To the Editor
Complex bacteriotherapy, such as fecal microbiota transplantation (FMT), is an emerging therapeutic modality for ulcerative colitis (UC) (1). FMT has been implicated in facilitating the withdrawal of conventional therapies in select patients (2). In our study, three immunotherapy (infliximab, 6-mercaptopurine, and steroid, respectively)-dependent pediatric (14–16 years of age) UC patients (Table 1) received a tapering course (22–30 treatments) of FMT delivered by means of colonoscopy and enemas during a 6–12-week period. The phase 1, open-label protocol was approved by the Institutional Review Board of Baylor College of Medicine (H-30591). The protocol is currently approved by the FDA (IND-15743; ClinicalTrials.gov number: NCT01947101). Filtered, frozen, and thawed stool specimens from a standardized single donor (a 37-year-old man) for all three patients was used, which provided a unique opportunity for examining microbial changes. Patients were concomitantly withdrawn from their conventional medications. Mucosal disease activity was assessed before and 2 weeks after the FMT series. Clinical disease activity was followed by the Pediatric Ulcerative Colitis Activity Index (PUCAI). The FMT series was well tolerated and transiently supported immunotherapy withdrawal (Supplementary Figure 1 online). FMT enabled all three patients to be symptom-free for at least 4 weeks following FMT and supported the withdrawal of immunotherapy (no treatment other than mesalamine) for more than 105 days in all. The number of FMT treatments significantly correlated with the time of being immunotherapy-free (r=0.998, P=0.04; Supplementary Figure 2). All patients were in endoscopic and histologic remission 2 weeks after the last FMT (Supplementary Figures 3–4).
Table 1. Patient characteristics and clinical outcomes after sequential FMT.
| Patients (age, gender) | Disease behavior | Mayo score | Tx after Dx | Mayo score | FMT # | Remission during FMT (in days) | Mayo score | Remission after last medication(days) | Remission after last FMT (days) | Tx following flare |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| At Dx | Before FMT | After FMT | ||||||||
| 1 (16y M) | Pancolitis | 2 | IFX | 0 | 30 | 65/70 (93%) | 0 | 261 | 126 | IFX |
| 2 (15y M) | Pancolitis | 2 | 6MP | 1 | 25 | 58/58 (100%) | 0 | 159 | 80 | PREDFMT |
| 3 (14y F) | Pancolitis | 3 | PRED | 0 | 22 | 36/36 (100%) | 0 | 105 | 79 | PREDFMT colectomy |
FMT, fecal microbiota transplantation; IFX, infliximab; 6MP, 6-mercaptopurine; PRED, prednisone.
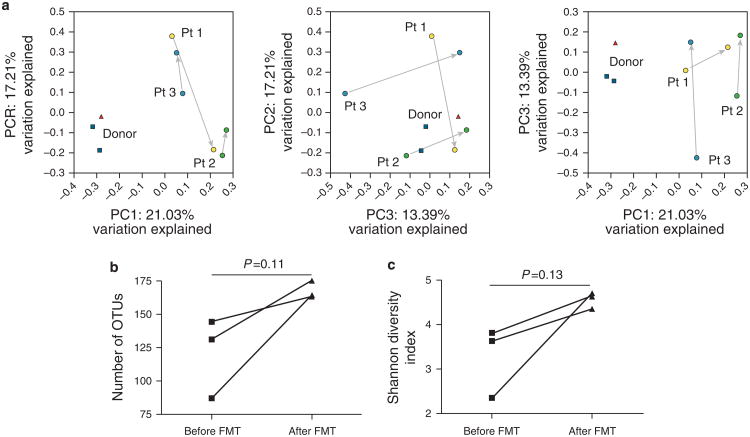
Fecal microbiomes were analyzed by massively parallel pyrosequencing of the V3V5 regions within the bacterial 16S rRNA gene. The nature of microbiota shifts differed, presumably because of differences in baseline composition of intestinal microbiota in each patient (detected by principal-coordinates-analysis, Figure 1a). Recipient microbiomes remained distinct from that of the anonymous donor. The FMT series appeared to induce a transient engraftment of the donor microbiome in a recipient (Supplementary Figure 5). Microbiome richness (Figure 1b) and diversity (Figure 1c) increased secondary to FMT. Fifteen operational taxonomic units (OTUs or bacterial taxa) consistently changed in relative abundance in all 3 patients following FMT (P<0.25; Supplementary Table 1). Six of 8 OTUs that increased in abundance were not detected in the donor or the recipient prior to FMT. Therefore, expansion of rare taxa may be functionally important in restoring colonic health, at least for short-term periods, following FMT. Of the OTUs that were increased in abundance 61.5% belonged to the Lachnospiraceae family. The abundance of Lachnospiraceae has been inversely correlated with UC disease activity (3), and those were more abundant in healthy members of monozygotic twin pairs discordant for UC, compared with control (4). At the genus level, only Coprococcus changed (increased) in abundance by more than twofold. The abundance of Coprococcus (a genus including butyrate-producing bacteria) has been found to be decreased in inflammatory bowel disorder (IBD) patients (5). Therefore, the increased abundance of Coprococcus and Lachnospiraceae upon the FMT series may have delivered beneficial effects to the colonic epithelium of the UC patients (recipients).
Figure 1.
Fecal microbiota shifts following fecal microbiota transplantation (FMT). Principle coordinates analysis of unweighted Unifrac distances (a) revealed that microbial community changes during FMT (arrows connect pre-FMT (within 24 h before first FMT) and post-FMT (2–3 weeks after last FMT) samples from each patient) were not consistent within each patient (did not shift in the same direction for each patient), and post-FMT fecal communities of recipients (patients) following FMT were dissimilar from that of the donor (▲: donor stool; ■: donor preparations from independent bowel movements on separate days). Microbial richness (b) and diversity (c) in terms of microbial taxa consistently increased following the FMT, although these trends did not reach statistical significance. OTU, operational taxonomic units.
Colonic mucosal gene expression profiles in response to the FMT series were studied by RNA sequencing. The expression of 742 genes decreased and that of 12 increased (>1.5-fold change in expression, false discovery rate<0.05) upon FMT therapy (Supplementary Table 2–3). Importantly, the suppression of human gene expression relevant in leukocyte activation and mitotic cell cycle progression was observed (Supplementary Figure 6–7). These molecular findings were associated with >50% decline in epithelial cell mitosis in two out of the three patients (Supplementary Figure 8).
In conclusion, this report describes high-intensity FMT as a strategy to reset the intestinal microbiota in pediatric IBD. Serial FMT in pediatric UC may induce beneficial changes in patient microbiota and colonic mucosa. Randomized trials will be required in the future to answer many challenging questions (donor selection, patient selection, number and length of FMT therapy required, and so on) with respect to the clinical application of this treatment.
Supplementary Material
Acknowledgments
We thank the patients and families for participating, the Texas Children's Hospital Research Resources Office (Supriya Parikh, Susan Blaney, and Lisa Bomgaars), the Gutsy Kids Fund, including philanthropic donation from the Karen and Brock Wagner family, and the support of the Houston Men of Distinction.
Guarantor of the article: Richard Kellermayer, MD, PhD.
Financial support: This study received support from the Gutsy Kids Fund including philanthropic donation from the Karen and Brock Wagner family, and support from the Houston Men of Distinction. The work in JV's laboratory is supported in part by research support from the National Institutes of Health (NIH; UH3 DK083990 and U01 CA170930). The authors also acknowledge support from the NIH for the Texas Medical Center Digestive Diseases Center (P30 DK56338).
Footnotes
Supplementary Material: is linked to the online version of the paper at http://www.nature.com/ajg
Specific author contributions: Study concept and design: RK, DN-S, RAL, MP, MAG, JV; acquisition of data: RK, DN-S, DS, SAVM, MEL; analysis and interpretation of data: RK, DN-S, RAH, RAL, JB, EBH, JV; drafting of the manuscript: RK, DN-S, EBH; critical revision of the manuscript for important intellectual content: RK, MEL, EBH, JB, JV; statistical analysis: RK, RAH, EBH; obtained funding: RK, JV; technical or material support: MP, JV; study supervision: RK, JV.
Conflict of Interest: Potential competing interests: None.
References
- 1.Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2014;8:1569–81. doi: 10.1016/j.crohns.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunde S, Pham A, Bonczyk S, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:597–601. doi: 10.1097/MPG.0b013e318292fa0d. [DOI] [PubMed] [Google Scholar]
- 3.Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108:1620–30. doi: 10.1038/ajg.2013.257. [DOI] [PubMed] [Google Scholar]
- 4.Lepage P, Hasler R, Spehlmann ME, et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology. 2011;141:227–36. doi: 10.1053/j.gastro.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Wang W, Zhou R, et al. Characteristics of fecal and mucosa-associated microbiota in chinese patients with inflammatory bowel disease. Medicine. 2014;93:e51. doi: 10.1097/MD.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.