Abstract
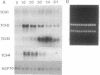
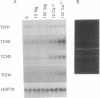
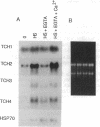
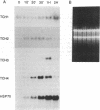
Expression of the calmodulin-related TCH genes of Arabidopsis is strongly and rapidly up-regulated in plants after a variety of stimuli, including touch. As an approach to investigating the mechanism(s) of TCH gene regulation, a manipulable cell culture system in which TCH gene expression is regulated has been developed. In response to increased external calcium or heat shock, TCH2, -3, and -4 mRNA levels significantly increased. Significantly, these two stimuli are known to result in cytoplasmic calcium increases, therefore implicating a role for calcium itself in the regulation of calmodulin-related genes. Further, external calcium is required for maximal heat-shock induction of expression of the TCH genes but not of the 70-kDa heat shock protein; therefore, there may exist at least two distinct mechanisms of heat shock induction of gene expression. Calcium ion regulation of genes encoding calcium-binding proteins may ensure the efficacy of calcium ion as a transient second messenger and the maintenance of cellular homeostasis. This possible regulatory circuit would likely be relevant not only for plant cells but also for the great variety of animal cells that transduce extracellular stimuli, such as hormones and electrical impulses, into calcium signals.
Full text
PDF
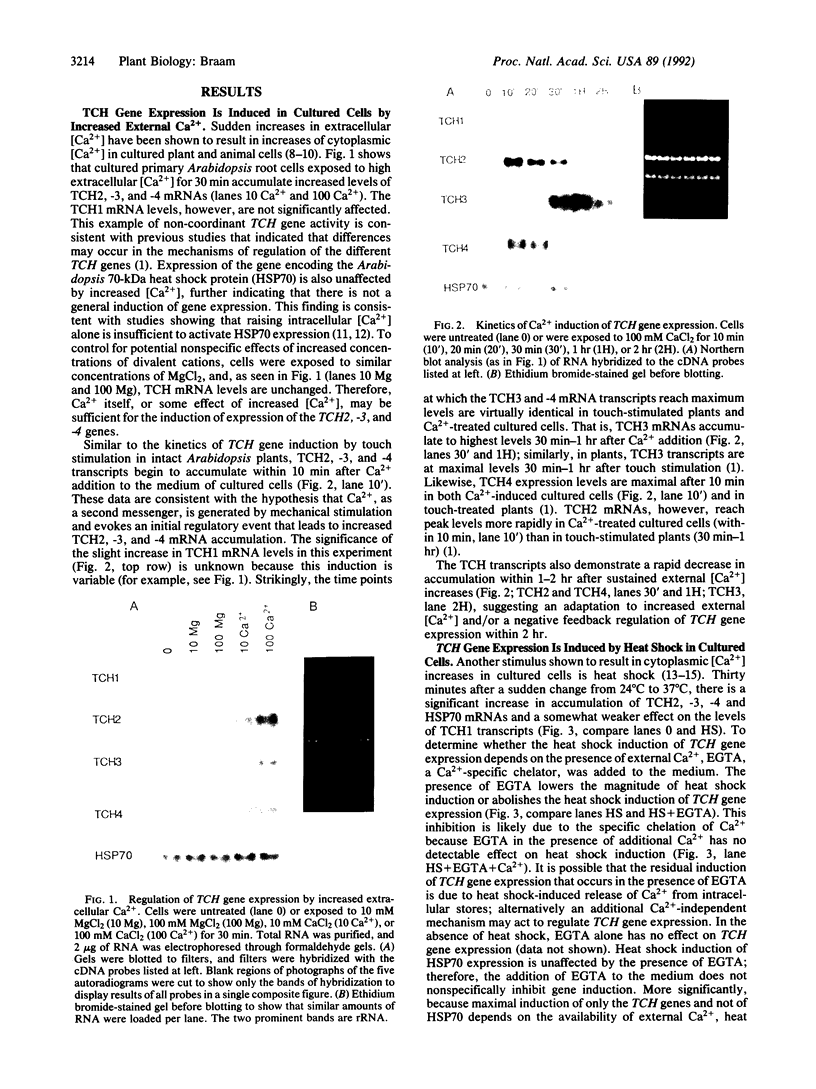
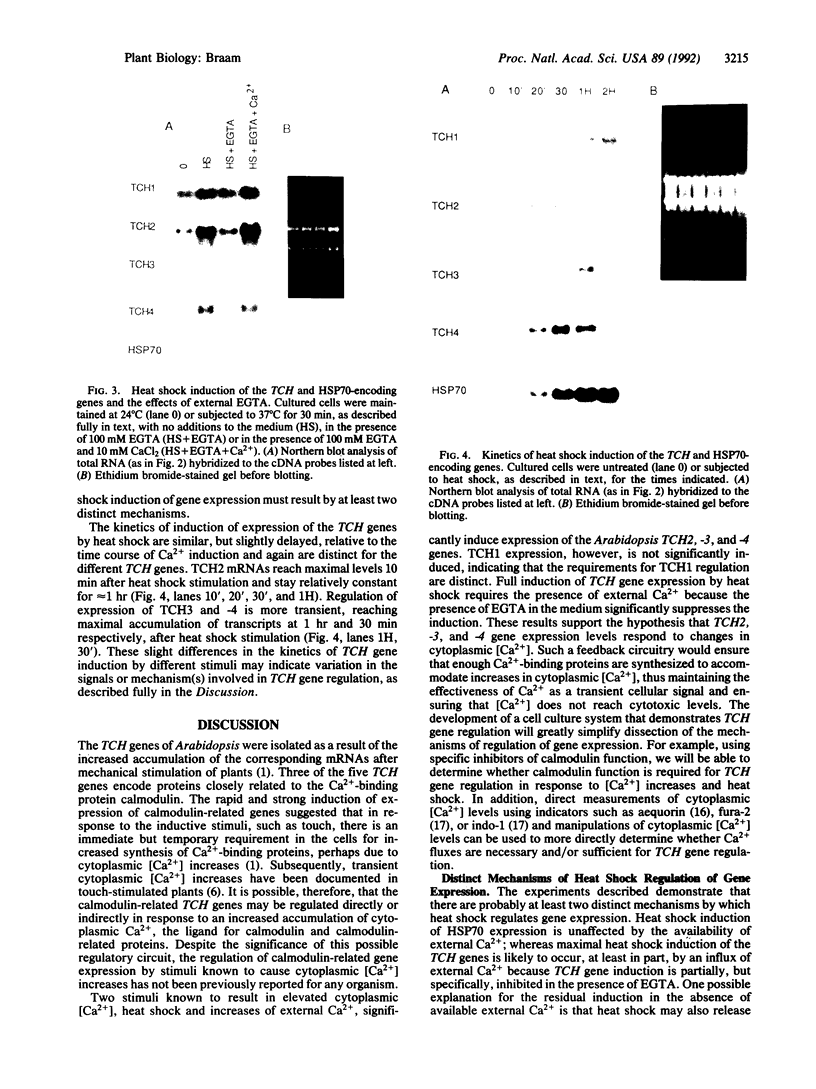

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Braam J., Davis R. W. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990 Feb 9;60(3):357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- Bush D. S., Jones R. L. Measuring intracellular ca levels in plant cells using the fluorescent probes, indo-1 and fura-2 : progress and prospects. Plant Physiol. 1990 Jul;93(3):841–845. doi: 10.1104/pp.93.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. K., Stevenson M. A., Hahn G. M. Effects of heat on cell calcium and inositol lipid metabolism. Radiat Res. 1988 Mar;113(3):414–425. [PubMed] [Google Scholar]
- Cheek T. R. Spatial aspects of calcium signalling. J Cell Sci. 1989 Jun;93(Pt 2):211–216. doi: 10.1242/jcs.93.2.211. [DOI] [PubMed] [Google Scholar]
- Drummond I. A., McClure S. A., Poenie M., Tsien R. Y., Steinhardt R. A. Large changes in intracellular pH and calcium observed during heat shock are not responsible for the induction of heat shock proteins in Drosophila melanogaster. Mol Cell Biol. 1986 May;6(5):1767–1775. doi: 10.1128/mcb.6.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S., Fricker M. D., Read N. D., Trewavas A. J. Role of Calcium in Signal Transduction of Commelina Guard Cells. Plant Cell. 1991 Apr;3(4):333–344. doi: 10.1105/tpc.3.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hensold J. O., Hunt C. R., Calderwood S. K., Housman D. E., Kingston R. E. DNA binding of heat shock factor to the heat shock element is insufficient for transcriptional activation in murine erythroleukemia cells. Mol Cell Biol. 1990 Apr;10(4):1600–1608. doi: 10.1128/mcb.10.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M. R., Campbell A. K., Smith S. M., Trewavas A. J. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991 Aug 8;352(6335):524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Larson J. S., Schuetz T. J., Kingston R. E. Activation in vitro of sequence-specific DNA binding by a human regulatory factor. Nature. 1988 Sep 22;335(6188):372–375. doi: 10.1038/335372a0. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Clapham D., Peralta E. Subcellular patterns of calcium release determined by G protein-specific residues of muscarinic receptors. Nature. 1991 Apr 11;350(6318):505–508. doi: 10.1038/350505a0. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Mosser D. D., Kotzbauer P. T., Sarge K. D., Morimoto R. I. In vitro activation of heat shock transcription factor DNA-binding by calcium and biochemical conditions that affect protein conformation. Proc Natl Acad Sci U S A. 1990 May;87(10):3748–3752. doi: 10.1073/pnas.87.10.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Reddy A. S. Calcium messenger system in plants. CRC Crit Rev Plant Sci. 1987;6(1):47–103. doi: 10.1080/07352688709382247. [DOI] [PubMed] [Google Scholar]
- Price B. D., Calderwood S. K. Ca2+ is essential for multistep activation of the heat shock factor in permeabilized cells. Mol Cell Biol. 1991 Jun;11(6):3365–3368. doi: 10.1128/mcb.11.6.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMOMURA O., JOHNSON F. H., SAIGA Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962 Jun;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- Stevenson M. A., Calderwood S. K., Hahn G. M. Effect of hyperthermia (45 degrees C) on calcium flux in Chinese hamster ovary HA-1 fibroblasts and its potential role in cytotoxicity and heat resistance. Cancer Res. 1987 Jul 15;47(14):3712–3717. [PubMed] [Google Scholar]
- Stevenson M. A., Calderwood S. K., Hahn G. M. Rapid increases in inositol trisphosphate and intracellular Ca++ after heat shock. Biochem Biophys Res Commun. 1986 Jun 13;137(2):826–833. doi: 10.1016/0006-291x(86)91154-x. [DOI] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidair C., Rubin H. Mg2+-sensitive alterations in Ca2+ regulation associated with cell transformation. J Cell Physiol. 1982 Dec;113(3):398–404. doi: 10.1002/jcp.1041130307. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Garrels J. I., Thomas G. P., Lin J. J., Feramisco J. R. Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose- and Ca2+-ionophore-regulated proteins. J Biol Chem. 1983 Jun 10;258(11):7102–7111. [PubMed] [Google Scholar]