Abstract
Introduction
Metabolic syndrome is comprised of a combination of the following states: increased insulin resistance, dyslipidemia, cardiovascular disease, and increased abdominal obesity. Women with polycystic ovary syndrome (PCOS) have an increased risk of developing metabolic syndrome over the course of their lives. Metabolic syndrome increases risk of major cardiovascular events, morbidity, quality of life, and overall health care costs. Though metabolic syndrome in women with PCOS is an area of great concern, there is no effective individual medical therapeutic to adequately treat this issue.
Areas Covered
This article will review key aspects of metabolic syndrome in PCOS. We will discuss classic and novel therapeutics to address metabolic syndrome in women with PCOS. We will conclude with the importance of developing strategic interventions to increase the compliance to lifestyle and dietary modification, in addition to appreciation of the emerging pharmaceutical therapeutics available.
Expert Opinion
Innovation in lifestyle modification, including diet, exercise, with and without dedicated stress reduction techniques is the future in treatment of metabolic syndrome in PCOS. Application of novel interventions, such as group medical care, may improve future adherence to lifestyle modification recommendations, in addition to or in combination with pharmaceutical therapeutics.
Keywords: group care, lifestyle modification, metabolic syndrome, polycystic ovary syndrome
1. Introduction
Polycystic ovary syndrome (PCOS) affects up to 10% of reproductive age women and is one of the most common endocrine disorders in this age group.[1–5] PCOS is a heterogeneous disease with neuroendocrine findings and metabolic sequelae characterized by menstrual irregularity and hyperandrogenism, with or without presence of polycystic ovarian morphology. PCOS presents with a broad spectrum of phenotypes. Insulin resistance is not universal in PCOS. However, certain phenotypes are associated with insulin resistance and an increased long-term risk of developing diabetes,[6,7] metabolic syndrome (MBS),[8,9] and cardiovascular disease,[10,11] which are compounded by concurrent obesity.[12,13]
1.1. Polycystic ovary syndrome
The studies on the prevalence of the disorder depend on the criteria used, with estimates ranging from 6 to 12%.[1,4,14] There is debate on what constitutes the required diagnostic criteria for PCOS. There are three main diagnostic criteria for PCOS reported by the National Institutes of Health, the Androgen Excess Society and the Rotterdam criteria.[15,16] The most commonly used criteria in clinical practice is the Rotterdam criteria, which requires two out of three of the following: oligo-ovulation or anovulation, clinical or biochemical evidence of androgen excess and ultrasonographic evidence of polycystic ovary morphology.[17] Evidence of biochemical or clinical hyperandrogenism is a key finding linked to PCOS and is related to its multiple metabolic sequelae.[18,19]
1.2. Metabolic syndrome
MBS is defined as a combination of abnormal glucose metabolism, elevated blood pressure, abnormal lipid profile and abdominal obesity. MBS is defined by distinct, diagnostic criteria set forth by several groups. The most commonly utilized criteria are the guidelines set by the National Cholesterol Education Program Adult Treatment Program III (ATP 3) in 2005. In the United States, these criteria state that three or more of the following abnormalities are required for the diagnosis of MBS in women: fasting plasma glucose ≥ 5.6 mmol/L (100 mg/ dL) or drug treatment for elevated blood glucose; serum high-density lipoprotein (HDL) < 1.3 mmol/L (50 mg/dL women) or drug treatment for low HDL; serum triglycerides ≥ 1.7 mmol/L (150 mg/dL) or drug treatment for elevated triglycerides; abdominal obesity defined as a waist circumference ≥88 cm and a blood pressure ≥ 130/85 mm/Hg or drug treatment for elevated blood pressure.[20] These guidelines are an updated version of the criteria established in 2001 to include a lower threshold for fasting plasma glucose (previously defined as ≥6.1 mmol/L (110 mg/dL)), as well as present drug treatment to meet the definitions of hyperglycemia, dyslipidemia and hypertension. The rationale behind these changes, as reported by the National Heart Lung and Blood Institute (NHLBI) in collaboration with the American Heart Association (AHA), is that multiple marginal abnormalities significantly increase cardiovascular disease risk.[20] Additionally, the lower cutpoint for fasting plasma glucose was implemented to match the American Diabetes Association-modified definition for prediabetes (impaired fasting glucose) or diabetes.[20,21] While not entirely defined in the 2005 ATP 3 guidelines, diagnostic criteria established by the International Diabetes Federation (IDF) consider ethnic variations in waist circumference. For example, the threshold for abdominal obesity in Asian, Ethnic South and Central American, Sub-Saharan Africans, Eastern Mediterranean, Arab and European women has been defined as a waist circumference ≥80 cm.[22] The higher cutpoint of 88 cm is currently used for all ethnic groups under the 2005 ATP 3 criteria; however, the NHLBI and AHA deem the lower cutpoint for Asian-Americans to be appropriate as this population has a relatively higher predisposition to insulin resistance, MBS and type 2 diabetes mellitus, with moderate increases in waist circumference.[20] In contrast, the IDF strongly recommends that ethnic group-specific cutpoints should be used for all subjects of epidemiological studies and clinical diagnoses regardless of their country of residence.[22] These diagnostic modifications complicate data comparisons between studies and overall prevalence estimates as the study population characteristics and the specific criteria used must be accounted for within each study. Based on data using the 2001 ATP 3 criteria and the revised glucose criterion, the prevalence of MBS in the United States from 1999 to 2000 in normal women aged 20–39 years old was approximately 19%.[23]
There are five applicable studies evaluating the prevalence of MBS in PCOS. Table 1 presents a summary of the original articles reporting prevalence estimates of PCOS with MBS with differing diagnostic criteria.[24] Of these studies, a retrospective study involving 106 women with PCOS presenting to an academic endocrinology clinic evaluated the prevalence of MBS in women with PCOS and found a twofold increased prevalence compared to healthy comparison groups (43% in women aged 20–39 vs 24% in the National Health and Nutrition Examination Survey III cohort, 1988–1994).[25] Furthermore, women with PCOS demonstrated markers of MBS earlier in life by 20 years compared to normal age-matched controls, with a 45% prevalence of MBS in PCOS in women aged 20–29 years, and of 53% in women aged 30 to 39 years. [25] Using the 2001 ATP 3 guidelines with elements of both the 2005 ATP 3 and World Health Organization criteria, an earlier case-control study with 129 cases of women with PCOS and 177 normally menstruating non-hirsute controls found comparable age-adjusted prevalence estimates of PCOS with MBS of 47.3% (95% confidence interval (CI) 35.3–56.9%) and in controls of 4.3% (95% CI 1.9–7.6%).[26] Another study using 2001 ATP 3 guidelines to evaluate the prevalence of MBS within a cohort of 394 women with PCOS that were enrolled in a therapeutic trial of troglitazone found that MBS was common (33.4%) and postulated that hyperinsulinemia was a common factor in PCOS and MBS, that increased BMI and insulinemia were correlated with MBS and that women with a BMI of 27 kg/m2 or less did not have MBS in their cohort.[27] As mentioned previously, the 2005 updates in the ATP 3 guidelines added and expanded inclusion factors for meeting criteria. As such, usage of the 2001 criteria likely excluded participants meeting the current diagnosis for MBS. Therefore, these reported prevalence estimates are likely to underestimate the true MBS prevalence within each study population. These estimates may also be limited in that they do not consider ethnic-specific values for waist circumference.[24]
Table 1.
A summary of the original articles describing prevalence estimates of polycystic ovary syndrome (PCOS) with metabolic syndrome (MBS).
| First author/year | Study design | Prevalence MBS/PCOS |
Metabolic Syndrome Factors |
|||
|---|---|---|---|---|---|---|
| Abnormal glucose metabolism |
Abnormal lipids | Abdominal obesity |
Elevated blood pressure |
|||
| Glueck 20031 | RC | 46% (64/138) |
11% (HI) | 56% HTRIG | 98% high WC | 70% HTN |
| Dokras 20052 | RC | 47% (61/129) |
12% (IFG) | 46% HTRIG 63% Low HDL-C |
72% high BMI | 29% HTN |
| Apridonidze 20053 | RC | 43% (46/106) |
20% (IFG, IGT, NIDDM) |
70% HTRIG 91% low HDL-C |
91% high BMI | 74% HTN |
| Vrbikova 20051 | CC | 1% (1/64) |
0 (IFG) | 5% HTRIG 34% low HDL-C |
11% high WC |
13% HTN |
| Ehrmann 20061 | MCT | 33% (123/368) |
5% (IFG) | 32% HTRIG 66% low HDL-C |
80% high WC |
21% HTN |
Notes: BMI = body mass index; CC = case control; HI = hyperinsulinemia; HTRIG = hypertriglyceridemia; HTN = hypertension; IFG = impaired fasting glucose;IGT = impaired glucose tolerance; MCT = multicenter clinical trial; NIDDM = non-insulin dependent diabetes mellitus; RC = retrospective cohort; WC = waist circumference.
2001 Adult Treatment Panel 3 (ATP 3) criteria.
Modified 2001 ATP 3 criteria that include the diagnosis of type 2 diabetes mellitus as meeting the criterion for abnormal glucose metabolism. World Health Organization criterion for abdominal obesity (BMI ≥ 30 kg/m2) used to substitute waist circumference measurement.
Modified 2001 ATP 3 criteria that substitute BMI ≥ 32 kg/m2 for waist circumference measurement to meet abdominal obesity criterion.
The earlier onset of MBS in women with PCOS may subsequently increase atherogenic cardiovascular disease. In one study using a risk factor model applied to 33 women with PCOS and 132 age-matched referents incorporated independent risks for myocardial infarction (age, hypertension, diabetes, central obesity and serum triglycerides) and found an increased relative risk (RR) of 7.4 for myocardial infarction among women with PCOS compared to their age-matched referents.[28] PCOS with MBS may manifest throughout the life cycle, thereby conveying cardiometabolic risk into pregnancy and menopause.[25,29–33]
Given that 10% of reproductive aged women may suffer from PCOS, and of those up to 53% in their fourth decade of life may develop MBS, based on the study previously described, PCOS with MBS should be prioritized as a major public health issue with implications on long-term cardiovascular, metabolic and reproductive health. Therapeutic targets are key in preventing and curbing the sequelae of MBS.
1.3. Pathophysiologic concept of polycystic ovary syndrome and metabolic syndrome
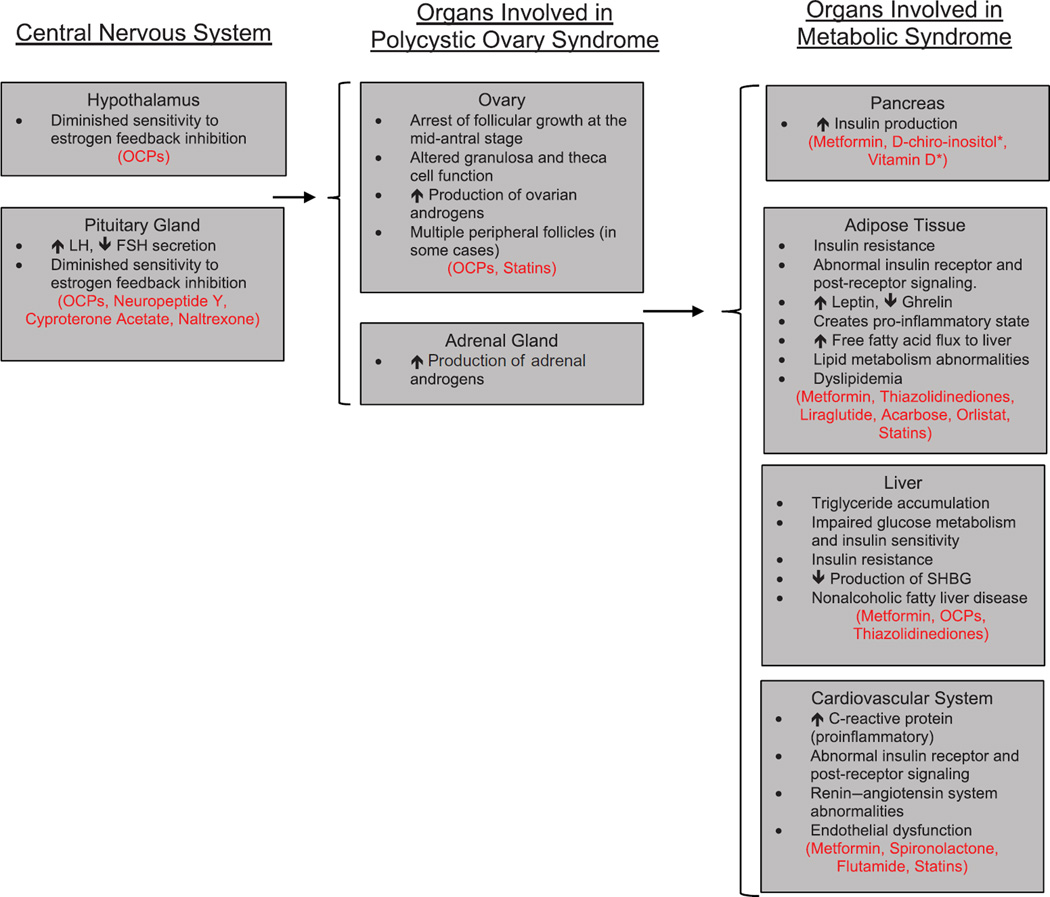
Though the exact etiology of PCOS syndrome is unknown, it has a complex evolving pathophysiology in which genetic and environmental parts are interweaving, while hormonal and metabolic abnormalities are contributing to a broad spectrum of phenotypes. Hypothalamic function is altered, with notable increased pulse frequency of luteinizing hormone (LH) secretion, and a diminished sensitivity to estrogen feedback inhibition. [34] At the level of the ovary, dysregulated folliculogenesis arrests follicular growth at the mid-antral stage. Additionally, there is granulosa cell dysfunction with suggestion of a decrease in cell death markers despite a thinning of the granulosa cell layer.[35] There is hypertrophy of the theca cells, which are stimulated by LH and responsible for production of ovarian androgens.[36] Both of these findings predispose to polycystic ovarian morphology with multiple peripheral follicles in a string of pearls appearance.[37] Granulosa cell function under the stimulation of follicle stimulating hormone (FSH) is altered due to relatively lower FSH concentrations in women with PCOS [38] with findings of decreased expression of aromatase for conversion of androgens to estrogens.[39] This in combination with increased theca cell function, stimulated by increased LH, promotes increased production of the ovarian androgens, testosterone and androstenedione. Adrenal androgen production is also increased in PCOS with elevated dehydroepiandrosterone compared to ovulatory non-hirsute controls and may be due to adrenal hyper-responsiveness to corticotropin (ACTH).[40,41] Aspects of MBS may increase the severity of biochemical or clinical androgen excess through reduction of sex hormone-binding globulin (SHBG) by obesity and hyperinsulinemia. Hyperinsulinemia also directly stimulates ovarian androgen production.[37] PCOS is a heterogenous disease with individual-specific pathophysiology dependent on the individual’s phenotype.[42,43]
1.4. Pathophysiologic concept of metabolic syndrome in PCOS
MBS is defined as a constellation of cardiovascular risks and insulin resistance, with altered values of serum lipids, abdominal adiposity, blood pressure and blood glucose. [20,21,44–49] It is estimated that obesity is common occurring in approximately 49% of women with PCOS. [50] Central to MBS in PCOS is abdominal obesity and insulin resistance, with an estimated prevalence of between 20 and 40%.[51,52] However, insulin resistance is also present in one-third of lean women with PCOS compared to age-matched controls.[53] PCOS is also associated with an increased lifetime risk of dyslipidemia,[54,55] cardiovascular disease,[56,57] and type 2 diabetes.[58] Cardiovascular risk seems to be elevated in women with the constellation of PCOS with obesity, insulin resistance and dyslipidemia.[59] Figure 1 summarizes the organs involved in PCOS with MBS as well as the corresponding treatment options.
Figure 1.
Organs involved in polycystic ovary syndrome with metabolic syndrome and corresponding treatment options.
*Sites of action at specific tissues are currently unknown.
1.5. Classic therapeutic options for PCOS and implications for MBS
Combined oral contraceptives (OCPs) and antiandrogens have been used as classical first-line treatments to target androgen excess in women with PCOS with effects at multiple sites.
Traditional use of OCPs has been considered first-line therapy in women with PCOS who are not attempting pregnancy and who do not have contraindications to the use of OCPs. The estrogen component in OCPs stimulates increased hepatic production of binding globulins, of which increased production of SHBG correlates with decreased free serum androgens.[60–64] A limitation of OCPs is that this may not be an acceptable therapeutic in PCOS with MBS comprised of hypertension and obesity due to the risk of deep-vein thrombosis and worsening hypertension.[65,66]
Antiandrogen therapy includes use of cyproterone acetate, spironolactone and flutamide. The antiandrogen cyproterone acetate has an untoward side effect of increasing insulin resistance.[67] Spironolactone, another antiandrogen, acting as a competitive inhibitor of the androgen receptor with some block of peripheral conversion of testosterone to dihydrotestosterone by 5-alpha reductase, has the additional benefit of increasing HDL and decreasing triglycerides in lean patients on long-term therapy.[68] However, one study suggested decreased HDL in short-term spironolactone therapy in conjunction with OCPs in lean women with PCOS.[69] Flutamide, a non-FDA-approved non-steroidal selective androgen receptor blocker, does have an additional beneficial effect on lipid profiles including decreased total cholesterol, low density lipoprotein and triglycerides.[70]
2. Metabolic abnormalities in PCOS
2.1. Obesity
Obesity is increasing in incidence and itself is a risk factor for MBS and may compound the risk of MBS in women with PCOS.[71] The prevalence of central obesity among women with PCOS is estimated to range between 20 and 85.5%.[50] Furthermore, in two large population-based cohort studies, body mass index (BMI) in reproductive aged women was correlated with increasing serum total testosterone and inversely correlated with SHBG concentrations in both ovulatory and oligo-ovulatory women.[72–74] Obesity also appears to modify the characteristics and severity of metabolic dysfunction in women with PCOS and thus may explain the metabolic heterogeneity seen in PCOS women with MBS. For example, lean women with PCOS more often present symptoms consistent with a hyperinsulinemia-driven pathophysiology in the absence of insulin resistance compared to obese women who primarily demonstrate a metabolic profile compatible with insulin resistance, most likely associated with visceral adiposity.[75]
Several recent review articles comment on the currently available therapeutic agents for MBS in PCOS [76,77], with note that no single drug/agent will target all aspects. Metformin, a biguanide insulin sensitizer, has been discussed as one such drug thought to impact nearly all aspects of MBS.[76] For some patients with PCOS and MBS, metfor-min has provided direct treatment of insulin resistance and weight loss.[4,78,79]
2.2. Insulin resistance
Insulin activity and glucose metabolism are aberrant in women with PCOS. Insulin resistance has been demonstrated in many target tissues such as skeletal muscle, fibro-blasts and adipose tissue with abnormalities suggested in insulin receptors and post-receptor signaling.[80,81] Counter to the insulin resistance noted in peripheral tissues, ovarian insulin responsiveness has remained intact in in vitro cell studies.[81,82] The prevalence of insulin resistance has been noted in 60–80% of women with PCOS and is compounded by obesity. Earlier studies reported a prevalence of impaired glucose tolerance of 35% and type 2 diabetes of 10% in women presenting with PCOS.[52] A more recent systematic review reported an increased odds of impaired glucose tolerance (OR: 2.54; 95% CI: 1.44, 4.47) and type 2 diabetes (OR: 4.00, 95% CI: 1.97, 8.10) among women with PCOS compared to BMI-matched controls.[83] Metabolic profile, more specifically insulin resistance, also appears to depend upon BMI status with age as the severity of insulin resistance has been shown to increase with age in PCOS women who are obese, but not in lean or overweight PCOS women.[84]
Classic treatment for insulin resistance includes peripheral insulin sensitizers, such as metformin [85], which decreases hepatic gluconeogenesis, increases peripheral glucose uptake and decreases gastrointestinal absorption of glucose.[86] Metformin has beneficial effects on inflammation and cardiovascular risk profile, such as improvement in endothelium-dependent vasodilation, endothelin-1, C-reactive protein (CRP), advanced glycosylation end points (AGEs) and adhesion molecules.[87,88] Further studies are needed to determine whether metformin provides long-term cardiovascular risk reduction in women with PCOS and markers of MBS with and without insulin resistance.
Other insulin sensitizers have been used in this population, such as the thiazolidinedione class of drugs like rosiglitazone and pioglitazone. The thiazolidinediones act through the peroxisome-proliferator-activated-gamma-receptor (PPAR-gamma) and improve insulin sensitivity; however, they are associated with weight gain.[60,89,90] Treatment of insulin resistance with thiazolidinediones are associated with a reduction in serum androgens and may also have beneficial effects at the level of ovarian steroidogenesis by altering the function of steroidogenic enzymes of 3β-hydroxysteroid dehydrogenase.[61–63] However, there have been no subsequent large randomized trials of thiazolidinediones in PCOS.[64] Additionally, a recent FDA advisory reported a linkage between pioglitazone to bladder cancer.[91] The risk–benefit ratio may also be less favorable for infertility because animal studies suggest that thiazolidinediones may be associated with fetal loss (FDA Pregnancy Category C).[92]
2.3. Dyslipidemia
Abnormalities in lipid metabolism and fasting lipid profiles in women with PCOS are variable and are due to a combination of insulin resistance,[93] obesity,[94] with additional modification by diet,[95–97] amount of exercise[98] and genetic predisposition.[99–103] The general trend is a lowering of HDL, elevation of total cholesterol, low-density lipoproteins and triglycerides.[104–106] One study reported presence of oxidized LDL as an early marker of altered metabolism in young women.[107]
It is notable that lean women with PCOS androgen excess have lipid profile abnormalities compared to normal women of lowered HDL.[108] However, in another study of obese women with PCOS, there was a slight but statistically significant increase in HDL.[54] Further research is needed to better understand the pathophysiology and early clinical biomarkers of abnormal lipid metabolism in PCOS with MBS.
2.4. Liver
Serum SHBG, produced by the liver, corresponds with the bioavailable concentration of androgens and is decreased by elevated circulating androgens and obesity.[109–111] Nonalcoholic fatty liver disease has been described occurring in 2–30% of women with PCOS; however screening guidelines do not recommend routine screening of liver enzymes or imaging of the liver.[112–115] Further studies are needed to accurately quantify this risk and guide clinical screening recommendations.[116]
2.5. Cardiovascular risk
Cardiovascular risk seems to be elevated in women with the constellation of PCOS with MBS, or obesity, insulin resistance/impaired glucose tolerance and dyslipidemia.[59] The absolute risk of cardiovascular disease is not well established. Cardiovascular risk may depend on the severity of PCOS, [117] as well as individual risk predictors such as BMI,[118] genetic predisposition, diet[95–97] and lifestyle factors. In a systematic review of the RR of fatal or non-fatal coronary heart disease (CHD) and stroke, women with PCOS had 1.55 times the risk of CHD or stroke compared to women without PCOS after adjusting for BMI.[119] Additionally, in one large prospective cohort study, menstrual irregularity was associated with an increased risk of non-fatal (age-adjusted RR: 1.25) and fatal (RR: 1.67) CHD.[57] Emerging literature demonstrates that women with PCOS have increased serum markers of inflammation such as CRP [120] and white blood cell count,[27] abnormalities in the renin–angiotensin system [28] and endothelial dysfunction [121–124], which may predispose to CHD seen in PCOS. At least two studies, Talbott and colleagues as well as Shroff et al., have suggested that the cardiovascular risk in women with PCOS may be mediated through early atherosclerotic disease.[125,126]
3. Novel therapeutic targets for PCOS with MBS
The ideal therapeutic for PCOS with MBS is one that addresses the underlying etiology to restore normal hypothalamic pituitary function, with return of normal steroidogenic function at the level of the ovary, and resultant normalization of androgen excess and insulin resistance. Ultimately, the normalization of the hypothalamic pituitary ovarian adrenal axis would lead to a normalization of the attributable component of MBS, notably obesity, insulin resistance, lipid profile, liver dysfunction and amelioration of cardiovascular risk. No single therapeutic has achieved this goal to date. First-line therapeutics, such as OCPs, antiandrogens and insulin sensitizers, have resulted in changes in the level of the hypothalamus–pituitary–ovarian axis (OCPs), decreased androgens (OCPs and antiandrogens) and improved glucose metabolism (metformin). Metformin has addressed several of these pathways but has fallen short as a panacea,[127,128] particularly in the reduction of androgen excess in obese individuals with PCOS.[86,129,130] Furthermore, women with PCOS-MBS who desire fertility pose an additional challenge to most of the mentioned therapeutics due to contraindications and safety in pregnancy. Despite these limitations, there are several emerging therapeutics for PCOS with MBS. Though most of these emerging therapeutics do not seem to address all pathways, addressing key factors of MBS may succeed in diminishing long-term cardiovascular risk. In this vein, acarbose and orlistat may improve dietary parameters influencing lipid profiles and obesity. Table 2 summarizes the associated pathophysiological effects and novel therapeutic targets for PCOS with MBS under each criterion defined by the 2005 ATP 3 guidelines for women.
Table 2.
Novel Therapeutic targets for polycystic ovary syndrome with metabolic syndrome.
| Metabolic syndrome criteria1 |
Definition/diagnosis | Associated pathophysiological effects |
Treatment options/therapeutic targets |
|---|---|---|---|
| Abnormal glucose metabolism |
Glucose ≥ 5.6 mmol/L (100 mg/dL) or drug treatment for elevated blood glucose |
Insulin resistance Hyperinsulinemia Increased androgen production Decreased SHBG |
Metformin, thiazolidinediones, D-chiro-inositol, naltrexone: improve insulin sensitivity Vitamin D: Associated with normal insulin metabolism |
| Abnormal lipid profile |
HDL cholesterol: <1.3 mmol/L (50 mg/ dL) or drug treatment for low HDL triglycerides ≥ 1.7 mmol/L (150 mg/dL) or drug treatment for elevated triglycerides |
Increased androgen production Insulin resistance Decreased SHBG |
Spironolactone: increases HDL and decreases triglycerides Flutamide: decreases total cholesterol, LDL and triglycerides Statins: inhibits cholesterol synthesis and improves lipid profiles |
| Abdominal obesity | Obesity with a waist circumference ≥ 88 cm |
Increased androgen production Insulin resistance Decreased SHBG production |
Metformin: incudes modest weight loss Liraglutide: induces weight loss Acarbose: decreases digestion and absorption of polysaccharides Orlistat: reduces dietary fat absorption Diet modification: reduces AGEs |
| Elevated blood pressure |
Blood pressure ≥ 130/85 mm/Hg or drug treatment for hypertension |
Increased C-reactive protein Endothelial dysfunction Pro-inflammatory state |
Metformin: Improves endothelium-dependent vasodilation |
Notes: ATP 3: Adult Treatment Panel III; HDL: high-density lipoprotein; LDL: low-density lipoprotein; SHBG: sex hormone-binding globulin, AGEs advanced glycosylation end products.
Based on the 2005 ATP 3 Guidelines for women.
3.1. Obesity
Acarbose is a complex oligosaccharide that inhibits alpha-glucosidase resulting in decreased digestion and absorption of polysaccharides. There are a few promising studies within the PCOS population, but further research is needed to address conflicting reports on lipid metabolism, insulin, BMI reduction and cardiovascular parameters.[131–135] Orlistat inhibits the hydrolysis of triglycerides and acts as a gastric and pancreatic lipase inhibitor, thereby reducing dietary fat absorption. Orlistat, which has been studied in PCOS-MBS, may hold promise as studies demonstrate reduction of AGEs, which are known to be elevated in PCOS and have associated cardiovascular risk.[96,136]
3.2. Insulin resistance
D-chiro-inositol, a newer insulin sensitizing agent, may ultimately prove to help women with PCOS and MBS. [137,138] More studies are required regarding the model of its action and its efficacy. Vitamin D, a key factor in calcium homeostasis and bone metabolism, has been evaluated in other populations, with note that normal levels of vitamin D are associated with normal insulin metabolism.[139] Two studies do suggest a potential benefit for vitamin D evaluation and treatment in PCOS-MBS,[140,141] but further research is needed regarding the utility of vitamin D supplementation or treatment in women with PCOS-MBS.
3.3. Dyslipidemia
After diet and exercise, statin therapy is a common therapeutic to address clinically relevant dyslipidemia in the adult population. Statin therapy, acting by inhibiting cholesterol synthesis at HMG co A reductase, improves lipid profiles and has demonstrated long-term treatment benefit in cardiovascular disease risk reduction. Some studies support pluripotent effects of hypolipidemic treatment on multiple organ systems including cardiovascular risk, hyperandrogenemia, insulin resistance and mood disturbance.[142] There are limitations to the use of statins in women who are attempting pregnancy, or who are pregnant, over concern of fetal teratogenicity [143] with the recommendation of stopping statin treatment prior to attempts at conception. In women with PCOS in whom pregnancy is not a current concern, and in whom therapy is warranted, statins may be considered. There are a few studies of statin therapy in women with PCOS with lipid abnormalities replicating this finding. [144,145] The additional benefits of statins include a reduction of ovarian androgen synthesis as noted by a study of atorvastatin on metabolic profile of women with PCOS.[146]
3.4. Other emerging therapeutics
Emerging therapeutics that may act at the level of the hypothalamus and pituitary are important to consider. Naltrexone, an opioid antagonist acting through increase of beta-endorphins and sympathetic nervous system activity, has been shown to normalize response to GnRH stimulation in women with PCOS [147] and improve insulin sensitivity.[148] Liraglutide, a glucagon-like peptide 1 (GLP-1) receptor agonist, has been shown to induce greater increases in weight loss compared to met-formin in a subset of PCOS women with a high-risk profile for MBS.[149] Targeting Neuropeptide Y, which has abnormal secretory profiles in women with PCOS in relation to ghrelin, may provide a novel future therapeutic.[150] Finally, there may be promise in evaluating the obesity-related hormones of leptin and ghrelin in targeting novel pathways for treatment of PCOS-MBS.[151,152]
In addition to the above therapeutic targets, lifestyle modification programs utilizing increased physical activity, techniques to decrease perceived stress and a group care model for women with PCOS-MBS may provide multiplicative benefit to use of a single or combination pharmaceutical therapeutic. Regarding stress reduction, studies incorporating low-frequency electroacupuncture (EA) may be useful in the treatment of hyperandrogenism and menstrual frequency. In a randomized controlled trial of 84 women with PCOS, women randomized to a 16-week regimen of EA had a 25% decrease in serum testosterone as well as an increase in menstrual frequency from 0.28 per month at baseline to 0.69 per month. Interestingly, the magnitudes of these changes in testosterone and menstrual frequency were significantly greater that what was seen in women randomized to a 16-week regimen of physical exercise (p = 0.038 and p = 0.018, respectively).[153] Group medical care and group-centered therapy have not yet been applied to women with PCOS-MBS, but have been evaluated in the prenatal population with interesting outcomes. In one early study, group prenatal care was compared to standard prenatal care with notable improvements in psychosocial aspects (prenatal education, preparedness for labor and delivery) and a 33% reduction in preterm birth in the group care participants; all outcomes were strengthened in the African American population of participants of that study.[154] A group care model, with a structured and varying exercise program, may prove critical for long-term treatment compliance and achievement of the greatest risk reduction in women with PCOS-MBS.
3.4.1. Evidence targeting lifestyle therapeutics
Lifestyle modification including diet, exercise and group-centered therapy may benefit women suffering from MBS with PCOS. The majority of current literature on diet and lifestyle modifications are focused on evaluating short-term reproductive outcomes such as clinical pregnancy rates and have secondary aims of metabolic parameters of a short time interval.[155,156] In a prospective intervention study comparing the effect of exercise between overweight women with and without PCOS, exercise training improved insulin resistance in women with PCOS by 16% and resulted in a more significant reduction in serum triglycerides compared to women without PCOS.[157] Another systematic review reported that exercise of any form, frequency or duration was associated with benefits including improved ovulation, weight loss and improved insulin resistance.[158] One main issue with lifestyle intervention is difficulty in long-term compliance.[159] Furthermore, there is a great need for future studies on diet/lifestyle modifications and their long-term impact on the reduction and prevention of MBS.
4. Conclusion
We feel that the beneficial effect of any therapeutic target will be enhanced by lifestyle modification. In addition to a selection of any particular individual pharmaceutical therapeutic, recommendation of lifestyle and diet modification is of critical importance in addressing MBS in PCOS women. Despite the trials of single or combination pharmacologic therapeutics, the literature has reiterated that lifestyle modification is superior to any single pharmacologic therapeutic alone.[160,161] In some instances, improved outcomes are noted when lifestyle interventions are combined with targeted pharmacologic therapeutics.[162–165]
5. Expert opinion
PCOS is the most common endocrinopathy affecting women of reproductive age, with a prevalence of 6–7%, and concern that the incidence, severity and risk of MBS may be increasing due to its relationship with obesity. However, no effective treatment options are currently available to target the constellation of symptoms found in PCOS-MBS. This may be due to the lack of a final common targetable cellular pathway, or that the pathways are complex and interrelated, requiring more than one intervention. Understanding the pathophysiology of PCOS with MBS is an area of importance for future research. Because the data on lifestyle intervention, including physical exercise, is so compelling for treatment benefit, we feel that the study of lifestyle interventions in combination with pharmacotherapy is critical in further addressing PCOS-MBS in a way that no single pharmacologic intervention can. Furthermore, if PCOS with MBS is not addressed or prevented at an early stage, significant morbidity at an earlier age is expected. In the worst-case scenario, treatment will consist of a pharmacopeia – including but not limited to insulin-sensitizing agents, anti-hypertensives and anti-hyperlipidemics. Therefore, early diagnosis, prevention and lifestyle modification along with judicious use of classic or novel therapeutics for PCOS-MBS tailored to each patient is recommended. Future studies combining the use of the mentioned novel therapeutics toward early treatment and PCOS-MBS risk reduction as well as studies elucidating the complex mechanisms of PCOS-MBS and physical activity-related life-style modification will be critical. Furthermore, continuing the search for modifiable early exposures that predispose to PCOS-MBS is also critical.
Article highlights.
No single drug/agent targeting all aspects of metabolic syndrome (MBS) in women with polycystic ovary syndrome (PCOS) is currently available.
Though most emerging therapeutics for PCOS with MBS do not appear to address all pathophysiological pathways, addressing key factors of MBS may succeed in diminishing long-term cardiovascular risk.
In addition to the discussed therapeutic targets, lifestyle modification programs and a group care model for women with PCOS-MBS may provide multiplicative benefit to use of a single or combination pharmaceutical therapeutic.
Acknowledgments
S Mahalingaiah is supported by the National Institutes of Health under the Reproductive Scientist Development Program, grant number K12 HD 00849, and by the American College of Obstetricians and Gynecologists for research on prenatal and early adult environmental exposures and polycystic ovary syndrome.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Knochenhauer ES, Key TJ, Kahsar-Miller M, et al. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern united states: A prospective study. J Clin Endocrinol Metab. 1998;83(9):3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 2.Farah L, Lazenby AJ, Boots LR, et al. Prevalence of polycystic ovary syndrome in women seeking treatment from community electrologists. Alabama professional electrology association study group. J Reprod Med. 1999;44(10):870–874. [PubMed] [Google Scholar]
- 3.Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 4.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, et al. A survey of the poly-cystic ovary syndrome in the Greek island of lesbos: Hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84(11):4006–4011. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 5.March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 6.Gambineri A, Patton L, Altieri P, et al. Polycystic ovary syndrome is a risk factor for type 2 diabetes: Results from a long-term prospective study. Diabetes. 2012;61(9):2369–2374. doi: 10.2337/db11-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yildir IC, Kutluturk F, Tasliyurt T, et al. Insulin resistance and cardiovascular risk factors in women with PCOS who have normal glucose tolerance test. Gynecol Endocrinol. 2013;29(2):148–151. doi: 10.3109/09513590.2012.730573. [DOI] [PubMed] [Google Scholar]
- 8.Huang G, Coviello A. Clinical update on screening, diagnosis and management of metabolic disorders and cardiovascular risk factors associated with polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2012;19(6):512–519. doi: 10.1097/MED.0b013e32835a000e. [DOI] [PubMed] [Google Scholar]
- 9.Hillman JK, Johnson LN, Limaye M, et al. Black women with polycystic ovary syndrome (PCOS) have increased risk for metabolic syndrome and cardiovascular disease compared with white women with PCOS [corrected] Fertil Steril. 2014;101(2):530–535. doi: 10.1016/j.fertnstert.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 10.Talbott EO, Zborowski JV, Sutton-Tyrrell K, et al. Cardiovascular risk in women with polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28(1):111–133. doi: 10.1016/s0889-8545(05)70189-3. vii. [DOI] [PubMed] [Google Scholar]
- 11.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18(6):774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 12. Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: A systematic review and meta-analysis. Obes Rev. 2013;14(2):95–109. doi: 10.1111/j.1467-789X.2012.01053.x. This is a comprehensive, systematic review of the effect of central obesity in women with and without polycystic ovary syndrome (PCOS). This extensive, robust meta-analysis of relevant literature provides key evidence to show that the increased prevalence of obesity among women with PCOS is likely responsible for its metabolic symptoms.
- 13. Ferrannini E, Natali A, Bell P, et al. Insulin resistance hypersecretion in obesity. European group for the study of insulin resistance (EGIR) J Clin Invest. 1997;100(5):1166–1173. doi: 10.1172/JCI119628. A large cohort study estimating the prevalence of insulin hypersecrtetion in obesity. This study illustrates that metabolic and cardiovascular adaptations to treatment as well as cardiovascular and diabetic risk may depend upon the presence of insulin resistance in the setting of obesity.
- 14. Asuncion M, Calvo RM, San Millan JL, et al. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85(7):2434–2438. doi: 10.1210/jcem.85.7.6682. A thorough estimation of the prevalence of polycystic ovary syndrome (PCOS) in a cohort of Caucasian women. The strength of this investigation is the prospective study design and method of population selection. This sufficiently eliminated selection bias allowed for a socioeconomically diverse study population.
- 15. Livadas S, Diamanti-Kandarakis E. Polycystic ovary syndrome: Definitions, phenotypes and diagnostic approach. Front Horm Res. 2013;40:1–21. doi: 10.1159/000341673. This is a well-compiled review of presented difficulties in PCOS diagnosis. Additionally, this is one of the few reviews to discuss the different phenotypes found in PCOS and their respective risk profiles.
- 16.Azziz R, Carmina E, Dewailly D, et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An androgen excess society guideline. J Clin Endocrinol Metab. 2006;91(11):4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 17.Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Velez LM, Motta AB. Association between polycystic ovary syndrome and metabolic syndrome. Curr Med Chem. 2014;21(35):3999–4012. doi: 10.2174/0929867321666140915141030. [DOI] [PubMed] [Google Scholar]
- 19.Caserta D, Adducchio G, Picchia S, et al. Metabolic syndrome and polycystic ovary syndrome: an intriguing overlapping. Gynecol Endocrinol. 2014;30(6):397–402. doi: 10.3109/09513590.2014.887673. [DOI] [PubMed] [Google Scholar]
- 20. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. This is a key report that details the most current, widely used diagnostic criteria for metabolic syndrome (MBS). Rationale for updates from the 2001 guidelines are also explained in depth.
- 21.Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: Report of the national heart, lung, and blood institute/american heart association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24(2):e13–e18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 22.Alberti KG, Zimmet P, Shaw J Group IDFETFC. The metabolic syndrome-a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. Adults. Diabetes Care. 2004;27(10):2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 24.Vrbikova J, Vondra K, Cibula D, et al. Metabolic syndrome in young Czech women with polycystic ovary syndrome. Hum Reprod. 2005;20(12):3328–3332. doi: 10.1093/humrep/dei221. [DOI] [PubMed] [Google Scholar]
- 25.Apridonidze T, Essah PA, Iuorno MJ, et al. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(4):1929–1935. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 26.Dokras A, Bochner M, Hollinrake E, et al. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol. 2005;106(1):131–137. doi: 10.1097/01.AOG.0000167408.30893.6b. [DOI] [PubMed] [Google Scholar]
- 27.Ehrmann DA, Liljenquist DR, Kasza K, et al. Group PCTS. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:148–153. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- 28.Dahlgren E, Janson PO, Johansson S, et al. Polycystic ovary syndrome risk for myocardial infarction. Evaluated from a risk factor model based on a prospective population study of women. Acta Obstet Gynecol Scand. 1992;71(8):599–604. doi: 10.3109/00016349209006227. [DOI] [PubMed] [Google Scholar]
- 29.Boomsma CM, Eijkemans MJ, Hughes EG, et al. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12(6):673–683. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 30.Boutzios G, Livadas S, Piperi C, et al. Polycystic ovary syndrome offspring display increased oxidative stress markers comparable to gestational diabetes offspring. Fertil Steril. 2013;99(3):943–950. doi: 10.1016/j.fertnstert.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 31.Naver KV, Grinsted J, Larsen SO, et al. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. Bjog. 2014;121(5):575–581. doi: 10.1111/1471-0528.12558. [DOI] [PubMed] [Google Scholar]
- 32.Glueck CJ, Papanna R, Wang P, et al. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism: Clin Exp. 2003;52(7):908–915. doi: 10.1016/s0026-0495(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 33.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall JE, Taylor AE, Hayes FJ, et al. Insights into hypothalamic-pituitary dysfunction in polycystic ovary syndrome. J Endocrinol Invest. 1998;21(9):602–611. doi: 10.1007/BF03350785. [DOI] [PubMed] [Google Scholar]
- 35.Das M, Djahanbakhch O, Hacihanefioglu B, et al. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(3):881–887. doi: 10.1210/jc.2007-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Ziegler D, Steingold K, Cedars M, et al. Recovery of hormone secretion after chronic gonadotropin-releasing hormone agonist administration in women with polycystic ovarian disease. J Clin Endocrinol Metab. 1989;68(6):1111–1117. doi: 10.1210/jcem-68-6-1111. [DOI] [PubMed] [Google Scholar]
- 37.Chang RJ. Chapter 22 - polycystic ovary syndrome and hyperandrogenic states. In: Strauss JF, Barbieri RL, editors. Yen & Jaffe’s reproductive endocrinology. Philadelphia: W.B. Saunders; 2014. pp. 485–511. e487. [Google Scholar]
- 38.Coffler MS, Patel K, Dahan MH, et al. Evidence for abnormal granulosa cell responsiveness to follicle-stimulating hormone in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(4):1742–1747. doi: 10.1210/jc.2002-021280. [DOI] [PubMed] [Google Scholar]
- 39.Wilson EA, Erickson GF, Zarutski P, et al. Endocrine studies of normal and polycystic ovarian tissues in vitro. Am J Obstet Gynecol. 1979;134(1):56–63. doi: 10.1016/0002-9378(79)90796-8. [DOI] [PubMed] [Google Scholar]
- 40.DeVane GW, Czekala NM, Judd HL, et al. Circulating gonadotropins, estrogens, and androgens in polycystic ovarian disease. Am J Obstet Gynecol. 1975;121(4):496–500. doi: 10.1016/0002-9378(75)90081-2. [DOI] [PubMed] [Google Scholar]
- 41.Azziz R, Black V, Hines GA, et al. Adrenal androgen excess in the polycystic ovary syndrome: Sensitivity and responsivity of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 1998;83(7):2317–2323. doi: 10.1210/jcem.83.7.4948. [DOI] [PubMed] [Google Scholar]
- 42.Welt CK, Arason G, Gudmundsson JA, et al. Defining constant versus variable phenotypic features of women with poly-cystic ovary syndrome using different ethnic groups and populations. Obstet Gynecol Surv. 2007;62(3):185–187. doi: 10.1210/jc.2006-1191. [DOI] [PubMed] [Google Scholar]
- 43.Welt CK, Gudmundsson JA, Arason G, et al. Characterizing discrete subsets of polycystic ovary syndrome as defined by the Rotterdam criteria: The impact of weight on phenotype and metabolic features. J Clin Endocrinol Metab. 2006;91(12):4842–4848. doi: 10.1210/jc.2006-1327. [DOI] [PubMed] [Google Scholar]
- 44.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 46.Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: Insights from therapeutic interventions. J Am Coll Cardiol. 2005;46(11):1978–1985. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 47.Lindsay RS, Howard BV. Cardiovascular risk associated with the metabolic syndrome. Curr Diab Rep. 2004;4(1):63–68. doi: 10.1007/s11892-004-0013-9. [DOI] [PubMed] [Google Scholar]
- 48.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 49.Reaven GM. Banting lecture 1988 Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 50.Lim SS, Davies MJ, Norman RJ, et al. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618–637. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 51. Legro RS, Kunselman AR, Dodson WC, et al. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: A prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84(1):165–169. doi: 10.1210/jcem.84.1.5393. One of the first studies to experimentally measure glucose tolerance in women with polycystic ovary syndrome (PCOS). These findings highlight the presence of glucose intolerance and type 2 diabetes mellitus in nonobese and young women with PCOS. Additionally, this study reports that PCOS-associated risk for impaired glucose tolerance and type 2 diabetes mellitus does not seem to be strongly modified by race and ethnicity.
- 52.Ehrmann DA, Barnes RB, Rosenfield RL, et al. Imperial J: Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22(1):141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 53.Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the poly-cystic ovary syndrome. Endocr Rev. 2012;33(5):812–841. doi: 10.1210/er.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111(8):607–613. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 55.Sam S, Legro RS, Essah PA, et al. Evidence for metabolic and reproductive phenotypes in mothers of women with polycystic ovary syndrome. Proc Natl Acad Sci U S A. 2006;103(18):7030–7035. doi: 10.1073/pnas.0602025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christian RC, Dumesic DA, Behrenbeck T, et al. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(6):2562–2568. doi: 10.1210/jc.2003-030334. [DOI] [PubMed] [Google Scholar]
- 57.Solomon CG, Hu FB, Dunaif A, et al. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87(5):2013–2017. doi: 10.1210/jcem.87.5.8471. [DOI] [PubMed] [Google Scholar]
- 58.Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: A consensus statement by the androgen excess and polycystic ovary syndrome (aepcos) society. J Clin Endocrinol Metab. 2010;95(5):2038–2049. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 59.Cussons AJ, Stuckey BG, Watts GF. Metabolic syndrome and cardiometabolic risk in pcos. Curr Diab Rep. 2007;7(1):66–73. doi: 10.1007/s11892-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 60.Akazawa S, Sun F, Ito M, et al. Efficacy of troglitazone on body fat distribution in type 2 diabetes. Diabetes Care. 2000;23(8):1067–1071. doi: 10.2337/diacare.23.8.1067. [DOI] [PubMed] [Google Scholar]
- 61.Gasic S, Nagamani M, Green A, et al. Troglitazone is a competitive inhibitor of 3beta-hydroxysteroid dehydrogenase enzyme in the ovary. Am J Obstet Gynecol. 2001;184(4):575–579. doi: 10.1067/mob.2001.111242. [DOI] [PubMed] [Google Scholar]
- 62.Arlt W, Auchus RJ, Miller WL. Thiazolidinediones but not metformin directly inhibit the steroidogenic enzymes p450c17 and 3beta -hydroxysteroid dehydrogenase. J Biol Chem. 2001;276(20):16767–16771. doi: 10.1074/jbc.M100040200. [DOI] [PubMed] [Google Scholar]
- 63.Mu YM, Yanase T, Nishi Y, et al. Insulin sensitizer, troglitazone, directly inhibits aromatase activity in human ovarian granulosa cells. Biochem Biophys Res Commun. 2000;271(3):710–713. doi: 10.1006/bbrc.2000.2701. [DOI] [PubMed] [Google Scholar]
- 64.Tang T, Lord JM, Norman RJ, et al. Insulin-sensitising drugs (metformin, rosi-glitazone, pioglitazone, d-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. The Cochrane Database Syst Rev. 2012;5:CD003053. doi: 10.1002/14651858.CD003053.pub5. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003053.pub5/abstract. [DOI] [PubMed] [Google Scholar]
- 65.Abdollahi M, Cushman M, Rosendaal FR. Obesity: Risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost. 2003;89(3):493–498. [PubMed] [Google Scholar]
- 66.Narkiewicz K, Graniero GR, D’Este D, et al. Ambulatory blood pressure in mild hypertensive women taking oral contraceptives. A case-control study. Am J Hypertens. 1995;8(3):249–253. doi: 10.1016/0895-7061(95)96212-3. [DOI] [PubMed] [Google Scholar]
- 67.Ajossa S, Guerriero S, Paoletti AM, et al. The treatment of polycystic ovary syndrome. Minerva Ginecol. 2004;56(1):15–26. [PubMed] [Google Scholar]
- 68.Zulian E, Sartorato P, Benedini S, et al. Spironolactone in the treatment of poly-cystic ovary syndrome: Effects on clinical features, insulin sensitivity and lipid profile. J Endocrinol Invest. 2005;28(1):49–53. doi: 10.1007/BF03345529. [DOI] [PubMed] [Google Scholar]
- 69.Harmanci A, Cinar N, Bayraktar M, et al. Oral contraceptive plus antiandrogen therapy and cardiometabolic risk in polycystic ovary syndrome. Clin Endocrinol. 2013;78(1):120–125. doi: 10.1111/j.1365-2265.2012.04466.x. [DOI] [PubMed] [Google Scholar]
- 70.Diamanti-Kandarakis E, Mitrakou A, Raptis S, et al. The effect of a pure anti-androgen receptor blocker, flutamide, on the lipid profile in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83(8):2699–2705. doi: 10.1210/jcem.83.8.5041. [DOI] [PubMed] [Google Scholar]
- 71.Alberti KG, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on E et al: Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 72.Taponen S, Martikainen H, Jarvelin MR, et al. Hormonal profile of women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland birth cohort 1966 study. J Clin Endocrinol Metab. 2003;88(1):141–147. doi: 10.1210/jc.2002-020982. [DOI] [PubMed] [Google Scholar]
- 73.Randolph JF, Jr, Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: Relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88(4):1516–1522. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 74.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 75.Escobar-Morreale HF, Samino S, Insenser M, et al. Metabolic heterogeneity in polycys-tic ovary syndrome is determined by obesity: Plasma metabolomic approach using gcms. Clin Chem. 2012;58(6):999–1009. doi: 10.1373/clinchem.2011.176396. [DOI] [PubMed] [Google Scholar]
- 76.Bargiota A, Diamanti-Kandarakis E. The effects of old, new and emerging medicines on metabolic aberrations in pcos. Ther Adv Endocrinol Metab. 2012;3(1):27–47. doi: 10.1177/2042018812437355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duleba AJ. Medical management of metabolic dysfunction in pcos. Steroids. 2012;77(4):306–311. doi: 10.1016/j.steroids.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Renato P. Metformin in women with pcos, pros. Endocrine. 2015;48(2):422–426. doi: 10.1007/s12020-014-0311-1. [DOI] [PubMed] [Google Scholar]
- 79.Gower BA, Goss AM. A lower-carbohydrate, higher-fat diet reduces abdominal and intermuscular fat and increases insulin sensitivity in adults at risk of type 2 diabetes. J Nutr. 2015;145(1):177S–183S. doi: 10.3945/jn.114.195065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12(7):324–332. doi: 10.1016/j.molmed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 81.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tosi F, Negri C, Perrone F, et al. Hyperinsulinemia amplifies GNRH agonist stimulated ovarian steroid secretion in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97(5):1712–1719. doi: 10.1210/jc.2011-2939. [DOI] [PubMed] [Google Scholar]
- 83.Moran LJ, Misso ML, Wild RA, et al. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycys-tic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–363. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 84.Livadas S, Kollias A, Panidis D, et al. Diverse impacts of aging on insulin resistance in lean and obese women with polycystic ovary syndrome: Evidence from 1345 women with the syndrome. Eur Journal Endocr/Eur Fed Endocr Soc. 2014;171(3):301–309. doi: 10.1530/EJE-13-1007. [DOI] [PubMed] [Google Scholar]
- 85.Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358(1):47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- 86.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome p450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335(9):617–623. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 87.Diamanti-Kandarakis E, Alexandraki K, Piperi C, et al. Effect of metformin administration on plasma advanced glyca-tion end product levels in women with polycystic ovary syndrome. Metabolism: Clin Exp. 2007;56(1):129–134. doi: 10.1016/j.metabol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 88.Diamanti-Kandarakis E, Alexandraki K, Protogerou A, et al. Metformin administration improves endothelial function in women with polycystic ovary syndrome. Eur Journal Endocr/Eur Fed Endocr Soc. 2005;152(5):749–756. doi: 10.1530/eje.1.01910. [DOI] [PubMed] [Google Scholar]
- 89.Iwamoto Y, Kosaka K, Kuzuya T, et al. Effects of troglitazone: A new hypoglyce-mic agent in patients with NIDDM poorly controlled by diet therapy. Diabetes Care. 1996;19(2):151–156. doi: 10.2337/diacare.19.2.151. [DOI] [PubMed] [Google Scholar]
- 90. Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351(11):1106–1118. doi: 10.1056/NEJMra041001. A systematic review of insulin-sensitizing pharmacopeia in improving metabolic and reproductive outcomes in polycystic ovary syndrome (PCOS). This review comprehensively analyzed randomized controlled trials of mainstay and relatively novel therapeutics and their effect on all metabolic parameters with some discussion on treatment of metabolic syndrome (MBS).
- 91.Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: Interim report of a longitudinal cohort study. Diabetes Care. 2011;34(4):916–922. doi: 10.2337/dc10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Froment P, Touraine P. Thiazolidinediones and fertility in polycystic ovary syndrome (pcos) PPAR Res. 2006;2006(73986):8. doi: 10.1155/PPAR/2006/73986. Available from: http://www.hindawi.com.ezproxy.bu.edu/journals/ppar/2006/073986/abs/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robinson S, Henderson AD, Gelding SV, et al. Dyslipidaemia is associated with insulin resistance in women with polycystic ovaries. Clin Endocrinol. 1996;44(3):277–284. doi: 10.1046/j.1365-2265.1996.674495.x. [DOI] [PubMed] [Google Scholar]
- 94.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26(5):968–976. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 95.Kandaraki E, Chatzigeorgiou A, Piperi C, et al. Reduced ovarian glyoxalase-i activity by dietary glycotoxins and androgen excess: A causative link to polycystic ovarian syndrome. Mol Medicine. 2012;18:1183–1189. doi: 10.2119/molmed.2012.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Diamanti-Kandarakis E, Piperi C, Kalofoutis A, et al. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin Endocrinol. 2005;62(1):37–43. doi: 10.1111/j.1365-2265.2004.02170.x. [DOI] [PubMed] [Google Scholar]
- 97.Tantalaki E, Piperi C, Livadas S, et al. Impact of dietary modification of advanced glycation end products (ages) on the hormonal and metabolic profile of women with polycystic ovary syndrome (pcos) Hormones. 2014;13(1):65–73. doi: 10.1007/BF03401321. [DOI] [PubMed] [Google Scholar]
- 98.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(19):1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 99.Hobbs HH, Russell DW, Brown MS, et al. The ldl receptor locus in familial hypercholesterolemia: Mutational analysis of a membrane protein. Annu Rev Genet. 1990;24:133–170. doi: 10.1146/annurev.ge.24.120190.001025. [DOI] [PubMed] [Google Scholar]
- 100.Ishibashi S, Brown MS, Goldstein JL, et al. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92(2):883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grossman M, Rader DJ, Muller DW, et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995;1(11):1148–1154. doi: 10.1038/nm1195-1148. [DOI] [PubMed] [Google Scholar]
- 102.Austin MA, Hutter CM, Zimmern RL, et al. Genetic causes of monogenic heterozygous familial hypercholesterolemia: A huge prevalence review. Am J Epidemiol. 2004;160(5):407–420. doi: 10.1093/aje/kwh236. [DOI] [PubMed] [Google Scholar]
- 103.Steinberg D, Parthasarathy S, Carew TE, et al. Beyond cholesterol Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 104.Legro RS, Azziz R, Ehrmann D, et al. Minimal response of circulating lipids in women with polycystic ovary syndrome to improvement in insulin sensitivity with troglitazone. J Clin Endocrinol Metab. 2003;88(11):5137–5144. doi: 10.1210/jc.2003-030044. [DOI] [PubMed] [Google Scholar]
- 105.Berneis K, Rizzo M, Lazzarini V, et al. Atherogenic lipoprotein phenotype and low-density lipoproteins size and subclasses in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(1):186–189. doi: 10.1210/jc.2006-1705. [DOI] [PubMed] [Google Scholar]
- 106.Phelan N, O’Connor A, Kyaw-Tun T, et al. Lipoprotein subclass patterns in women with polycystic ovary syndrome (pcos) compared with equally insulin-resistant women without pcos. J Clin Endocrinol Metab. 2010;95(8):3933–3939. doi: 10.1210/jc.2009-2444. [DOI] [PubMed] [Google Scholar]
- 107.Macut D, Damjanovic S, Panidis D, et al. Oxidised low-density lipoprotein concentration - early marker of an altered lipid metabolism in young women with pcos. Eur Journal Endocr/Eur Fed Endocr Soc. 2006;155(1):131–136. doi: 10.1530/eje.1.02187. [DOI] [PubMed] [Google Scholar]
- 108.Conway GS, Agrawal R, Betteridge DJ, et al. Risk factors for coronary artery disease in lean and obese women with the polycystic ovary syndrome. Clin Endocrinol. 1992;37(2):119–125. doi: 10.1111/j.1365-2265.1992.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 109.Pugeat M, Nader N, Hogeveen K, et al. Sex hormone-binding globulin gene expression in the liver: Drugs and the metabolic syndrome. Mol Cell Endocrinol. 2010;316(1):53–59. doi: 10.1016/j.mce.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 110.Moll GW, Jr, Rosenfield RL. Testosterone binding and free plasma androgen concentrations under physiological conditions: Characterization by flow dialysis technique. J Clin Endocrinol Metab. 1979;49(5):730–736. doi: 10.1210/jcem-49-5-730. [DOI] [PubMed] [Google Scholar]
- 111.Rosenfield R, Moll G. The role of proteins in the distribution of plasma androgens and estradiol. New York (NY): Androgenization in Women Raven Press; 1983. pp. 25–45. [Google Scholar]
- 112.Schwimmer JB, Khorram O, Chiu V, et al. Abnormal aminotransferase activity in women with polycystic ovary syndrome. Fertil Steril. 2005;83(2):494–497. doi: 10.1016/j.fertnstert.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 113.Setji TL, Holland ND, Sanders LL, et al. Nonalcoholic steatohepatitis and nonalcoholic fatty liver disease in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(5):1741–1747. doi: 10.1210/jc.2005-2774. [DOI] [PubMed] [Google Scholar]
- 114.Cerda C, Perez-Ayuso RM, Riquelme A, et al. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. J Hepatol. 2007;47(3):412–417. doi: 10.1016/j.jhep.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 115.Erbey JR, Silberman C, Lydick E. Prevalence of abnormal serum alanine aminotransferase levels in obese patients and patients with type 2 diabetes. Am J Med. 2000;109(7):588–590. doi: 10.1016/s0002-9343(00)00602-1. [DOI] [PubMed] [Google Scholar]
- 116.Economou F, Xyrafis X, Livadas S, et al. In overweight/obese but not in normal-weight women, polycystic ovary syndrome is associated with elevated liver enzymes compared to controls. Hormones. 2009;8(3):199–206. doi: 10.14310/horm.2002.1236. [DOI] [PubMed] [Google Scholar]
- 117. Jovanovic VP, Carmina E, Lobo RA. Not all women diagnosed with pcos share the same cardiovascular risk profiles. Fertil Steril. 2010;94(3):826–832. doi: 10.1016/j.fertnstert.2009.04.021. One of the few studies to examine the available literature relevant to risk profile differences among polycystic ovary syndrome (PCOS) phenotypes. This review offers insight into the consideration of PCOS phenotype when assessing metabolic risk.
- 118.Wang ET, Cirillo PM, Vittinghoff E, et al. Menstrual irregularity and cardiovascular mortality. J Clin Endocrinol Metab. 2011;96(1):E114–E118. doi: 10.1210/jc.2010-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Groot PC, Dekkers OM, Romijn JA, et al. Pcos, coronary heart disease, stroke and the influence of obesity: A systematic review and meta-analysis. Hum Reprod Update. 2011;17(4):495–500. doi: 10.1093/humupd/dmr001. [DOI] [PubMed] [Google Scholar]
- 120.Boulman N, Levy Y, Leiba R, et al. Increased c-reactive protein levels in the polycystic ovary syndrome: A marker of cardiovascular disease. J Clin Endocrinol Metab. 2004;89(5):2160–2165. doi: 10.1210/jc.2003-031096. [DOI] [PubMed] [Google Scholar]
- 121.Tarkun I, Arslan BC, Canturk Z, et al. Endothelial dysfunction in young women with polycystic ovary syndrome: relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab. 2004;89(11):5592–5596. doi: 10.1210/jc.2004-0751. [DOI] [PubMed] [Google Scholar]
- 122.Kravariti M, Naka KK, Kalantaridou SN, et al. Predictors of endothelial dysfunction in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(9):5088–5095. doi: 10.1210/jc.2005-0151. [DOI] [PubMed] [Google Scholar]
- 123.Carmina E, Orio F, Palomba S, et al. Endothelial dysfunction in pcos: Role of obesity and adipose hormones. Am J Med. 2006;119(4):e351–e356. doi: 10.1016/j.amjmed.2005.10.059. [DOI] [PubMed] [Google Scholar]
- 124.Diamanti-Kandarakis E, Spina G, Kouli C, et al. Increased endothelin-1 levels in women with polycystic ovary syndrome and the beneficial effect of metformin therapy. J Clin Endocrinol Metab. 2001;86(10):4666–4673. doi: 10.1210/jcem.86.10.7904. [DOI] [PubMed] [Google Scholar]
- 125.Talbott EO, Zborowski JV, Rager JR, et al. Evidence for an association between metabolic cardiovascular syndrome and coronary and aortic calcification among women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(11):5454–5461. doi: 10.1210/jc.2003-032237. [DOI] [PubMed] [Google Scholar]
- 126.Shroff R, Kerchner A, Maifeld M, et al. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J Clin Endocrinol Metab. 2007;92(12):4609–4614. doi: 10.1210/jc.2007-1343. [DOI] [PubMed] [Google Scholar]
- 127.Legro RS. Metformin during pregnancy in polycystic ovary syndrome: another vitamin bites the dust. J Clin Endocrinol Metab. 2010;95(12):5199–5202. doi: 10.1210/jc.2010-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diamanti-Kandarakis E, Christakou CD, Kandaraki E, et al. Metformin: an old medication of new fashion: Evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur Journal Endocr/Eur Fed Endocr Soc. 2010;162(2):193–212. doi: 10.1530/EJE-09-0733. [DOI] [PubMed] [Google Scholar]
- 129.Azziz R, Ehrmann D, Legro RS, et al. Group PCTS. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: A multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2001;86:41626–1632. doi: 10.1210/jcem.86.4.7375. [DOI] [PubMed] [Google Scholar]
- 130.Dunaif A, Scott D, Finegood D, et al. The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81(9):3299–3306. doi: 10.1210/jcem.81.9.8784087. [DOI] [PubMed] [Google Scholar]
- 131.Ciotta L, Calogero AE, Farina M, et al. Clinical, endocrine and metabolic effects of acarbose, an alpha-glucosidase inhibitor, in pcos patients with increased insulin response and normal glucose tolerance. Hum Reprod. 2001;16(10):2066–2072. doi: 10.1093/humrep/16.10.2066. [DOI] [PubMed] [Google Scholar]
- 132.Penna IA, Canella PR, Reis RM, et al. Acarbose in obese patients with polycystic ovarian syndrome: A double-blind, randomized, placebo-controlled study. Hum Reprod. 2005;20(9):2396–2401. doi: 10.1093/humrep/dei104. [DOI] [PubMed] [Google Scholar]
- 133.Tugrul S, Kutlu T, Pekin O, et al. Clinical, endocrine, and metabolic effects of acarbose, a alpha-glucosidase inhibitor, in overweight and nonoverweight patients with polycystic ovarian syndrome. Fertil Steril. 2008;90(4):1144–1148. doi: 10.1016/j.fertnstert.2007.07.1326. [DOI] [PubMed] [Google Scholar]
- 134.Hanjalic-Beck A, Gabriel B, Schaefer W, et al. Metformin versus acarbose therapy in patients with polycystic ovary syndrome (pcos): A prospective randomised double-blind study. Gynecol Endocrinol. 2010;26(9):690–697. doi: 10.3109/09513591003686379. [DOI] [PubMed] [Google Scholar]
- 135.Araujo Penna I, Canella PR, Vieira CS, et al. Cardiovascular risk factors are reduced with a low dose of acarbose in obese patients with polycystic ovary syndrome. Fertil Steril. 2007;88(2):519–522. doi: 10.1016/j.fertnstert.2006.11.073. [DOI] [PubMed] [Google Scholar]
- 136.Diamanti-Kandarakis E, Katsikis I, Piperi C, et al. Effect of long-term orlistat treatment on serum levels of advanced glyca-tion end-products in women with polycystic ovary syndrome. Clin Endocrinol. 2007;66(1):103–109. doi: 10.1111/j.1365-2265.2006.02693.x. [DOI] [PubMed] [Google Scholar]
- 137. Pizzo A, Lagana AS, Barbaro L. Comparison between effects of myo-inositol and d-chiro-inositol on ovarian function and metabolic factors in women with pcos. Gynecol Endocrinol. 2014;30(3):205–208. doi: 10.3109/09513590.2013.860120. A novel investigation into the efficacy of myo-inositol and D-chiro-inositol in improving ovarian function and metabolic profiles among women with poly-cystic ovary syndrome (PCOS). This investigation sheds light on the differential activity of these isoforms and how they may be useful in the management of metabolic syndrome (MBS) and infertility in PCOS.
- 138.Lagana AS, Barbaro L, Pizzo A. Evaluation of ovarian function and metabolic factors in women affected by polycystic ovary syndrome after treatment with d-chiro-inositol. Arch Gynecol Obstet. 2015;291(5):1181–1186. doi: 10.1007/s00404-014-3552-6. [DOI] [PubMed] [Google Scholar]
- 139.Kotsa K, Yavropoulou MP, Anastasiou O, et al. Role of vitamin d treatment in glucose metabolism in polycystic ovary syndrome. Fertil Steril. 2009;92(3):1053–1058. doi: 10.1016/j.fertnstert.2008.07.1757. [DOI] [PubMed] [Google Scholar]
- 140.Selimoglu H, Duran C, Kiyici S, et al. The effect of vitamin d replacement therapy on insulin resistance and andro-gen levels in women with polycystic ovary syndrome. J Endocrinol Invest. 2010;33(4):234–238. doi: 10.1007/BF03345785. [DOI] [PubMed] [Google Scholar]
- 141.Oulmouden A, Karst F. Isolation of the erg12 gene of saccharomyces cerevisiae encoding mevalonate kinase. Gene. 1990;88(2):253–257. doi: 10.1016/0378-1119(90)90039-t. [DOI] [PubMed] [Google Scholar]
- 142.Economou F, Xyrafis X, Christakou C, et al. The pluripotential effects of hypolipidemic treatment for polycystic ovary syndrome (pcos): Dyslipidemia, cardiovascular risk factors and beyond. Curr Pharm Des. 2011;17(9):908–921. doi: 10.2174/138161211795428821. [DOI] [PubMed] [Google Scholar]
- 143.Hosokawa A, Bar-Oz B, Ito S. Use of lipid-lowering agents (statins) during pregnancy. Can Fam Physician Medecin De Famille Canadien. 2003;49:747–749. [PMC free article] [PubMed] [Google Scholar]
- 144.Sun J, Yuan Y, Cai R, et al. An investigation into the therapeutic effects of statins with metformin on polycystic ovary syndrome: A meta-analysis of randomised controlled trials. BMJ Open. 2015;5(3):e007280. doi: 10.1136/bmjopen-2014-007280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eyvazzadeh AD, Pennington KP, Pop-Busui R, et al. The role of the endogenous opioid system in polycystic ovary syndrome. Fertil Steril. 2009;92(1):1–12. doi: 10.1016/j.fertnstert.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 146.Sathyapalan T, Kilpatrick ES, Coady AM, et al. The effect of atorvastatin in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled study. J Clin Endocrinol Metab. 2009;94(1):103–108. doi: 10.1210/jc.2008-1750. [DOI] [PubMed] [Google Scholar]
- 147.Lanzone A, Apa R, Fulghesu AM, et al. Long-term naltrexone treatment normalizes the pituitary response to gonado-tropin-releasing hormone in polycystic ovarian syndrome. Fertil Steril. 1993;59(4):734–737. doi: 10.1016/s0015-0282(16)55851-8. [DOI] [PubMed] [Google Scholar]
- 148.Guido M, Romualdi D, Lanzone A. Role of opioid antagonists in the treatment of women with glucoregulation abnormalities. Curr Pharm Des. 2006;12(8):1001–1012. doi: 10.2174/138161206776055895. [DOI] [PubMed] [Google Scholar]
- 149.Jensterle M, Kravos NA, Pfeifer M, et al. A 12-week treatment with the long-acting glucagon-like peptide 1 receptor agonist liraglutide leads to significant weight loss in a subset of obese women with newly diagnosed polycystic ovary syndrome. Hormones. 2015;14(1):81–90. doi: 10.1007/BF03401383. [DOI] [PubMed] [Google Scholar]
- 150.Romualdi D, De Marinis L, Campagna G, et al. Alteration of ghrelin-neuropeptide y network in obese patients with polycystic ovary syndrome: role of hyperinsulinism. Clin Endocrinol. 2008;69(4):562–567. doi: 10.1111/j.1365-2265.2008.03204.x. [DOI] [PubMed] [Google Scholar]
- 151.Kupeli Akkol E, Ilhan M, Ayse Demirel M, et al. Thuja occidentalis l. And its active compound, alpha-thujone: Promising effects in the treatment of polycystic ovary syndrome without inducing osteoporosis. J Ethnopharmacol. 2015;168:25–30. doi: 10.1016/j.jep.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 152.Vazquez MJ, Romero-Ruiz A, Tena-Sempere M. Roles of leptin in reproduction, pregnancy and polycystic ovary syndrome: Consensus knowledge and recent developments. Metabolism: Clin Exp. 2015;64(1):79–91. doi: 10.1016/j.metabol.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 153.Jedel E, Labrie F, Oden A, et al. Impact of electro-acupuncture and physical exercise on hyperandrogenism and oligo/amenor-rhea in women with polycystic ovary syndrome: A randomized controlled trial. Am J Physiol Endocrinol Metabol. 2011;300(1):E37–E45. doi: 10.1152/ajpendo.00495.2010. [DOI] [PubMed] [Google Scholar]
- 154.Novick G, Reid AE, Lewis J, et al. Group prenatal care. Model fidelity and outcomes. Am J Obstet Gynecol. 2013;2112(209):e111–e116. doi: 10.1016/j.ajog.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Haqq L, McFarlane J, Dieberg G, et al. Effect of lifestyle intervention on the reproductive endocrine profile in women with polycystic ovarian syndrome: A systematic review and meta-analysis. Endocr Connections. 2014;3(1):36–46. doi: 10.1530/EC-14-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Florakis D, Diamanti-Kandarakis E, Katsikis I, et al. Effect of hypocaloric diet plus sibutramine treatment on hormonal and metabolic features in overweight and obese women with polycystic ovary syndrome: A randomized, 24-week study. Int J Obes (Lond) 2008;32(4):692–699. doi: 10.1038/sj.ijo.0803777. [DOI] [PubMed] [Google Scholar]
- 157. Hutchison SK, Stepto NK, Harrison CL, et al. Effects of exercise on insulin resistance and body composition in overweight and obese women with and without poly-cystic ovary syndrome. J Clin Endocrinol Metab. 2011;96(1):E48–E56. doi: 10.1210/jc.2010-0828. This study offers insight into the pathophysiological role of insulin resistance in the development of central obesity in women with polycystic ovary syndrome (PCOS). The findings also highlight the efficacy of exercise in improving metabolic profile among women with both PCOS and insulin resistance.
- 158.Harrison CL, Lombard CB, Moran LJ, et al. Exercise therapy in polycystic ovary syndrome: A systematic review. Hum Reprod Update. 2011;17(2):171–183. doi: 10.1093/humupd/dmq045. [DOI] [PubMed] [Google Scholar]
- 159.Mahoney D. Lifestyle modification intervention among infertile overweight and obese women with polycystic ovary syndrome. J Am Assoc Nurse Pract. 2014;26(6):301–308. doi: 10.1002/2327-6924.12073. [DOI] [PubMed] [Google Scholar]
- 160.Costello MF, Ledger WL. Evidence-based lifestyle and pharmacological management of infertility in women with polycystic ovary syndrome. Women’s Health (Lond Engl) 2012;8(3):277–290. doi: 10.2217/whe.12.14. [DOI] [PubMed] [Google Scholar]
- 161.Karimzadeh MA, Javedani M. An assessment of lifestyle modification versus medical treatment with clomiphene citrate, metformin, and clomiphene citrate-metformin in patients with polycystic ovary syndrome. Fertil Steril. 2010;94(1):216–220. doi: 10.1016/j.fertnstert.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 162.Panidis D, Tziomalos K, Papadakis E, et al. The role of orlistat combined with lifestyle changes in the management of overweight and obese patients with polycystic ovary syndrome. Clin Endocrinol. 2014;80(3):432–438. doi: 10.1111/cen.12305. [DOI] [PubMed] [Google Scholar]
- 163.Rajagopal G, Reddy AP, Venkata Harinarayan C, et al. Effect of lifestyle modification and metformin therapy on emerging cardiovascular risk factors in overweight Indian women with polycystic ovary syndrome. Metab Syndr Relat Disord. 2012;10(4):273–279. doi: 10.1089/met.2011.0127. [DOI] [PubMed] [Google Scholar]
- 164.Bates GW, Legro RS. Long-term management of polycystic ovarian syndrome (pcos) Mol Cell Endocrinol. 2013;373(1– 2):91–97. doi: 10.1016/j.mce.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hoeger K, Davidson K, Kochman L, et al. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93(11):4299–4306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]