Summary
Burn wound infection is a major cause of morbidity and mortality in burn victims. Pseudomonas and Acinetobacter species are among the most common organisms complicating burn wounds. Presence of extended spectrum ß-lactamase (ESBL) and metallo-ß-lactamase (MBL) genes plays an important role in spreading ß-lactam resistant strains of these organisms and is a serious condition in the treatment of the affected patients. As a result, we aimed to determine the prevalence of SHV, TEM, PER and VIM ß-lactamases in Pseudomonas and Acinetobacter species isolates from burn wound swabs of children with burn injury. In this descriptive observational study, 107 Pseudomonas and Acinetobacter isolates collected from burn patients were subjected to PCR assay. Using PCR method and DNA sequencing, the existence of SHV-, TEM-, PER- and VIM-type ß-lactamase encoding genes were determined. Out of the 107 Pseudomonas and Acinetobacter isolates, 66 (77.6%) were ESBL positive, 26.2% were positive for SHV gene, 37.4% were positive for TEM gene, 14% were positive for PER gene and 15.9% of them harbored VIM gene. More than half of the Pseudomonas and Acinetobacter strains in our pediatric burn unit harbor ß-lactamase encoding genes that make them resistant to a wide range of ß-lactam antibiotics. Consequently, it is suggested to choose an appropriate antibiotic regimen based on the antibiogram pattern of the strains.
Keywords: Acinetobacter species, burn wound, extended-spectrum ß-lactamases, metallo-ß-lactamase, Pseudomonas species
Abstract
Les infections cutanées sont une cause majeure de morbidité et de mortalité chez les brûlés. Pseudomonas et Acinetobacter sont parmi les micro-organismes les plus communs chez les brûlés. La présence des gènes codant les ß-lactamases à spectre étendu (BLSE) et métallo-ß-lactamases (MBL) joue un rôle important dans la dissémination des souches résistantes et obère le traitement des patients infectés. Nous avons étudié la prévalence des gènes encodant pour des enzymes des groupes SHV, TEM, PER et VIM dans des isolats de Pseudomonas et Acinetobacter chez les brûlés pédiatriques, grâce à des techniques de PCR. Dans cette étude observationnelle descriptive, 107 isolats de Pseudomonas et Acinetobacter, obtenus chez des patients brûlés ont été étudiés. Plus des 3/4 des souches de Pseudomonas et Acinetobacter expriment une BLSE (26.2% SHV; 37.4% TEM; 14% PER; 15.9% VIM), ce qui les rend résistants à de nombreuses ß-lactamines. Il est donc suggéré de choisir un traitement antibiotique approprié, basé sur l’antibiogramme des souches infectantes.
Introduction
Despite improvements in the medical and surgical care of burn patients, no significant improvement has been documented on the mortality of burn patients in developing countries. Infection is a major complication of burn injury. Disruption of the normal skin barrier and compromised immune responses increase the vulnerability of burn patients to serious infections. Furthermore, burn wounds contain a large amount of necrotic tissue and protein-rich exudates which provide a rich growth medium for colonized organisms.1,2 Burn wound infection is responsible for 50 to 75% of inhospital deaths. Moreover, infection of burn wounds leads to graft failure, increased tissue necrosis, and scarring.3-5
Serious infections caused by Pseudomonas aeruginosa and Acinetobacter spp. are common complications in burn pediatric patients leading to substantial morbidity and mortality. Pseudomonas aeruginosa and Acinetobacter baumannii are two important nosocomial pathogens that are highly amenable to multidrug resistance. Moreover, Gramnegative bacteria including Pseudomonas and Acinetobacter species (spp.) are usually the predominant etiologies of bloodstream and wound infections in the pediatric population.6,7
Pseudomonas aeruginosa is an opportunistic Gramnegative organism. Several mechanisms including beta-lactamase production, up regulation of efflux systems, and decreased outer membrane permeability result in beta-lactam resistance in P. aeruginosa and render the organism very difficult to treat or eradicate, consequently predisposing the burn wound and medical equipment and devices in burn units to Pseudomonas contamination and infection. 8,9
Recently, Acinetobacter spp. has emerged as a main cause of nosocomial infections associated with considerable morbidity and mortality.10,11 Acinetobacter spp. exhibit multidrug resistance through production of ß-lactamases, alterations in outer membrane proteins (OMPs) and penicillin- binding proteins (PBPs), and increased activity of efflux pumps.11,12-15 It is believed that Acinetobacter spp. are resistant to almost all currently available antimicrobial agents, including the aminoglycosides, the quinolones, and broad-spectrum ß-lactams.16 The broad spectrum of antibiotic resistance in these organisms in association with their unique survival capabilities represents a major challenge in the treatment of these types of infections in hospitals, especially burn units. Most strains of Acinetobacter are resistant to cephalosporins and resistance to carbapenems is increasingly common.17,18
Spread of beta-lactamase producing mediated antibiotic resistance in P. aeruginosa and Acinetobacter spp. is a major concern in pediatric burn units. As a result, in the present investigation, we aimed to assess the presence of beta lactamase producing genes in Pseudomonas and Acinetobacter strains isolated from the burn wounds of pediatric burn patients.
Patients and methods
This cross-sectional study was conducted at the Pediatric ward of Motahari Burn center in Tehran, Iran from September 2012 to June 2013. Hospitalized patients aged 1 to 18 years old with burn injury were enrolled if their burn wound culture was positive either for Pseudomonas or Acinetobacter strains.
The study was reviewed and approved by the Institutional Review Board at Iran University of Medical Sciences and a written informed consent was obtained from parents or guardians of all participants.
On admission, patients with burns greater than 20% of the Total Body Surface Area (TBSA) were resuscitated with intravenous fluid (20 mL/kg per hour) and a Foley catheter was placed and urine output was monitored hourly, the goal being 1.0 mL/kg per hour. Once the extent of the burn was estimated according to Lund–Browder chart, resuscitation continued using the Parkland formula (approximately 6 mL/kg per percent TBSA of burn). The burn depth was estimated clinically by an experienced trauma surgeon. A topical anti-microbial agent (preferentially silver sulphadiazine) was applied to burn wounds and cotton gauze dressings used for all patients were changed daily. Sampling from burn wounds was carried out using swabs from various parts of the wound before cleansing and application of the topical antimicrobial. Swabs were transferred into sterile tubes immediately following sampling and sent to a microbiology laboratory. Identification of isolates was performed according to the conventional bacteriologic methods or using commercial identification kits. In cases with Pseudomonas spp. or Acinetobacter spp. positive cultures, samples were subjected to PCR analysis to examine the ß- lactamase producing genes including PER, SHV, TEM (extended- spectrum ß-lactamase encoding genes) and VIM (a metallo-ß-lactamase encoding gene, also known as carbapenemase). Patients received antibiotic therapy only if there was a clinical suspicion of infection (i.e. change in appearance or color of wound or positive blood or wound cultures) at the discretion of the treating surgeon.
Statistical analysis
All analyses were conducted by Statistical Package for Social Sciences (SPSS) software, version 19 (SPSS Inc., Chicago, IL, USA). Continuous data are presented as mean± SD. Unpaired t-test was used for comparison of age of patients between Pseudomonas and Acinetobacter positive cultures. Categorical data were given as frequencies and percentages. A P value of <0.05 was considered statistically significant.
Results
There were 107 patients including 84 boys (78.5%) and 23 (21.5%) girls. The mean age was 3.8± 2.5 years. The burn size in 43 patients (40.2%) was less than 30% TBSA, in 29 patients (27.1%) between 31-40% of TBSA, in 17 (15.9%) patients it was between 41-50% and in 18 patients (16.8%) the burn size was more than 50% TBSA.
In 55 patients (51.5%), depth and severity of burn were at stage IIb, in 27 (25.2%) patients at stages III to IV, in 12 (11.2%) at a combination of II and III, and in the remaining 13 (12.1) patients at a combination of stage III and IV. The isolated strains included Pseudomonas spp. in 76 (71%) patients and Acinetobacter spp. in 31 (29%) patients. No significant difference was seen between the isolated strains from burn wounds with respect to the patients` age (p=0.622, Fig. 1).
Fig. 1. No significant difference was seen in the result of burn wound swab cultures and age of the participants (p=0.62).
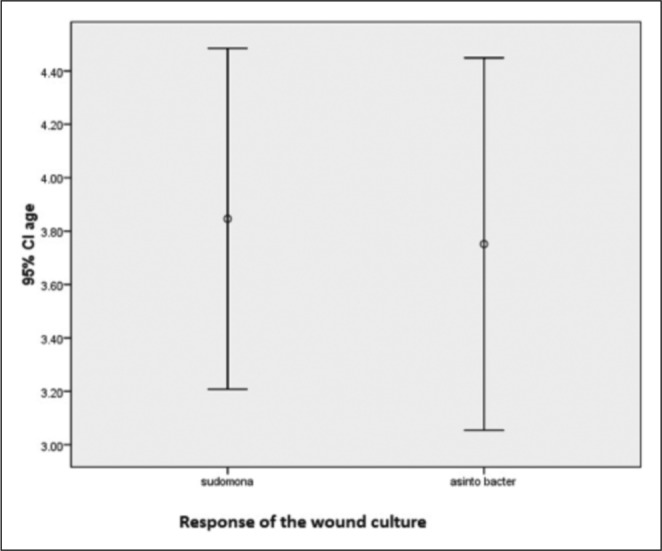
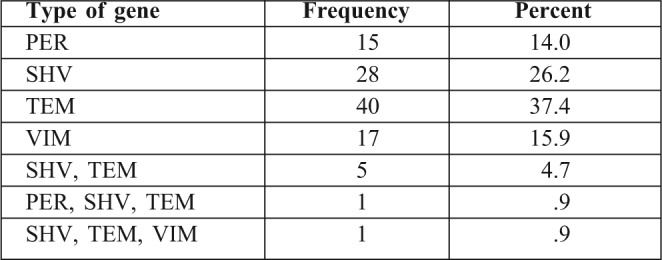
Table I shows the frequency of each of the beta-lactamase producing genes (PER, SHV, TEM and VIM) in the isolated strains of Pseudomonas and Acinetobacter.
Table I. Distribution of ß-lactamase producing genes.
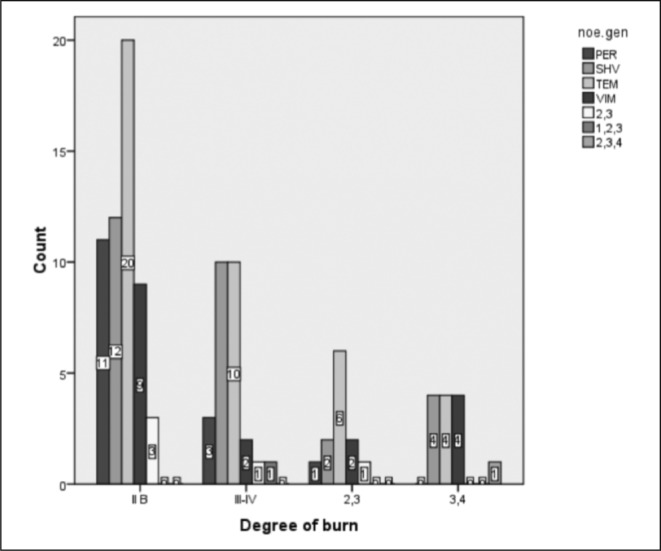
The distribution of ß-lactamase producing genes in the isolated strains of Pseudomonas and Acinetobacter based on gender is shown in Fig. 2. Figs. 3 and 4 also demonstrate the distribution of ß-lactamase producing genes in the isolated strains of Pseudomonas and Acinetobacter based on the size and degree of the burn, respectively.
Fig. 2. The distribution of ß-lactamase producing genes in the isolated strains of Pseudomonas and Acinetobacter is shown based on the gender of participants.
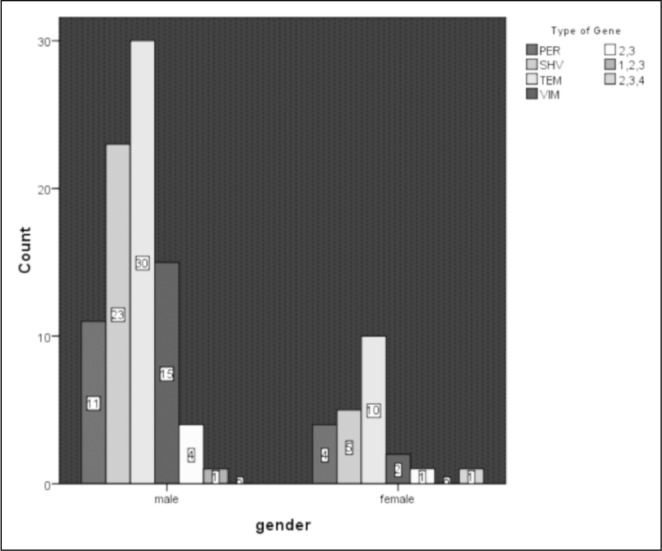
Fig. 3. The distribution of ß-lactamase producing genes in the isolated strains of Pseudomonas and Acinetobacter is shown based on the extent of burn as % of Total Body Surface Area (TBSA).
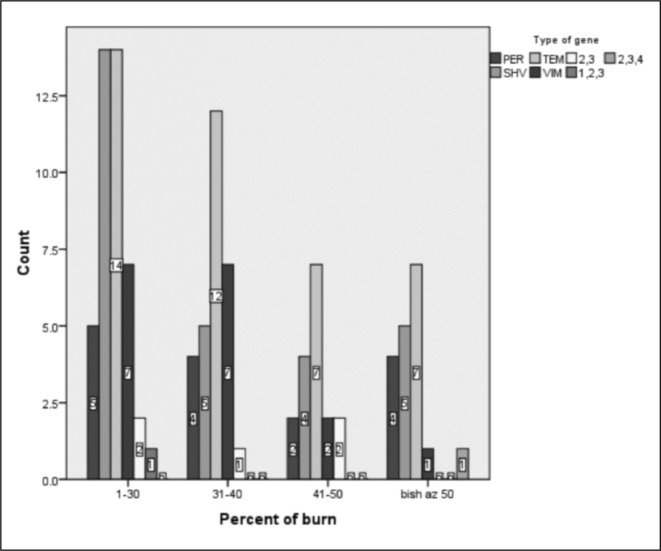
Fig. 4. The distribution of ß-lactamase producing genes in the isolated strains of Pseudomonas and Acinetobacter is shown based on the degree of burn.
Discussion
Burn wounds represent the most extensive surface wounds seen in surgery. Though the treatment of burn wounds has progressed over time, their management still provides considerable challenges. To date, there is still a remarkable mortality rate in burn victims in developing countries.
With improved resuscitation of the burn patients, the major etiologies of mortality in this population include inhalation injury, adult respiratory distress syndrome (ARDS) and sepsis ranging from systemic inflammatory response syndrome (SIRS) to frank septic shock.19 Wound infection, especially by nosocomial and opportunistic infections, are the main cause of sepsis in burned children. As a result, a growing cause of concern in burn victims is colonization of burn wounds by multidrug resistant organisms.
In a survey based on the data from 176 burn care centers in North America, Pseudomonas species was found to be the most important cause of life threatening infections in patients with thermal injuries.20 Similarly, McManus et al. in a 25-year review of Pseudomonas bacteremia in burn patients demonstrated that bacteremia due to P. aeruginosa caused an overall burn mortality of 77% which was 28% higher than predicted.21 Revathi et al. also reported their experience in 600 infected burn patients.22 They showed that Pseudomonas spp. and Staphylococcus aureous caused the most common and severe infections followed by other Gram-negative organisms. In a study by Gastmeier on pediatric burn patients with hospital acquired infections, the most frequently isolated pathogens were Staphylococcus aureous (20.7%) and Pseudomonas spp. (14.6%), followed by Enterococcus spp. and Escherichia coli (both 12.2%).23
In a study by Ganesamoni et al. conducted on burn patients, Pseudomonas aeroginosa was the most common organism identified followed by Acinetobacter species. They also showed that these strains were resistant to the majority of the commonly used antibiotics.24 According to their results, amikacin and ceftriaxone had only less than 20% in vivo activity and only around one third of isolates of P. aeroginosa and a similar percentage of Acinetobacter spp. were sensitive to meropenem. This study emphasized the increasing prevalence of Pseudomonas colonization in burn wounds compared to the previously reported series. Furthermore, prevalence of Acinetobacter has also been reported to have increased in western studies.22,25-30 However, Acinetobacter infection has not been demonstrated to affect mortality independently.31
In this study, 15.9% of P. aeroginosa and Acinetobacter isolates were found to produce carbapenemase i.e. VIM (a metallo-beta-lactamase, MBL). Also, 63% of P. aeroginosa and Acinetobacter isolates harbored SHV and TEM-typed ESBL while only 14% of isolates harbored PER, as an ESBL. Approximately 5% of isolates had both “class A” ESBLs, (i.e. SHV and TEM).
The present study revealed a high prevalence of ß-lactamase producing genes in burn wounds colonized by Pseudomonas or Acinetobacter species. It seems that extended spectrum ß-lactamase producing genes, including TEM-type ESBL encoding genes followed by SHV genes, are responsible for the majority of emerging resistance to ß-lactams in the pediatric burn unit of our hospital. The roles of PER-type ESBL and VIM (a MBL) are less conspicuous.
Sepehriseresht et al. evaluated vim1, vim2, ipm1 and ipm2 metallo-ß-lactamase encoding genes in imipenem-resistant and intermediate P. aeruginosa strains isolated from burn wounds.32 Among the 483 isolates evaluated in their study, 272 (56%) were imipenem-resistant with a growth zone of < 13mm, 63 (13%) isolates had intermediate pattern in their imipenem antibiogram with a growth zone between 13-15mm, and 148 (31%) isolates were susceptible to imipenem with a growth zone of > 15 mm. PCR assay for vim1, vim2, ipm1 and ipm2 genes were done for the isolates with resistant and intermediate patterns to imipenem. Results of DNA sequencing by PCR showed that 54 (16.1%), 7 (2.1%), 22 (6.6%), and 11 (3.3%) of these isolates had vim1, vim2, ipm1 and ipm2 genes, respectively. The incidences of vim1, vim2, ipm1 and ipm2 genes among the imipenem-resistant isolates were 15.4%, 1.8%, 6.6%, and 3.3%, respectively; while intermediate isolates, had frequencies of 4.8%, 3.1%, 6.3%, and 3.1%, respectively. However, in another study conducted in our hospital, Saderi et al. found that 38.28% of 128 clinical isolates of P. aeruginosa were resistant to imipenem.33 Shahcheraghi et al. assessed 350 clinical isolates of P. aeruginosa from two general hospitals in Tehran, Iran and demonstrated that only 5% of the isolates were imipenem-resistant.34
According to our study, and also in comparison with the study by Sepehriseresht et al., it seems that prevalence of VIM-typed MBL genes in the isolates of P. Aeruginosa has been in a steady state from 2008 to 2012.32 However, data regarding the time-dependent changes in the prevalence of ESBL genes in P. aeruginosa and Acinetobacter spp. and also of MBL encoding genes in Acinetobacter spp. are less available. However, Rasmussen and Bush believed that as carbapenems use goes up, an increase in MBL-producing organisms would be inevitable.35 Consistently, a study by Lee et al. revealed that after nine years use of carbapenems in Korea, the imipenem-resistance rate of P. aeruginosa rapidly raised from 6% in 1996 to 19% in 2001.36 Shibata et al. demonstrated that 35%, 0.5%, and 64.5% of 180 isolates of P. aeroginosa had vim2, ipm1, and ipm2- typed MBL genes, respectively.37 Laupland et al. also found a 92% prevalence of vim2 positive isolates of P. aeroginosa isolates in the Calgary Health Region in Canada between May 2002 and April 2004.38 Lee et al. showed prevalences of 1.7% and 0% of vim2 and imp1 MBLs among 415 clinical isolates of P. aeroginosa in Korea, respectively.39 These findings reinforce the fact that geographical conditions, hygienic states and chromosomal and plasmid differences of the isolated organism lead to different prevalence of MBLs all over the world.
Azimi et al. evaluated the prevalence of Carbapenemase (KPC) among isolated Pseudomonas aeruginosa and Acinetobacter spp. at a tertiary burn hospital in Tehran, Iran.40 They isolated 64 strains from 20 patients with TBSA burns of 15-40% and an age range of 0-6 years. The isolated strains included 28 Gram-positive bacteria, 16 Enterobacteriacea, 12 Pseudomonas and 7 Acinetobacter baumannii. Among 36 Gram-negative isolates, 10 isolates were resistant to all tested antibiotics except Colistin and 10 isolates were susceptible to at least one aminoglycoside. Moreover, 15 out of 36 isolates including 6 Pseudomonas aeruginosa, 6 Acintobacter baumannii and 3 Klebsiella pneumonia strains were found to be imipenem-resistant. Finally, 13 out of 15 imipenem-resistant strains were confirmed as KPC-producing organisms. In detail, four of six imipenem- resistant Pseudomonas strains, all six imipenem-resistant Acinetobacter baumannii strains and all three imipenem- resistant Klebsiella pneumoniae strains were producing KPC. Only six out of 36 isolated strains were ESBL producers; while four strains were both KPC and ESBL positive.
Similarly, Shakibaei et al. evaluated the presence of SHV, TEM and PER type extended-spectrum ß-lactamase genes among clinical strains of Pseudomonas aeroginosa isolated from burnt patients at Shafa hospital, Kerman, Iran.41 Amongst 120 isolates of P. aeroginosa collected from 245 patients in the burn unit, 41 isolates (34%) were found to be ESBL producers; while none of the isolates produced MBL. The PCR assay of these ESBL producing isolates of P. aeroginosa demonstrated prevalences of 6.6%, 4.1% and 2.5% for SHV, PER and TEM type ESBL genes, respectively. Furthermore, Shojapour et al assessed the prevalence of TEM-1 type ESBL gene in Pseudomonas aeroginosa strains isolated from burn wounds in Shahrekord, Iran.42 They found 66 (37.7%) ESBL positive isolates among all 175 studied Pseudomonas aeruginosa isolates; and 15.15% of them were positive for TEM-1 gene. Similarly, Ranjbar et al also evaluated the characteristics of Pseudomonas aeruginosa strains isolated from burned patients hospitalized in our hospital.43 They studied a total of 70 P. aeruginosa isolates obtained from different clinical origins. They showed that 100% of P. aeruginosa strains were resistant to cefoxitin, 97% to cefotetan, 93% to ticarcillin, 89% to ticarcillin/clav, 76% to gentamicin and imipenem, 63% to piperacillin, 49% to tetracycline, and 20% to meropenem. However, they did not assess the prevalence of beta-lactamase producing genes in their Pseudomonas aeruginosa strains.
Although the antibiotic resistance of Acinetobacter spp. has been assessed in isolates recovered from general hospitals, especially ICUs, in Iran,44,45 there is limited information regarding the antibiotic resistance of Acinetobacter spp. isolated from burn patients in Iran. It has been reported that most of the VIM-positive isolates (76%) of Acinetobacter spp. were multidrug-resistant. While it has recently been found that increased expression of chromosomal genes for efflux systems plays a major role in multidrug resistance, the role of beta-lactamase producing genes in the emerging resistance of Acinetobacter spp should also be seriously considered, as shown previously.46,47
Schlager et al. showed a significantly higher rate of burn wound infection in patients with >20% TBSA as compared to the patients with <20% TBSA burn injury.48 Their results were in accordance with those reported by Weber et al. and Gastmeier et al., who also found a close association between the burn size and burn wound infection. The effects of TBSA on the development of burn wound infections could be explained by the fact that larger burn wounds increase the likelihood of wound contamination. 23,49-50
Our study has several limitations which need to be discussed. First, we used a relatively small sample size. Second, we did not assess the antibiogram pattern of the strains to decide the in vitro consequences of the presence of extended- spectrum ß-lactamase-producing and carbapenemaseproducing genes. Furthermore, there is a need for assessment of the clinical outcome of the patients affected by these strains that would reflect an in vivo reflection of the effects of these genes in morbidity and mortality of burn victims.
Conclusion
Our findings show that more than half of the Pseudomonas and Acinetobacter strains in our pediatric burn unit harbor ß-lactamase encoding genes which make them resistant to a wide range of antibiotics. This might independently affect the outcome of our patients. Increased morbidity, mortality and also high treatment costs are consequences of considerable burn wound infections which should be addressed in future studies with larger sample sizes.
Acknowledgments
Acknowledgements. none.
Financial Disclosure. none.
Funding/Support. none.
Conflict of interest. the authors of this paper hereby declare that they have no conflict of interest
References
- 1.Manson AD, McManus AT, Pruitt BA. Association of burn mortality and bacteremia, a 25-year review. Arch Surg. 1986;121:1027–31. doi: 10.1001/archsurg.1986.01400090057009. [DOI] [PubMed] [Google Scholar]
- 2.Pruitt Jr BA, McManus AT, Kim SH, Goodwin CV. Burn wound infections: Current status. World J Surg. 1998;22:135–45. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 3.Curreri WP, Luterman A, Braun DW, Shires GT. Burn injury: Analysis of survival and hospitalization time for 937 patients. Ann Surg. 1980;192:472–8. doi: 10.1097/00000658-198010000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polk HC. Consensus summary on infection. J Trauma. 1979;19:894–5. [PubMed] [Google Scholar]
- 5.Linares HA. A report of 115 consecutive autopsies in burned children: 1966-80. Burns lncl Therm lnj. 1982;8:263–70. doi: 10.1016/0305-4179(82)90007-9. [DOI] [PubMed] [Google Scholar]
- 6.Al-Zamil FA. Bacteremia in children at the University Hospital in Riyadh, Saudi Arabia. World J Pediatr. 2008;4:118–22. doi: 10.1007/s12519-008-0023-9. [DOI] [PubMed] [Google Scholar]
- 7.Azimi L, Motevalian A, Ebrahimzadeh Namvar A, et al. Nosocomial infections in burned patients in Motahari hospital, Tehran, Iran. Dermatol Res Pract. 2011;436952:1–4. doi: 10.1155/2011/436952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roe MT, Pillai SD. Monitoring and identifying antibiotic resistance mechanisms in bacteria. Poult Sci. 2003;82:622–6. doi: 10.1093/ps/82.4.622. [DOI] [PubMed] [Google Scholar]
- 9.Hogan D, Kolter R. Why are bacteria refractory to antimicrobials? Curr Opin Microbiol. 2002;5:472–7. doi: 10.1016/s1369-5274(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 10.Joly-Guillou ML. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect. 2005;11:868–73. doi: 10.1111/j.1469-0691.2005.01227.x. [DOI] [PubMed] [Google Scholar]
- 11.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–84. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Looveren M, Goosens H. ARPAC Steering Group. Antimicrobial resistance of Acinetobacter spp. in Europe. Clin Microbiol Infect. 2004;10:684–704. doi: 10.1111/j.1469-0691.2004.00942.x. [DOI] [PubMed] [Google Scholar]
- 13.Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin Microbiol Infect. 2006;12:826–36. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 14.Bonomo RA, Szabo D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis. 2006;43:49–56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- 15.Vila J, Marti S, S´anchez-C´espedes J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J Antimicrob Chemother. 2007;59:1210–5. doi: 10.1093/jac/dkl509. [DOI] [PubMed] [Google Scholar]
- 16.Shakibaie MR, Adeli S, Salehi MH. Antimicrobial resistance and infection control. 2012 doi: 10.1186/2047-2994-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim C, Lee Y, Lee H, Woo J, Song W, Kim M, Leeh W, Jung SH, Lee K, Chong Y. Prevalence and diversity of carbapenemases among imipenem-nonsusceptible Acinetobacter isolates in Korea: emergence of a novel OXA-182. Diagn Microbiol Infect Dis. 2010;68:432–8. doi: 10.1016/j.diagmicrobio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi A, Yomod AS, Kobayashi I, Okubo T, Tsunoda M, Iyobe S. Detection of carbapenemase-producing Acinetobacter baumannii in a hospital. Antimicrob Agents Chemother. 2000;38:526–9. doi: 10.1128/jcm.38.2.526-529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gullo A. Sepsis and organ dysfunction/failure. An overview. Minerva Anestesiol. 1999;65:529–40. [PubMed] [Google Scholar]
- 20.Shankowsky HA, Callioux LS, Tredget EE. North American survey of hydrotherapy in modern burn care. J Burn Care Rehabil. 1994;15:143–6. doi: 10.1097/00004630-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 21.McManus AT, et al. Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur J Clin Microbiol. 1985;4:219–23. doi: 10.1007/BF02013601. [DOI] [PubMed] [Google Scholar]
- 22.Revathi G, Puri J, Jain BK. Bacteriology of burns. Burns. 1998;24:347–9. doi: 10.1016/s0305-4179(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 23.Gastmeier P, Weigty O, RuÈden H. Comparison of hospital-acquired infection rates in paediatric burn patients. Journal of Hospital Infection. 2002;52:161–5. doi: 10.1053/jhin.2002.1292. [DOI] [PubMed] [Google Scholar]
- 24.Ganesamoni S, Kate V, Sadasivan J. Epidemiology of hospitalized burn patients in a tertiary care hospital in South India. Burns. 2010;36:422–9. doi: 10.1016/j.burns.2009.06.212. [DOI] [PubMed] [Google Scholar]
- 25.Mayhall CG. The epidemiology of burn wound infections: Then and now. Clin Inf Dis. 2003;37:543–50. doi: 10.1086/376993. [DOI] [PubMed] [Google Scholar]
- 26.Pruitt BA, McManus AT, Kim SH, Goodwin CW. Burn wound infections: Current status. World J Surg. 1998;22:135–45. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- 27.Agnihotri N, Gupta V, Joshi RM. Aerobic bacterial isolates from burn wound infections and their antibiograms – a five-year study. Burns. 2004;30:241–3. doi: 10.1016/j.burns.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Tredget EE, Shankowsky HA, Rennie R, Burrell RE, Logsetty S. Pseudomonas infections in the thermally injured patient. Burns. 2004;0:3–26. doi: 10.1016/j.burns.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Altoparlak U, Erol S, Akcay MN, Celebi F, Kadanali A. The timerelated changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns. 2004;30:660–4. doi: 10.1016/j.burns.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19-2:403–34. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albrecht MA, Griffith ME, Murray CK, Chung KK, Horvath EE, Ward JA, et al. Impact of Acinetobacter infection on the mortality of burn patients. J Am Coll Surg. 2006;203:546–50. doi: 10.1016/j.jamcollsurg.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Sepehriseresht S, Boroumand MA, Pourgholi L, Sotoudeh Anvari M, Habibi E, Sattarzadeh Tabrizi M. Detection of vim- and ipmtype metallo-beta-lactamases in Pseudomonas aeruginosa clinical isolates. Arch Iran Med. 2012;15:670–3. [PubMed] [Google Scholar]
- 33.Saderi H, Karimi Z, Owlia P, Bahar MA, Akhavi Rad SMB. Phenotypic detection of metallo-beta-lactamase producing Pseudomonas aeruginosa strains isolated from burned patients. Iranian J Pathol. 2008;3:20–4. [Google Scholar]
- 34.Shacheraghi F, Nikbin VS. Metallo betalactamases and determination of ceftazidim and imipenem resistance rate between resistant Pseudomonas aeruginosa clinical isolates isolated from Imam Khomeini hospital and Tehran Children Center during 2005. Iranian J Infect Dis. 2005;12:19–22. [Google Scholar]
- 35.Rasmussen BA, Bush K. Carbapenem-hydrolysing ß-lactamases. Antimicrob Agent Chemother. 1997;41:223–32. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee K, Lee WG, Uh Y, Ha GY, Cho J, Chong Y. VIM and IMP-type metallo-ß-lactamase producing Pseudomonas spp. and Acinetobacter spp. in Korean Hospitals. Emerg Infect Dis. 2003;9:868–71. doi: 10.3201/eid0907.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata N, Doi Y, Yamane K, Yagi T, Kurokawa H, Shibayama K, et al. PCR typing of genetic determinants for metallo-ß-lactamases and integrases carried by Gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J Clin Microbiol. 2003;41:5407–13. doi: 10.1128/JCM.41.12.5407-5413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laupland KB, Parkins MD, Church DL, Gregson DB, Louie TJ, Conly JM, et al. Population-based epidemiological study of infections caused by carbapenem-resistant Pseudomonas aeruginosa in the Calgary Health Region: Importance of metallo-ß-lactamase (MBL)-producing strains. J Inf Dis. 2005;192:1606–12. doi: 10.1086/444469. [DOI] [PubMed] [Google Scholar]
- 39.Lee K, Park AJ, Kim MY, Lee HJ, Cho JH, Kang Jo, et al. Metallo-ß-lactamase-producing Pseudomonas spp. in Korea: High prevalence of isolates with VIM-2 type and emergence of isolates with IMP-1 type. Yonsei Med J. 2009;50:335–9. doi: 10.3349/ymj.2009.50.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azimi L, Rastegar Lari A, Alaghehbandan R, Alinejad F, Mohammadpoor M, Rahbar M. KPC-producer gram negative bacteria among burned infants in Motahari hospital, tehran: First report from Iran. Annals of Burns and Fire Disasters. 2012;25:2. [PMC free article] [PubMed] [Google Scholar]
- 41.Shakibaei MR, Shahcheraghi F, Hashemi A, Adeli NS. Detection of TEM, SHV, and PER type extended-spectrum ß-lactamase genes among clinical strains of Pseudomonas aeruginosa isolated from burnt patients at Shafa-hospital, Kerman, Iran. Iranian journal of basic medical sciences. 2008;1:104–11. [Google Scholar]
- 42.Shojapour M, Shariati L, Karimi A, Zamanzad B. Prevalence of TEM-1 type beta-lactmase genes in Pseudomonas aeruginosa strains isolated from burn infections using Duplex PCr in Shahrekord, 2008. Arak Medical University Journal (AMUJ) 2011;14:55–61. [Google Scholar]
- 43.Ranjbar R, Owlia P, Saderi H, Mansouri S, Jonaidi-Jafari N, Izadi M, Farshad S, Arjomandzadegan M. Characterization of Pseudomonas aeruginosa strains isolated from burned patients hospitalized in a major burn center in tehran, Iran. Acta Med Iran. 2011;49:675–9. [PubMed] [Google Scholar]
- 44.Khosroshahi N, Sharifi M. Isolation of A. baumannii resistant to carbapenems from ICU patients. Iran J Med Microbiol. 2009;1:33–8. [Google Scholar]
- 45.Farahani R, Moniri R, Shajari G, Nazem Shirazi MH, Musavi G, Ghasemi A, Haj Aghazadeh S. Antibiotic resistance of Acinetobacter sp. isolated from Shahid Beheshti Hospital, Kashan. Faiz. 2009;4:60–6. [Google Scholar]
- 46.Sinha M, Srinivasa H, Macaden R. Antibiotic resistance profile & extended spectrum beta-lactamase (eSBL) production in Acinetobacter species. Indian J Med Res. 2007;126:63–7. [PubMed] [Google Scholar]
- 47.Takahashi A, Yomod AS, Kobayashi I, Okubo T, Tsunoda M, Iyobe S. Detection of carbapenemase-producing Acinetobacter baumannii in a hospital. Antimicrob Agents Chemother. 2000;38:526–9. doi: 10.1128/jcm.38.2.526-529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlager T, Sadler J, Weber D, Donowitz L, Johr J. Hospital-acquired infections in pediatric burn patients. South Med J. 1994;87:481–4. doi: 10.1097/00007611-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Weber J, Sheridan R, Pasternack M, Tompkins R. Nosocomial infections in pediatric patients with burns. Am J Infect Control. 1997;25:195–201. doi: 10.1016/s0196-6553(97)90004-3. [DOI] [PubMed] [Google Scholar]
- 50.Mayhall C, editor. Hospital epidemiology and Infection Control. Philadelphia: Lippincott Williams & Wilkins; 1999. Nosocomial burn wound infections; pp. 275–286. [Google Scholar]


