Summary
Keloid scars continue to pose a challenge to clinicians as the treatment armamentarium lacks a formidable agent to tackle them. We have undertaken an in vitro study based on the mechanism of action of Vitamin D3 and quercetin on isolated keloid fibroblasts. Dose-dependent action on the reduction of cellular proliferation, collagen synthesis and induction of apoptosis by Vitamin D3 and quercetin are analyzed and probable mechanism of action is elaborated. This study thus opens up newer avenues in tackling keloid scars effectively.
Keywords: keloid fibroblasts, vitamin D3, quercetin, apoptosis
Abstract
Les cicatrices chéloïdes restent un défi pour les cliniciens car il n’existe pas encore un agent efficace pour les traiter. Pour cette raison nous avons entrepris une étude in vitro sur le mécanisme d’action de la vitamine D3 et la quercétine sur des fibroblastes isolées à partir de chéloïdes. Nous avons analysé l’effet dose dépendant de la vitamine D3 et la quercétine sur la diminution de la prolifération cellulaire, la synthèse du collagène et de l’induction de l’apoptose, et élaboré le mécanisme probable de cette action. Notre étude ouvre ainsi des voies nouvelles dans la lutte contre les cicatrices chéloïdes.
Introduction
Both hypertrophic and keloid scars are caused by abnormal wound healing, the key feature being excess collagen secretion by fibroblasts. Both keloid and hypertrophic scars are considered dermal fibro proliferative diseases that differ both clinically and histopathologically.1 Keloids are benign, fibroproliferative lesions that result in excessive fibrosis. They are composed of mainly type III (early) or type I (late) collagen. Some of the symptoms include pruritus, tenderness, and pain. Often, they are very difficult to treat and recurrence is very common. In contrast to hypertrophic scars, keloids extend beyond the original confines of the wound.2 Because of the central role played by collagen in the wound healing process, control of collagen synthesis by fibroblasts has been of paramount importance in abrogating abnormal wound healing to prevent the development of hypertrophic scarring and keloids. Multiple treatments have been advocated in the past with varied success.3
Treatment of keloid scars currently includes surgery, intralesional corticosteroids, pressure garments, tamoxifen, and interferon therapy, with different treatment regimens resulting in varying degrees of recurrence. Most of the literature on keloid treatment suggests that a high rate of recurrence (50%-70%) prevails during their management.4-8
Recent in vitro studies on novel therapeutic approaches for treating keloids suggest that Vitamin D3 and quercetin may prove to play a significant role in managing them.9,10 Hence we explored whether Vitamin D3 and quercetin could control the fibrosis induced by keloid fibroblasts. In addition to this, a more extensive and deeper understanding of the mechanisms responsible for keloid formation is required to develop robust therapies.
Materials and methods
Materials
All chemicals used were procured from Sigma Chemical Co., USA. Vitamin D3 was used in our experiments. Other chemicals, unless specified, were of analytical grade. Fetal calf serum (In vitrogen), Mouse Anti-Human Bcl-2 primary antibody (Santa Cruz), Goat Anti-Mouse FITC conjugated secondary antibody (Genie), cDNA directTM KIT, and (Genie) Gene JET RNA purification kit (Thermo Scientific) were used.
A total of five keloids of the ear lobules, post traumatic in origin due to ear boring in childhood, were obtained during cosmetic reconstruction. The biopsies were sent for culture studies. Approval for this research work was obtained from the Institutional Review Board and Ethics Committee. As this was purely an in vitro study, we did not treat any of our patients with Vitamin D3 and quercetin.
Isolation of fibroblasts
Freshly collected keloid biopsies were thoroughly washed in PBS then incubated in 10X antibiotic solution with fungizone for 15 minutes, before being washed in 1X antibiotic solution with fungizone. Biopsies were incubated in 0.3% dispase overnight at 4ºC. Post incubation biopsies were washed in PBS and the dermis was separated from the epidermis. The thick dermal/keloid portion was sliced into small pieces and suspended in collagenase for 1-2 hours at 37oC. The released cells were spun at 1500 rpm for 5 minutes and replated in DMEM with 10% FBS. Cells were allowed to grow to 80% confluency. Subculture was done by trypsinisation and plating. The experiments were carried out during 3-5 passages. Fibroblast cultures were performed in triplicate in all five biopsy samples.
Keratinocyte isolation
The epidermis separated from the dermis was taken for keratinocyte isolation. We added 1 ml of keratinocyte growth medium (KGM) to the epidermis and transferred it into a sterile plate before treating it with 0.1% trypsin for 5 minutes. Cells were allowed to release from the epidermis, suspended in KGM, spun at 2000 rpm for 10 minutes. The pellet was resuspended in KGM and left to grow for 15 days to make a passage. Cells in passages 2 and 4 were used for the present study. Keratinocyte cultures were performed in all five biopsy samples in triplicate.
MTT assay
Fibroblasts (2.5X104 ) were seeded into a 24 well plate, incubated at 37°C and 5% CO2 overnight. Cells were treated with different concentrations of Vitamin D3 (5-50 M/l) (5-50 µg) or quercetin for 24 and 48 hours. Cell viability was assessed using mitochondrial dependent reduction of (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan. Cells were incubated with MTT solution for 3-4 hours followed by solubilisation in DMSO.11 Cell viability was quantitated by measuring optical density at 570 nm (Tecan-Infinite 50-Megallan) and was expressed as percentage of untreated controls. Proliferation studies were carried out in triplicate in all the five biopsy samples in both fibroblasts and keratinocytes.
Statistics
Statistical analysis was performed using Student’s ttest. P values ≤ 0.05 were considered to be statistically significant. Error bars represent Mean ± Standard Deviation.
RNA Extraction and Analysis
Total RNA was extracted from fibroblasts after 4 hrs of treatment with Vitamin D3 or quercetin by using the Gene JET RNA purification kit (Thermo Scientific) according to the manufacturer’s instructions. Total RNA (5ng) was reverse transcribed to cDNA using cDNA directTM KIT (Genie) and then amplified using Semi-quantitative reverse transcription-polymerase chain reaction (Agilent Technologies Surecycler 8800). All reagents, primers and probes were purchased from Fermentas. Each sample was assayed in triplicate on 0.8 % agarose. Results were analyzed by using Genie-UVTECH Cambridge and software (DMAX) -Fire Reader. The housekeeping gene GAPDH was used to normalize RNA concentration.
Immunocytochemistry
Glass cover slips of 13mm diameter were autoclaved followed by coating with poly-l-lysine by immersion in 1mg/ml solution of the same for 30min before being air dried. Keloid scar fibroblasts were trypsinised and plated onto the cover slip and allowed to adhere overnight, followed by treatment with quercetin and Vitamin D3. After 4 hours of treatment, the cells were fixed in 2% paraformaldehyde for 30 minutes and permeabilized for 10 minutes in the buffer (PBS, 0.2% BSA, 0.1% TritonX- 100). Once they were washed in the aforementioned buffer, the cells were incubated with primary antibodies in 0.2% BSA for 1 hour, and then with a fluorochrome- conjugated secondary antibody.12 Nikon Eclipse T100 inverted microscope was used for imaging the stained cells.
Results
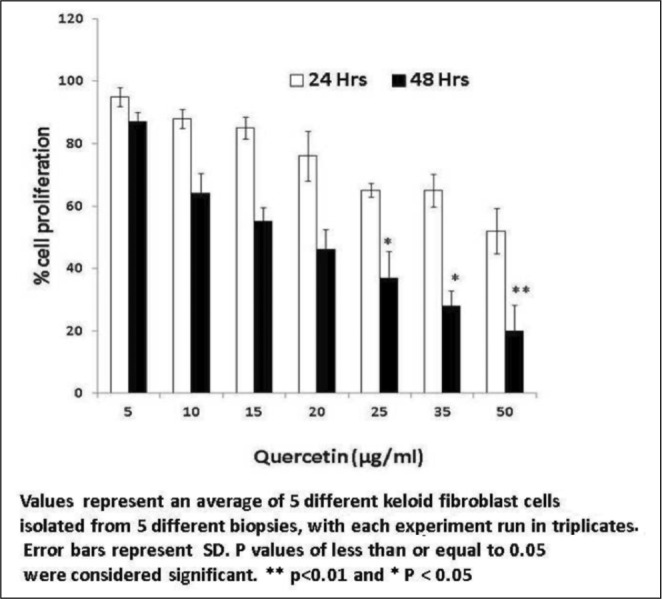
Keloid fibroblasts treated with Vitamin D3 showed a dose-dependent reduction in cell proliferation in the tested range of concentrations after 48 hours (5-50 ng/ml) with morphological changes. After 24 hours of treatment there was a negligible reduction in proliferation (Fig. 1). Similar experiments with kertinocytes did not show any effect. Quercetin treatment of fibroblasts (5-50 µg/ml) showed a similar trend with a substantial decrease in cell proliferation without any noticeable morphological changes (Fig. 2). When quercetin was used with kertinocytes no change in proliferation was observed.
Fig. 1. Percentage reduction in fibroblasts proliferation by Vitamin D3.
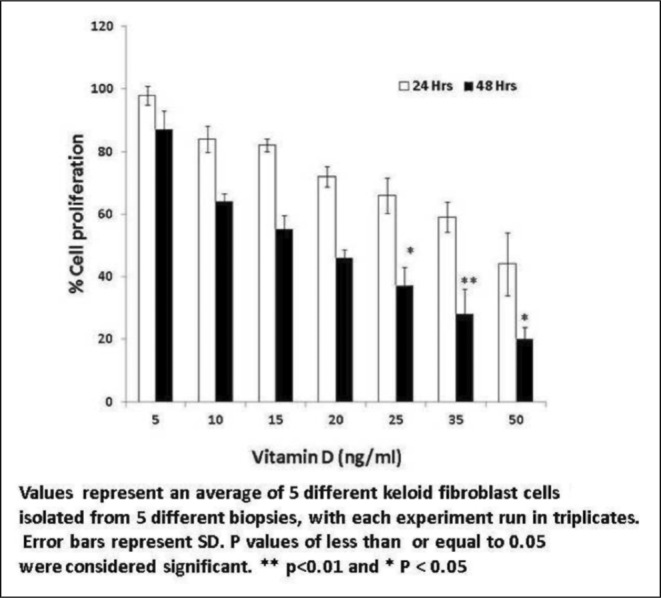
Fig. 2. Percentage reduction in fibroblast proliferation by Quercetin.
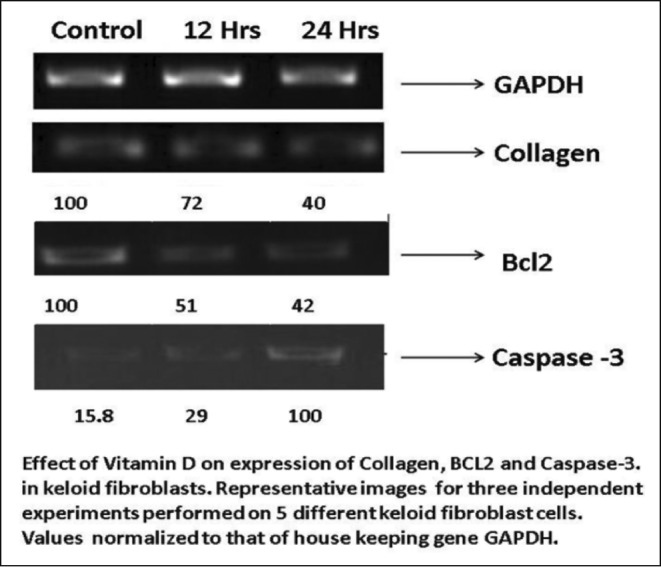
In comparison to untreated controls, collagen I expression showed a three-fold gradual decline in samples treated with Vitamin D3 (20ng/ml), demonstrating the role of Vitamin D3 in keloid regression. The gene expression pattern of Bcl-2 in keloid fibroblasts treated with 20ng/ml Vitamin D3 at different time points (12 hours and 24 hours) drastically reduced the levels of Bcl-2 in a time-dependent manner. Caspase-3 levels remained undetectable in untreated fibroblasts, but was raised five-fold after 24 hours of treatment with Vitamin D3 at 20ng/ml (Fig. 3).
Fig. 3. Expression collagen, BCL2 and caspase-3 by fibroblasts treated with Vitamin D3.
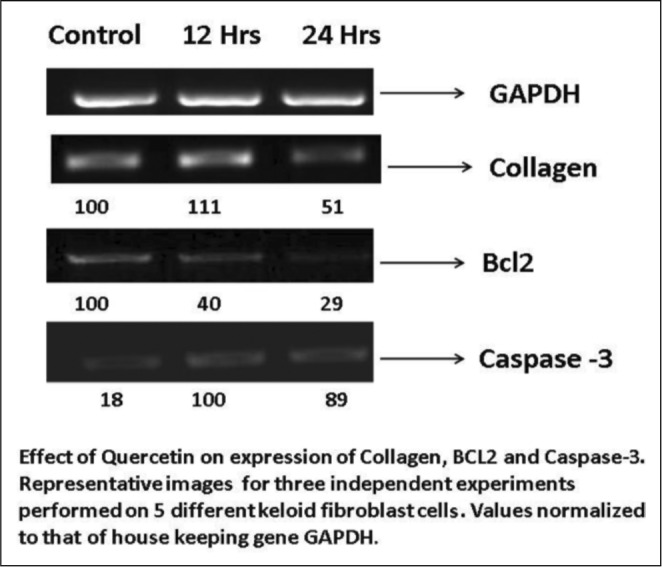
Expression of collagen I was observed in abundance in the untreated keloid fibroblasts compared to a three-fold decrease of the same in the cells treated with quercetin at 25 µg/ml after 48 hours. When fibroblasts were treated with quercetin at 12 hours and 24 hours at the concentration of 50µg/ml, collagen I level was decreased. Quercetin treatment also showed an increase in caspase-3 expression at 25 µg/ml after both 12 and 24 hours (Fig. 4). All of the polymerase chain reaction experiments to assess the expression of collagen, Bcl-2 and caspase-3 levels were carried out 3 times.
Fig. 4. Expression collagen, BCL2 and caspase-3 by fibroblasts treated with quercetin.
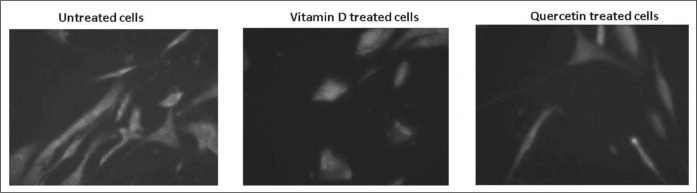
Immuno-staining of keloid fibroblasts treated with Vitamin D3 and quercetin showed decreased Bcl-2 expression but the cellular morphology was altered in Vitamin D3 treated cells. Treatment with quercetin did not alter the fibroblasts’ morphology (Fig. 5).
Fig. 5. Immunostaining of fibroblasts with BCL 2 antibody.
Discussion
Pathological scarring, such as keloids, can lead to severe functional impairment and psychological morbidity, translating into a considerable financial burden for the patient. In the past, treatment methods have primarily addressed factors that operate on keloids and not during the initial onset of keloid formation. The proposed mechanisms which underlie the pathogenesis of keloids include excessive inflammation, excessive angiogenesis, altered levels of matrix metalloproteinases, growth factors, and delayed apoptosis of fibrotic myofibroblasts.13,14
Whether a skin injury leads to a hypertrophic scar or keloid depends on the duration of fibroblast exposure to the micro environment which influences and alters cellular and molecular processes, thereby leading to increased collagen production. It is also reported that keloid fibroblasts are more resistant to programmed cell death than normal skin fibroblasts.15
In our study we investigated the effect of Vitamin D3 and quercetin on keloid fibroblasts. Both the agents decreased cellular proliferation and collagen synthesis. The reason for decreased proliferation may be attributed to increased apoptosis since antiapoptotic factor Bcl-2 expression showed a decline in the treated keloid fibroblasts. To support this observation the expression of caspase-3 was analyzed. This showed an increase when keloid fibroblasts were treated with Vitamin D3 and quercetin. Therefore our in-vitro study suggests that Vitamin D3 and quercetin may have an important role to play in arresting keloid formation. It was interesting to observe that Vitamin D3 and quercetin did not show any effect on kercinocyte proliferation. Thus our study has brought to light a combination approach that switches on apoptotic factors while being firmly grounded in tackling cellular proliferation and synthesis of collagen.
Elucidation of the molecular pathways leading to keloid formation will undoubtedly open up a host of opportunities for therapeutic intervention. Prevention is paramount, but combination therapy will most likely prove to be effective over a single modality in the management of keloids as a whole.
References
- 1.Meenakshi J, Jayaraman V, Rmakrishnan KM, Babu M. Ultrastructural differentiation of abnormal scars. Annals of Burns and Fire Disasters. 2005;18:83–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Robles DT, Berg D. Abnormal wound healing: Keloids. Clinics in Dermatology. 2007;25:26–32. doi: 10.1016/j.clindermatol.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Tuan Tl, Nichter S. The molecular basis of keloids and hyper trophic scar formation. Mol Med today. 1998;1:19–24. doi: 10.1016/S1357-4310(97)80541-2. [DOI] [PubMed] [Google Scholar]
- 4.Shaffer JJ, Taylor SC, Cook-Bolden F. Keloidal scars: A review with a critical look at therapeutic options. J Am Acad Dermatol. 2002;46:63–97. doi: 10.1067/mjd.2002.120788. [DOI] [PubMed] [Google Scholar]
- 5.Ledon JA, Savas J, Franca K, Chacon A, Nouri K. Intralesional treatment for keloids and hypertrophic scars: A review. Dermatol Surg. 2013;39:1745–57. doi: 10.1111/dsu.12346. [DOI] [PubMed] [Google Scholar]
- 6.Suzawa H, Kikuchi S, Arai N, Koda A. The mechanism involved in the inhibitory action of tranilast on collagen biosynthesis of keloid fibroblasts. JPN J Pharmacol. 1992;60:91–6. doi: 10.1254/jjp.60.91. [DOI] [PubMed] [Google Scholar]
- 7.Fulton JE. Silicone gel sheeting for the prevention and management of evolving hypertrophic and keloid scars. Dermatol Surg. 1995;21:947–51. doi: 10.1111/j.1524-4725.1995.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee EC, Doong H, Jellema A. The response of burn scars to intralesional verapamil. Arch Surg. 1994;129:107–11. doi: 10.1001/archsurg.1994.01420250119015. [DOI] [PubMed] [Google Scholar]
- 9.Atiyeh BS. Nonsurgical management of hypertrophic scars: evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast Surg. 2007;31:468–92. doi: 10.1007/s00266-006-0253-y. [DOI] [PubMed] [Google Scholar]
- 10.Long X, Zeng X, Zhang FQ, Wang XJ. Influence of quercetin and X-ray on collagen synthesis of cultured human keloid-derived fibroblasts. Chin Med Sci J. 2006;21:179–83. [PubMed] [Google Scholar]
- 11.Zhang GY, Cheng T, Luan Q, et al. Vitamin D: A novel therapeutic approach for keloid, an in vitro analysis. British Journal of Dermatology. 2011;164:729–37. [Google Scholar]
- 12.Arnold JT, Le H, McFann KK, et al. Comparative effects of DHeA vs. testosterone, dihydrotestosterone, and estradiol on proliferation and gene expression in human LNCaP prostate cancer cells. Am J Physiol Endocrinol Metab. 2005;288:573–84. doi: 10.1152/ajpendo.00454.2004. [DOI] [PubMed] [Google Scholar]
- 13.Chauhan S, Kunz S, Davis K, et al. Androgen control of cell proliferation and cytoskeletal reorganization in human fibrosarcoma cells: role of rhoB signaling. J Biol Chem. 2004;79:937–44. doi: 10.1074/jbc.M311325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the tGF-ß family in wound healing, burns and scarring: A review. Int J Burns Trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 15.Messadi DV, Le A, Berg S, Jewett A, et al. Expression of apoptosis-associated genes by human dermal scar fibroblasts. Wound Rep Reg. 1999;7:511–7. doi: 10.1046/j.1524-475x.1999.00511.x. [DOI] [PubMed] [Google Scholar]