Abstract
The extracellular matrix is a structural support network made up of diverse proteins, sugars and other components. It influences a wide number of cellular processes including migration, wound healing and differentiation, all of which is of particular interest to researchers in the field of tissue engineering. Understanding the composition and structure of the extracellular matrix will aid in exploring the ways the extracellular matrix can be utilised in tissue engineering applications especially as a scaffold. This review summarises the current knowledge of the composition, structure and functions of the extracellular matrix and introduces the effect of ageing on extracellular matrix remodelling and its contribution to cellular functions. Additionally, the current analytical technologies to study the extracellular matrix and extracellular matrix–related cellular processes are also reviewed.
Keywords: Extracellular matrix, ageing, wound healing, tissue engineering, connective tissue, scaffolds
Introduction
The extracellular matrix (ECM) is responsible for the physical maintenance1 of all cells. However, the concept that the ECM has a passive role to play in cellular activity has been refuted. It is now known to play a part in numerous cellular processes including cell proliferation,1 differentiation2 and migration.3 The ECM can be defined as the non-cellular component of tissues, which has been likened to ‘glue’ that binds cells together in connective tissues, where it is a major constituent of the tissue.4
Research into the ECM has existed for decades, and our current understanding of the ECM is that it influences cellular activity and responses. The cell reciprocates through maintenance and assembly of the ECM. Most recently, there has been great interest in matrix biology in two main areas. First, it has been demonstrated that the ECM is influential at a biomechanical level and molecular level, in particular the importance of its stiffness5 and how this impacts on the cell differentiation profiles.6 Second, the part it plays in anchoring the cells via mechanisms such as integrins.7,8 These will form the main sections of the review, as outlined below.
Initially, this review will explore the present knowledge of the composition and structure of the ECM. The key components from proteins like collagen and fibronectin, to matrix metalloproteinases (MMPs) will be detailed. Moreover, the significance of cell–matrix interactions will be examined, particularly in relation to how the elasticity of the ECM regulates cell behaviour.
Another area that the ECM has been shown to impact is wound healing processes and the apparent differences that occur as a result of age. Numerous studies have illustrated at the foetal stage that the reaction to injury is remarkably contrasting to the response in adult tissue.4,9,10 However, the role of the ECM in this has not been fully addressed; therefore, the age-related changes in the ECM of connective tissues, such as tendon and skin, along with skeletal muscle will be detailed, and implications of the ageing ECM to the wound healing process. Both these topics are becoming increasingly relevant, primarily due to the growing numbers of the elderly population, and second, the problems associated with reparative wound healing in adults remain a significant burden on clinicians.
The next section of the review will focus upon the numerous techniques that have been employed by researchers, providing both quantitative and qualitative information of the ECM. Such techniques include immunostaining, atomic force microscopy (AFM) and confocal microscopy. The general principles of these methods as well as their advantages and disadvantages will be critiqued. Additionally, their role in the analysis of the ECM will be evaluated. Other lesser-known methods for ECM analysis such as Raman spectroscopy and second-harmonic generation microscopy (SHGM) will also be appraised.
The final section of the review will detail how the area of tissue engineering is being explored as a potential solution to encourage scarless healing of soft tissue injuries in adults. The ECM is one of the key factors to consider for tissue engineering applications. Research has focused largely on either the way the matrix can manipulate differentiation of stem cells or how it serves as a scaffold towards cells, providing much needed structural integrity to the cellular environment.
Composition of the ECM
Proteins
The ECM can be considered as being constructed from multiple matrix proteins, forming the principal component of the ECM (Table 1). It is these proteins that provide the necessary support to cells and tissues.20 The proteins that constitute the ECM can be categorised as either structural or non-structural (also known as glycoproteins), depending on their function. Examples of structural proteins include collagens and elastin,21 with fibronectin,22 laminin23 and tenascin24 instances of non-structural proteins.25 Other important components of the ECM include integrins,26 growth factors (GFs)27 and a group of MMPs.28
Table 1.
Components of the ECM, their structure, function and significance to tissue engineering.
| ECM component | Structure | Function | Importance to tissue engineering |
|---|---|---|---|
| Collagens | Formed as fibrils within the ECM (Types I, II, III, V and XI) | Provide tensile strengthInfluence cell processes, for example, adhesion and migration | Collagen type I is often used as a coating on gel scaffolds to promote cell adhesion, in addition to its ability to stimulate myogenic and osteogenic differentiation of stem cells6Also frequently used as scaffold material for cells11,12 both as a single material and within a composite scaffold. As a natural material, it seldom initiates an inflammatory response from the host |
| Elastin | Composed of single tropoelastin subunits cross-linked with an outer layer of fibrillin microfibrils making up an elastic fibre | Closely linked to collagensAllows tissues such as the skin and tendon to recover/recoil | Being investigated as a biomaterial for use in tissue engineering, particularly in vascular tissue engineering due to its importance in blood vessels13 |
| Fibronectin | Two forms either plasma (within blood) or cellular protein (created by fibroblasts)Arranged into a mesh of fibrils similar to collagen and is linked to cell surface receptors (integrins) | Found in the basement membrane of the ECMPlays a role in cell adhesion, embryonic development and the healing process following wound injury | Importance of fibronectin in embryonic development and wound healing demonstrate that it needs to play a key role in tissue engineering applications. Especially, the RGD sequence that is critical in ensuring cell adhesion, transforming substrates allowing for cell attachment14,15 |
| Laminins | Laminins are another type of glycoprotein, with a trimeric structure. They are made up of three different chains, α, β and γ which exist in various genetically distinct forms | Reside in the basement membraneExpressed by various tissue types including both muscle and epithelial cellsPlay a vital part in several cell processes including differentiation and migration via their integrins | Similarly to fibronectin, laminins have the capacity for cell binding and are another option to be used to enhance cell adhesion in culture conditions16 |
| Tenascins | Tenascins are a group of ECM proteins and exist as five different manifestations, TN-C, TN-R, TN-W, TN-X and TN-Y | Linked to mechanical activityTypically found within connective tissues where load bearing is required, although they also occur within the skin and brain | During embryonic development and tissue repair, TN-C is highly expressed, it is also typically found within stem cell niches. However, it prevents cell adhesion when used as a protein coating for cell culture substrates17 |
| Growth factors | Tied to the ECM through either heparan or heparan sulphate | They can be linked to tissues with their names. For example, vascular endothelial growth factor stimulates the formation of blood vesselsTriggered into action by a variety of processes (not necessarily in a soluble form) including wound healing and tissue remodelling | Multiple growth factors demonstrated as being crucial to development and differentiation of many tissues. Their use is being explored within tissue engineering, for example, improving wound healing of tendon tissue18 |
| MMPs | Structure occurs as zinc-dependent endopeptidases19 | Capable of disintegrating the ECM, associated with many different processes including angiogenesis and wound repair | MMPs are key modulators for tissue remodelling; their expression can be useful indicators of cellular behaviour for tissue engineering investigations |
ECM: extracellular matrix; MMP: matrix metalloproteinase.
Collagens
Collagen is the most abundant protein within the body26 and is found amassed in the ECM of connective tissues such as tendon and skin.27 Collagens are the predominant form of structural proteins found within the ECM providing not only tensile strength but also play a role in other cell processes such as adhesion and migration.29 There are almost 30 types of collagen that have been distinguished,30 although not all are isolated to the ECM.
Here, the collagen is arranged into fibrils to provide the required structural integrity for the tissues. Specifically, this fibril formation is restricted to collagen types I, II, III, V and XI. The fibril arrangement imparts tensile strength to connective tissues that are required to withstand different mechanical stresses like tension, shear and pressure.31 There are indications that collagen fibril formation is strictly regulated by other ECM molecules such as decorin and fibromodulin. Included in this process are also cell surface receptors like integrins. Integrins are made up of an alpha (α) and beta (β) subunit which combine to form a heterodimer; among the various types of integrins are four known collagen receptors; two of these receptors α1β1 and α2β1 are well recognised. The roles of these receptors have been established, with α1β1 responsible for controlling production of collagen through negative feedback, whereas α2β1 has the opposite effect of increasing collagen matrix synthesis and turnover.32
Collagen type I is the dominant form found extensively in almost all tissues, particularly in tendons and the skin. The other forms of collagen occur in defined areas, for example, type II collagen is found in cartilage and the cornea, while collagen type III is the principle form within the walls of blood vessels. Collagen is produced not only by fibroblasts but also endothelial cells and epithelial cells.28
Elastin
Elastin is the other structural protein, with its role closely linked with collagen. It provides tissue such as the dermis of the skin20 with the ability to recover from continuous stretching,33 alongside the glycoproteins including fibrillin and fibulin.20 However, in tendons, it only forms approximately 2% of their dry weight.34 Despite this, it plays a crucial role in allowing the crimp structure of tendon to be stretched and recoil, which is crucial to the function of tendon and other tissues such as the artery vessels. Its structure is composed of single tropoelastin subunits which are cross-linked with an outer layer of fibrillin microfibrils that make up an elastic fibre.
Fibronectin
Fibronectin has been studied to a lesser degree than collagen; it is located within the basement membrane (BM) of the ECM and it has been determined as having a key role in both cell adhesion26 and the wound healing response to injury. Additionally, fibronectin is critical to other processes in vivo such as embryonic development, as an absence of fibronectin has been observed as fatal in mice.35 The protein exists in two different forms, either as plasma that circulates in the blood and is one of the first components delivered by blood plasma to the site of injury20 or as a cellular protein that is created by fibroblasts.
Fibronectin is arranged into a mesh of fibrils similar to collagen and is linked to cell surface receptors (integrins). It is expressed by various cell types and is not unique to connective tissues. Fibronectin is produced in the form of a disulphide-bonded dimer that can be broken down into subunits, of which there are three different types (types I, II and III) that are repeated within each subunit.35
The integrin α5β1 is the principal receptor involved in the process of fibronectin matrix assembly which together with the RGD region (abbreviation for the tripeptide sequence of Arg-Gly-Asp) of fibronectin promotes binding of cells to the protein.36 The fibronectin matrix is associated with the actin cytoskeleton of cells through integrin activity. This connection is key to successful matrix formation37 and progress can be easily tracked via immunofluorescence staining of cells. Upon formation, fibronectin develops into fibrils that can differ widely in their thickness between 10 and 1000 nm.38
Laminins
Laminins are another type of glycoprotein, with a trimeric structure. They are made up of three different chains, α, β and γ which exist in various genetically distinct forms.39 Through evolution, they have advanced from a sole laminin heterotrimer within low multicellular organisms to an excess of 16 unique trimeric isoforms in complex vertebrates.40 They were first recognised as a constituent of the ECM of murine Engelbreth–Holm–Swarm (EHS) sarcoma (Matrigel).26,28
They are involved in cell adhesion, expressed by various tissue types including both muscle and epithelial cells. Laminins play a vital part in several cell processes including differentiation and migration20 via their integrins and they intercede between cells and the underlying matrix, namely, the BM where they reside. Laminins are among the first proteins of the ECM to appear in embryos. As mentioned, they are crucial to differentiation, to the degree that a defect in the genes that code for laminins can either have fatal results in the embryo or manifest as serious conditions that affect multiple organs. This is supported by the fact that laminins play an important role in different tissues such as nerves and blood vessels.41
Tenascins
Tenascins are a group of ECM proteins and exist as five different manifestations, TN-C, TN-R, TN-W, TN-X and TN-Y. Tenascins are typically found within connective tissues where load bearing is required, although they also occur within the skin and brain.28 Consequently, tenascin levels have been linked to mechanical activity. Its expression is also influenced by the changes brought about by disease and development.42 In conjunction with this, absence of tenascin-C interferes with regeneration processes, as it appears to be upregulated within the interim matrix following an injury;20 typically, it is not expressed in ‘normal adult tissues’.28
Other components
Growth Factors (GFs)
The ECM has been referred to as a ‘reservoir’ for GFs;20,27 a number of ECM components alter the actions of GFs within cell pathways accordingly. This will be briefly discussed here, however; several comprehensive reviews on GFs can be found elsewhere.43,44 Vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and transforming growth factor β (TGF-β) are examples of GFs that are tied to the ECM through either heparan or heparan sulphate.1
GFs are triggered into action by a variety of processes (not necessarily in a soluble form)27 including wound healing and tissue remodelling.20 As mentioned, mechanical stimulus induces the protein tenascin-C; there has been evidence that GFs are also responsible for its activation alongside mechanical stimulation.42 The crucial role of GFs in wound healing has been acknowledged by researchers, as demonstrated by the interest in exploring how they may positively impact on tendon repair in adults.45,46 However, GFs are vital to the development and differentiation of many other tissue types; they can be linked to tissues with their names. For example, VEGF stimulates the formation of blood vessels and nerve GF is involved in the growth of neurons.47,48
Matrix Metalloproteinases (MMPs)
MMPs are the key group of protease enzymes involved in disintegration of the ECM. Presently, dozens of MMPs have been identified; they are effective in their capacity to breakdown the matrix, as they ‘overlap in substrate specificity’.28 This breakdown of the matrix forms part of the on-going remodelling of the ECM. This transformation process is critical for the ECM of many tissues to undergo and, for example, occurs during neovascularisation and bone remodelling.49,50
When an increase in cytokine and GF activity occurs as a result of tissue repair, MMPs are triggered into action. As the actions of MMPs are detrimental to the ECM, they are tightly regulated using three mechanisms: primarily by managing them at transcription, by preserving them in a quiescent state prior to activation and finally having tissue inhibitors of metalloproteinases (TIMPs) to counteract unwarranted damage to the matrix.27
Structure and function of the ECM
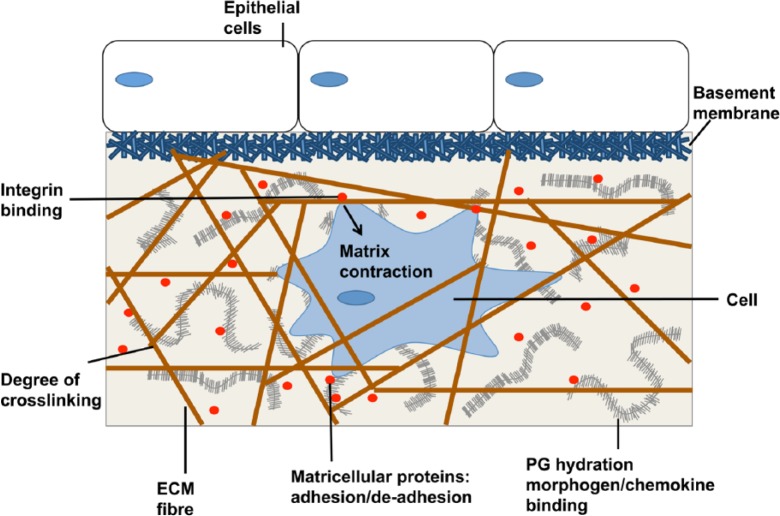
The different components of the ECM are organised into a recognisable three-dimensional (3D) structure, which can be split into two main forms, the BM and interstitial matrix. Although differences exist between these two forms, the basic structural outline is alike. Various collagens form the framework of both and the non-structural proteins bind to this scaffold, communicating with surrounding cells via integrins (Figure 1).28
Figure 1.
Schematic of the composition and assembly of the ECM adapted from Griffith and Swartz.51
ECM: extracellular matrix.
Basement Membrane (BM)
The main elements of the BM include collagen type IV, laminins and fibronectin, the latter of which equips the overlying tissue with some tensile strength. It is known to be more dense and ‘less porous’ than the interstitial matrix.52 Perlecan is an important heparan sulphate proteoglycan (HSPG) of the BM binding to FGF and VEGF, thus influencing angiogenic processes. Hence, the BM is found in blood vessels in addition to epithelial and endothelial tissues, forming a highly organised matrix, with the epithelium ‘critically dependent’ on the BM to conduct its expected role.27 Integrins between the BM and the cells above convey messages regarding ‘cell shape and motility’.26
Interstitial matrix
Interstitial matrix occurs in locations where the BM is found; however, in addition, it also appears between connective tissue cells such as tenocytes (within tendon). The major elements that form this ECM include collagens, elastin and fibronectin, creating a ‘3D amorphous gel’.53 Despite collagens constituting the majority of the fibrous proteins within the matrix, it is fibronectin that dictates the organisation of the matrix structure. Each tissue within the body has its own distinctive ECM that differs in both composition and topographical features.33
The ECM is now acknowledged as an active environment, which is constantly experiencing changes in composition and structure. This occurs in response to the actions of surrounding cells. Consequently, the communication between cells and the ECM is essential to understanding how the ECM and cells learn to adapt to each other.
Functions of the ECM
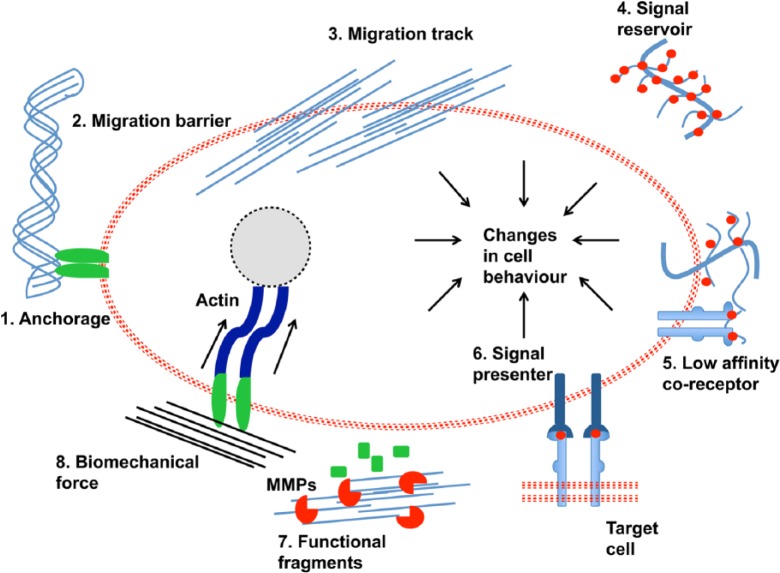
There are multiple functions that the ECM conducts (Figure 2).52 For example, its role in cell migration is complex as it can provide itself as an anchorage site. Anchorage to the BM is critical for the process of cell division in stem cells. Furthermore, it can either help or hinder migration of cells by acting to facilitate migration tracking or act as an obstruction to migration of cells. These functions arise from the physical characteristics of the ECM.
Figure 2.
Various functions of the ECM adapted from Lu et al.52
ECM: extracellular matrix.
The biochemical properties of the ECM allow for it to directly and indirectly influence the way cells interrelate with their environment through different signal pathways, including behaving as a reservoir for GF signalling molecules. Particular ECM components such as proteoglycans will bind with GFs becoming either low-affinity co-receptors for signalling or signal presenters and impact on communications between cells. Another way in which the ECM passes signals to cells is through functional fragments that have first been managed by MMPs. Recently, the biomechanical properties of the ECM have been of specific interest to researchers and the way these properties change cellular behaviour in response to the degree of elasticity of the ECM, especially the process of cell differentiation. This will be explored in further detail later in the review.
Role of mechanobiology
The relationship between cells and the underlying matrix is commonly referred to as mechanobiology.34 There are several different components involved in these interactions. The main form of communication between cells and the ECM is through focal adhesions.42 They can be divided into two different categories, cytoskeletal proteins including talin and filamin and regulatory proteins such as kinase and paxillin.54
Focal adhesions are characteristic of cells seeded onto stiff substrates while dynamic adhesion complexes occur with cells on softer substrates.3 These dynamic adhesion complexes occur during cell migration in phases of formation and disassembly, as the leading edge of the cells (lamellipodia) propels the cells forward. Once cell migration ends, the dynamic adhesion complexes are disassembled and the cells begin to formulate more stable and conspicuous focal adhesions.55,56 Focal adhesions provide the vector to transmit mechanical signals to cells from the ECM. Cells are known to respond accordingly to this mechanical strain by either increasing or limiting ECM production, alter their cytoskeletal structure and reorder the forces they exert. The principal receptors that deliver these signals between cells and the ECM are integrins.
Integrins
Attachment of cells to a particular matrix is determined by ‘specific integrin expression patterns’.27 This is conducted through integrins binding to ECM proteins such as fibronectin; these ligands attach themselves to the ‘extracellular domains’ of the integrins. Integrins are classified as transmembrane heterodimers, and they are involved in a number of cell signalling pathways for cell processes such as propagation and motility.7,8
As integrins are linked with a cell’s cytoskeleton, attention has been drawn to their role in mechanical stimulation of cells, with researchers suggesting they act as ‘stretch sensors’,42 resulting in signalling pathways being triggered in response through different mechanisms. For example, ‘phosphorylation of intracellular linker proteins or lipid second messengers and small GTPases’.20 They have also been described as offering ‘mechanical continuity’ between the inner and outer environment of the cells.26,28
Despite not being catalysts strictly speaking, integrins do form part of a network of other matrix components that are involved with the ‘transduction of mechanical forces’ including enlisting and prompting signalling molecules such as G proteins, FAK, receptor tyrosine kinases (RTKs), and mitogen-activated protein kinases (MAPKs);34 in addition to focal adhesion proteins, for example, vinculin and talin. Recently, the linker proteins talin and kindlin have been identified as being liable for triggering integrin activity.20 Talin is involved with the initial step of activating the integrins that are otherwise dormant and kindlin is required to assure ‘maximal integrin activation’.27
Cytoskeleton
Critical to this network is the cellular cytoskeleton, which adjusts in accordance with mechanical forces exerted on cells by the ECM. The cytoskeleton is made up of various microfilaments and microtubules. The microfilaments are composed of actin and have a fluid relationship to corresponding constituents of the intracellular environment.
The importance of cell–matrix interactions and their involvement in the mechanotransduction of cells has grown over the years since they were originally uncovered. Their vital role in many processes has been highlighted; they are not only required for normal processes like development, but additionally have a function to play in the advance of diseases such as cancer.28 The elasticity of the matrix is now known to influence stem cell differentiation towards particular lineages and cell–matrix interactions are central to uncovering the steps of this process.
How the ECM of cells is altered with age
As cells naturally undergo age-related changes, the BM underlying many tissues deteriorates. This is due to the effects of fewer BM proteins being formed and increased levels of MMP resulting in more proteins being broken down.33 Along with this, the cells experience higher levels of fibronectin, GFs, interleukins and cytokines. It has been observed by many researchers that aged cells become impeded within the cell cycle to proliferating; this has been linked to telomere shortening.21,33,57
As with elastin, the decline in the amount of collagen in tissues is associated with ageing, weakening ‘tissue integrity and strength’.58 Greater stiffness observed in ageing tissue has been attributed to further and unnecessary collagen cross-linking, resulting in compromised biomechanical properties and therefore ‘compromise ECM organisation and function’.33 Additionally, the arrangement of the (type I) collagen fibres becomes less organised and more loose and fragmented.
This is observed in most aged tissues, particularly the skin.59 The alteration in the arrangement of the collagen fibres results in the cells being less able to bind to the ECM, which has a negative impact on the cell–matrix interactions.60 These differences in the organisation of type I collagen fibres have been observed in tendons extracted from tails of young and old adult mice.58
Furthermore, alterations in the composition of the ECM of rat tail tendon have been detected from being low in collagen content and highly cellular in young rats following birth and 3 months of age.61 In older rats, the collagen content of the tendon is much higher and this results in the tissue displaying a greater stiffness.62
As with other connective tissues, the balance between matrix synthesis and matrix breakdown in the skin shifts towards matrix breakdown with the ageing process.63 This is demonstrated by a loss in the elasticity of the skin, also known as wrinkling.64,65 The loss in elasticity is due to the action of MMPs degrading the main constituents of the ECM such as types I and III collagen, proteoglycans and glycosaminoglycans (GAGs).66 The loss of these ECM components together with increased cross-linking of collagens results in reduced biomechanical properties of the tissue with age.67
In contrast to this, the levels of fibronectin (FN) have been shown to increase with age,63 as demonstrated with fibronectin isolated from skin tissue of mice. This alte-ration in fibronectin expression has a detrimental effect on integrin–ECM binding, thus impacting on the ability of cells to respond to environmental change, especially with age.
Within skeletal muscle, the ECM plays a critical role in the transfer of force and response of the tissue to mechanical loading.68 Animal studies conducted in rats reflected similar findings to other ageing tissues, in that the collagen content of limb skeletal muscle increased exponentially with age.69 In contrast to this, these studies when repeated in humans showed that the collagen content of skeletal muscle remained relatively stable with age.70 This highlights that age-related changes within the ECM varies across tissue types and the ECM is modified in distinct ways between different species.
More recently, there has been renewed interest in the subject; first, this can be linked with attempting to understand the conceivable relationship linking age-related changes in cells and the advance of diseases such as cancer.1,27,71 Second, to investigate the differences behind reparative wound healing in adults, in contrast to regenerative wound healing as seen in the foetus and how age-related decline in the ECM of soft tissue has bearing on these disparities in wound healing.72,73
How ageing ECM affects wound healing
The ECM experiences a multitude of changes during wound healing in an adult from the initial steps instantly following injury to the establishment of the collagenous scar tissue that signals the end of the healing process. Wound healing in a foetus occurs in an opposing fashion, and this has been confirmed in many tissue types, most notably the skin, but also tendon, articular cartilage and bone.73–77 Differences in the healing models of adults and foetuses have been observed in terms of the inflammatory reaction, GF levels and gene expression of proteins along with ECM constituents.4,75 This regenerative capability of the foetal tissue is confirmed to be inherent to the tissue.73,78 Thus, the age-related changes to the ECM and its impact on cell–matrix interactions are vital to understand.
Foetal wound healing
Most of the evidence gathered on regenerative healing in foetuses has focused on the skin. In foetal models, the dermis and epidermis are fully restored upon injury, including complete repair of the structure, strength and function of the ECM.4,9,10 Additionally, higher levels of collagen type III than collagen type I exist in foetal skin, where it amounts to between 30% and 60% of the total sum of collagen, compared to adult skin79,80 which holds between 10% and 20% of collagen type III.9
Along with variations in the amount of collagens present, other age-related differences between the wound healing processes have been noted. Hyaluronic acid (HA) is present in high levels in foetal skin wounds, but is present for longer lengths of time in adult skin wounds.75,81 The presence of HA is important in enabling the migration and proliferation of multiple cell types after injury in a foetus.4 HA is also considered significant for collagen synthesis; this is corroborated by the reduction in scar tissue that forms during adult wound healing when HA levels are increased.82
Similarly, the levels of different proteoglycans such as decorin and fibromodulin have been linked to the scarred phenotype seen in healed adult skin and scarless phenotype of foetal repaired skin. Lower expression of decorin in adult skin is linked to scar formation,83 and lower levels of fibromodulin are seen in adult wounds than foetal wounds. This is verified by a reduction in the extent of fibromodulin synthesis with age.84 Additionally, the ratio of MMP to TIMP activity seems to be key in understanding the disparity between scarred and scarless healing. In foetal skin wounds, the level of MMPs to TIMPs is much higher,85 particularly MMP-1 and MMP-9. Contrastingly, more MMP-2 and less TIMPs are expressed during adult wound healing.10
Adult wound healing
In adults, the ECM plays an active part in the proliferative phase and remodelling phase of the wound healing process.86 During the proliferative phase, fibroblasts initiate deposition of the ECM, following their migration into the wound site. This is referred to as the provisional matrix. Tenascin-C of the tenascin family of matricellular proteins plays a key part in encouraging fibroblasts to the injury site to generate the provisional matrix made up of fibrin and fibronectin.87 The provisional matrix allows for cell adhesion and migration to occur88 and granulation tissue replaces the interim matrix.
This granulation tissue is abundant in fibronectin and provides a vascular system for collagen to be laid down.89 Collagen forms the main component of the final ECM and is commonly known as scar tissue. The collagenous matrix is formed in the remodelling stage and increased cross-linking of the collagen results in a stiffer matrix.90 Although collagen is crucial to restore the structure and function of the tissue at the wound site, excess collagen is detrimental to the tissue, causing a destabilised structure due to the presence of a fibrotic scar that replaces the former tissue.91 This scarred tissue is an issue for clinicians, as the function of the tissue is hampered, and currently, no successful solution exists to restore the former properties of the healed tissue.10
Therefore, understanding the changes to the ECM that occur with age that result in the different healing mechanisms between adult and foetal healing is critical to developing a way of healing adult wounds through a regenerative process. Revealing these age-related differences in the ECM could result in new methods being created through tissue engineering techniques to encourage adult injuries to repair without scar formation. This will be explored in further detail in a later section.
Techniques to analyse the ECM
There are several techniques that can be utilised for analysing the ECM; some of these are well-established, for example, confocal microscopy and electron microscopy.58,92 Other methods such as Raman spectroscopy93 are being explored as new ways to examine ECM structure and composition, requiring little or no sample preparation. These approaches allow for the composition, structure and biomechanical properties of the ECM to be examined. A selection of these will be detailed here with a brief outline of their basic principles and what they can reveal about the ECM.
AFM
The elastic modulus (E) is the standard way of calculating the amount of stretching of a substrate in response to a given level of stress. Researchers have employed AFM in the past few years as a reliable method to measure the mechanical properties in terms of stiffness (Young’s modulus) of a matrix.2 Attention has been brought to the way that the mechanical properties of the ECM influence the differentiation profiles of mesenchymal stem cells (MSCs). More recently, researchers confirmed AFM as a valid method to monitor differentiation of stem cells into tenocytes, by measuring the changes that occurred in elastic modulus at a cellular level.94 It has been demonstrated that the mechanical properties of a substrate have consequences in the cell ‘contractility, motility and spreading’, for example, cells on a soft gel substrate form ‘dynamic adhesions’, whereas on stiffer substrates they formulate ‘static focal adhesions’.2
The technique works through two main components, a sensor and detector. The detector calculates the sensor’s reaction to forces; this sensor is in the form of a cantilever beam that responds to the forces it receives by contorting, and the detector measures the deflection of the cantilever tip.95,96 Furthermore, there are three key modes of operation for AFM, contact, non-contact and tapping mode. For the interest of this review, tapping mode will only be discussed.
Tapping mode was specifically developed to surmount the problems with contact mode and biological samples, including samples being damaged by the cantilever tip.96,97 It is this mode of AFM that is used in calculating the elastic modulus of ECM specimens, during which the contact between the tip and samples is kept to a minimum, thus averting damage to softer substrates. Additionally, samples with either wet or dry surfaces can be tested.96
Further advantages of AFM in ECM analysis include the ability to take measurements on three separate axis (x, y and z) providing 3D images of a sample surface.96 It is also a technique that does not require any prior sample preparation or particular environment in order to operate such as a vacuum. It has become a vital tool in revealing information on the study of mechanobiology and the relationship between cells and ECM stiffness.98
For example, it has been successfully employed to determine the changes that occur in the elastic modulus of osteoblast cells, as a result of adhesion to various ECM proteins such as FN. Moreover, AFM imaging experiments were utilised to demonstrate how binding to different substrates caused this increase in elastic modulus. For instance, the plating of osteoblasts on FN was found to be associated with ample fibre formation and remodelling of the cytoskeleton, which lead to an increase in the modulus of osteoblasts.98
Immunostaining
Immunostaining of the ECM takes two main forms either as immunohistochemical (IHC) staining or immunocytochemical (ICC) staining. Immunostaining is an effective qualitative way of evaluating the presence of individual proteins expressed within the ECM.99 It relies on specific antibody and antigen relationships via a visualisation tag to identify the expression of definitive proteins.
The sample preparation method differs between the two techniques; tissue samples for IHC need to be embedded in resin or paraffin and cut it into thin sections prior to staining. One of the most common ways to conduct IHC staining is to use haematoxylin and eosin (H&E) staining. Throughout the literature, there are several proteins that are surveyed, typically they include collagen type I, laminin and fibronectin. In contrast to this, to examine ECM proteins at a cellular level, ICC staining is employed.100
IHC staining has been successfully exercised in tendon fibroblasts to determine the expression levels of proliferation cell nuclear antigen (PCNA) and α-smooth muscle actin (α-SMA), which are released by healing tendon fibroblasts. The fibroblasts are responsible for synthesising the ECM during the wound healing process. IHC was able to measure the increasing levels of PCNA and α-SMA post injury.101 Additionally, IHC has been utilised to examine the composition of the ECM in human coronary arteries where, for example, collagen type IV has been detected to be underlying the endothelium equivalent to the position of the basal lamina. Moreover, it efficiently detected the presence of proteoglycans such as biglycan and decorin, which are crucial components of tissues in blood vessels and are implicated in vascular disease.102
ICC staining examines the composition of the ECM at a cellular level. Similar to IHC, ICC staining was carried out on tendon fibroblasts observing expression levels of PCNA and α-SMA, although the tendon cells were inspected as per the basic principle. This enabled the demonstration of the differences in tendon fibroblasts that were synthesised from an intact tendon to a tendon post injury undergoing the wound healing process.101 Recently, this staining technique has been utilised to observe the modifications, in protein expression of the cartilage matrix proteins in tenocytes during development, which include collagen type I and aggrecan.103
Confocal microscopy
Confocal microscopy is typically used in conjunction with immunostaining; one of the most important aspects of this imaging technique is that it allows for 3D analysis of samples. A confocal microscope generates sharp images of a sample that would appear blurred if they viewed via a conventional light microscope.104 These 3D images are attributed to the confocal microscope’s ability to significantly reduce the light from out-of-focus planes. Moreover, this results in enhanced lateral resolution. It is particularly useful for examining the 3D architecture of the ECM through z-stacked two-dimensional (2D) images.105
Confocal microscopy was used to examine the general composition and structure of the fibroblast-synthesised ECM by obtaining z-stacked images to display the conformation, distribution and orientation of proteins including FN, collagen type VI and elastin. These images were fairly detailed, highlighting the x- and y-axis projections of the z-stacked images where spatial overlap between the proteins was displayed. Moreover, quantitative differences of the various protein networks (elastin network and FN network) were detected. Overall, these findings demonstrated confocal microscopy can be used not merely to determine the structure of ECM constituents but also their role in the organisation of ECM components.105
This enables high-resolution 3D images of the ECM and its various constituents to be built up, allowing researchers to discern more information about the ECM environment, namely, the organisation and distribution of proteins and determining the level of protein deposition. Thus, it could provide important structural insight into how this environment is altered by age-related changes.
Tandem mass spectroscopy
Tandem mass spectroscopy or Mass Spectroscopy/Mass Spectroscopy (MS/MS) is being an increasingly popular way of characterising the profile of ECM proteins by examining the individual peptides that constitute them and can be viewed as a complementary quantitative technique to immunostaining. MS/MS incorporates a combination of two mass spectrometers where the first spectrometer selects an individual mass (precursor), which represents an analyte in the mixture. Mass selected ions subsequently pass through a region where they are activated to produce fragment (product) ions, which is carried out via the collision of ions with a neutral gas in a process termed collisional activation. The second-stage mass spectrometer discerns the fragment ions depending on mass, thus generating an MS/MS spectrum, which consists of merely product ions from the precursor selected.106
It can provide detailed analysis of overlapping proteins that are shared between differing sources of ECM and highlight any unique proteins present in individual cases.107,108 In terms of quantitative results, MS/MS was utilised in humans to detect 300 peptides where 25 of these exhibited the existence of ECM proteins such as FN, fibrillin and an ECM protein known as frasl.108 Frasl is known to have an essential role in structure and cell signalling.109 MS/MS has been employed to detect other ECM proteins through identification of peptides including fibrillin-1, laminin, elastin and lumican, which is a keratin sulphate proteoglycan.107
Transmission electron microscopy/scanning electron microscopy
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are opposing microscopy techniques that both work on the principle of using electrons to visualise a substrate. Briefly, SEM focuses on the surface to build up a 3D image of a substrate’s topography; whereas in TEM, as the name suggests, it involves transmitting electrons at a sample, which pass straight through providing a 2D image of the inner structure. In typical analysis of the ECM, researchers have favoured using TEM in order to view the ultrastructure.110
SEM has been employed to investigate the ECM of rat tail tendon. The conformation of the ECM in tendon could be detected where the GAGs were seen interwoven with the collagen fibrils. Additionally, it could also be demonstrated that while some GAG filaments merely interacted with one fibril, others made contact with many fibrils simultaneously.111 SEM has also been previously used effectively to observe the shape and organisation of laminin and FN where, for instance, laminin was found to be imaged as a ‘rigid asymmetric cross consisting of a long and three identical short arms’ and FN consisting of two identical strands with single peptide chains.112 Furthermore, the SEM has been successfully utilised to detect synthesis of and thus image ECM material.113
TEM has been effectively applied in order to quantify the differences between young and aged 3D collagen gels in mice tail tendons. It was used to directly assess the substantial modifications that occur in fibril diameter and density as a result of the transition from a young mice phenotype to an aged mice phenotype. Both these quantities exhibited a decrease with age highlighting the inability of senescent collagen to form a 3D matrix in comparison to young collagen.58 Moreover, these findings were corroborated by previous evidence where reduction in collagen fibril density corresponds to the tensile strength of collagen polymerised in vitro.114 These are a few examples of the extensive use of the TEM to characterise many aspects of the ECM as well as the modifications it undergoes.
Gelatin substrate zymography
Gelatin substrate zymography (GSZ) is usually used in detection of MMP, in particular MMP-2 and MMP-9. MMPs are enzymes that are known to degrade the ECM;115 this procedure takes advantage of the fact that MMPs are recognised by their molecular weight and the ‘degradation of their preferential substrate’.116 This method enables one to determine whether the MMPs are in an active or latent form. Generally, the most frequently employed substrate is gelatin for MMP-2 and MMP-9.19
It is based on the sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), which consists of a substrate co-polymerised with polyacrylamide gel in order to observe enzyme activity based on molecular weight separation. Substrate zymography is generally considered to be the most suitable method to use in order to detect MMP activity from a variety of biological samples as it can be used for screening drugs and as mentioned discerns the latent MMPs from the activated MMPs. However, it does have a fairly length protocol (2 days), but nevertheless it is still broadly used for observation of MMP activity.19
Raman spectroscopy
One of the biggest advantages of Raman Spectroscopy is that unlike all the techniques mentioned above, it does not require any prior sample preparation. Despite this, it is still a relatively new analysis method for characterising ECM.93 Raman spectroscopy can be used to evaluate the rate of ECM synthesis and the rate of the biochemical components of the ECM synthesised. Its non-invasive nature enables modulation of the growth and progression of the ECM by numerous cells on an array of scaffold materials bared to stimuli including GFs and mechanical forces.117 Again, it is typically used in conjunction with other analysis methods to confirm the presence of specific components of the ECM.
The operation of Raman spectroscopy is based on the traditional ‘Raman effect’.118 Incident light or photons are scattered from a sample in an inelastic manner and shifted infrequency via the distinguishing vibrational energy of the molecular bond.119 Molecular bonds are probed concurrently while a Raman spectrum is produced, which acts as a ‘molecular fingerprint’ representing the total biochemical components of the sample. The Raman signals generated from the spectrometer are linearly correlated with the number of molecular bonds that occur in the sample; thus, quantitative data can be obtained from the spectrum.120
SHGM
SHGM works on the basis of ‘nonlinear optical effect’ as a result of photons coming into contact with a nonlinear substance and merging to create new photons with half the wavelength and twice the frequency of before. It is a three-level process and the second-harmonic photons are produced virtually instantly in order to generate a clear second-harmonic signal that is discharged primarily in the forward direction.121 In the same way that Raman spectroscopy does not require sample preparation, SHGM is an emerging technique that has been effectively used in other applications such as drug delivery,122 and now attention has turned to it being used for identifying collagen fibres within cells and tissue.
SHGM was employed to demonstrate the differences in collagen bundle distribution, length and packing between normal skin and burn scar tissues. This was shown in human, ovine and porcine dermal tissue. SHGM displayed an increased resolution of individual collagen bundles in scar tissue.123 This study was important as it showed that SHGM can be proficiently used to inspect the collagen architecture in tissues and it can also be done without any prior staining. SHGM has been associated with several dermatological studies over the last decade.124,125 Overall, this technique is clearly vital and efficient for observing fibrillar collagen structures both in a normal state and as a result of pathological changes that occur.
Overall, there is clearly an extensive range of analytical techniques for characterisation in the ECM, and these are not merely limited to determining the composition and structure but also be utilised to determine the modifications that occur as a result of diseases, wound healing and age-dependent processes. Moreover, these techniques are often not only used exclusively but also in conjunction with others, as some may provide qualitative observations whereas some are prominent in terms of quantitative results. Furthermore, these techniques vary in terms of their reputation for characterising the ECM and when they were utilised to do this. Some of the more recent techniques including Raman spectroscopy may be even more favourable to use due to their non-invasive properties. However, this is also dependent on the application and what aspect of the ECM that is required to be characterised.
Ways the ECM is employed in tissue engineering
Influence on differentiation of MSCs
The importance of the ECM in instructing differentiation of stem cells has been demonstrated at the organ level.64 Nevertheless, the ability of the ECM to do this is compromised with age. Ageing of the ECM can have detrimental effects via ‘imbalanced proteolytic degradation and the release of free radicals’.64 It has been shown that ECM derived from young MSCs displays significantly higher levels of protein expression, in contrast to ECM taken from aged MSCs. It is evident that the age of ECM impacts on MSCs, as older MSCs expressed greater pluripotency when seeded onto young ECM.57 However, the mechanism of why this occurs is still to be answered.
Recently, it has been reported that ECM put down by foetal stem cells was superior to ECM generated by adult stem cells in encouraging cell expansion and chondrogenic differentiation.126 Such findings indicate that the age of the ECM may impact upon the behaviour of cells. Many other properties of the ECM have been shown to influence the behaviour of cells. Scaffolds with a nanoscale diameter resulted in tenocytes producing their own ECM that resembled the matrix seen during the reparative healing process. Contrastingly, scaffolds with a microscale diameter caused tenocytes to form matrix similar to that seen during the regenerative healing process.127
Furthermore, it has been indicated that the ECM plays a significant role in various cellular activities including cell migration and proliferation.33 It has been found that ECM can manipulate the differentiation of MSCs through its stiffness with stem cells changing their properties according to the degree of stiffness of the underlying substrate.2,6 This highlights the critical nature of the mechanical signals that are passed on to cells from the ECM, and the importance of the relationship between cells and their environment in determining the commitment to a certain cell lineage. Understanding more about the mechanisms behind this relationship could reveal more detail regarding the age-related differences displayed during the wound healing process.
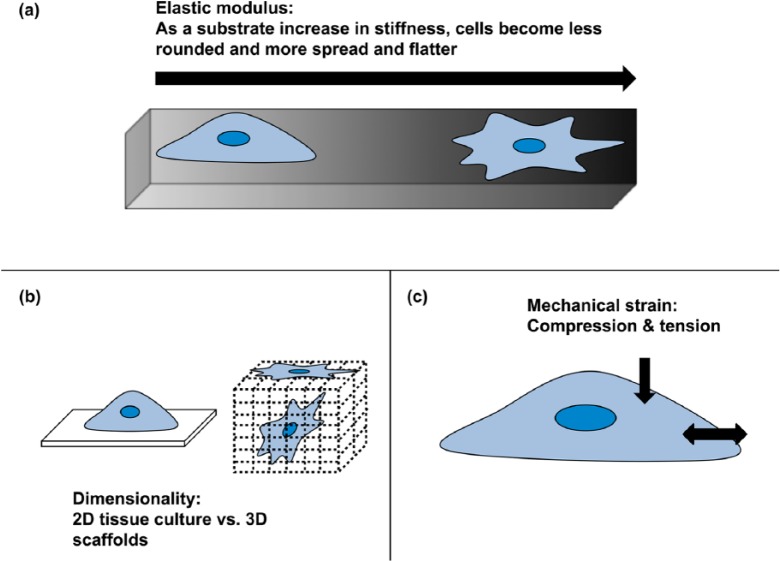
There has been much interest in the influence of different aspects of substrate features over stem cell differentiation (Figure 3), in particular the stiffness or elasticity of a substrate. This relationship between cells and the underlying matrix is referred to as mechanobiology.34 As mentioned earlier, as part of its role, tendon responds to mechanical load. It does this in a variety of ways at the cellular level, changing its ‘gene expression, protein synthesis and phenotype’.34 The components involved in the mechanobiology of the cells and their interactions were detailed further above in a previous section.
Figure 3.
Physical substrate features that can influence cellular behaviour: (a) stiffness (elastic modulus), (b) dimensionality and (c) mechanical strain of compression and/or stretch adapted from Brafman.128
2D: two-dimensional; 3D: three-dimensional.
These components, in particular the cell’s contractile forces, are exerted through its cytoskeleton, using this to anchor and pull on a substrate. These cellular contractile forces are engaged in a feedback loop with the elastic modulus (E) of the surrounding substrate. This has been demonstrated with cells on substrates with a variation in elasticity. On soft gels (E = 1 kPa), cells presented with disperse adhesions, whereas cells seeded on substrates with a higher stiffness (E= 30–100 kPa) demonstrated strong adhesions, similar to those found in cells attached to glass.2
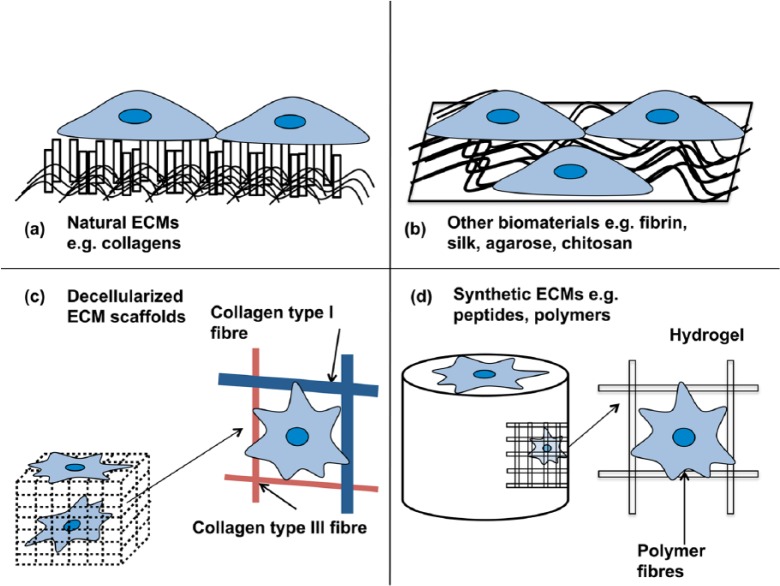
Use as a scaffold
A few other alternatives exist to surgical intervention such as graft products made by companies including DePuy, Zimmer and Wright Medical. These manufactured graft materials are categorised either as biological or synthetic (Figure 4). All but one of the biological scaffolds commercially available is derived from a xenogeneic source, albeit with Food and Drug Administration (FDA) approval.129 These biological scaffolds appear to be most effective at treating rotator cuff tendon injuries. Synthetic scaffolds have been constructed from numerous materials including polyester and polyacrylamide, although these have been reported with limited success, due to problems with resorption of the graft materials from the injury site and lack of adequate biocompatibility.129,130 Consequently, their use peaked over 20 years ago.
Figure 4.
Different types of ECM scaffolds available to use for tissue engineering applications: (a) natural ECM, for example, collagen; (b) other biomaterials, for example, fibrin, silk (typically in the form of electrospun fibres or as a coating on a 2D substrate); (c) decellularised ECM (stripped of all previous cellular material) and (d) synthetic ECM, for example, polymers (usually in fibres or hydrogels) adapted from Brafman.128
ECM: extracellular matrix; 2D: two-dimensional.
Regenerative medicine is increasingly becoming a potential therapy to replace original tissue, with tissue engineering providing the method to produce prospective constructs for this therapy. The concept involves implantation of cells within a scaffold construct along with the required biomolecules. Presently, there are a few examples of such applications in a clinical setting, including artificial skin46 and the graft materials mentioned. The necessary components for tendon tissue engineering demand specific sourcing of cells, scaffold material and biomolecules; of particular importance for vascular tissues is the issue of mass transport of oxygen and nutrients.131
Conclusion
This review demonstrates that the ECM is not purely a dynamic non-cellular constituent but also one that is susceptible to substantial modifications as a function of the normal ageing process. Its components regulate various processes including cell proliferation, survival, differentiation and migration. Furthermore, the ECM can also be defined as dynamic as it is subject to constant remodelling; also the 3D architecture of the ECM regulates the mechanical properties of the cells and is crucial for the outline of connective tissues.
The ageing effects of the ECM have been explored in many ways in several connective tissues with varying results. For instance, the general consensus is that collagen type I decreases in content with age in tissues such as skin in addition to a more disorganised distribution. However, with the ECM becoming a highly collagenous tissue with age, this has implications for mechanical properties, for example, an increase in stiffness. These modifications have crucial implications in numerous processes particularly for the wound healing process.
The age-related modifications, as mentioned, have implications with the transition from a scarless to a scarred phenotype during wound healing, indicating that scar formation is attributed to numerous complex changes. All the topics discussed with reference to ECM composition, age-related changes and contrasting healing properties in foetal versus adult phenotypes would not be possible without the analytical techniques employed to examine the ECM. A variety of analytical techniques are utilised scrutinising various parts of the ECM ranging from the observation of MMP activities via GSZ to quantitative measurement of matrix stiffness via AFM.
Research has highlighted that stiffness of matrices influences the stem cell lineage. Moreover, the interactions between the cell and matrix are characterised by the stiffness of the matrix with focal adhesions for soft matrices and dynamic adhesions for stiff matrices. Moreover, the types of stem cells to be seeded onto scaffolds have been investigated predominantly using MSCs with varying extents of success. Nevertheless, knowledge of the age-related changes in the ECM and its implications for the wound healing process as well as potential of using the ECM as a scaffold for regenerative healing is only at the tip of the iceberg. Thus, the on-going trend of ECM research will prevail in the next few decades.
Footnotes
Declaration of conflicting interests: None of the authors have any potential conflicts of interest to declare.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009; 326: 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 2005; 310: 1139–1143. [DOI] [PubMed] [Google Scholar]
- 3. Pelham RJ, Wang Y-L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 1997; 94: 13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rolfe KJ, Grobbelaar AO. A review of fetal scarless healing. ISRN Dermatol 2012; 2012: 698034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma RI, Snedeker JG. Biochemical and biomechanical gradients for directed bone marrow stromal cell differentiation toward tendon and bone. Biomaterials 2010; 31: 7695–7704. [DOI] [PubMed] [Google Scholar]
- 6. Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126: 677–689. [DOI] [PubMed] [Google Scholar]
- 7. Larsen M, Artym VV, Green JA, et al. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol 2006; 18: 463–471. [DOI] [PubMed] [Google Scholar]
- 8. Mould AP, Humphries MJ. Regulation of integrin function through conformational complexity: not simply a knee-jerk reaction? Curr Opin Cell Biol 2004; 16: 544–551. [DOI] [PubMed] [Google Scholar]
- 9. Bullard KM, Longaker MT, Lorenz HP. Fetal wound healing: current biology. World J Surg 2003; 27: 54–61. [DOI] [PubMed] [Google Scholar]
- 10. Colwell AS, Longaker MT, Lorenz HP. Fetal wound healing. Front biosci 2003; 8: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 11. Hakkinen KM, Harunaga JS, Doyle AD, et al. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng Part A 2011; 17: 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Young RG, Butler DL, Weber W, et al. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res 1998; 16: 406–413. [DOI] [PubMed] [Google Scholar]
- 13. Daamen WF, Veerkamp J, Van Hest J, et al. Elastin as a biomaterial for tissue engineering. Biomaterials 2007; 28: 4378–4398. [DOI] [PubMed] [Google Scholar]
- 14. Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci 2002; 115: 3861–3863. [DOI] [PubMed] [Google Scholar]
- 15. Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater 2009; 8: 457–470. [DOI] [PubMed] [Google Scholar]
- 16. Rosso F, Giordano A, Barbarisi M, et al. From cell-ECM interactions to tissue engineering. J Cell Physiol 2004; 199: 174–180. [DOI] [PubMed] [Google Scholar]
- 17. Chiquet-Ehrismann R, Tucker RP. Tenascins and the importance of adhesion modulation. Cold Spring Harb Perspect Biol 2011; 3: a004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan BP, Fu S, Qin L, et al. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta Orthop Scand 2000; 71: 513–518. [DOI] [PubMed] [Google Scholar]
- 19. Kupai K, Szucs G, Cseh S, et al. Matrix metalloproteinase activity assays: importance of zymography. J Pharmacol Toxicol Methods 2010; 61: 205–209. [DOI] [PubMed] [Google Scholar]
- 20. Eckes B, Nischt R, Krieg T. Cell-matrix interactions in dermal repair and scarring. Fibrogenesis Tissue Repair 2010; 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Labat-Robert J, Robert AM, Robert L. Aging of the extracellular matrix. Méd Longév 2012; 4: 3–32. [Google Scholar]
- 22. Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 2010; 26: 397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tzu J, Marinkovich MP. Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol 2008; 40: 199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiquet-Ehrismann R, Tucker RP. Connective tissues: signalling by tenascins. Int J Biochem Cell Biol 2004; 36: 1085–1089. [DOI] [PubMed] [Google Scholar]
- 25. Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am 2005; 87: 187–202. [DOI] [PubMed] [Google Scholar]
- 26. Tanzer ML. Current concepts of extracellular matrix. J Orthop Sci 2006; 11: 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 2011; 209: 139–151. [DOI] [PubMed] [Google Scholar]
- 28. Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol 2003; 200: 423–428. [DOI] [PubMed] [Google Scholar]
- 29. Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol 2010; 341: 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heino J. The collagen family members as cell adhesion proteins. Bioessays 2007; 29: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 31. Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater 2009; 5: 1–13. [DOI] [PubMed] [Google Scholar]
- 32. White DJ, Puranen S, Johnson MS, et al. The collagen receptor subfamily of the integrins. Int J Biochem Cell Biol 2004; 36: 1405–1410. [DOI] [PubMed] [Google Scholar]
- 33. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010; 123: 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang JH. Mechanobiology of tendon. J Biomech 2006; 39: 1563–1582. [DOI] [PubMed] [Google Scholar]
- 35. Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol 2005; 24: 389–399. [DOI] [PubMed] [Google Scholar]
- 36. Fogerty FJ, Akiyama SK, Yamada KM, et al. Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (alpha 5 beta 1) antibodies. J Cell Biol 1990; 111: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu C, Keivens VM, O’Toole TE, et al. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell 1995; 83: 715–724. [DOI] [PubMed] [Google Scholar]
- 38. Chen LB, Murray A, Segal RA, et al. Studies on intercellular LETS glycoprotein matrices. Cell 1978; 14: 377–391. [DOI] [PubMed] [Google Scholar]
- 39. Aumailley M, Bruckner-Tuderman L, Carter WG, et al. A simplified laminin nomenclature. Matrix Biol 2005; 24: 326–332. [DOI] [PubMed] [Google Scholar]
- 40. Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 2011; 3: a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Domogatskaya A, Rodin S, Tryggvason K. Functional diversity of laminins. Annu Rev Cell Dev Biol 2012; 28: 523–553. [DOI] [PubMed] [Google Scholar]
- 42. Chiquet M, Renedo AS, Huber F, et al. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol 2003; 22: 73–80. [DOI] [PubMed] [Google Scholar]
- 43. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009; 17: 153–162. [DOI] [PubMed] [Google Scholar]
- 44. Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. FASEB J 1997; 11: 51–59. [DOI] [PubMed] [Google Scholar]
- 45. Lui PPY, Rui YF, Ni M, et al. Tenogenic differentiation of stem cells for tendon repair – what is the current evidence? J Tissue Eng Regen Med 2011; 5: e144–e163. [DOI] [PubMed] [Google Scholar]
- 46. Yin Z, Chen X, Chen J, et al. Stem cells for tendon tissue engineering and restoration. Expert Opin Biol Ther 2010; 10: 689–700. [DOI] [PubMed] [Google Scholar]
- 47. Petersen W, Pufe T, Kurz B, et al. Angiogenesis in fetal tendon development: spatial and temporal expression of the angiogenic peptide vascular endothelial cell growth factor. Anat Embryol 2002; 205: 263–270. [DOI] [PubMed] [Google Scholar]
- 48. Foley JD, Grunwald EW, Nealey PF, et al. Cooperative modulation of neuritogenesis by PC12 cells by topography and nerve growth factor. Biomaterials 2005; 26: 3639–3644. [DOI] [PubMed] [Google Scholar]
- 49. Streuli C. Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol 1999; 11: 634–640. [DOI] [PubMed] [Google Scholar]
- 50. Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 2007; 8: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 2006; 7: 211–224. [DOI] [PubMed] [Google Scholar]
- 52. Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012; 196: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mitchell RS, Kumar V, Abbas AK, et al. Robbins basic pathology. Philadelphia, PA: Saunders, 2007. [Google Scholar]
- 54. Petit V, Thiery JP. Focal adhesions: structure and dynamics. Biol Cell 2000; 92: 477–494. [DOI] [PubMed] [Google Scholar]
- 55. Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 2010; 11: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zaidel-Bar R, Cohen M, Addadi L, et al. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans 2004; 32: 416–420. [DOI] [PubMed] [Google Scholar]
- 57. Choi HR, Cho KA, Kang HT, et al. Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix. Aging Cell 2011; 10: 148–157. [DOI] [PubMed] [Google Scholar]
- 58. Damodarasamy M, Vernon RB, Karres N, et al. Collagen extracts derived from young and aged mice demonstrate different structural properties and cellular effects in three-dimensional gels. J Gerontol A Biol Sci Med Sci 2010; 65: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol 2008; 144: 666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sprenger CC, Plymate SR, Reed MJ. Aging-related alterations in the extracellular matrix modulate the microenvironment and influence tumor progression. Int J Cancer 2010; 127: 2739–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lavagnino M, Gardner K, Arnoczky SP. Age-related changes in the cellular, mechanical, and contractile properties of rat tail tendons. Connect Tissue Res 2013; 54: 70–75. [DOI] [PubMed] [Google Scholar]
- 62. Goh K, Holmes D, Lu H, et al. Ageing changes in the tensile properties of tendons: influence of collagen fibril volume fraction. J Biomech Eng 2008; 130: 021011. [DOI] [PubMed] [Google Scholar]
- 63. Labat-Robert J. Cell-matrix interactions in aging: role of receptors and matricryptins. Ageing Res Rev 2004; 3: 233–247. [DOI] [PubMed] [Google Scholar]
- 64. Kurtz A, Oh SJ. Age related changes of the extracellular matrix and stem cell maintenance. Prev Med 2012; 54(Suppl.): S50–S56. [DOI] [PubMed] [Google Scholar]
- 65. Mancini M, Saintigny G, Mahé C, et al. MicroRNA-152 and -181a participate in human dermal fibroblasts senescence acting on cell adhesion and remodeling of the extra-cellular matrix. Aging 2012; 4: 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Carrino DA, Önnerfjord P, Sandy JD, et al. Age-related changes in the proteoglycans of human skin: specific cleavage of decorin to yield a major catabolic fragment in adult skin. J Biol Chem 2003; 278: 17566–17572. [DOI] [PubMed] [Google Scholar]
- 67. Vedrenne N, Coulomb B, Danigo A, et al. The complex dialogue between (myo)fibroblasts and the extracellular matrix during skin repair processes and ageing. Pathol Biol 2012; 60: 20–27. [DOI] [PubMed] [Google Scholar]
- 68. Kjær M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 2004; 84: 649–698. [DOI] [PubMed] [Google Scholar]
- 69. Ramaswamy KS, Palmer ML, van der Meulen JH, et al. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol 2011; 589: 1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haus JM, Carrithers JA, Trappe SW, et al. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 2007; 103: 2068–2076. [DOI] [PubMed] [Google Scholar]
- 71. Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474: 179–183. [DOI] [PubMed] [Google Scholar]
- 72. Coolen NA, Schouten KC, Boekema BK, et al. Wound healing in a fetal, adult, and scar tissue model: a comparative study. Wound Repair Regen 2010; 18: 291–301. [DOI] [PubMed] [Google Scholar]
- 73. Favata M, Beredjiklian PK, Zgonis MH, et al. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J Orthop Res 2006; 24: 2124–2132. [DOI] [PubMed] [Google Scholar]
- 74. Weinzweig J, Panter KE, Pantaloni M, et al. The fetal cleft palate: I. Characterization of a congenital model. Plast Reconstr Surg 1999; 103: 419–428. [DOI] [PubMed] [Google Scholar]
- 75. Lo DD, Zimmermann AS, Nauta A, et al. Scarless fetal skin wound healing update. Birth Defects Res C Embryo Today 2012; 96: 237–247. [DOI] [PubMed] [Google Scholar]
- 76. Lin K, Posnick J, al-Qattan M, et al. Fetal nerve healing: an experimental study. Plast Reconstr Surg 1994; 93: 1323–1333. [PubMed] [Google Scholar]
- 77. Slate KR, Posnick JC, Wells MD, et al. Fetal tibial bone healing in utero: the effects of miniplate fixation. Plast Reconstr Surg 1993; 92: 874–883. [PubMed] [Google Scholar]
- 78. Longaker MT, Whitby DJ, Ferguson M, et al. Adult skin wounds in the fetal environment heal with scar formation. Ann Surg 1994; 219: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lovvorn HN, III, Cheung DT, Nimni ME, et al. Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J Pediatr Surg 1999; 34: 218–223. [DOI] [PubMed] [Google Scholar]
- 80. Knight KR, Lepore DA, Horne R, et al. Collagen content of uninjured skin and scar tissue in foetal and adult sheep. Int J Exp Pathol 1993; 74: 583–591. [PMC free article] [PubMed] [Google Scholar]
- 81. Sawai T, Usui N, Sando K, et al. Hyaluronic acid of wound fluid in adult and fetal rabbits. J Pediatr Surg 1997; 32: 41–43. [DOI] [PubMed] [Google Scholar]
- 82. Hu M, Sabelman EE, Cao Y, et al. Three-dimensional hyaluronic acid grafts promote healing and reduce scar formation in skin incision wounds. J Biomed Mater Res B Appl Biomater 2003; 67: 586–592. [DOI] [PubMed] [Google Scholar]
- 83. Sayani K, Dodd C, Nedelec B, et al. Delayed appearance of decorin in healing burn scars. Histopathology 2000; 36: 262–272. [DOI] [PubMed] [Google Scholar]
- 84. Soo C, Hu F-Y, Zhang X, et al. Differential expression of fibromodulin, a transforming growth factor-β modulator, in fetal skin development and scarless repair. Am J Pathol 2000; 157: 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Peled ZM, Phelps ED, Updike DL, et al. Matrix metalloproteinases and the ontogeny of scarless repair: the other side of the wound healing balance. Plast Reconstr Surg 2002; 110: 801–811. [DOI] [PubMed] [Google Scholar]
- 86. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010; 89: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Midwood KS, Schwarzbauer JE. Tenascin-C modulates matrix contraction via focal adhesion kinase- and Rho-mediated signaling pathways. Mol Biol Cell 2002; 13: 3601–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 2004; 36: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 89. Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature 2008; 453: 314–321. [DOI] [PubMed] [Google Scholar]
- 90. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004; 9: 283–289. [DOI] [PubMed] [Google Scholar]
- 91. Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen 1994; 2: 165–170. [DOI] [PubMed] [Google Scholar]
- 92. Duncan NA, Bruehlmann SB, Hunter CJ, et al. In situ cell-matrix mechanics in tendon fascicles and seeded collagen gels: implications for the multiscale design of biomaterials. Comput Methods Biomech Biomed Eng 2014; 17: 39–47. [DOI] [PubMed] [Google Scholar]
- 93. Votteler M, Carvajal Berrio DA, Pudlas M, et al. Raman spectroscopy for the non-contact and non-destructive monitoring of collagen damage within tissues. J Biophotonics 2012; 5: 47–56. [DOI] [PubMed] [Google Scholar]
- 94. Morita Y, Mukai T, Ju Y, et al. Evaluation of stem cell-to-tenocyte differentiation by atomic force microscopy to measure cellular elastic moduli. Cell Biochem Biophys 2013; 66: 73–80. [DOI] [PubMed] [Google Scholar]
- 95. Binning G, Rohrer H, Gerber C, et al. Surface studies by scanning tunneling microscopy. In: Neddermeyer H. (ed.) Scanning tunneling microscopy. Amsterdam: Springer, 1993, pp. 31–35. [Google Scholar]
- 96. Blanchard CR. Atomic force microscopy. Chem Educ 1996; 1: 1–8. [Google Scholar]
- 97. Magonov SN, Whangbo M-H. Surface analysis with STM and AFM: experimental and theoretical aspects of image analysis. New York: Wiley, 2008. [Google Scholar]
- 98. Takai E, Costa KD, Shaheen A, et al. Osteoblast elastic modulus measured by atomic force microscopy is substrate dependent. Ann Biomed Eng 2005; 33: 963–971. [DOI] [PubMed] [Google Scholar]
- 99. Bronckers A, Lyaruu D, Wöltgens J. Immunohistochemistry of extracellular matrix proteins during various stages of dentinogenesis. Connect Tissue Res 1989; 22: 691–696. [PubMed] [Google Scholar]
- 100. Zheng J. Immunohistochemistry and immunocytochemistry. In: Bird I. (ed.) Phospholipid signaling protocols. New York: Humana Press, 1998, pp. 307–314. [Google Scholar]
- 101. Fu SC, Cheuk YC, Chan KM, et al. Is cultured tendon fibroblast a good model to study tendon healing? J Orthop Res 2008; 26: 374–383. [DOI] [PubMed] [Google Scholar]
- 102. Riessen R, Isner JM, Blessing E, et al. Regional differences in the distribution of the proteoglycans biglycan and decorin in the extracellular matrix of atherosclerotic and restenotic human coronary arteries. Am J Pathol 1994; 144: 962–974. [PMC free article] [PubMed] [Google Scholar]
- 103. Stoll C, John T, Endres M, et al. Extracellular matrix expression of human tenocytes in three-dimensional air-liquid and PLGA cultures compared with tendon tissue: implications for tendon tissue engineering. J Orthop Res 2010; 28: 1170–1177. [DOI] [PubMed] [Google Scholar]
- 104. Semwogerere D, Weeks ER. Confocal microscopy. In: Wnek G, Bowlin G. (eds) Encyclopedia of biomaterials and biomedical engineering. New York: Taylor & Francis, 2005, pp. 705–714. [Google Scholar]
- 105. Petrini S, D’Amico A, Rizza T, et al. Two and three dimensional imaging in confocal laser scanning microscopy – applications for collagen VI defect studies. In: Méndez-Vilas A, Díaz J. (eds) Microscopy: science, technology, applications and education. Formatex Research Centre, 2010, Vol. 1, pp. 649–657. [Google Scholar]
- 106. JEOL. Tandem mass spectrometry (Mass spectrometry – essays and tutorials). 2006, http://www.jeolusa.com/DesktopModules/Bring2mind/DMX/Download.aspx?EntryId=78
- 107. DeQuach JA, Mezzano V, Miglani A, et al. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One 2010; 5: e13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lavigne D, Guerrier L, Gueguen V, et al. Culture of human cells and synthesis of extracellular matrix on materials compatible with direct analysis by mass spectrometry. Analyst 2010; 135: 503–511. [DOI] [PubMed] [Google Scholar]
- 109. McGregor L, Makela V, Darling SM, et al. Fraser syndrome and mouse blebbed phenotype caused by mutations in FRAS1/Fras1 encoding a putative extracellular matrix protein. Nat Genet 2003; 34: 203–208. [DOI] [PubMed] [Google Scholar]
- 110. Faltin R, Faltin K, Sander F, et al. Ultrastructure of cementum and periodontal ligament after continuous intrusion in humans: a transmission electron microscopy study. Eur J Orthod 2001; 23: 35–49. [DOI] [PubMed] [Google Scholar]
- 111. Raspanti M, Congiu T, Guizzardi S. Structural aspects of the extracellular matrix of the tendon: an atomic force and scanning electron microscopy study. Arch Histol Cytol 2002; 65: 37–43. [DOI] [PubMed] [Google Scholar]
- 112. Engel J, Odermatt E, Engel A, et al. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol 1981; 150: 97–120. [DOI] [PubMed] [Google Scholar]
- 113. Hawser S, Baillie G, Douglas LJ. Production of extracellular matrix by Candida albicans biofilms. J Med Microbiol 1998; 47: 253–256. [DOI] [PubMed] [Google Scholar]
- 114. Bradshaw AD, Puolakkainen P, Dasgupta J, et al. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol 2003; 120: 949–955. [DOI] [PubMed] [Google Scholar]
- 115. Iyer RP, Patterson NL, Fields GB, et al. The history of matrix metalloproteinases: milestones, myths, and misperceptions. Am J Physiol Heart Circ Physiol 2012; 303: H919–H930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Snoek-van Beurden P, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques 2005; 38: 73–83. [DOI] [PubMed] [Google Scholar]
- 117. Jell G, Swain R, Stevens M. Raman spectroscopy: a tool for tissue engineering. In: Matousek P, Morris MD. (eds) Emerging Raman applications and techniques in biomedical and pharmaceutical fields. Berlin, Heidelberg: Springer, 2010, pp. 419–437. [Google Scholar]
- 118. Raman C, Krishnan K. A new type of secondary radiation. Nature 1928; 121: 501–502. [Google Scholar]
- 119. Kneipp K, Kneipp H, Itzkan I, et al. Ultrasensitive chemical analysis by Raman spectroscopy. Chem Rev 1999; 99: 2957–2976. [DOI] [PubMed] [Google Scholar]
- 120. Chan JW, Lieu DK. Label-free biochemical characterization of stem cells using vibrational spectroscopy. J Biophotonics 2009; 2: 656–668. [DOI] [PubMed] [Google Scholar]
- 121. Gauderon R, Lukins P, Sheppard C. Optimization of second-harmonic generation microscopy. Micron 2001; 32: 691–700. [DOI] [PubMed] [Google Scholar]
- 122. Campagnola PJ, Millard AC, Terasaki M, et al. Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys J 2002; 82: 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Chen AC, McNeilly C, Liu AP, et al. Second harmonic generation and multiphoton microscopic detection of collagen without the need for species specific antibodies. Burns 2011; 37: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 124. Yeh AT, Kao B, Jung WG, et al. Imaging wound healing using optical coherence tomography and multiphoton microscopy in an in vitro skin-equivalent tissue model. J Biomed Opt 2004; 9: 248–253. [DOI] [PubMed] [Google Scholar]
- 125. Da Costa V, Wei R, Lim R, et al. Nondestructive imaging of live human keloid and facial tissue using multiphoton microscopy. Arch Facial Plast Surg 2008; 10: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Li J, Hansen KC, Zhang Y, et al. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials 2014; 35: 642–653. [DOI] [PubMed] [Google Scholar]
- 127. Erisken C, Zhang X, Moffat KL, et al. Scaffold fiber diameter regulates human tendon fibroblast growth and differentiation. Tissue Eng Part A 2012; 19: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Brafman DA. Constructing stem cell microenvironments using bioengineering approaches. Physiol Genomics 2013; 45: 1123–1135. [DOI] [PubMed] [Google Scholar]
- 129. Chen J, Xu J, Wang A, et al. Scaffolds for tendon and ligament repair: review of the efficacy of commercial products. Expert Rev Med Devices 2009; 6: 61–73. [DOI] [PubMed] [Google Scholar]
- 130. Longo UG, Lamberti A, Petrillo S, et al. Scaffolds in tendon tissue engineering. Stem Cells Int 2011; 2012: 517165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lovett M, Lee K, Edwards A, et al. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev 2009; 15: 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]