Abstract
Cytomegalovirus (CMV) is a ubiquitous human herpes virus, which, after often asymptomatic primary infection, establishes a lifelong latent infection that can periodically be reactivated in both immunocompetent and immunosuppressed carriers. Whereas the diagnostic approach in case of a suspicion of CMV reactivation is well defined, the indication for antiviral therapy can often only be made in the context of an extent of organ involvement, the immune status, and comorbidities of the patient. This article reviews the epidemiology, diagnosis, and therapy of CMV reactivation with a focus on inflammatory bowel diseases and potentially different diagnostic and therapeutic approaches in Asia and the Western world.
Key Words: Cytomegalovirus, Inflammatory bowel diseases, Immunosuppression, Antiviral therapy, Anti-TNF therapy
Introduction
Human cytomegalovirus (CMV; human β-herpesvirus type 5), which has a genome with the size of 235 kbp, is transmitted through blood, body fluids, or transplanted organs. Infection may also be acquired transplacentally or during birth [1, 2]. A primary infection in children or adults is often asymptomatic or only accompanied by mild mononucleosis-like symptoms. Like other members of the Herpesvirus family, CMV establishes a latent infection after the resolution of the acute infection. A productive (lytic) infection leads to the synthesis of immediate-early, early, and late viral proteins [3].
Viral DNA has been detected in monocytes, dendritic cells, megakaryocytes, and myeloid progenitor cells in the bone marrow [4]. In general, there have been many reports regarding manifestations of CMV infection in immunocompromised hosts, but little attention has been paid to these manifestations in immunocompetent patients because acquired infections (reactivation) are often asymptomatic [5]. Reactivation of CMV may occur at any time during the life of the human host, although the risk is higher in the setting of systemic immunosuppression, either iatrogenic or secondary to underlying medical conditions, such as acquired immunodeficiency syndrome (AIDS), a status after organ transplantation or inflammatory bowel diseases (IBD). Under conditions of reduced immune responses, CMV can cause acute systemic infections with replication in virtually any organ. Fibroblasts, epithelial cells, smooth muscle cells, macrophages, and endothelial cells were found to be the predominant target of the CMV infection in the lung, gastrointestinal and placental tissues. In gastrointestinal CMV disease, the pathogenesis of intestinal lesions associated with CMV infection is very complicated because the endoscopic appearance and location of the lesions are similar despite the cause of the host's immunodeficiency. Particularly, many physicians focus on CMV reactivation in patients with IBD because CMV may play a crucial role in triggering the onset of intestinal inflammation. However, it is well known that the role of CMV activation as a major trigger of inflammation or as a merely innocent bystander is still debated [6]. This review highlights diagnostic and therapeutic strategies for CMV infections in Asia and the Western hemisphere, but with a main focus on CMV colitis.
CMV Epidemiology
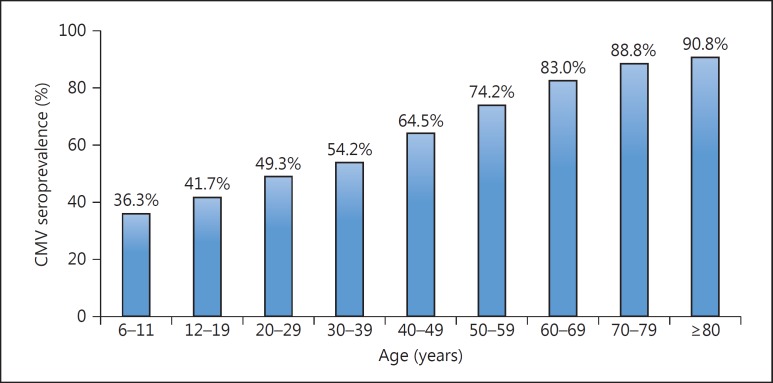
CMV has been cultured from multiple sites, including the urine, blood, throat, cervix, semen, stool, tears, and breast milk [7, 8]. Transmission can occur via multiple routes: sexual exposure, close contact, blood, tissue exposure, occupational exposure, and perinatal exposure. It is generally considered that CMV infection increases with age, and that 40–100% of adults are carriers of CMV (fig. 1). A positive CMV seroprevalence also varies by race, ethnicity, and socioeconomic status. In a recent review of the literature about the worldwide CMV seroprevalence, the highest rates can be found in South America, Africa, and Asia and the lowest in Western Europe and in the United States [9]. In the US, a positive CMV serostatus (IgG) was found to be more prevalent among Mexican American children with foreign-born householders than among children with native-born householders [10]. A country's socioeconomic development correlates inversely with CMV seroprevalence, with the highest rates observed in developing countries throughout Africa and Asia [11]. In Japan, an interesting study has recently assessed CMV seroprevalence in pregnant women [12]. This cross-sectional study included pregnant Japanese women who gave birth between 2003 and 2012 at the hospital (n = 15,616). Data showed that the overall CMV IgG prevalence rate was 66.0%. CMV IgG prevalence significantly decreased over the course of the 10 years from 2003 to 2012 (from 69.9% in 2003 to 65.2% in 2012; p < 0.001). They concluded that calendar year, maternal age, and parity were significantly associated with changes in CMV seroprevalence among this population. The percentage of patients who are seropositive for CMV in Japan might decrease little by little.
Fig. 1.
Age-adjusted CMV seroprevalence in the noninstitutionalized, civilian population of the US (n = 21,639) published in the third National Health and Nutrition Examination Survey (1988–1994) [78].
CMV Diagnosis Methods
There are several possible diagnostic approaches for suspected CMV infection. Each approach has advantages and disadvantages (table 1).
Table 1.
Characteristics, costs, and problems of diagnostic tests for the detection of CMV (modified, based on Kandiel and Lashner [57])
| Test | Test material | Sensitivity, % | Specificity, % | Cost | Problem |
|---|---|---|---|---|---|
| Histology (H&E) | Biopsy | 10–87 | 92–100 | $ | Sampling error |
| Histology (IHC) | Biopsy | 78–93 | 92–100 | $$ | Sampling error |
| CMV culture | Blood, tissue, urine, saliva, or respiratory swabs | 45–78 | 89–100 | $$ | 1–3 weeks incubation time |
| CMV-DNA PCR | Plasma, stool, biopsy | 65–100 | 40–92 | $$$ | Qualitative PCR: differentiating between CMV infection and disease is difficult |
| Quantitative PCR: no standardized technique, cutoff not defined | |||||
| CMV IgM (serum) | Blood | 100 | 99 | $$ | May not be present in immunocompromised patients |
| CMV IgG (serum) | Blood | 98–100 | 96–99 | $$ | Fourfold increase between two separate titers |
| CMV antigen | Blood, cerebrospinal fluid | 60–100 | 83–100 | $ | Semiquantitative |
H&E = Hematoxylin and eosin stain.
1 Isolation culture: this is a direct culture system with human fetal lung fibroblasts used to proof CMV infection by visualizing typical cytopathic effects of the virus. The problem with this procedure is that cytopathic effects often evolve very slowly and it can take up to 21 days to visualize these structural changes in culture cells. Also, it is well known that this kind of technique lacks sensitivity [13].
2 Serology: CMV infection is diagnosed by comparing the antibody titer at the acute stage with the titer of the recovery stage. CMV-specific IgM titers will rise 2–6 weeks after primary infection. The titer of IgM antibody usually decreases 2–3 months after the acute primary infection in healthy people; however, it rarely reappears during CMV reactivation (the frequency is 0.1–2%). A more than fourfold increase in CMV-specific IgG antibody is considered to be one of the criteria for the diagnosis of CMV infection or reactivation and, therefore, paired serum samples obtained at least 2–4 weeks apart are needed [14, 15]. Generally, the measurement of CMV-specific antibodies is of limited value in the evaluation of CMV reactivation from CMV carriers.
3 CMV antigenemia: this method detects the antigen of CMV in the specimen without separating CMV from tissues. The infected cells are detected by an immunofluorescent technique using the antibody against the immediate-early antigen and the CMV pp65 antigen of CMV [14]. The CMV antigenemia method has a sensitivity of 60–100% and a specificity of 83–100% [16]. In general, the detection of antigen (pp65)-positive cells in peripheral blood cells reflects active reactivation of CMV; however, the positive finding of CMV antigenemia does not necessarily reflect CMV infection-induced organ damages. Therefore, the positivity of antigenemia does not necessarily reflect the necessity of anti-viral treatments either.
4 Histopathological diagnosis of CMV: the gold standard of diagnosing CMV infection in each organ is to ascertain the presence of cytomegalic cells on histology. Typical CMV-infected cells increase in size and contain cytomegalic inclusion bodies with a halo, which gives the cell an owl's eye appearance [17, 18, 19]. It is well known that CMV infection not only results in an inclusion body in the nucleus, but a similar inclusion body can also be found in the cytoplasm (cytoplasmic inclusion body). The sensitivity of CMV detection in tissue specimens has been improved by immunoperoxidase or immunofluorescence staining for CMV antigens using monoclonal antibodies and/or in situ DNA hybridization.
5 Diagnosis of CMV by polymerase chain reaction (PCR) analysis: qualitative and quantitative PCR has been used to identify CMV-DNA in the urine, stool, blood, and tissues [20, 21, 22, 23, 24]. The PCR analysis has a higher sensitivity for diagnosing CMV infection and monitoring the viral load than CMV antigenemia. However, the high sensitivity of the PCR assay may result in low specificity for diagnosing active CMV infection because already very few copies of CMV-DNA can be detected, which may have no clinical significance but only points towards a local low level reactivation [25].
In Japan, many physicians use the pp65 antigen assay and histologic examination for the diagnosis of CMV infection in clinical practice, whereas the use of blood and tissue PCR for the diagnosis of CMV is limited. In the US, the diagnostic approach depends on the suspected organ involvement. If systemic CMV infection is suspected, quantitative PCR in plasma is currently considered the gold standard. In cases of questionable organ involvement, such as the liver or gut, most of the time immunohistochemistry (IHC) on biopsy specimen is performed, but in patients with IBD in clinical practice, qualitative PCR for CMV-DNA is increasingly executed on colonic biopsies. However, in IBD the most accurate approach for the detection of clinically significant CMV infection has not been firmly established and current guidelines are not consistent on this topic [26, 27, 28].
CMV Disease
The spectrum of human illness caused by CMV is diverse and dependent on the host. CMV infections in immunocompromised patients cause substantial morbidity and mortality. On the other hand, infection in the immunocompetent host is generally asymptomatic or may present as a mononucleosis syndrome. Disease localized to a single organ has been described in immunocompetent hosts, even presenting as a fulminant, multisystem disorder. However, these cases are rare and limited to small series and case reports [29, 30]. In general, most of the time, CMV disease results from reactivation of latent disease in the immunocompromised host. However, we should keep in mind that reactivation of CMV can result in the shedding of the virus in the urine, stool, or other body fluids, but does not always indicate disease. For that reason, in the literature, a distinction is made between CMV infection where a person is CMV positive on PCR or serological testing, and CMV disease where clinical symptoms are manifest [31].
CMV Hepatitis
The clinical picture is often not specific, but prolonged unexplained fever and malaise may accompany CMV hepatitis. Liver function test abnormalities are characterized by mild transaminitis with increases in aspartate aminotransferase, alanine aminotransferase and lactate dehydrogenase, whereas the bilirubin can be completely normal or only mild-to-moderately elevated. Portal vein thrombosis has also been described as a rare complication of acute CMV-associated hepatitis [32, 33]. CMV infection is also a differential diagnosis in cases of granulomatous hepatitis [34, 35].
CMV Colitis
Gastrointestinal involvement with CMV is uncommon in immunocompetent hosts, while it can cause significant morbidity and mortality in immunocompromised host [36, 37, 38]. CMV colitis is the second most common manifestation of end-organ disease following CMV retinitis. Most of the time, CMV colitis is due to the reactivation of a latent infection in immunosuppressed patients, but can also occur in the immunocompetent host in the setting of a primary infection.
The clinical manifestation of CMV colitis is associated with low-grade fever, weight loss, anorexia, malaise, and abdominal pain. Watery diarrhea and hematochezia are common. Extensive mucosal hemorrhage and perforation related to CMV infection can be life-threatening complications. Therefore, antiviral treatment should be considered for CMV-related colitis in immunocompromised hosts, particularly AIDS patients.
Clinical Characteristics of CMV Colitis in the Immunocompetent Host
Klauber et al. [39] reported 15 immunocompetent adults with CMV colitis. Seven patients had probable primary CMV infection based upon seroconversion at the time of the diagnosis, detection of CMV-specific IgM antibodies, or positive viral cultures in the absence of detectable antibodies. Diarrhea, fever, and abdominal pain were common presenting symptoms. Among patients complaining of diarrhea, grossly hematochezia was seen in 53%. Therefore, we must deliberately diagnose CMV colitis in older adult patients presenting with bloody diarrhea and abdominal pain, which could mimic clinical features of ischemic colitis. Seo et al. [40] reported endoscopic findings of 12 immunocompetent adults with CMV colitis. The most commonly identified endoscopic abnormalities in this paper were defined as well-demarcated ulcerations (50%), ulcero-infiltrative changes (25%), and formation of pseudomembranes (25%). Since the majority of immunocompetent patients with CMV disease recover without intervention, it is very difficult to prove whether antiviral therapy has a significant impact on the clinical outcome as well as on IBD patients (as mentioned below). Thus, the severity and potential morbidity of CMV disease must be balanced against the risk of medication toxicity in deciding whether or not to use antivirals in an individual immunocompetent patient [41, 42, 43].
Prevalence of CMV Infection in IBD, Particularly Ulcerative Colitis
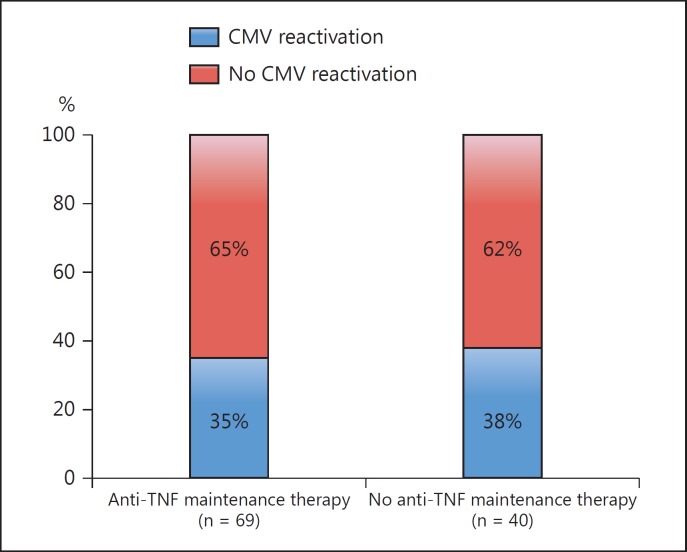
CMV infection is considered to be an important exacerbating factor in ulcerative colitis (UC) patients, but can also occur in patients with Crohn's disease [44, 45, 46, 47]. The first case with an association between UC and CMV infection was described by Powell et al. [48] in 1961. Since then, numerous case reports and many studies suggested the association of CMV infection with a flare of UC [46, 49]. Retrospective studies reported the strong association of a steroid-refractory condition in UC with CMV infection [31]. A case-control study showed that the ratio of positive CMV findings by IHC in the surgical specimens of steroid-refractory UC patients and nonrefractory UC were 25 and 2.5%, respectively [50]. Hommes et al. [51] reported that CMV in intestinal tissue biopsies was approximately 20 times more likely in UC patients than in controls with noninflammatory disease. Yoshino et al. [52] demonstrated that 56.7% of UC patients refractory to immunosuppressive therapies were positive for CMV-DNA in colonic mucosa. Fukuchi et al. [53] reported that 29.4% of active UC patients without receiving any immunosuppressive therapies were positive for CMV-DNA in their colonic mucosa. In addition, analysis in a newly established mouse model, which resembles human UC with concomitant CMV infection, strongly suggests that CMV infection can trigger intestinal inflammation [54]. Overall, diagnostic methods and selection bias of patients in published CMV case series influence the prevalence results of CMV infection in UC. However, the debate is still open if the detection of CMV in the colon in patients with severe colitis represents a mere bystander reactivation, which clinically has no significance [25]. The clinical data and those from the mouse model suggest that preceding mucosal inflammation in UC is strongly involved in the induction of CMV reactivation in intestinal tissue [54]. Also, it is speculated that disease activity together with the use of immunosuppressive drugs, specifically steroids, can predispose UC patients to colonic reactivation of CMV. Interestingly, anti-TNF therapy seems to have no or very little effect on CMV reactivation in patients with UC (fig. 2) [55].
Fig. 2.
Prevalence of colonic CMV infection as determined by quantitative PCR in patients with active UC (n = 109) depending on baseline immunosuppressive therapy [55].
Antigenimia Assay, Endoscopy, Histology, or CMV-DNA PCR: Which Is the Best Modality for Diagnosing CMV Colitis in UC Patients?
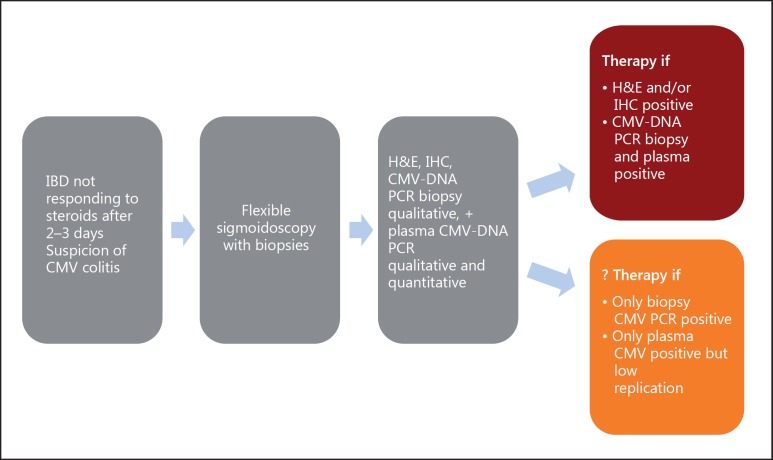
The detection of CMV antigen (pp65 antigenemia assay) or CMV-DNA (by PCR) in the peripheral blood, which can quantify viral load and be generally applied for the diagnosis of systemic CMV infection, is not necessarily useful for diagnosing CMV colitis, which can occur even if there is no detection of CMV in the blood [56]. Gastroenterologists agree on the necessity to perform an endoscopic examination for the evaluation of disease activity in patients with a steroid-refractory UC flare (fig. 3). Unfortunately, there are no characteristic endoscopic findings, which could point towards a CMV infection since ulcers can occur in active UC independent of CMV reactivation [57, 58]. In general, it is recommended to take biopsy specimens from the ulcer base for detecting CMV-positive cells compared with specimens from the ulcer edge. The detection of CMV in biopsy specimens by histologic examinations, such as the detection of inclusion bodies, and IHC is the gold standard for the diagnosis of the involvement of CMV in gastrointestinal diseases. An alternative is qualitative or quantitative PCR. Interestingly, even a high CMV-DNA load in colonic tissue does not often correlate with the occurrence or number of CMV-positive cells on histology or IHC [59]. The significance of this difference is not well understood. Very recently, McCurdy et al. [60] reported that 11 biopsies in UC were required to achieve an 80% probability of a positive biopsy. However, one can question the clinical significance of CMV infection of the colon, if such a number of biopsies are necessary to prove it. The European Crohn's and Colitis Organization guidelines recommend the use of tissue PCR as an alternative to IHC for the detection of CMV infection [26]. In Japan, the PCR method is only employed if CMV-positive cells and inclusion bodies on histology are negative, but a CMV infection is strongly suspected in patients with a severe UC flare. In the US, CMV testing is recommended in patients with steroid refractory disease. There is no uniform approach to the diagnosis of CMV colitis, and IHC and/or CMV PCR are used equally. While quantitative PCR, which is routinely performed for the systemic detection of CMV-DNA in plasma specimen, from mucosal specimens is not yet a standardized approach for the diagnosis of CMV infection, studies indicate that qualitative or quantitative PCR is the most sensitive method for the detection of CMV in different tissues [24, 47, 52, 61, 62, 63, 64, 65].
Fig. 3.
Proposed diagnostic and therapeutic algorithm for patients with IBD and suspected and/or proven CMV colitis. The indication for therapy in the setting of qualitative PCR CMV-DNA and negative histology or quantitative PCR of plasma and low CMV-DNA titer as well as negative findings on tissue PCR is debatable. H&E = Hematoxylin and eosin stain.
Treatment of CMV Infection
In severely immunocompromised patients (e.g. AIDS, status past organ transplant) and in immunocompetent patients with CMV organ disease, such as colitis or retinitis, antiviral therapy with gancyclovir or valgancyclovir is recommended. However, in patients with IBD, no recommendation or guideline defines when to initiate antiviral therapy. Whereas reports and case series describe the efficacy of an antiviral therapy in steroid-refractory IBD patients with concurrent CMV, there is still an ongoing debate whether an optimal control of intestinal inflammation also reduces IBD disease activity by itself [25]. Nevertheless, in the US and Europe, antiviral therapy with gancyclovir or valgancyclovir is recommended in patients with active IBD and proven CMV disease on histology and/or positive CMV-DNA shown by PCR in biopsies and plasma (fig. 3). With regard to drug, dose, and duration, ganciclovir 5 mg/kg i.v. every 12 h for 3–5 days and then a switch to oral valganciclovir for a total of 2–3 weeks is recommended [26, 57]. If ganciclovir resistance or intolerance (e.g. myelotoxicity) is encountered, foscarnet (for 2–3 weeks) is a possible alternative therapeutic approach [26].
The reason for the absence of firm recommendations for antiviral therapy in CMV-positive IBD patients is due to the multitude of single-center retrospective case series, which comprise different patient populations and different diagnostic and concomitant therapeutic approaches [66]. Prospective standardized trials with standardized diagnostic and therapeutic approaches are currently not available. A recent systematic review and meta-analysis by Shukla et al. [67] demonstrated that antiviral therapy resulted in a significantly lower risk of colectomy in patients with steroid-refractory disease (odds ratio, 0.20; 95% confidence interval, 0.08–0.49) but not in the overall population of patients with UC (odds ratio, 0.92; 95% confidence interval, 0.31–2.76). They concluded that antiviral therapy may benefit a subgroup of patients with UC who are refractory to steroids. However, other retrospective studies and another meta-analysis of all available studies have not been able to confirm a beneficial effect of antiviral therapy, suggesting that CMV infection or reactivation is rather a bystander in the setting of severe intestinal inflammation and disappears under appropriate immunosuppressive therapy [56, 68, 69].
Another approach to evaluate the severity of CMV infection and to decide about the need for antiviral therapy is the use of quantitative PCR, which, as mentioned above, is currently not yet standardized. Recently, a correlation of the viral load and colectomy has been shown [70]. A French study employing quantitative colonic PCR in UC patients demonstrated a correlation between higher viral load and resistance to immunosuppressive therapy with significant differences found when employing a cutoff viral load of >250/mg tissue [64]. After patients resistant to immunosuppression were treated with antiviral therapy, initial remission was seen in 7 of 8 patients and sustained remission in 5 of 8. Ciccocioppo et al. [59] proposed that refractory IBD patients whose mucosal viral load has DNA peak values ≥103 copies/105 cells require antiviral treatments. The authors also proposed a detailed treatment algorithm based on the quantitative CMV-DNA number incorporating not only antiviral therapy but also the elimination of steroids from the therapy based on different cutoff values [71].
A recent study from the Mayo clinic followed a slightly different approach and grouped patients with CMV-positive colonic tissue in high-grade and low-grade disease depending on the number of CMV inclusion bodies [72]. No difference in 1-year colectomy-free survival was found in the CMV-positive group compared to controls. Still, when comparing only the groups with CMV high- and low-grade disease, patients with low numbers of CMV inclusion bodies were at a significant risk to undergo a surgical intervention compared to the high-grade disease (≥5 inclusion bodies) patients. However, all of the high-grade CMV disease patients had been treated with gancyclovir and/or valganciclovir, whereas this was not always the case in the low-grade CMV disease group. When further analyzing the low-grade disease CMV group, there was also a benefit of antiviral therapy in this group compared to no therapy, thus supporting the need for antiviral therapy in every patient with conventionally or immunohistochemically proven tissue CMV reactivation.
Apart from antiviral therapy, intestinal inflammation in CMV-positive IBD patients should also be controlled with immunosuppressive therapy. At the same time, the continuation or initiation of steroids, azathioprine, or anti-TNF agents in IBD patients with a disease flare-up and CMV positivity may be antagonistic. The ‘Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease’ recommends discontinuation of all immunosuppressive therapeutic regimen in patients treated with antiviral therapy. In a Japanese study with 69 moderate-to-severely active UC patients, steroid therapy with concomitant immunosuppression with cyclosporine and/or 6-MP had no influence on clinical outcomes including rates of remission and colectomy among the CMV reactivation-positive, -negative, and CMV IgG-negative groups [56]. Interestingly, in a prospective French single-center study, the initiation of anti-TNF therapy had no adverse impact on colonic CMV-DNA load as measured by quantitative PCR, and the success of anti-TNF therapy was not affected by concurrent CMV infection [55]. Similarly, in a small Italian case series including 3 patients, the application of infliximab did not affect the colonic CMV-DNA viral load and therapeutic outcome [73].
In Japan, granulocyte and monocyte adsorptive apheresis (GMAA) with adacolumn (JIMRO, Gunma, Japan) is available [74]. As a result of the removal of peripheral granulocytes and monocytes, GMAA reduces the production of inflammatory cytokines such as TNF-α, interleukin (IL)-6, and IL-8. This anti-inflammatory mechanism of GMAA comprises a promising treatment for UC patients with concomitant CMV infection because production of proinflammatory cytokines, particularly TNF-α, is strongly associated with colonic CMV reactivation. In fact, 2 Japanese studies reported that GMAA does not induce colonic CMV reactivation and leads to disappearance of colonic CMV replication in previously PCR CMV-DNA-positive UC patients [53, 75]. Thus, GMAA therapy offers optimal inflammatory control without the need for steroids. Of note, whereas this therapy is successfully used in Japan, placebo-controlled trials in the US and Canada did not show a significant therapeutic benefit compared to placebo [76, 77].
Conclusion
Given the ubiquitous latent infection with CMV, reactivation should always be considered as the cause of unexplained malaise and, in case of IBD patients, exacerbation of the underlying disease. The diagnosis of an organ infection is still based on histology and IHC, but especially quantitative PCR is an interesting and promising alternative approach to better define the degree of CMV reactivation. Whereas antiviral therapy is indicated in most cases of CMV disease such as CMV hepatitis, in IBD, a general recommendation is still controversial. However, more recent findings suggest a benefit of antiviral therapy in patients with histologically proven CMV colitis and/or high colonic CMV load as determined by quantitative PCR. To finally solve the current debate of the most accurate diagnostic and therapeutic approach in IBD patients with concomitant CMV infection, multicenter randomized, prospective studies are necessary.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgement
H.H. is supported by National Institutes of Health grant 5U01DK092239-03.
References
- 1.Pass RF. Epidemiology and transmission of cytomegalovirus. J Infect Dis. 1985;152:243–248. doi: 10.1093/infdis/152.2.243. [DOI] [PubMed] [Google Scholar]
- 2.Plosa EJ, Esbenshade JC, Fuller MP, et al. Cytomegalovirus infection. Pediatr Rev. 2012;33:156–163. doi: 10.1542/pir.33-4-156. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. 2004;4:725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- 4.Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 5.Ko JH, Peck KR, Lee WJ, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60:e20–e26. doi: 10.1093/cid/ciu969. [DOI] [PubMed] [Google Scholar]
- 6.Nakase H, Honzawa Y, Toyonaga T, et al. Diagnosis and treatment of ulcerative colitis with cytomegalovirus infection: importance of controlling mucosal inflammation to prevent cytomegalovirus reactivation. Intest Res. 2014;12:5–11. doi: 10.5217/ir.2014.12.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans AS. Infectious mononucleosis and related syndromes. Am J Med Sci. 1978;276:325–339. doi: 10.1097/00000441-197811000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Handsfield HH, Chandler SH, Caine VA, et al. Cytomegalovirus infection in sex partners: evidence for sexual transmission. J Infect Dis. 1985;151:344–348. doi: 10.1093/infdis/151.2.344. [DOI] [PubMed] [Google Scholar]
- 9.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 10.Staras SA, Flanders WD, Dollard SC, et al. Cytomegalovirus seroprevalence and childhood sources of infection: a population-based study among pre-adolescents in the United States. J Clin Virol. 2008;43:266–271. doi: 10.1016/j.jcv.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Ho M. Epidemiology of cytomegalovirus infections. Rev Infect Dis. 1990;12((suppl 7)):S701–S710. doi: 10.1093/clinids/12.supplement_7.s701. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi K, Watanabe N, Sato A, et al. Changes in cytomegalovirus seroprevalence in pregnant Japanese women – a 10-year single center study. J Clin Virol. 2014;59:192–194. doi: 10.1016/j.jcv.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Rothbarth PH, Diepersloot RJ, Metselaar HJ, et al. Rapid demonstration of cytomegalovirus in clinical specimens. Infection. 1987;15:228–231. doi: 10.1007/BF01644118. [DOI] [PubMed] [Google Scholar]
- 14.Rowshani AT, Bemelman FJ, van Leeuwen EM, et al. Clinical and immunologic aspects of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2005;79:381–386. doi: 10.1097/01.tp.0000148239.00384.f0. [DOI] [PubMed] [Google Scholar]
- 15.Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev. 2002;15:680–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 17.Hinnant KL, Rotterdam HZ, Bell ET, et al. Cytomegalovirus infection of the alimentary tract: a clinicopathological correlation. Am J Gastroenterol. 1986;81:944–950. [PubMed] [Google Scholar]
- 18.Culpepper-Morgan JA, Kotler DP, Scholes JV, et al. Evaluation of diagnostic criteria for mucosal cytomegalic inclusion disease in the acquired immune deficiency syndrome. Am J Gastroenterol. 1987;82:1264–1270. [PubMed] [Google Scholar]
- 19.Wilcox CM, Diehl DL, Cello JP, et al. Cytomegalovirus esophagitis in patients with AIDS. A clinical, endoscopic, and pathologic correlation. Ann Intern Med. 1990;113:589–593. doi: 10.7326/0003-4819-113-8-589. [DOI] [PubMed] [Google Scholar]
- 20.Rogers BB, Alpert LC, Hine EA, et al. Analysis of DNA in fresh and fixed tissue by the polymerase chain reaction. Am J Pathol. 1990;136:541–548. [PMC free article] [PubMed] [Google Scholar]
- 21.Chang MH, Huang HH, Huang ES, et al. Polymerase chain reaction to detect human cytomegalovirus in livers of infants with neonatal hepatitis. Gastroenterology. 1992;103:1022–1025. doi: 10.1016/0016-5085(92)90038-z. [DOI] [PubMed] [Google Scholar]
- 22.Persons DL, Moore JA, Fishback JL. Comparison of polymerase chain reaction, DNA hybridization, and histology with viral culture to detect cytomegalovirus in immunosuppressed patients. Mod Pathol. 1991;4:149–153. [PubMed] [Google Scholar]
- 23.Chen YT, Mercer GO, Cheigh JS, et al. Cytomegalovirus infection of renal allografts. Detection by polymerase chain reaction. Transplantation. 1992;53:99–102. doi: 10.1097/00007890-199201000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Herfarth HH, Long MD, Rubinas TC, et al. Evaluation of a non-invasive method to detect cytomegalovirus (CMV)-DNA in stool samples of patients with inflammatory bowel disease (IBD): a pilot study. Dig Dis Sci. 2010;55:1053–1058. doi: 10.1007/s10620-010-1146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis. 2010;16:1620–1627. doi: 10.1002/ibd.21275. [DOI] [PubMed] [Google Scholar]
- 26.Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 28.Turner D, Travis SP, Griffiths AM, et al. Consensus for managing acute severe ulcerative colitis in children: a systematic review and joint statement from ECCO, ESPGHAN, and the Porto IBD Working Group of ESPGHAN. Am J Gastroenterol. 2011;106:574–588. doi: 10.1038/ajg.2010.481. [DOI] [PubMed] [Google Scholar]
- 29.Eddleston M, Peacock S, Juniper M, et al. Severe cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 1997;24:52–56. doi: 10.1093/clinids/24.1.52. [DOI] [PubMed] [Google Scholar]
- 30.Lancini D, Faddy HM, Flower R, et al. Cytomegalovirus disease in immunocompetent adults. Med J Aust. 2014;201:578–580. doi: 10.5694/mja14.00183. [DOI] [PubMed] [Google Scholar]
- 31.Ayre K, Warren BF, Jeffery K, et al. The role of CMV in steroid-resistant ulcerative colitis: a systematic review. J Crohns Colitis. 2009;3:141–148. doi: 10.1016/j.crohns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Abgueguen P, Delbos V, Ducancelle A, et al. Venous thrombosis in immunocompetent patients with acute cytomegalovirus infection: a complication that may be underestimated. Clin Microbiol Infect. 2010;16:851–854. doi: 10.1111/j.1469-0691.2009.03022.x. [DOI] [PubMed] [Google Scholar]
- 33.Ladd AM, Goyal R, Rosainz L, et al. Pulmonary embolism and portal vein thrombosis in an immunocompetent adolescent with acute cytomegalovirus hepatitis. J Thromb Thrombolysis. 2009;28:496–499. doi: 10.1007/s11239-008-0303-1. [DOI] [PubMed] [Google Scholar]
- 34.Tjwa M, De Hertogh G, Neuville B, et al. Hepatic fibrin-ring granulomas in granulomatous hepatitis: report of four cases and review of the literature. Acta Clin Belg. 2001;56:341–348. doi: 10.1179/acb.2001.051. [DOI] [PubMed] [Google Scholar]
- 35.Bonkowsky HL, Lee RV, Klatskin G. Acute granulomatous hepatitis. Occurrence in cytomegalovirus mononucleosis. JAMA. 1975;233:1284–1288. [PubMed] [Google Scholar]
- 36.Siegal DS, Hamid N, Cunha BA. Cytomegalovirus colitis mimicking ischemic colitis in an immunocompetent host. Heart Lung. 2005;34:291–294. doi: 10.1016/j.hrtlng.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Patra S, Samal SC, Chacko A, et al. Cytomegalovirus infection of the human gastrointestinal tract. J Gastroenterol Hepatol. 1999;14:973–976. doi: 10.1046/j.1440-1746.1999.01986.x. [DOI] [PubMed] [Google Scholar]
- 38.Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med. 1993;119:924–935. doi: 10.7326/0003-4819-119-9-199311010-00010. [DOI] [PubMed] [Google Scholar]
- 39.Klauber E, Briski LE, Khatib R. Cytomegalovirus colitis in the immunocompetent host: an overview. Scand J Infect Dis. 1998;30:559–564. doi: 10.1080/00365549850161098. [DOI] [PubMed] [Google Scholar]
- 40.Seo TH, Kim JH, Ko SY, et al. Cytomegalovirus colitis in immunocompetent patients: a clinical and endoscopic study. Hepatogastroenterology. 2012;59:2137–2141. doi: 10.5754/hge10825. [DOI] [PubMed] [Google Scholar]
- 41.Dieterich DT, Rahmin M. Cytomegalovirus colitis in AIDS: presentation in 44 patients and a review of the literature. J Acquir Immune Defic Syndr. 1991;4((suppl 1)):S29–S35. [PubMed] [Google Scholar]
- 42.Whitley RJ, Jacobson MA, Friedberg DN, et al. Guidelines for the treatment of cytomegalovirus diseases in patients with AIDS in the era of potent antiretroviral therapy: recommendations of an international panel. International AIDS Society-USA. Arch Intern Med. 1998;158:957–969. doi: 10.1001/archinte.158.9.957. [DOI] [PubMed] [Google Scholar]
- 43.Orenstein JM, Dieterich DT. The histopathology of 103 consecutive colonoscopy biopsies from 82 symptomatic patients with acquired immunodeficiency syndrome: original and look-back diagnoses. Arch Pathol Lab Med. 2001;125:1042–1046. doi: 10.5858/2001-125-1042-THOCCB. [DOI] [PubMed] [Google Scholar]
- 44.Wakefield AJ, Fox JD, Sawyerr AM, et al. Detection of herpesvirus DNA in the large intestine of patients with ulcerative colitis and Crohn's disease using the nested polymerase chain reaction. J Med Virol. 1992;38:183–190. doi: 10.1002/jmv.1890380306. [DOI] [PubMed] [Google Scholar]
- 45.Adani GL, Avital I, Ferraresso C, et al. CMV infection in severe refractory ulcerative and Crohn's colitis. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96:3464–3465. doi: 10.1111/j.1572-0241.2001.05360.x. [DOI] [PubMed] [Google Scholar]
- 46.Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn's colitis. Am J Gastroenterol. 2001;96:773–775. doi: 10.1111/j.1572-0241.2001.03620.x. [DOI] [PubMed] [Google Scholar]
- 47.Roblin X, Pillet S, Berthelot P, et al. Prevalence of cytomegalovirus infection in steroid-refractory Crohn's disease. Inflamm Bowel Dis. 2012;18:E1396–E1397. doi: 10.1002/ibd.21907. [DOI] [PubMed] [Google Scholar]
- 48.Powell RD, Warner NE, Levine RS, et al. Cytomegalic inclusion disease and ulcerative colitis; report of a case in a young adult. Am J Med. 1961;30:334–340. doi: 10.1016/0002-9343(61)90105-x. [DOI] [PubMed] [Google Scholar]
- 49.Cooper HS, Raffensperger EC, Jonas L, et al. Cytomegalovirus inclusions in patients with ulcerative colitis and toxic dilation requiring colonic resection. Gastroenterology. 1977;72:1253–1256. [PubMed] [Google Scholar]
- 50.Criscuoli V, Casa A, Orlando A, et al. Severe acute colitis associated with CMV: a prevalence study. Dig Liver Dis. 2004;36:818–820. doi: 10.1016/j.dld.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Hommes DW, Sterringa G, van Deventer SJ, et al. The pathogenicity of cytomegalovirus in inflammatory bowel disease: a systematic review and evidence-based recommendations for future research. Inflamm Bowel Dis. 2004;10:245–250. doi: 10.1097/00054725-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Yoshino T, Nakase H, Ueno S, et al. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007;13:1516–1521. doi: 10.1002/ibd.20253. [DOI] [PubMed] [Google Scholar]
- 53.Fukuchi T, Nakase H, Matsuura M, et al. Effect of intensive granulocyte and monocyte adsorptive apheresis in patients with ulcerative colitis positive for cytomegalovirus. J Crohns Colitis. 2013;7:803–811. doi: 10.1016/j.crohns.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Matsumura K, Nakase H, Kosugi I, et al. Establishment of a novel mouse model of ulcerative colitis with concomitant cytomegalovirus infection: in vivo identification of cytomegalovirus persistent infected cells. Inflamm Bowel Dis. 2013;19:1951–1963. doi: 10.1097/MIB.0b013e318293c5bf. [DOI] [PubMed] [Google Scholar]
- 55.Pillet S, Jarlot C, Courault M, et al. Infliximab does not worsen outcomes during flare-ups associated with CMV infection in patients with ulcerative colitis. Inflamm Bowel Dis. 2015;21:1580–1586. doi: 10.1097/MIB.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 56.Matsuoka K, Iwao Y, Mori T, et al. Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol. 2007;102:331–337. doi: 10.1111/j.1572-0241.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 57.Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol. 2006;101:2857–2865. doi: 10.1111/j.1572-0241.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 58.Korkmaz M, Kunefeci G, Selcuk H, et al. The role of early colonoscopy in CMV colitis of transplant recipients. Transplant Proc. 2005;37:3059–3060. doi: 10.1016/j.transproceed.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 59.Ciccocioppo R, Racca F, Paolucci S, et al. Human cytomegalovirus and Epstein-Barr virus infection in inflammatory bowel disease: need for mucosal viral load measurement. World J Gastroenterol. 2015;21:1915–1926. doi: 10.3748/wjg.v21.i6.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCurdy JD, Enders FT, Jones A, et al. Detection of cytomegalovirus in patients with inflammatory bowel disease: where to biopsy and how many biopsies? Inflamm Bowel Dis. 2015;21:2833–2838. doi: 10.1097/MIB.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 61.Boeckh M, Boivin G. Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin Microbiol Rev. 1998;11:533–554. doi: 10.1128/cmr.11.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cotte L, Drouet E, Bailly F, et al. Cytomegalovirus DNA level on biopsy specimens during treatment of cytomegalovirus gastrointestinal disease. Gastroenterology. 1996;111:439–444. doi: 10.1053/gast.1996.v111.pm8690210. [DOI] [PubMed] [Google Scholar]
- 63.Kou T, Nakase H, Tamaki H, et al. Cytomegalovirus infection in patients with ulcerative colitis diagnosed by quantitative real-time PCR analysis. Dig Dis Sci. 2006;51:1052–1055. doi: 10.1007/s10620-006-8006-y. [DOI] [PubMed] [Google Scholar]
- 64.Roblin X, Pillet S, Oussalah A, et al. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011;106:2001–2008. doi: 10.1038/ajg.2011.202. [DOI] [PubMed] [Google Scholar]
- 65.Dimitroulia E, Spanakis N, Konstantinidou AE, et al. Frequent detection of cytomegalovirus in the intestine of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:879–884. doi: 10.1097/01.mib.0000231576.11678.57. [DOI] [PubMed] [Google Scholar]
- 66.Wu XW, Yang MF, Li N, et al. Unfavorable outcome of antiviral therapy in cytomegalovirus-positive ulcerative colitis may be due to inappropriate study inclusion in meta-analysis. World J Gastroenterol. 2015;21:1689–1690. doi: 10.3748/wjg.v21.i5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shukla T, Singh S, Loftus EV, et al. Antiviral therapy in steroid-refractory ulcerative colitis with cytomegalovirus. Inflamm Bowel Dis. 2015;21:2718–2725. doi: 10.1097/MIB.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 68.Delvincourt M, Lopez A, Pillet S, et al. The impact of cytomegalovirus reactivation and its treatment on the course of inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:712–720. doi: 10.1111/apt.12650. [DOI] [PubMed] [Google Scholar]
- 69.Kopylov U, Eliakim-Raz N, Szilagy A, et al. Antiviral therapy in cytomegalovirus-positive ulcerative colitis: a systematic review and meta-analysis. World J Gastroenterol. 2014;20:2695–2703. doi: 10.3748/wjg.v20.i10.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Onyiah JC, Long MD, Miller M, et al. Su1386 is the cytomegalovirus (CMV) viral load in colonic biopsies associated with a higher risk of colectomy in hospitalized patients with inflammatory bowel diseases (IBD)? Gastroenterology. 2014;146:S454–S455. [Google Scholar]
- 71.Ciccocioppo R. Letter: cytomegalovirus infection in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;42:127. doi: 10.1111/apt.13234. [DOI] [PubMed] [Google Scholar]
- 72.Jones A, McCurdy JD, Loftus EV, et al. Effects of antiviral therapy for patients with inflammatory bowel disease and a positive intestinal biopsy for cytomegalovirus. Clin Gastroenterol Hepatol. 2015;13:949–955. doi: 10.1016/j.cgh.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 73.D'Ovidio V, Vernia P, Gentile G, et al. Cytomegalovirus infection in inflammatory bowel disease patients undergoing anti-TNFalpha therapy. J Clin Virol. 2008;43:180–183. doi: 10.1016/j.jcv.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 74.Saniabadi AR, Tanaka T, Ohmori T, et al. Treating inflammatory bowel disease by adsorptive leucocytapheresis: a desire to treat without drugs. World J Gastroenterol. 2014;20:9699–9715. doi: 10.3748/wjg.v20.i29.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshino T, Nakase H, Matsuura M, et al. Effect and safety of granulocyte-monocyte adsorption apheresis for patients with ulcerative colitis positive for cytomegalovirus in comparison with immunosuppressants. Digestion. 2011;84:3–9. doi: 10.1159/000321911. [DOI] [PubMed] [Google Scholar]
- 76.Sands BE, Sandborn WJ, Feagan B, et al. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology. 2008;135:400–409. doi: 10.1053/j.gastro.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 77.Sands BE, Katz S, Wolf DC, et al. A randomised, double-blind, sham-controlled study of granulocyte/monocyte apheresis for moderate to severe Crohn's disease. Gut. 2012;62:1288–1294. doi: 10.1136/gutjnl-2011-300995. [DOI] [PubMed] [Google Scholar]
- 78.Staras SA, Dollard SC, Radford KW, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]