. doi: 10.1002/art.21655. [DOI] [PubMed] [Google Scholar]
- 16.Blum MA, Koo D, Doshi JA. Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic review. Clin Ther. 2011;33:901–913. doi: 10.1016/j.clinthera.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Yazici Y, Shi N, John A. Utilization of biologic agents in rheumatoid arthritis in the United States: analysis of prescribing patterns in 16,752 newly diagnosed patients and patients new to biologic therapy. Bull NYU Hosp Jt Dis. 2008;66:77–85. [PubMed] [Google Scholar]
- 18.Salt E, Frazier SK. Adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a narrative review of the literature. Orthop Nurs. 2010;29:260–275. doi: 10.1097/NOR.0b013e3181e5c2c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8:337–351. doi: 10.1586/eci.12.23. [DOI] [PubMed] [Google Scholar]
- 20.Viller F, Guillemin F, Briancon S, Moum T, Suurmeijer T, van den Heuvel W. Compliance to drug treatment of patients with rheumatoid arthritis: a 3 year longitudinal study. J Rheumatol. 1999;26:2114–2122. [PubMed] [Google Scholar]
- 21.Tuncay R, Eksioglu E, Cakir B, Gurcay E, Cakci A. Factors affecting drug treatment compliance in patients with rheumatoid arthritis. Rheumatol Int. 2007;27:743–746. doi: 10.1007/s00296-006-0299-9. [DOI] [PubMed] [Google Scholar]
- 22.Waimann CA, Marengo MF, de Achaval S, et al. Electronic monitoring of oral therapies in ethnically diverse and economically disadvantaged patients with rheumatoid arthritis: consequences of low adherence. Arthritis Rheum. 2013;65:1421–1429. doi: 10.1002/art.37917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Thurah A, Norgaard M, Johansen MB, Stengaard-Pedersen K. Methotrexate compliance among patients with rheumatoid arthritis: the influence of disease activity, disease duration, and co-morbidity in a 10-year longitudinal study. Scand J Rheumatol. 2010;39:197–205. doi: 10.3109/03009740903251318. [DOI] [PubMed] [Google Scholar]
- 24.Rau R, Herborn G. Benefit and risk of methotrexate treatment in rheumatoid arthritis. Clin Exp Rheumatol. 2004;22(5 Suppl 35):S83–S94. [PubMed] [Google Scholar]
- 25.Neame R, Hammond A. Beliefs about medications: a questionnaire survey of people with rheumatoid arthritis. Rheumatol (Oxf) 2005;44:762–767. doi: 10.1093/rheumatology/keh587. [DOI] [PubMed] [Google Scholar]
- 26.Kristensen LE, Saxne T, Nilsson J, Geborek P. Impact of concomitant DMARD therapy on adherence to treatment with etanercept and infliximab in rheumatoid arthritis. Results from a six-year observational study in southern Sweden. Arthritis Res Ther. 2006;8:R174. doi: 10.1186/ar2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyrich KL, Symmons DP, Watson KD, Silman AJ, British Society for Rheumatology Biologics Register Comparison of the response to infliximab or etanercept monotherapy with the response to cotherapy with methotrexate or another disease-modifying antirheumatic drug in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:1786–1794. doi: 10.1002/art.21830. [DOI] [PubMed] [Google Scholar]
- 28.Kristensen LE, Gulfe A, Saxne T, Geborek P. Efficacy and tolerability of anti-tumour necrosis factor therapy in psoriatic arthritis patients: results from the South Swedish Arthritis Treatment Group register. Ann Rheum Dis. 2008;67:364–369. doi: 10.1136/ard.2007.073544. [DOI] [PubMed] [Google Scholar]
- 29.Zink A, Listing J, Kary S, et al. Treatment continuation in patients receiving biological agents or conventional DMARD therapy. Ann Rheum Dis. 2005;64:1274–1279. doi: 10.1136/ard.2004.031476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buch MH, Bingham SJ, Bryer D, Emery P. Long-term infliximab treatment in rheumatoid arthritis: subsequent outcome of initial responders. Rheumatol (Oxf) 2007;46:1153–1156. doi: 10.1093/rheumatology/kem075. [DOI] [PubMed] [Google Scholar]
- 31.Emery P, Sebba A, Huizinga TW. Biologic and oral disease-modifying antirheumatic drug monotherapy in rheumatoid arthritis. Ann Rheum Dis. 2013;72:1897–1904. doi: 10.1136/annrheumdis-2013-203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heiberg MS, Koldingsnes W, Mikkelsen K, et al. The comparative one-year performance of anti-tumor necrosis factor α drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum. 2008;59:234–240. doi: 10.1002/art.23333. [DOI] [PubMed] [Google Scholar]
- 33.Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–1550. doi: 10.1016/S0140-6736(13)60250-0. [DOI] [PubMed] [Google Scholar]
- 34.de Achaval S, Suarez-Almazor ME. Treatment adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosus. Int J Clin Rheumatol. 2010;5:313–326. doi: 10.2217/ijr.10.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermeer M, Kuper HH, Bernelot Moens HJ, et al. Adherence to a treat-to-target strategy in early rheumatoid arthritis: results of the DREAM remission induction cohort. Arthritis Res Ther. 2012;14:R254. doi: 10.1186/ar4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barton JL, Imboden J, Graf J, Glidden D, Yelin EH, Schillinger D. Patient-physician discordance in assessments of global disease severity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62:857–864. doi: 10.1002/acr.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.