Abstract
Circulating antibodies can access most tissues to mediate surveillance and elimination of invading pathogens. Immunopriviledged tissues such as the brain and the peripheral nervous system, are shielded from plasma proteins, by the blood-brain barrier1 and blood nerve barrier2, respectively. Yet, circulating antibodies must somehow gain access to these tissues in order to mediate their antimicrobial functions. Here, we examine the mechanism by which antibodies gain access to neuronal tissues to control infection. Using mouse model of genital herpes infection, we demonstrate that both antibodies and CD4 T cells are required to protect the host following immunization at a distal site. We show that memory CD4 T cells migrate to the dorsal root ganglia (DRG) and spinal cord in response to HSV-2 infection. Once inside these neuronal tissues, CD4 T cells secrete interferon (IFN)-γ and mediate local increase in vascular permeability, enabling antibody access for viral control. A similar requirement for CD4 T cells for antibody access to the brain was observed following intranasal challenge with vesicular stomatitis virus. Our results reveal a previously unappreciated role of CD4 help in mobilizing antibodies to the peripheral sites of infection where they help to limit viral spread.
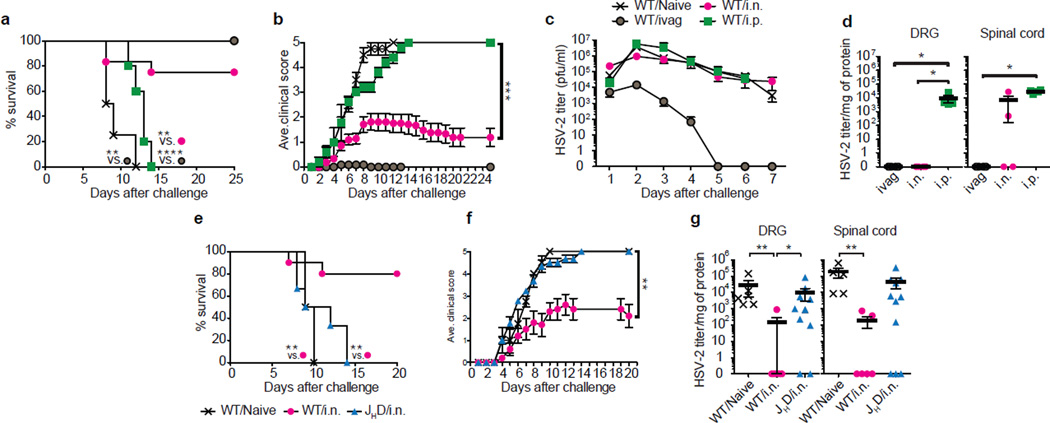
To investigate the mechanism of antibody-mediated protection within the barrier-protected tissues, we employed a mouse model of genital herpes infection. Herpes simplex virus type 2 (HSV-2) enters the host through the mucosal epithelia, and infects the innervating neurons in the DRG to establish latency3,4. Vaginal immunization by an attenuated HSV-2 with deletion of the thymidine kinase gene (TK− HSV-2) provides complete protection from lethal disease following genital challenge with wild type HSV-2 (Ref.5) by establishing tissue-resident memory T cells (TRM)6. In vaginally immunized mice, IFN-γ-secretion by CD4 T cells, but not antibodies, are required for protection7,8. In contrast, distal immunization with the same virus fails to establish TRM and provides only partial protection6. Nevertheless, of the distal immunization routes tested, intranasal immunization with TK− HSV-2 provided the most robust protection against intravaginal challenge with WT HSV-2, whereas intraperitoneal immunization provided the least protection (Fig. 1a–d)9,10. As shown previously6, intransal immunization did not establish TRM in the genital mucosa (Extended Data Fig. 1a&b), despite generating comparable circulating memory T cell pool (Extended Data Fig. 1c&d). Following vaginal HSV-2 challenge, mice that were immunized intranasally with TK− HSV-2 were unable to control viral replication within the vaginal mucosa (Fig. 1c), but had significantly reduced viral replication in the innervating neurons of the dorsal root ganglia (DRG) (Fig. 1d). Notably, we found that protection conferred by intranasal immunization required B cells, as JHD mice (deficient in B cells) were not protected by intranasal immunization (Fig. 1e–g). In the absence of B cells, intranasal immunization was unable to control viral replication in the DRG and spinal cord (Fig. 1g).
Figure 1. Intranasal immunization confers B cell-dependent neuron protection following genital HSV-2 challenge.
(a–d) C57/BL6 mice were immunized with TK− HSV-2 (105 pfu) via the intranasal (i.n.; n=12), intraperitoneal (i.p.; n=5) or intravaginal (ivag; n=11) route. Five to six weeks later, these mice and naïve mice (n=4) were challenged with a lethal dose of WT HSV-2 (104 pfu). Mortality (a), clinical score (b) and virus titer in vaginal wash (c) were measured on indicated days after challenge. Six days after challenge, virus titer in tissue homogenates including DRG and spinal cord was measured (d). (e–g) Balb/c mice (n=10) or B cell-deficient JHD mice (n=6) were immunized i.n. with TK− HSV-2 (5×104 pfu). Six weeks later, these mice and naïve mice (n=4) were challenged with lethal WT HSV-2 (105 pfu). Mortality (e) and clinical score (f) were measured. Six days after challenge, virus titer in tissue homogenates including DRG and spinal cord was measured by plaque assay (g). Data are means ± s.e.m. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001 (Unpaired student t-test).
In mice immunized intranasally with TK− HSV-2, no evidence of infection in the DRG or the spinal cord was found (Extended Data Fig. 1e). Moreover, the intranasal route of immunization was not unique in conferring protective response, as parabiotic mice sharing circulation with intravaginally immunized partners were also partially protected from vaginal challenge with WT HSV-2 in the absence of TRM6 (Extended Data Fig. 1f–h). We found that the B cells in the immunized partners were required to confer protection in the naïve conjoined mice, as partners of immunized µMT mice were unprotected (Extended Data Fig. 1f–h). Moreover, antigen-specific B cells were required to confer protection, as ivag immunized partner whose B cells bearing an irrelevant B cell receptor (against hen egg lysozyme (HEL)) were unable to confer protection in the conjoined naïve partner (Extended Data Fig. 1f–h). As observed for the intranasal immunization, viral control conferred by the immunized parabiotic partner was not observed in the vaginal mucosa (Extended Data Fig. 1h), suggesting that protection occurs in the innervating neurons.
Next, we investigated the basis for superior protection by antibodies following different routes of immunization. Intravaginal, intranasal and intraperitoneal routes of immunization with TK− HSV-2 results in comparable circulating CD4 T cell memory responses6. While no differences were seen for other isotypes, the intranasal and intravaginal routes of immunization were superior to intraperitoneal route in generating higher levels of systemic HSV-2-specific IgG2b and IgG2c responses (Extended Data Fig. 2). These results indicated that higher levels of circulating virus-specific IgG2b and IgG2c correlate with protection against vaginal HSV-2 challenge.
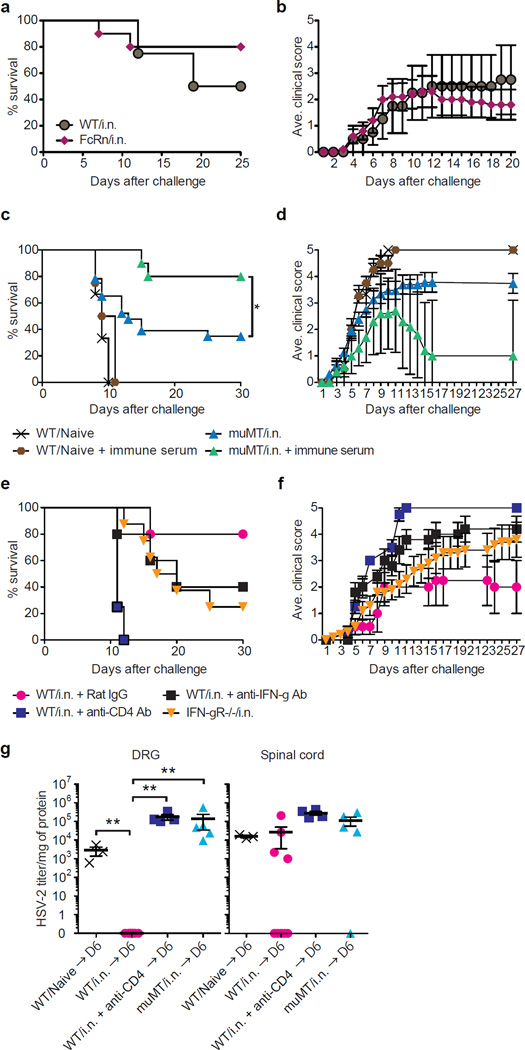
We next examined how antibody access to the DRG and spinal cord is mediated. Even though the peripheral nervous tissues are protected from antibody diffusion through blood nerve barrier, it was formally possible that secretion of antibody into the tissue occurs through transport of serum antibody by FcRn11 expressed on the endothelial cells within the infected tissues. However, we found that mice deficient in FcRn immunized intranasally with TK− HSV-2 were equally protected as the WT counterpart from vaginal HSV-2 infection (Fig. 2a,b). Thus, circulating HSV-2-specific antibodies are somehow mobilized to the neuronal tissues following local viral infection in FcRn-independent manner, and are required for protection of the host.
Figure 2. Antibody-mediated neuroprotection depends on CD4 T cells but not FcRn mediated transport.
(a&b) C57/BL6 (WT) mice (n=4) and FcRn−/− (n=10) mice were immunized i.n. with TK− HSV-2 (105 pfu), and six weeks later, challenged with lethal dose of WT HSV-2 (104 pfu). Mortality (a) and clinical score (b) were measured. (c&d) µMT mice were immunized with TK− HSV-2 (105 pfu) intranasally. Five to six weeks later, naïve mice (n=3), naïve mice receiving immune serum i.v. (n=4), µMT mice (n=23) and µMT mice receiving immune serum i.v. (n=10) were challenged with a lethal dose of WT HSV-2, and mortality (c) and clinical score (d) were assessed. Immune serum prepared from mice immunized 4 wks prior with TK− HSV-2 (200 µl/mouse) was injected 3 hr prior to challenge, 3 and 6 days after challenge. (e&f) WT C57/BL6 mice (n=5) and IFN-γR−/− mice (n=8) immunized i.n. with TK− HSV-2 (105 pfu) six weeks prior were challenged with a lethal dose of WT HSV-2, and mortality (e) and clinical score (f) were assessed. Depletion of CD4 T cells (n=4) or neutralization of IFN-γ (n=5) was performed on days −4, and −1, 2 and 4 after challenge by i.v. injection of anti-CD4 (GK1.5) or anti-IFN-γ (XMG1.2), respectively. (g) Six days after challenge, virus titer in tissue homogenates including DRG and spinal cord was measured by plaque assay (e). Data are means ± s.e.m. **: p<0.01 (Unpaired student t-test).
If circulating antibodies are sufficient, passive transfer of HSV-2-specific antibodies alone should be able to protect the host. However, we and others12,13 have found that intravenous injection of HSV-2-specific antibodies alone fails to protect naïve mice against HSV-2 challenge (Fig. 2c,d). In contrast, consistent with a previous study13, we found that B cell deficient µMT mice immunized intranasally with TK− HSV-2 given systemic administration of HSV-2 specific antiserum were protected (Fig. 2c,d). Thus, these results demonstrated that it is the secreted antibodies, and not B cells themselves, in concert with non-B cell immune cells, likely T cells induced by immunization, appear to be required for protection. To test this possibility, we depleted CD4 T cells from mice previously immunized intranasally just prior to ivag HSV-2 challenge. In this setting, differentiation of B cells and antibody responses were allowed to occur fully in the presence of CD4 T cell help for six weeks. Mice acutely depleted of CD4 T cells succumbed to infection with HSV-2 (Fig. 2e,f), whereas depletion of CD8 T cells and NK cells had no effect9. Moreover, neutralization of IFN-γ prior to challenge, or genetic deficiency in IFN-γR also rendered intranasally immunized mice more susceptible to ivag HSV-2 challenge (Fig. 2e,f). Of note, depletion of CD4 T cells from i.n. immunized mice just before the viral challenge rendered mice incapable of viral control in the DRG, to the extent similar to the immunized B-cell deficient µMT mice (Fig. 2g). We observed that i.n. immunization conferred near complete protection from HSV-2 in the DRG but variable protection in the spinal cord (Fig. 1d, 2g). Because HSV-2 can differentially seed the DRG and spinal cord through sensory neurons and autonomic neurons14, these data suggest that the efficacy of antibody-mediated protection may depend on the route of viral entry. Further, these results indicate that circulating antibodies, CD4 T cells, and IFN-γ collectively mediate neuroprotection against HSV-2.
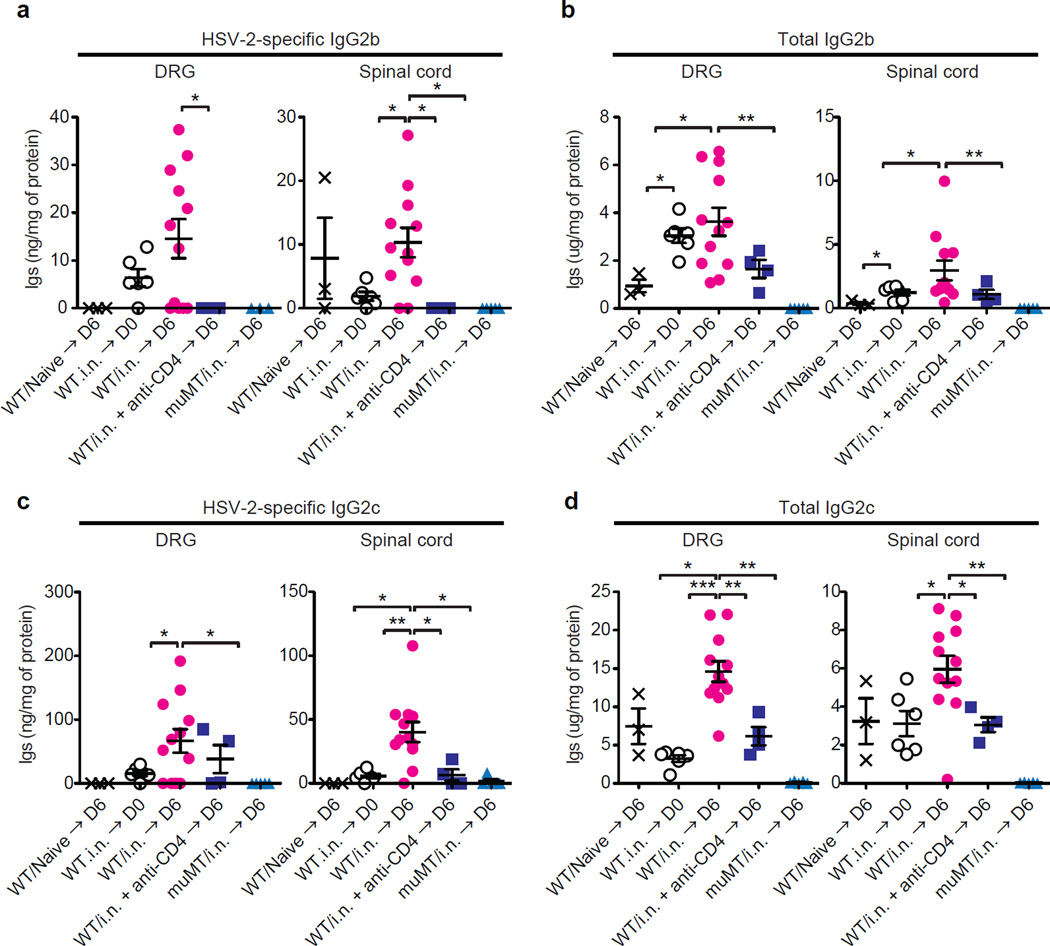
Given that antibody-mediated protection occurs at the level of the innervating neurons and not within the vagina (Figs. 1c and Extended Data Fig. 1h), we hypothesized that CD4 T cells might control delivery of antibodies to the tissue parenchyma through secretion of IFN-γ. We detected only low levels of virus-specific and total antibodies in the DRG or spinal cord at steady state in immunized mice (Fig. 3; WT/i.n.→ D0), and undetectable levels of antibodies in these tissues in previously unimmunized mice six days after an acute infection with HSV-2 (Fig. 3; WT/Naïve → D6). However, in mice immunized intranasally with TK− HSV-2 six weeks earlier, increase in the levels of antibodies was detected six days post ivag HSV-2 challenge within the DRG and in the spinal cord (Fig. 3; WT/i.n. → D6). Moreover, CD4 T cells were required for access of virus-specific antibodies to the restricted tissue such as the DRG, as depletion of CD4 T cells completely diminished antibody levels in this tissue and spinal cord (Fig. 3d; WT/i.n. + anti-CD4 → D6). Further, similar requirement for CD4 T cells (Fig. 3b,d) and IFN-γ (Extended Data Fig. 3) was found for diffusion of total IgG2b and IgG2c isotypes into the DRG, indicating that the delivery mechanism does not discriminate virus-specificity of the antibodies. In contrast to the neuronal tissues, acute depletion of CD4 or IFN-γ blockade once antibody responses are established had no significant impact on the serum levels of anti-HSV-2 or total antibodies (Extended Data Fig. 4a,b). To examine whether antigen-specific memory CD4 T cells was required to mediate antibody access to the neuronal tissues, mice were primed intranasally with a heterologous virus, influenza A virus, and four weeks later, were challenged with HSV-2 intravaginally. In contrast to mice harboring cognate memory CD4 T cells, antibody access to neuronal tissues following ivag HSV-2 challenge was not observed in mice that have irrelevant memory CD4 T cells (against influenza A virus) (Extended Data Fig. 5). These data indicate that antigen-specific memory CD4 T cells are required for antibody access to the neuronal tissues.
Figure 3. Memory CD4+ T cells are required for Ab access to neuronal tissues.
(a–d) Naïve WT mice or WT and µMT mice intranasally immunized with TK− HSV-2 (105 pfu) six weeks earlier were challenged with a lethal dose of WT HSV-2 intravaginally. Six days after the challenge, after extensive perfusion, HSV-2-specific (a&c) and total Ig (b&d) levels in tissue homogenates of DRG and spinal cord were analyzed by ELISA. To deplete CD4 T cells, CD4-specific antibody was injected on days −4, and −1, 2 and 4 days after challenge. Data are means ± s.e.m. *: p<0.05; **: p<0.01 (Unpaired student t-test).
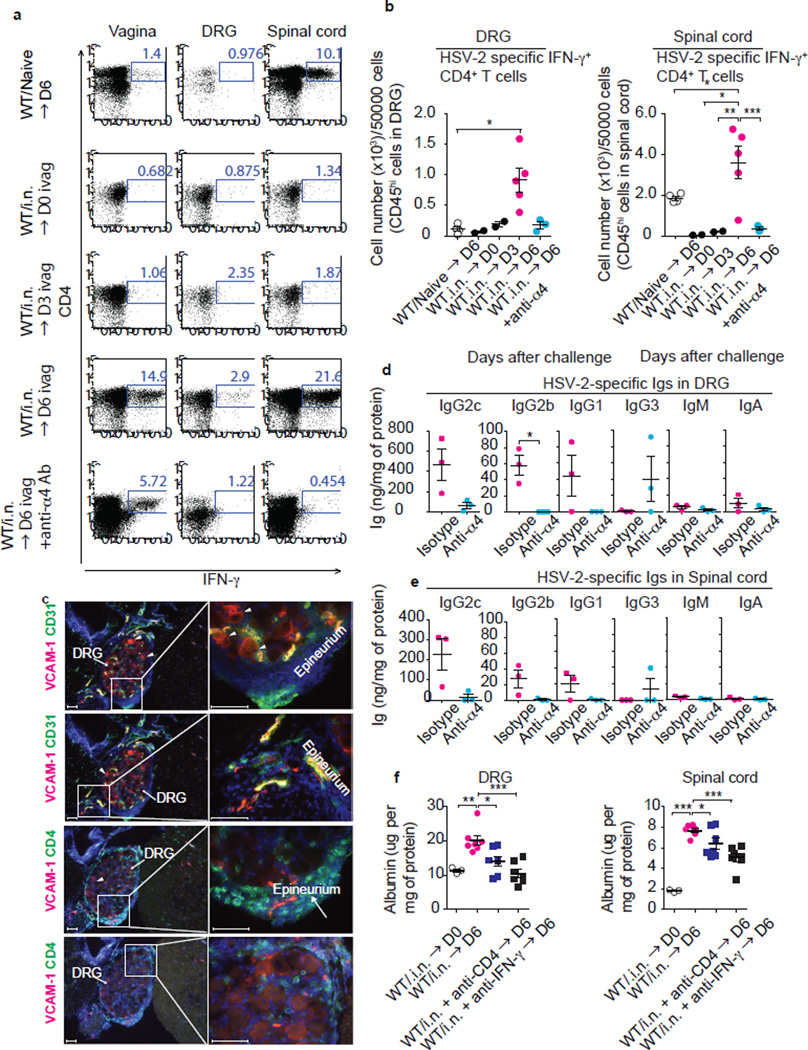
We hypothesized that memory CD4 T cell might enter the barrier-protected tissues and mobilize antibody access through local secretion of IFN-γ. In support of this idea, we found that IFN-γ-secreting HSV-2-specific CD4 T cells entered the DRG and spinal cord around 6 days post genital HSV-2 challenge in mice that received intranasal immunization six weeks prior (Fig. 4a,b; WT/i.n. → D6). Some increase in innate leukocytes bearing CD11b, Ly6G or MHCII was observed in DRG and spinal cord 6 days post challenge (Extended Data Fig. 6a). IFN-γ secretion was confined to the memory CD4 T cell population within the DRG (Fig. 4a). Moreover, entry of effector CD4 T cells to the DRG and spinal cord at 6 days post primary vaginal HSV-2 infection was much less efficient than their memory counterpart (Fig. 4a,b; WT/Naïve → D6), suggesting T cell intrinsic ability to migrate into these neuronal tissues enhance with memory development.
Figure 4. α4-integrin-dependent recruitment of memory CD4+ T cells is required for antibody access to neuronal tissues.
WT mice immunized i.n. with TK− HSV-2 six weeks earlier were challenged with a lethal dose of WT HSV-2. Neutralization of α4-integrin was performed on days 2 and 4 after challenge by i.v. injection of anti-α4 integrin (CD49d) Ab. Six days after challenge, after extensive perfusion, HSV-2-specific IFN-γ+ CD4+ T cells in DRG and spinal cord were detected by flow cytometry (a). The number of IFN-γ-secreting CD4 T cells among 50,000 cells of CD45hi leukocytes in DRG and spinal cord is depicted (b). (c) Frozen sections of DRG were stained with antibodies against CD4, VCAM-1 or CD31. Nuclei are depicted by DAPI stain (blue). Images were captured using a 10× or 40× objective lens. Scale bars indicate 100 µm. Arrowhead indicates VCAM-1+ cells in parenchyma of DRG. These data are representative of at least three similar experiments. HSV-2-specific Abs in the DRG (d) and the spinal cord (e) were analyzed by ELISA. Albumin level in tissue homogenates was analyzed by ELISA (f). Depletion of CD4 T cells or neutralization of IFN-γ was performed on days −4, and −1, 2 and 4 days after challenge by i.v. injection of anti-CD4 (GK1.5) or anti-IFN-γ (XMG1.2), respectively. Data are means ± s.e.m. *: p<0.05; **: p<0.01; ***: p<0.001 (two-tailed Paired t-test).
Interaction of α4β1 (or VLA4) and VCAM-1 contributes to T cell recruitment across the blood brain barrier15. Memory CD4 T cells generated against HSV-2 expresses CD49d which is the integrin α4 subunit6. We found that the entry of memory CD4 T cells into the nervous tissue was strictly dependent on α4 integrin, as antibody blockade of α4 prevented their entry into the DRG and spinal cord (Fig. 4a,b). We observed expression of ligand for α4β1, VCAM-1, in the endothelium of DRG and spinal cord in immune challenged mice (Fig. 4c & Extended Data Fig. 6b). Further, analysis of tissue sections revealed that the CD4 T cells were found in the parenchyma of the DRG and spinal cord, as well as within their epineurium and meninges, but not within the vasculature (Fig. 4c, Extended Data Fig. 6a,b). Notably, many CD4 T cells were found adjacent to the cell body of neurons within the DRG. Some VCAM-1 staining was found in the cytosol of neuronal cell bodies (arrowhead Fig. 4c). Additionally, intravascular staining16 with antibody to CD90.2 revealed that vast majority of the CD4 T cells in the DRG and spinal cord are sequestered from circulation (Extended Data Fig. 7a,b). Thus, CD4 T cells recruited to the neuronal tissues access the parenchyma of the DRG and spinal cord. Notably, α4 integrin blockade of CD4 T cell recruitment resulted in diminished access of virus-specific antibody to the DRG and spinal cord (Fig. 4d&e), with no effect on blood levels of virus-specific antibody (Extended Data Fig. 4c) or the total antibody levels of various isotypes in circulation (Extended Data Fig. 4d). Collectively, these data indicate that memory CD4 T cells enter into the neuronal tissue and secrete IFN-γ to promote antibody access to the DRG and spinal cord.
How might IFN-γ secreted by CD4 T cells enable circulating antibody access to the neuronal tissues? It is well known that IFN-γ acts on the endothelial cells to remodel tight junctions and increase permeability17. We observed that recombinant IFN-γ injected intravaginally was sufficient to enable antibody access to the vaginal lumen, suggesting that IFN-γ is sufficient to induce both vascular permeability and epithelial permeability in peripheral tissues (Extended Data Fig. 8a) and to enhance VCAM-1 expression on endothelial cells (Extended Data Fig. 8b). To assess whether antibody access to the neuronal tissues mediated by CD4 T cells and IFN-γ is through increase in vascular permeability, we measured release of blood albumin into the neuronal tissue following genital HSV-2 challenge in intranasally immunized mice. Notably, we observed that vascular permeability occurred in the DRG and the spinal cord in CD4 T cell- and IFN-γ-dependent manner, as measured by leakage of blood albumin to the neuronal tissues by ELISA and immunohistochemical analysis (Fig. 4f & Extended Fig. 9a). We confirmed CD4-dependent vascular permeability to the DRG and the spinal cord using intravenous injection of 70 kDa FITC-dextran, which has a similar size to IgG (Extended Data Fig. 9b). Collectively, our results support the notion that CD4 T cells enable antibody delivery to the sites of infection by secreting IFN-γ and enhancing microvascular permeability. This mechanism of antibody delivery is crucial for host immune protection, as depletion of CD4 T cells, inhibition of CD4 T cell migration into the neuronal tissues, or neutralization of IFN-γ renders immune mice susceptible to infection.
To determine whether our findings extend beyond HSV-2, we examined antibody access to the neuronal tissue following a different neurotropic virus, vesicular stomatitis virus (VSV), a negative sense RNA virus of the Rhabdoviridae family. Upon i.n. inoculation, VSV infects olfactory sensory neurons in the nasal mucosa and enters the CNS through the olfactory bulb18. In contrast, i.v. infection with VSV is well tolerated, and generates robust T and B cell responses (Extended Fig. 10)19. To determine whether antibody access to the brain requires memory CD4 T cells, we immunized mice with VSV intravenously. Five weeks later, immunized mice were challenged with VSV intranasally. Entry of VSV-specific antibodies was monitored in the brain six days after intranasal challenge. Consistent with the data obtained from HSV-2 infection, we observed a striking dependence on CD4 T cells of antibody access to the brain (Extended Fig. 10b). Further, anti-α4 Ab treatment of mice immediately prior to intranasal VSV challenge also diminished antibody access to the brain, without impacting VSV-specific antibodies in circulation (Extended Fig. 10c). Furthermore, we observed that vascular permeability to the brain was dependent on α4 integrin, as antibody blockade of α4 integrin resulted in diminished albumin leakage to the brain (Extended Fig. 10d). Taken together, these results indicate that the requirement for α4-integrin and memory CD4 T cells for antibody access applies to two distinct neurotropic viruses, HSV-2 and VSV, and suggest a general mechanism of antibody access to the immunopriviledged tissues protected by the blood nerve barriers.
We have demonstrated a role of CD4 T cells in controlling antibody access to neuronal tissues through local migration and secretion of IFN-γ. Circulating CD4 memory T cells effectively target antibody delivery to the sites of infection through their secretion of IFN-γ, presumably upon recognition of cognate antigenic peptides presented by local antigen presenting cells23,20. These results indicate the requirement for CD4 T cell help at the effector phase of the antibody response, and add to the growing appreciation of CD4 T cells in paving the way to other effector cell types such as CD8 T cells21–23. We believe that the requirement for CD4 T cells for antibody access in neuronal tissue reflects an additional layer of control imposed by the immunopriviledged sites. In accessible tissues, inflammatory leukocytes can migrate to and in response to PAMPs secrete cytokines such as TNF-α that is sufficient to trigger vascular permeability independently of CD4 T cells. However, after neurotropic viral infections, the infected neurons are expected to be poor at producing inflammatory cytokines that remodel vascular tight junctions. At the same time, recruitment of innate leukocytes is blocked by shut down of specific chemokines in the ganglia of HSV-1-infected mice24. Curiously, expression of T cell-trophic chemokines, CXCL9 and CXCL10, were preserved in the DRG of infected mice24, suggesting that access by lymphocytes is permitted. Thus, in neuronal tissues, the entry of viral specific CD4 T cells is crucial to provide cytokines that permit antibodies through the induction of vascular permeability.
On the other hand, aberrant entry and activation of CD4 T cells predispose immunopriviledged tissues for access to autoantibodies and tissue damage25,26. Thus, this mode of targeted release of circulating antibodies not only provides a rapid and efficient mechanism of pathogen control, but may also restrict antibody release to irrelevant sites to limit immunopathology. Our results implicate that antibody-based vaccines or treatment against neurotropic viruses would benefit from generating robust circulating CD4 T memory responses. Conversely, treatment of autoantibody mediated neuropathies including chronic inflammatory demyelinating polyneuropathy and Guillain–Barré syndrome might benefit from preventing the accessibility of autoantibodies to target neurons enabled by CD4 T cells.
Methods
Mice
Six to eight-week old female C57BL/6 (CD45.2+) and congenic C57BL6 B6.SJL-PtprcaPep3b/BoyJ (B6.Ly5.1) (CD45.1+) mice, B6.129S2-IghtmICgn/J (µMT) mice, anti-HEL B-cell receptor (BCR)-transgenic C57BL/6-TgN (IghelMD4) (HELTg) mice, CBy.PL(B6)-Thy1a/ScrJ (Thy1.1+ Balb/c) mice and B6.129X1-Fcgrttm1Dcr/DcrJ (FcRn−/−) mice were purchased from the National Cancer Institute and Jackson Laboratory. JHD mice (B-cell deficient on Balb/c background) were obtained from Taconic Animal Models. All procedures used in this study complied with federal guidelines and institutional policies by the Yale animal care and use committee.
Viruses
HSV-2 strains 186syn− TK− and 186syn+ were generous gifts of Dr. David Knipe (Harvard Medical School, Boston, MA). These viruses were propagated and titered on Vero cells (ATCC CCL-81) as previously described20. Influenza virus A/Puerto Rico/3/334 (A/PR8: H1N1) and WT/VSV was propagated as previously described20,27.
Virus infection
Six- to eight-week-old female mice injected s.c. with Depo Provera (Pharmacia Upjohn, 2 mg per mouse) were immunized intravaginally, intraperitoneally or intranasally with 105 PFU of HSV-2 (186syn− TK−) as previously described6. For secondary challenge, immunized mice were challenged vaginally with 104 pfu of WT HSV-2 (186syn+) (100% lethal dose for naïve mice). In the case of Balb/c and JHD mice, these mice were immunized with 5 × 104 to 105 PFU of HSV-2. For secondary challenge, immunized mice were challenged with 105 pfu of WT HSV-2 (100% lethal dose for naïve mice). The severity of disease was scored as ; 0, no sign; 1, slight genital erythema and edema; 2, moderate genital inflammation; 3, purulent genital lesions; 4, hind-limb paralysis; 5, pre-moribund20. Due to humane concerns, the animals were euthanized prior to reaching moribund state. To measure virus titer in peripheral tissues, vaginal tissues, dorsal root ganglia and spinal cord were harvested in ABC buffer (0.5 mM MgCl26H2O, 0.9 mM CaCl22H2O, 1% glucose, 5% HI FBS and penicillin-streptomycin) including 1% amphotericin-B (Sigma). Thereafter, these tissues were homogenized by lysing matrix D (MP Biomedicals), followed by clarifying by centrifugation. Viral titers were obtained by titration of tissue samples on Vero cell monolayer. Protein concentration in tissue homogenates was measured by DC™ protein assay kit (Bio-Rad Laboratories Inc.). C57/BL6 mice were immunized i.v. with WT/VSV (2 × 106 pfu/mouse) or i.n. with influenza A/PR8 (10 pfu/mouse). For secondary challenge, VSV-immunized mice were re-infected i.n. with WT/VSV (1 × 107 pfu/mouse).
Antibodies
Anti-CD90.2 (30-H12), anti-CD90.1 (OX-7), anti-CD45.2 (104), anti-CD45.1 (A20), anti-CD4 (GK1.5, RM4-5 and RM4-4), anti-CD19 (6D5), anti-CD45R/B220 (RA3-6B2), anti-MHC class II (I-A/I-E, M5/114.15.2), anti-CD69 (H1.2F3), anti-CD44 (IM7), anti-CD49d (R1-2), anti-NKp46 (29A1.4), anti-IFN-γ (XMG1.2 and R4-6A2) were purchased from e-Bioscience or Biolegend.
Isolation of leukocytes from peripheral tissues
The genital tracts of vaginal tissues treated with Depo-Provera were dissected from the urethra and cervix. Prior to collection of neuronal tissues, mice were perfused extensively using transcardiac perfusion and perfusion through inferior vena cava and great saphenous vein with more than 30 mL of PBS. The dorsal root ganglia and the adjacent region of the spinal cord were harvested in PBS for flow cytometry or ABC buffer for tissue homogenization. The tissues in PBS were then incubated with 0.5 mg/mL Dispase II (Roche) for 15 min at 37 °C. Thereafter, vaginal tissues were digested with 1 mg/mL collagenase D (Roche) and 30 µg/mL DNase I (Sigma-Aldrich) at 37 °C for 25 min. The resulting cells were filtered through a 70-µm filter28,29.
Flow cytometry
Preparation of single cell suspensions from spleen, draining LNs (inguinal LN and iliac LNs), vagina and neuronal tissues were described previously. Multiparameter analyses were carried out on the LSR II flow cytometer (Becton Dickinson) and were analyzed using the FlowJo software (Tree Star, Ashland, OR). To detect HSV-2-speific CD4+ T cells or VSV-specific CD4+ T cells (CD45.1+ or CD45.2+), single cell suspensions from vaginal tissues of TK− HSV-2 immunized mice or VSV immunized mice were stimulated in the presence of 5 µg/ml Brefeldin A with naïve splenocytes (CD45.1+ CD45.2+) loaded with heat-inactivated HSV-2 antigen, heat-inactivated WT VSV and heat-inactivated influenza virus A/PR8 for around 12 hr6. To detect HSV-2-specific CD4+ T cells in Balb/c and JHD mice, single cell suspensions (CD90.2+) from vaginal tissues of TK− HSV-2 immunized mice were stimulated with naïve splenocytes (CD90.1+) loaded with heat-inactivated HSV-2 antigen.
In vivo treatment with neutralizing/depleting antibodies
C57/BL6 mice or Balb/c mice were immunized with TK− HSV-2 virus. Five to eight weeks later, these mice were injected i.v. (tail vain) with 300 µg of anti-CD4 (GK1.5; BioXCell) or anti-IFN-γ (XMG1.2; BioXCell) Ab at days −4, −1, 2 and 4 after HSV-2 challenge. In vivo depletion for CD4 was confirmed by FACS analysis of the cell suspension from spleen. For the neutralization of α4-integrin, purified anti-mouse α4 integrin/CD49d (PS/2; SouthernBiotech) was given a tail vain injection of 300 µg Ab at days 2 and 4 after challenge.
Parabiosis
Parabiosis was performed as previously described with slight modifications6. Naïve or immunized C57/BL6 mice, HELTg and µMT mice were anesthetized with a mixture of Ketamine/Xylazine (100 mg/kg and 10 mg/kg respectively). After shaving the corresponding lateral aspects of each mouse, matching skin incisions were made from behind the ear to hip and sutured together with Chromic Gut (4-0, HENRY SCHEIN, UK) absorbable suture, then these areas were clipped with 7-mm stainless-steel wound clips (ROBOZ).
Measurement of virus-specific Igs and total Igs in serum and tissue homogenates
Ninety six-well EIA/RIA plate wells were filled with 100 µl of heat-inactivated purified HSV-2 (104 to 105 pfu equivalent per 100 µl) or heat-inactivated purified VSV (5 × 105 pfu equivalent per 100 µl) for virus-specific Ig measurement or goat anti-mouse Ig (1:1000; SouthernBiotech, 1010-01) for total Ig measurement in carbonate buffer (pH 9.5) and then incubated overnight at 4°C. On the following day, these plates were washed with PBS-Tween 20 and blocked 2hr with 5% FBS in PBS. Tissue samples and serum samples in ABC buffer were then plated in the wells and incubate for at least four hours at ambient temperature. After washing in PBS-Tween 20, HRP-conjugated anti-mouse IgG1, IgG3, IgM, IgA, IgG2a, IgG2b or IgG2c (SouthernBiotech) was added in the wells for 1 h, followed by washing and adding TMB solution (eBioscience). Reactions were stopped with 1N H2SO4 and absorbance was measured at 450 nm. The sample Ab titers were defined by using Ig standard (C57BL/6 Mouse Immunoglobulin Panel; SouthernBiotech) or mouse IgG2a (HOPC-1; SouthernBiotech).
Albumin ELISA
Using tissue homogenates (DRG and spinal cord) prepared after extensive perfusion, albumin ELISA (Genway) was performed according to instruction.
Immunofluorescence staining
Frozen sections 8 µm in thickness were cut, fixed, left to dry at ambient temperature. These tissues were stained with the Abs (anti-CD4 [H129.19], anti–MHC class II [M5/114.15.2] anti-VCAM-1 [429/MVCAM.A], anti-CD31 [390 and MEC13.3], anti-Ly6G [1A8], anti-CD11b [M1/70] and anti-mouse albumin [Goat pAb/BETHYL Laboratories Inc. TX]) as previously described6. These slides were washed and incubated with DAPI and mounted with Fluoromount-G (SouthernBiotech). These slides were analyzed by fluorescence microscopy (BX51; Olympus).
Vascular permeability assays
Spinal column was harvested from intranasal TK− HSV-2 immunized mice 45 min after tail vein injection with 200 µl of 5 mg/ml Oregon Green 488-conjugated dextran (70kDa, D7173, Thermo Fisher Scientific, MA) in PBS. Spine was then fixed with 4% paraformaldehyde in PBS overnight, and cut frozen sections (8 µm in thickness) for immunohistochemical analysis30.
DNA isolation from tissues
C57/BL6 mice were immunized intranasally with TK− HSV-2. Six weeks later, vaginal tissues, DRG and spinal cord of these mice were lysed in 10 mg/ml Proteinase K (Roche) to isolate DNA at 55 °C overnight. After removing these tubes, phenol equilibrated with Tris pH 8.0 was added. Thereafter, upper aqueous phase was added to phenol/chloroform (1:1). The upper aqueous phase was re-suspended with sodium acetate, pH 6.0 and 100% ethanol at RT. After shaking and centrifuging the concentration of isolated DNA pellet was measured. Level of HSV-2 genoimc DNA in peripheral tissues based on HSV-2 gD (Forward primer: agcgaggataacctgggatt, Reverse primer: gggataaagcggggtaacat) was analyzed by qPCR using purified viral DNA genome as standard.
Statistical Analysis
Survival curve was analyzed using the log-rank test. For other data, normally distributed continuous variable comparisons were performed using two-tailed unpaired t-test or paired t-test using Prism software. For comparison of two nonparametric datasets, the Mann-Whitney U-test was used.
Extended Data
Extended Data Fig. 1. In the absence of TRM, B cells are required for protection of the host against genital HSV-2 challenge.
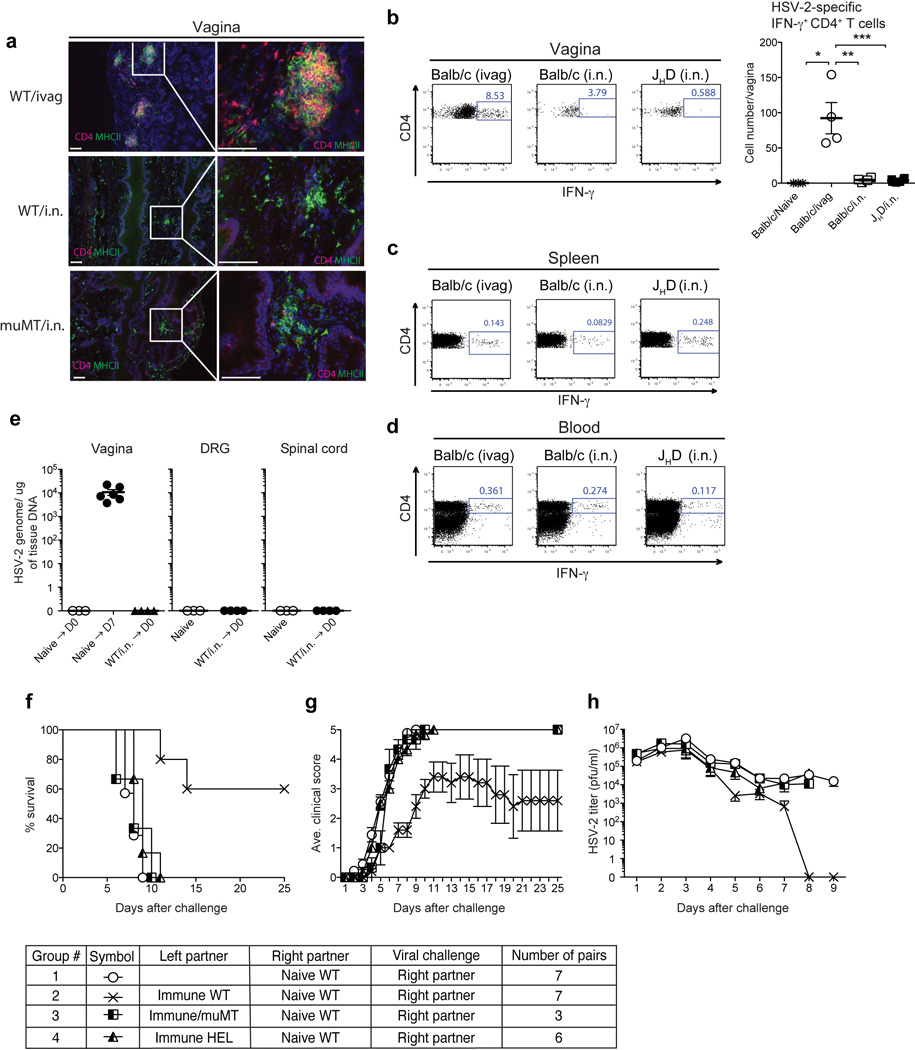
(a) C57/BL6 mice and µMT mice were immunized ivag or i.n. with TK− HSV-2. Five weeks later, vaginal tissue sections were stained for CD4+ cells (red) and MHC class II+ cells (green). Blue labeling depicts nuclear staining with DAPI (blue). Images were captured using a 10× or 40× objective lens. Scale bars indicate 100 µm. These data are representative of three similar experiments. (b–d) Balb/c mice and JHD mice were immunized with TK− HSV-2 (105 pfu) via i.n. or ivag route. Six weeks later, the number of total CD4+ T cells and HSV-2-specific IFN-γ+ CD4+ T cells in the vagina (b), spleen (c), and peripheral blood (d) were analyzed by flow cytometry. Percentages and total number of IFN-γ+ cells among CD4+CD90.2+ cells are shown. Data are means ± s.e.m. *, p<0.05; **, p<0.001; ***, p<0.001 (unpaired Student t-test). (e) C57/BL6 mice were immunized intravaginally (Naïve → D7) or intranasally (WT/i.n. → D0) with TK− HSV-2 virus. At the indicated time points (D7: 7 days after immunization; WT/i.n. → D0: 6 weeks after immunization), total viral genomic DNA in the vaginal tissues, DRG and spinal cord were measured by quantitative PCR. (f–h) Intravaginally immunized C57/BL6 (WT), µMT and HEL-BCR Tg mice (left partner) were surgically joined with naïve WT mice (right partner). Three weeks after parabiosis, the naive partner was challenged with a lethal dose of WT HSV-2 intravaginally. Mortality (e), clinical score (f) and virus titer in vaginal wash (g) following viral challenge are depicted.
Extended Data Fig. 2. Mucosal TK− HSV-2 immunization generates higher levels of virus-specific IgG2b and IgG2c compared to intraperitoneal immunization.
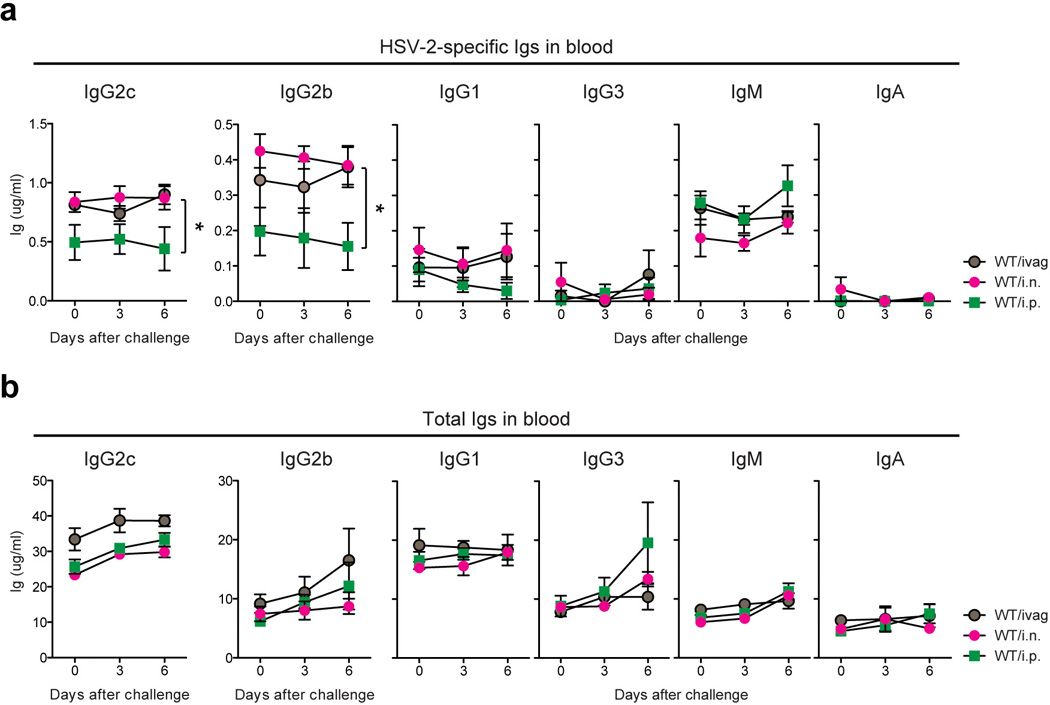
WT mice were immunized with TK− HSV-2 (105 pfu/mouse) via the intravaginal (ivag), intraperitoneal (i.p.) or intranasal (i.n.) route. Six weeks later, these mice were challenged with a lethal dose of WT HSV-2 intravaginally. At the indicated days after challenge, HSV-2-specific Ig (a) and total Ig (b) in serum were analyzed by ELISA. Data are means ± s.e.m. *: p<0.05 (Mann-Whitney U test)
Extended Data Fig. 3. IFN-γ enhances Ab access to the DRG.
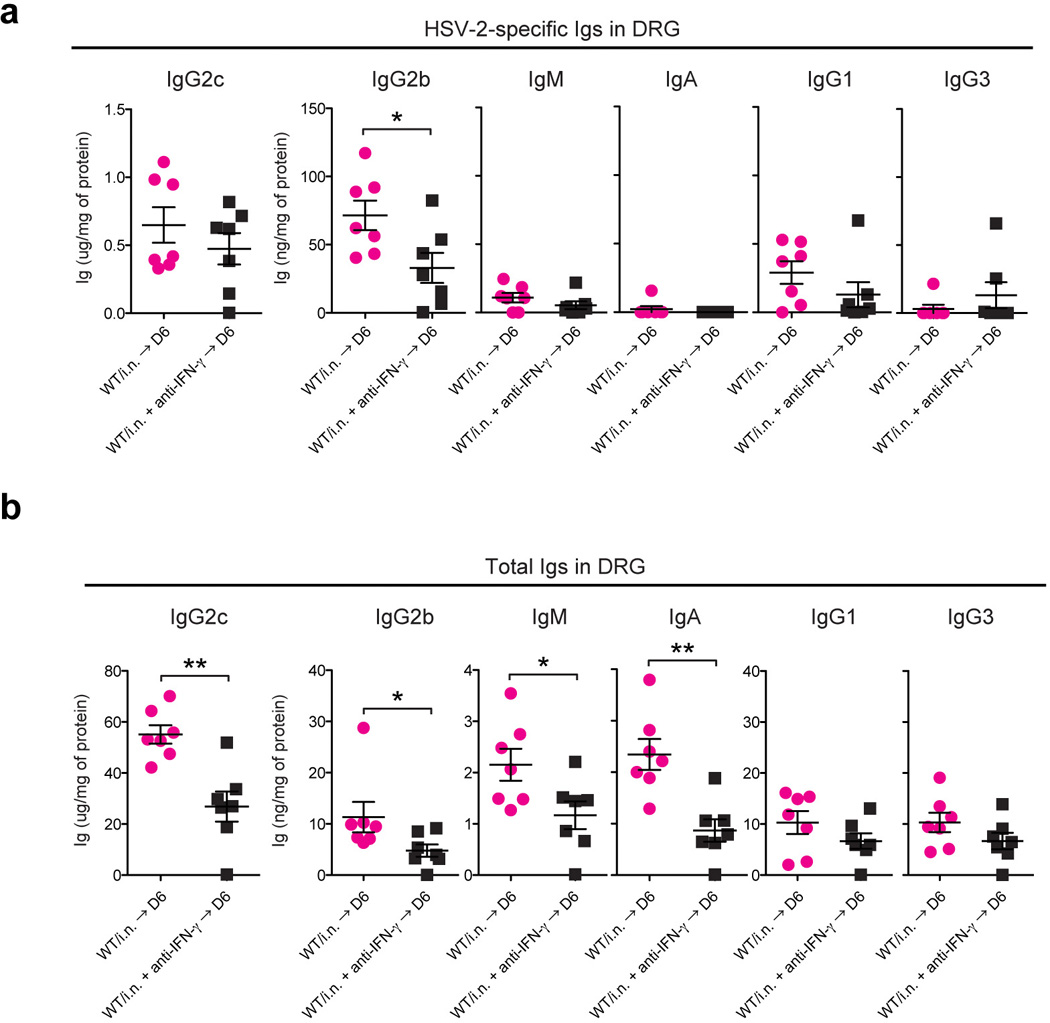
WT mice immunized with TK− HSV-2 (105 pfu/mouse) intranasally six weeks earlier were challenged with a lethal dose of WT HSV-2 intravaginally. Six days after challenge, after extensive perfusion, HSV-2-specific (a) and total Ig (b) in DRG homogenates were analyzed by ELISA. Depletion of CD4 T cells or neutralization of IFN-γ was performed on days −4, and −1, 2 and 4 days after challenge by i.v. injection of anti-CD4 (GK1.5) or anti-IFN-γ (XMG1.2), respectively. Data are means ± s.e.m. *: p<0.05; **, p<0.001 (Unpaired student t-test).
Extended Data Fig. 4. Neutralization of IFN-γ, α4-integrin, or depletion of CD4 T cells has no impact on circulating immunoglobulin levels.
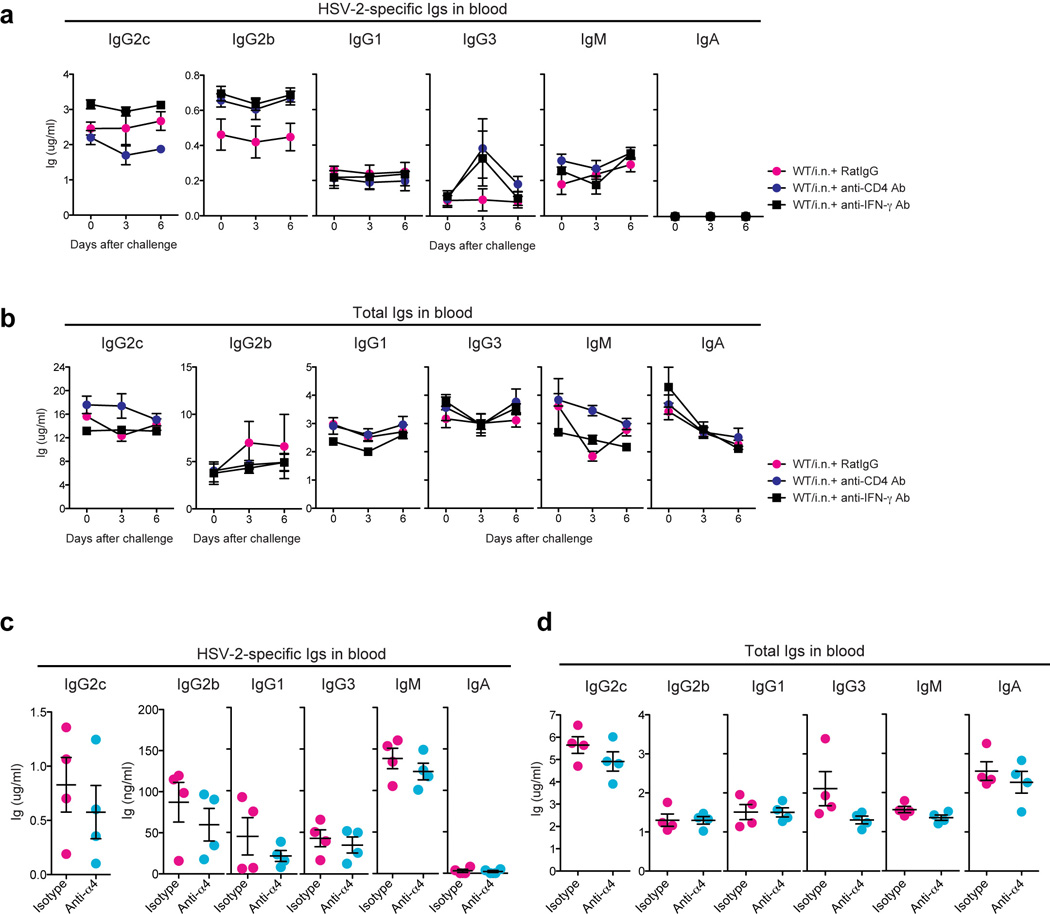
(a&b) WT mice immunized i.n. with TK− HSV-2 six to eight weeks earlier were challenged with a lethal dose of WT HSV-2. Depletion of CD4 T cells or neutralization of IFN-γ was performed on days −4, and −1, 2 and 4 days after challenge by i.v. injection of anti-CD4 (GK1.5) or anti-IFN-γ (XMG1.2), respectively. At time points indicated, HSV-2-specific Ig in the blood (n=4) (a) and total Ig in the blood (n=4) (b) were measured. (c&d) WT mice immunized i.n. with TK− HSV-2 six weeks earlier were challenged with a lethal dose of WT HSV-2. Neutralization of α4-integrin was performed on day 2 and 4 after challenge by i.v. injection of anti–α4-integrin/CD49b Ab. Six days later, HSV-2-specific Ab (c) and total Ab (d) in the blood were measured. These data are representative of three similar experiments.
Extended Data Fig. 5. An irrelevant immunization fails to increase the levels of total Abs in neuronal tissues.
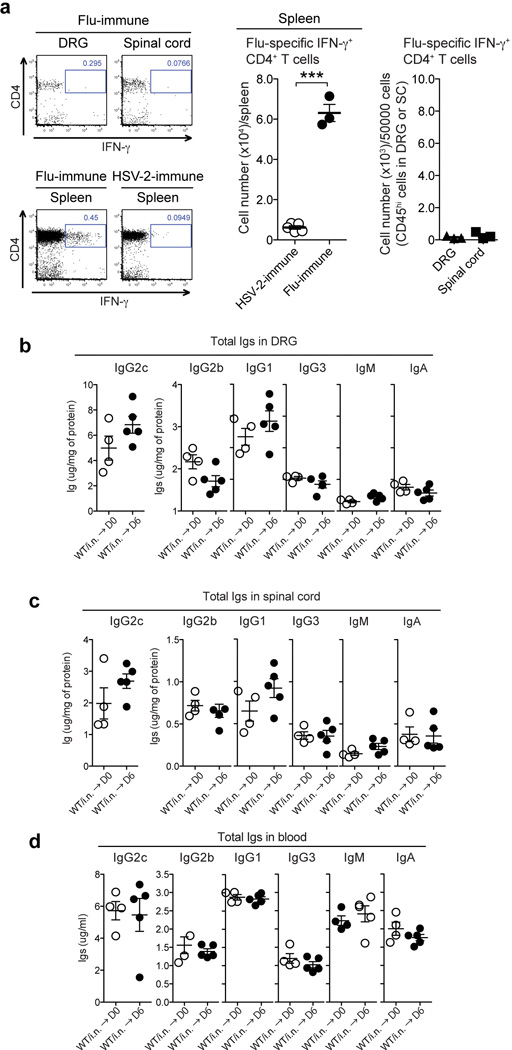
(a) C57/BL6 mice were immunized with a sublethal dose of influenza A/PR8 virus (10 pfu/mouse) via intranasal (i.n.) route. Three weeks later, Flu-specific IFN-γ+ CD4+ T cells in spleen and neuronal tissues (DRG & spinal cord)(CD45.2+) following co-culture with HI-Flu/PR8 loaded splenocytes (CD45.1+) were analyzed by flow cytometry. As a control, lymphocytes isolated from spleen of TK− HSV-2 i.n.-immunized mice six weeks post vaccination were used for co-culture. (***: p<0.001; Unpaired student t-test). (b–d) C57/BL6 mice were immunized with a sublethal dose of influenza A/PR8 virus (10 pfu/mouse). Four weeks later, these mice were challenged with a lethal dose of WT HSV-2 (104 pfu/mouse) intravaginally. Six days after challenge, total Abs in lysate in DRG (b), spinal cord (c) and blood (d) were measured by ELISA. Data are means ± s.e.m. ***: p<0.001; (Unpaired student t-test).
Extended Data Fig. 6. Majority of CD4 T cells recruited to the DRG and spinal cord of immunized mice is localized in the parenchyma of neuronal tissues.
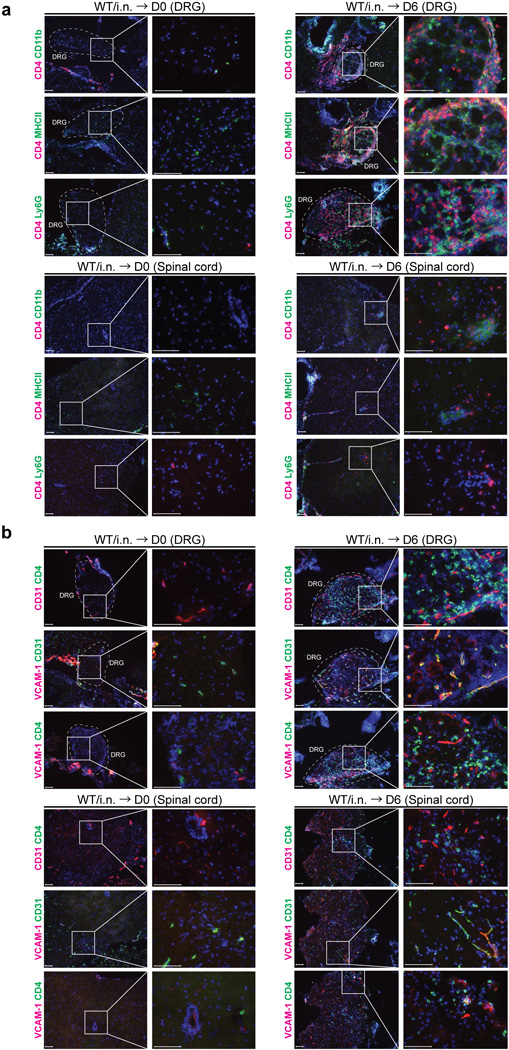
(a) C57/BL6 mice were immunized i.n. with TK− HSV-2. Six days after challenge of immunized mice six weeks prior, neuronal tissue sections (DRG and spinal cord) were stained for CD4+ cells and VCAM-1+ cells or CD31+ cells (red or green). Blue labeling depicts nuclear staining with DAPI (blue). Images were captured using a 10× or 40× objective lens. Scale bars indicate 100 µm. (b) C57/BL6 mice were immunized i.n. with TK− HSV-2. Six weeks later, mice were challenged with WT HSV-2 ivag and neuronal tissues were collected 6 days later. DRG and spinal cord were stained for CD4+ cells (red) and MHC class II+ cells, CD11b+ cells or Ly6G+ cells (green). Blue labeling depicts nuclear staining with DAPI (blue). Images were captured using a 10× or 40× objective lens. Scale bars indicate 100 µm. These data are representative of at least three similar experiments.
Extended Data Fig. 7. Intravascular staining reveals localization of CD4 T cells in the parenchyma of neuronal tissues.
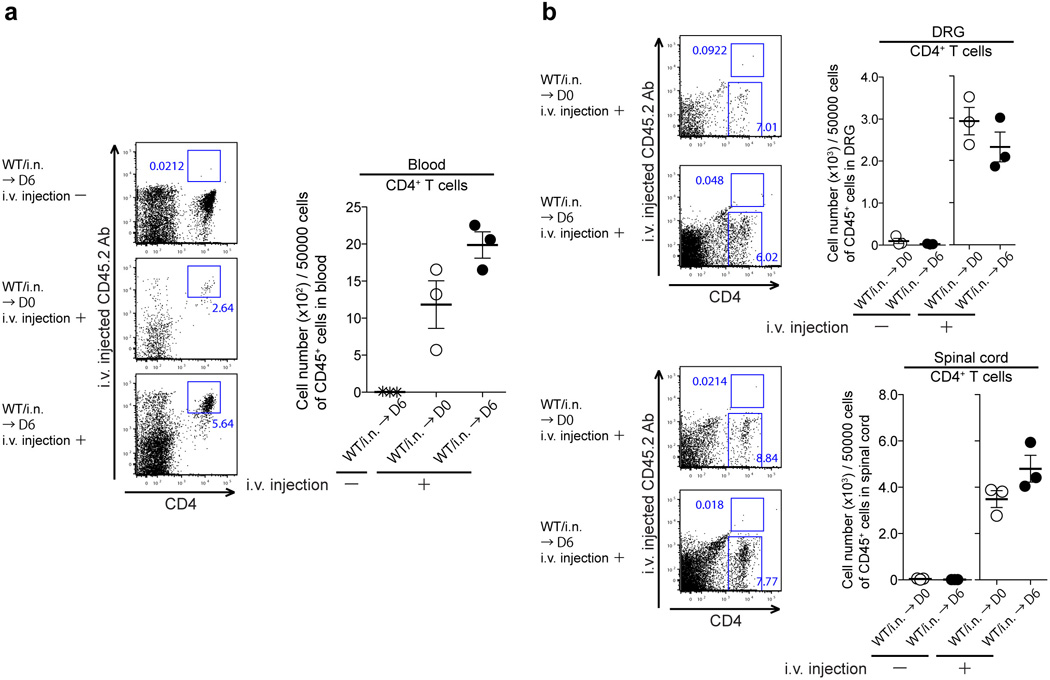
(a&b) C57/BL6 mice immunized i.n. with TK− HSV-2 six weeks prior were challenged with lethal WT HSV-2. Six days after challenge, Alexa Fluor® 700 conjugated anti-CD90.2 Ab (3 µg per mouse) was injected i.v. (tail vain) into immunized mice. Five minutes later, these mice were sacrificed for FACS analysis of intravascular vs. extravascular lymphocytes.
Extended Data Fig. 8. Recombinant IFN-γ is sufficient to increase epithelial and vascular permeability in vaginal tissues.
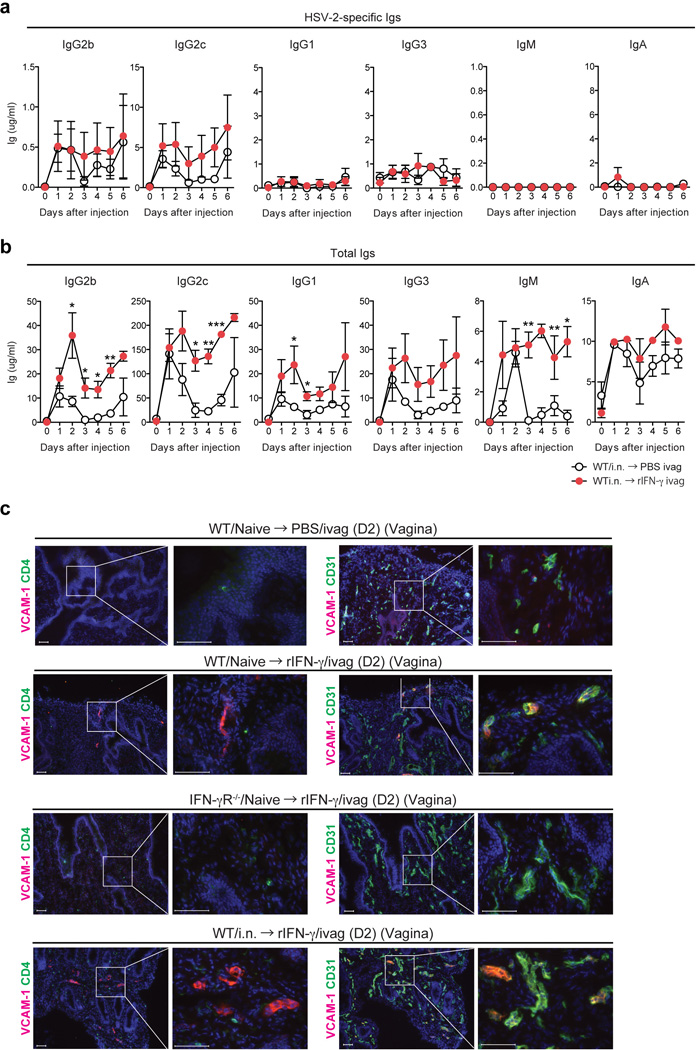
(a) WT mice immunized with TK− HSV-2 (105 pfu) intranasally six weeks earlier were injected intravaginally with recombinant mouse IFN-γ (10 µg per mouse) (n=3) or PBS (n=3). At the indicated time points, HSV-2-specific Ig (a) and total Ig (b) in vaginal wash were measured by ELISA. (c) Two days after rIFN-γ treatment, vaginal tissue sections were stained for VCAM-1+ cells (red) or CD4+ cells (green) and CD31+ cells (green). Blue labeling depicts nuclear staining with DAPI (blue). Images were captured using a 10× or 40× objective lens. Scale bars indicate 100 µm. These data are representative of at least three similar experiments.
Extended Data Fig. 9. Vascular permeability in DRG and spinal cord is augmented following WT HSV-2 challenge.
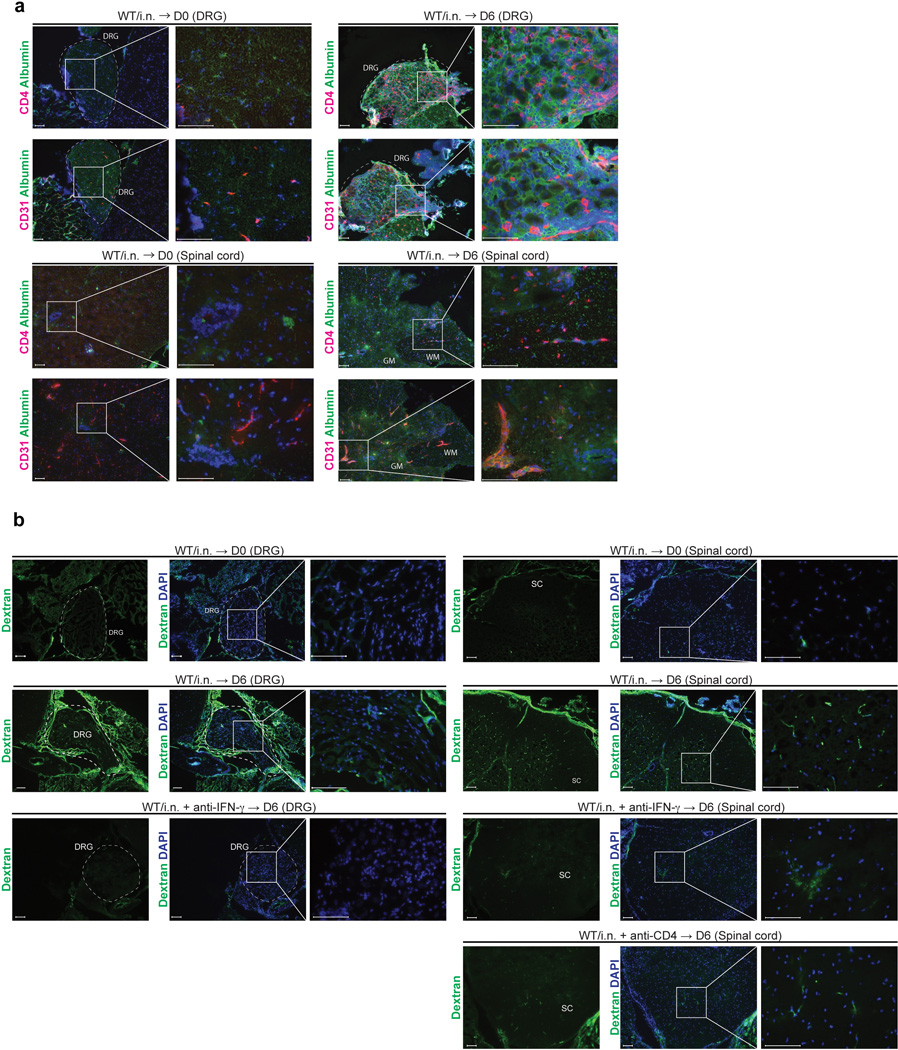
(a) C57/BL6 mice were immunized i.n. with TK− HSV-2. Six days after challenge of immunized mice six weeks prior, neuronal tissue sections (DRG and spinal cord) were stained for CD4+ cells (red) and mouse albumin (green). Blue labeling depicts nuclear staining with DAPI (blue). (b) C57/BL6 mice were immunized i.n. with TK− HSV-2. Six weeks later, these mice were challenged with lethal WT HSV-2. Six days after challenge, Oregon green 488-conjugated dextran (70 kDa) (5 mg/ml, 200 µl/mouse) was injected i.v. into intranasal immunized mice. Forty five minutes later, these mice were sacrificed for immunohistochemical analysis. GM; gray matter, WM; white matter. These data are representative of three similar experiments.
Extended Data Fig. 10. Memory CD4+ T cells are required for the increase in antibody levels and vascular permeability in the brain following VSV immunization and challenge.
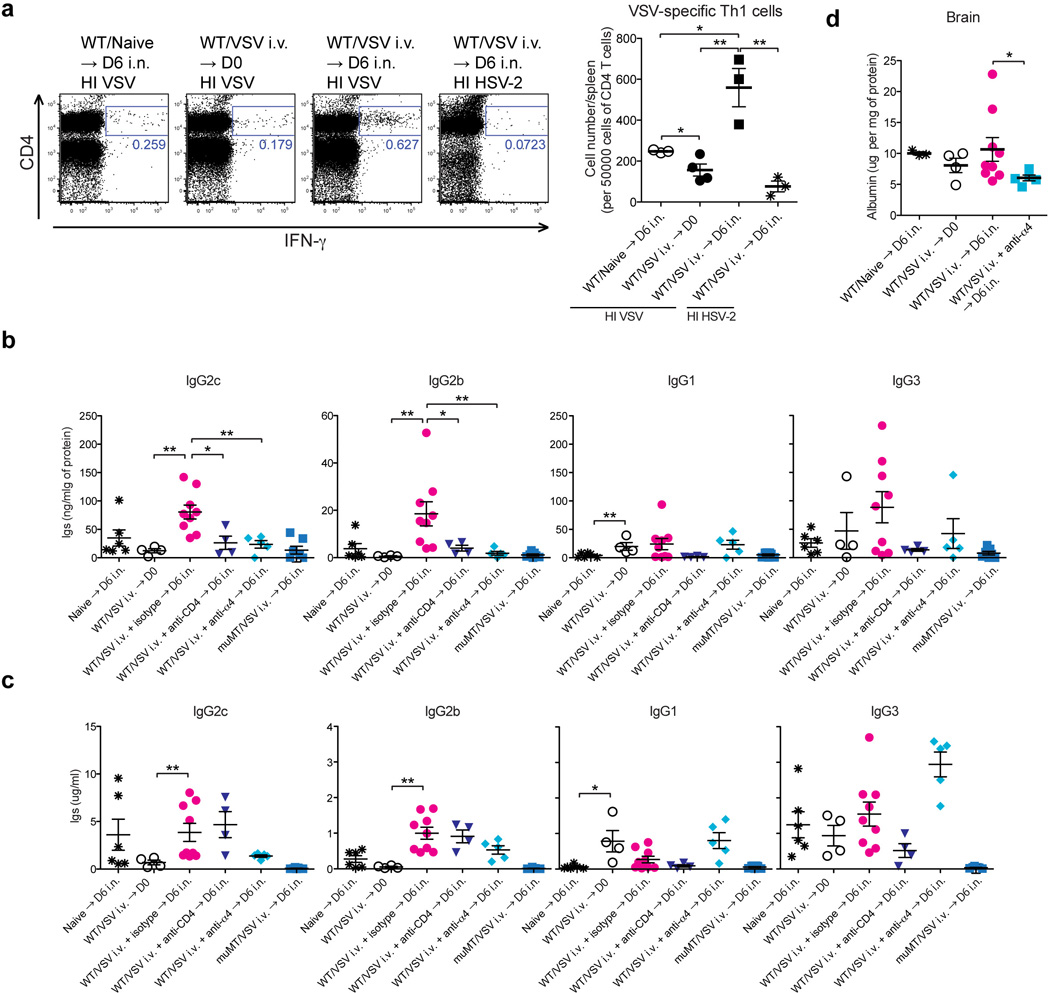
(a) C57/BL6 mice were immunized i.v. with WT VSV (2 × 106 pfu/mouse). Five weeks later, these mice were challenged intranasally with WT VSV (1 × 107 pfu/mouse). Six days after challenge, VSV-specific IFN-γ+ CD4+ T cells in spleen (CD45.2+) following co-culture with HI-VSV loaded splenocytes (CD45.1+) or HI HSV-2 loaded splenocytes were analyzed by flow cytometry. (b,c) Five weeks after VSV immunization, these mice were challenged intranasally with WT VSV (1 × 107 pfu/mouse). Six days after challenge, VSV-specific Abs and total Abs in lysate of brain (b) and serum (c) were measured by ELISA. Depletion of CD4 T cells was performed on −4, −1, 2 and 4 days after challenge by i.v. injection of anti-CD4 (GK1.5). (d) Albumin levels in tissue homogenates were analyzed by ELISA (Data are means ± s.e.m. *: p<0.05; *: p<0.01; ***: P<0.001 (Mann-Whitney U-test)).
Acknowledgments
We thank Huiping Dong, Susan L. Fink and Kazue Hashimoto-Torii for animal care support and technical help. We thank Ruslan Medzhitov for helpful discussions. This study was supported by awards from NIH AI054359, AI062428, AI064705 (to A.I.) A.I. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Contributions
N.I. and A.I. planned the project, designed experiments, analysed and interpreted data and wrote the manuscript. N.I. performed experiments.
Competing financial interests
None
References
- 1.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacological reviews. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 2.Weerasuriya A, Mizisin AP. The blood-nerve barrier: structure and functional significance. Methods Mol Biol. 2011;686:149–173. doi: 10.1007/978-1-60761-938-3_6. [DOI] [PubMed] [Google Scholar]
- 3.Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med. 2008;59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 4.Knipe DM, Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol. 2008;6:211–221. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 5.Parr MB, et al. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–380. [PubMed] [Google Scholar]
- 6.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–6100. [PubMed] [Google Scholar]
- 8.Parr MB, Parr EL. Immunity to vaginal herpes simplex virus-2 infection in B-cell knockout mice. Immunology. 2000;101:126–131. doi: 10.1046/j.1365-2567.2000.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato A, et al. Vaginal memory T cells induced by intranasal vaccination are critical for protective T cell recruitment and prevention of genital HSV-2 disease. Journal of virology. 2014;88:13699–13708. doi: 10.1128/JVI.02279-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones CA, Taylor TJ, Knipe DM. Biological properties of herpes simplex virus 2 replication-defective mutant strains in a murine nasal infection model. Virology. 2000;278:137–150. doi: 10.1006/viro.2000.0628. [DOI] [PubMed] [Google Scholar]
- 11.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 12.McDermott MR, Brais LJ, Evelegh MJ. Mucosal and systemic antiviral antibodies in mice inoculated intravaginally with herpes simplex virus type 2. The Journal of general virology. 1990;71:1497–1504. doi: 10.1099/0022-1317-71-7-1497. [DOI] [PubMed] [Google Scholar]
- 13.Morrison LA, Zhu L, Thebeau LG. Vaccine-induced serum immunoglobin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. Journal of virology. 2001;75:1195–1204. doi: 10.1128/JVI.75.3.1195-1204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohashi M, Bertke AS, Patel A, Krause PR. Spread of herpes simplex virus to the spinal cord is independent of spread to dorsal root ganglia. Journal of virology. 2011;85:3030–3032. doi: 10.1128/JVI.02426-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Man S, Ubogu EE, Ransohoff RM. Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain pathology (Zurich, Switzerland) 2007;17:243–250. doi: 10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson KG, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capaldo CT, et al. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol Biol Cell. 2014;25:2710–2719. doi: 10.1091/mbc.E14-02-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiss CS, Plakhov IV, Komatsu T. Viral replication in olfactory receptor neurons and entry into the olfactory bulb and brain. Ann N Y Acad Sci. 1998;855:751–761. doi: 10.1111/j.1749-6632.1998.tb10655.x. [DOI] [PubMed] [Google Scholar]
- 19.Thomsen AR, et al. Cooperation of B cells and T cells is required for survival of mice infected with vesicular stomatitis virus. Int Immunol. 1997;9:1757–1766. doi: 10.1093/intimm/9.11.1757. [DOI] [PubMed] [Google Scholar]
- 20.Iijima N, et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. The Journal of experimental medicine. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nature immunology. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 23.Laidlaw BJ, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 2014;41:633–645. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stock AT, Smith JM, Carbone FR. Type I IFN suppresses Cxcr2 driven neutrophil recruitment into the sensory ganglia during viral infection. The Journal of experimental medicine. 2014;211:751–759. doi: 10.1084/jem.20132183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westland KW, et al. Activated non-neural specific T cells open the blood-brain barrier to circulating antibodies. Brain : a journal of neurology. 1999;122(Pt 7):1283–1291. doi: 10.1093/brain/122.7.1283. [DOI] [PubMed] [Google Scholar]
- 26.Pollard JD, et al. Activated T cells of nonneural specificity open the blood-nerve barrier to circulating antibody. Ann Neurol. 1995;37:467–475. doi: 10.1002/ana.410370409. [DOI] [PubMed] [Google Scholar]
- 27.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iijima N, Mattei LM, Iwasaki A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc Natl Acad Sci U S A. 2011;108:284–289. doi: 10.1073/pnas.1005201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson AJ, Chu CF, Milligan GN. Effector CD4+ T-cell involvement in clearance of infectious herpes simplex virus type 1 from sensory ganglia and spinal cords. Journal of virology. 2008;82:9678–9688. doi: 10.1128/JVI.01159-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowland D, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82:603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]