Abstract
Improved understanding of the role of inflammation in tendon disease is required to facilitate therapeutic target discovery. We studied supraspinatus tendons from patients experiencing pain before and after surgical subacromial decompression treatment. Tendons were classified as having early, intermediate or advanced disease and inflammation was characterized through activation of pathways mediated by Interferon, NF-κB, glucocorticoid receptor and STAT-6. Inflammation signatures revealed expression of genes and proteins induced by Interferon and NF-κB in early stage disease and genes and proteins induced by STAT-6 and glucocorticoid receptor activation in advanced stage disease. The pro-resolving proteins FPR2/ALX and ChemR23 were increased in early stage disease compared to intermediate-advanced stage disease. Patients who were pain-free post-treatment had tendons with increased expression of CD206 and ALOX15 mRNA compared to tendons from patients who continued to experience pain post-treatment, suggesting that these genes and their pathways may moderate tendon pain. Stromal cells from diseased tendons cultured in vitro showed increased expression of NF-κB and Interferon target genes after treatment with lipopolysaccharide or IFNγ compared to stromal cells derived from healthy tendons. We identified 15-epi Lipoxin A4, a stable lipoxin metabolite derived from aspirin treatment, as potentially beneficial in the resolution of tendon inflammation.
Introduction
Pathology of musculoskeletal soft tissues is a common and significant healthcare problem both in sports injuries and in the ageing population. Shoulder pain is the third commonest orthopedic problem to present to clinicians (1, 2). Shoulder soft tissue pathology such as rotator cuff tears cause pain, loss of function, joint failure and development of secondary osteoarthritis with a substantial social and economic burden. Rotator cuff pathology can be classified as early stage disease in intact tendons or intermediate to advanced stage disease in torn tendons. Inflamed musculoskeletal soft tissues heal by the formation of a repair scar, however, the normal architecture, composition and function of the tissues are not fully restored (3, 4) thus increasing susceptibility to re-injury. Multiple therapies have been advocated in patients with rotator cuff tendinopathy (most frequently affecting the supraspinatus tendon) with mixed results, including physiotherapy, non steroidal anti-inflammatory drugs (NSAIDs), and local injections of glucocorticoids and platelet-rich plasma. Surgical subacromial decompression and rotator cuff tear repair are performed in patients with persistent symptoms (approximately 25,000 subacromial decompression surgeries and 10,000 repairs in the UK, and 300,000 repairs in the US annually) (5). Surgical repair of tendon tears is associated with high post-operative failure rates of approximately 40% (6, 7). Whilst the effects of repetitive wear and tear, daily exercise, ageing and genetic factors are cited as important contributing factors (8–11), our incomplete understanding of the mechanisms underpinning tendon pathology hampers the development of efficacious new therapies.
The identification of key immune cell populations as master regulators of inflammation has advanced our understanding of pathogenic mechanisms in other rheumatic diseases such as rheumatoid arthritis and the spondyloarthropathies (12–15). Growing evidence supports the contribution of inflammation to the development of tendinopathy (16–21). Whilst animal models of induced tendinopathy have improved our understanding of acute inflammatory processes (22), the phenotypes of the key cells orchestrating inflammation and fibrosis in human soft tissue pathologies such as tendinopathy and enthesopathy have not been fully characterized. Macrophages are known to play an essential role in homeostasis and pathology, orchestrating inflammation and repair in many tissues. The signaling pathways underpinning activation of macrophages to become M1 or M2 subtypes (23–25) have been revised to highlight receptors and key signaling mediators in common and distinct pathways. These include pro-inflammatory pathways containing Interferon (IFN) and NF-κB, pro-fibrotic pathways containing STAT-6 and inflammation resolving pathways involving glucocorticoid receptor activation (26).
Recent studies highlight the importance of tissue microenvironments and the innate immune response in perpetuating the inflammatory process. Non-myeloid cell populations such as resident stromal cells also play a prominent role in the generation and maintenance of chronic inflammation (27, 28). Cancer-associated fibroblasts are known to become “polarized” within the tumor microenvironment. This “polarization” reflects the altered activation status and phenotype of these cells. The interactions between tumor-associated macrophages and stromal cells have been implicated in chronic inflammation (29, 30). However inflammation activation pathways and the interplay between myeloid and stromal cells are understudied in diseased human musculoskeletal soft tissues from patients with tendinopathy or enthesopathy.
Inflammation resolution is known to be a highly active and co-ordinated process (31). In health, a repertoire of pro-resolving lipids and proteins derived from myeloid and stromal cells promote the timely resolution of the inflammatory response and restoration of tissue homeostasis (32–36). Inadequate or dysregulated resolution is thought to contribute to the development of many systemic chronic inflammatory diseases (37, 38). Resolution has been well studied in experimental mouse models of inflammation (39–42), but not in diseased human tendons.
In this study we characterized inflammation activation pathways in diseased human supraspinatus tendons. We studied tendon tissue samples from a well phenotyped longitudinal cohort of symptomatic patients with early to advanced disease before and after surgical subacromial decompression treatment. In some patients the symptoms resolved after treatment, whereas other patients remained symptomatic; the post-treatment samples allowed us to compare the inflammatory signatures from these two groups. We also compared inflammation activation pathways in stromal cells derived from healthy asymptomatic human tendons and pathological painful human tendons. We hypothesized that inflammation signatures would differ throughout the spectrum of supraspinatus tendon pathology and after the resolution of clinical symptoms. We also tested whether stromal cells from diseased tendons exhibited a more pro-inflammatory phenotype after cytokine stimulation compared to cells derived from healthy tendons.
Results
Diseased tendons show increased numbers of CD14+ and CD68+ myeloid cells
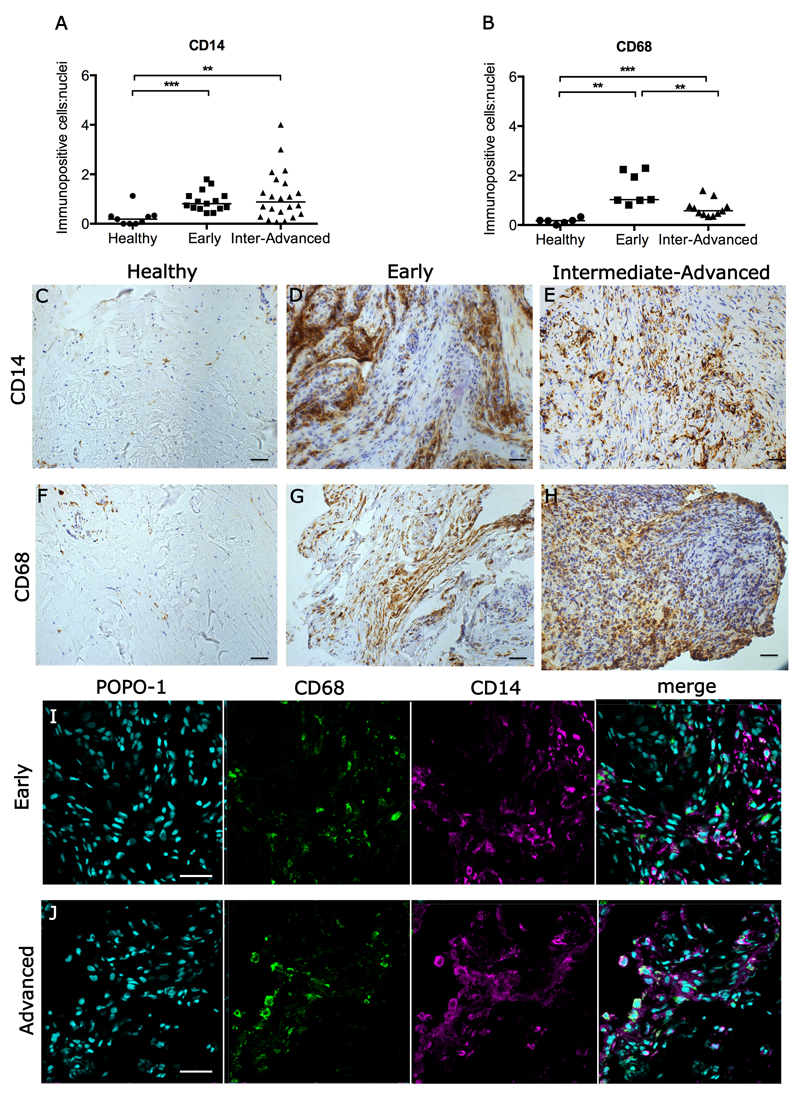
Resident macrophage populations in diseased human tendons are not well studied and little is known of their phenotype. The macrophage infiltrate in diseased tendons could derive from the adult axis of inflammatory CD14+ monocytes that extravasate and mature into CD68+ macrophages. Immunohistochemistry was performed to determine if monocytes (CD14+ cells) or tissue resident macrophages (CD68+ cells) were present in samples of early, intermediate and advanced stages of disease in supraspinatus tendons and was compared to healthy supraspinatus tendons. Morphologically, diseased samples showed a marked increase in cellularity compared to healthy tendons as shown by quantitative analyses (Figure 1A,B) of immunopositive staining (Fig. 1C-H). There was an increase in CD14+ monocytes/macrophages in early (p=0.0004) and intermediate-advanced disease tendons (p=0.0026) compared to healthy tendon samples (Figure 1A). There was also an increase in CD68+ macrophages in early (p=0.0012) and intermediate-advanced disease tendons (p=0.0002) compared to healthy samples; CD68+ macrophages were increased in early compared to intermediate-advanced disease tendons (p=0.004) (Figure 1B). The proportion of myeloid cells expressing both CD14 and CD68 was 13.4% (+/- 5%), suggesting that the majority of monocytes and macrophages in diseased tendons were distinct populations (Figure 1I,J).
Figure 1.
Immunohistochemistry showing CD14+ and CD68+ monocytes/macrophages in healthy and diseased human supraspinatus tendons. (A, B) Graphs show quantitative analysis of CD14+ cells (monocytes) and CD68+ cells (macrophages) in healthy tendons, and in early and intermediate-advanced stage diseased tendons. Bar shows median values. Statistically significant differences were calculated using Kruskal-Wallis with pairwise post-hoc Mann Whitney U tests. **p<0.01, ***p<0.001. Panel shows representative images of 3,3’-Diaminobenzidine immunostaining (brown) for CD14 and CD68 in healthy (C,F), early (D,G) and intermediate-advanced stages of tendon disease (E,H). Nuclear counterstain is haematoxylin. Scale bar=50µm. (I,J) Representative confocal immunofluorescence images showing dual labelling for CD14 (purple) and CD68 (green) in sections of early and advanced stage diseased tendons. Cyan represents POPO-1 nuclear counterstain. Scale bar = 20µm.
Inflammation activation signatures are altered in different stages of tendon disease
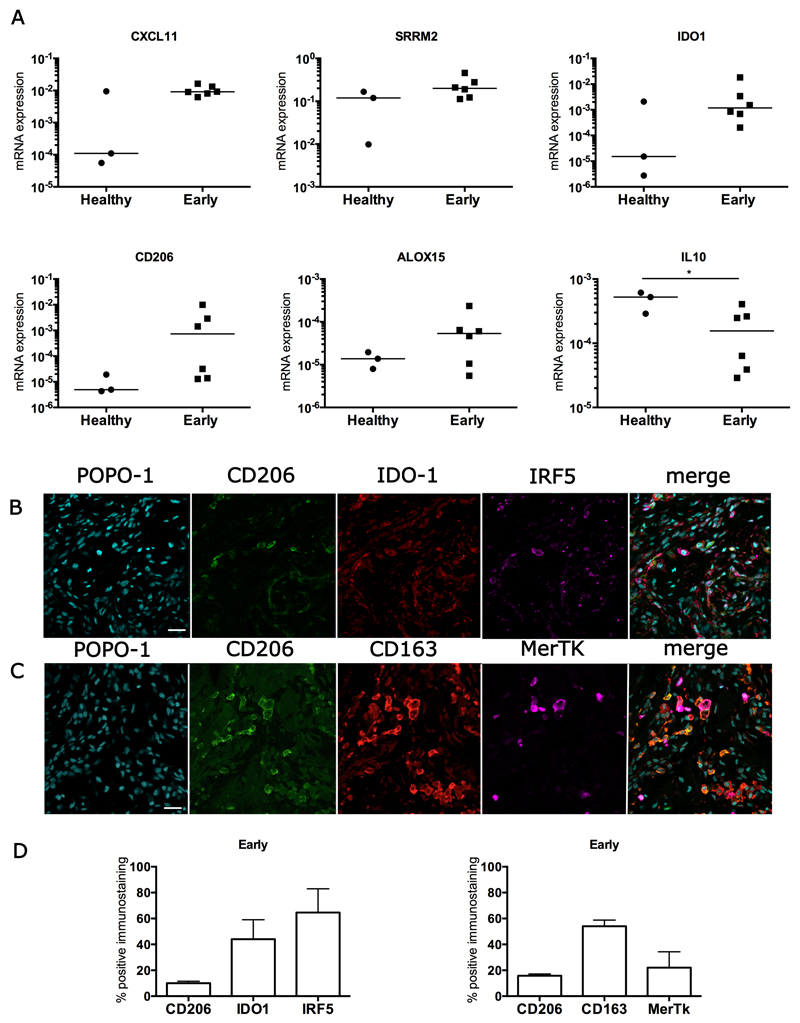
Having demonstrated the presence of CD14+ and CD68+ macrophages in diseased human tendons we investigated if products associated with macrophage activation could be identified in whole tendon tissue derived from patients with tendon pathology. To study gene expression we used a panel of genes including those known to be involved in activation of macrophages such as Interferon (IFN), NF-κB, interleukin (IL)-13, IL-4, STAT-6 and the genes encoding the glucocorticoid receptor activation pathways (Table S1). We investigated these inflammation activation pathways in samples of early, intermediate, and advanced stage tendon disease compared to healthy tendons and also in samples collected from patients after surgical subacromial decompression treatment who showed resolution of their clinical symptoms. Changes in inflammation activation gene expression signatures in diseased tendons relative to healthy tendons are shown in Table S2. Supraspinatus tendon samples from patients with early stage disease prior to surgical subacromial decompression treatment (n=6) showed a mixed inflammation gene signature including activation of IFN, NF-κB and STAT-6 pathways compared to subscapularis tendons from healthy patients (Figure 2A). There was increased expression of the IFN-induced genes CXCL11 and Serine/Arginine Repetitive Matrix 2 (SRRM2), of NF-κB-induced genes Indoleamine 2,3-dioxgenase 1 (IDO1) and STAT-6-induced genes (CD206 and ALOX15) in early stage supraspinatus tendons compared to healthy subscapularis tendons (Figure 2A). The IFN-induced genes vesicle-associated membrane protein 5 (VAMP5) and tryptophanyl-tRNA synthetase (WARS) were over represented in early compared to advanced stage disease tendons (Table S2). IL-10 mRNA expression was reduced in early disease tendons compared to healthy tendons (p=0.047, 2.7-fold decrease) (Figure 2A). A mixed protein signature was also observed in sections of tendons with early stage disease, including IRF5 (interferon regulatory factor 5; a marker of IFN activation), IDO-1 (NF-κB activation), CD206 (STAT-6 activation) and CD163 (glucocorticoid receptor activation) (Figure 2 B, C). Quantitative analysis of immunostaining showed an IFN and NF-κB activation signature, with 64% and 44% of immunopositive cells expressing IRF5 and IDO-1 proteins, respectively (Figure 2D).
Figure 2.
Expression of inflammation activation pathway genes and proteins in early stage diseased supraspinatus tendons compared to healthy subscapularis tendons. (A) A mixed gene expression signature was present in pre-treatment tendon samples with early disease (n=6) compared to healthy subscapularis control tendons (n=3). Shown is expression of genes induced by IFN including CXCL11, and Serine/arginine repetitive matrix 2 (SRRM2); induced by NF-κB including Indoleamine 2,3-dioxygenase 1 (IDO1) and induced by STAT-6 ALOX15 and CD206. Fold changes in gene expression are shown in Table S2. There was greater expression of IFN-induced genes including CXCL11 and SRRM2, and the NF-κB-induced gene IDO1 in early stage disease supraspinatus tendons compared to healthy subscapularis control tendons. Gene expression is normalized to β-actin; bar shows median value. (B, C) Representative immunofluorescence images of sections of pre-treatment early stage disease tendons stained for inflammation activation markers including those in the STAT-6 pathway (CD206, green), the glucocorticoid receptor pathway (CD163 red), the IFN pathway (IRF5, purple) and the NF-κB pathway (IDO-1, red). MerTK (purple) represents Mer tyrosine kinase, a tissue resident macrophage marker. Cyan represents POPO-1 nuclear counterstain. Scale bar = 20µm. (D) Quantitative analysis showing percentage of immunopositive staining for inflammation activation markers. Data are shown as mean and SEM.
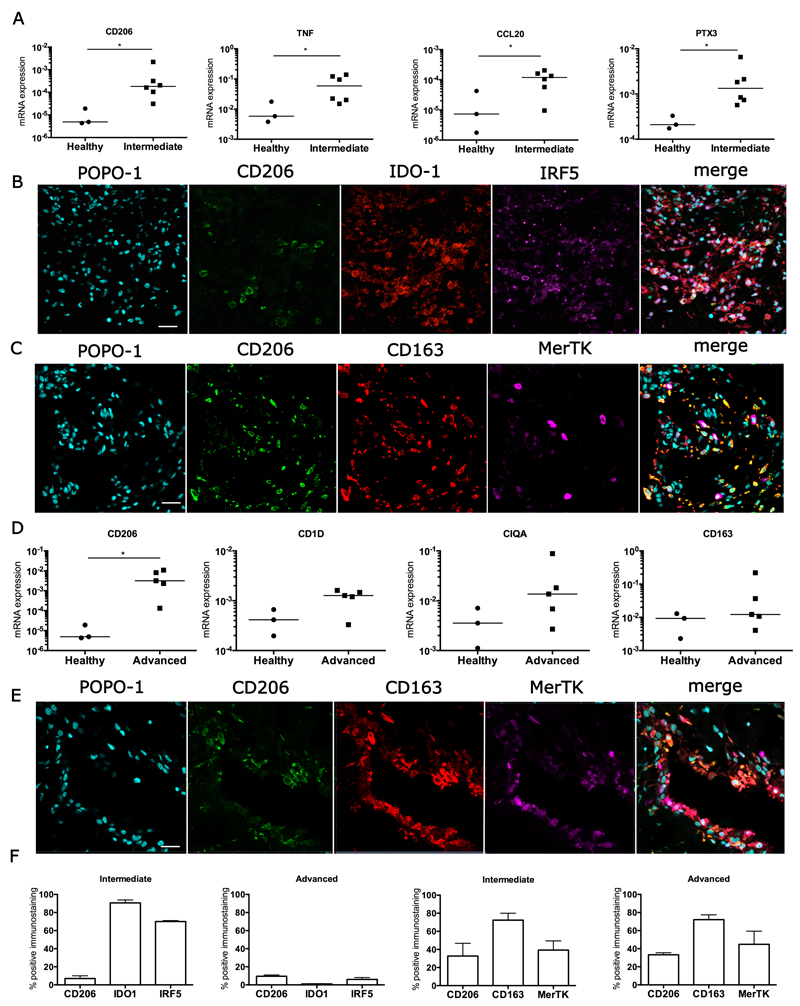
A mixed inflammation activation gene signature was also seen in intermediate stage samples of diseased supraspinatus tendons (Figure 3). These samples showed an NF-κB activation gene signature with increased expression of NF-κB pathway genes including Tumor Necrosis Factor (TNF) mRNA (p=0.047, mean 7.5-fold increase) and CCL20 mRNA (p=0.047, mean 6.5-fold increase) compared to healthy subscapularis tendons (Figure 3A). Genes expressed in the STAT-6 pathway (CD206, p=0.024, mean 53-fold increase) and glucocorticoid receptor activation pathway (pentraxin 3, p=0.024, mean 8.9-fold increase) were also increased in these samples compared to healthy tendons (Figure 3A). In support of this, a mixed protein signature was observed in intermediate stage tendon disease with increased expression of IRF5 (Interferon activation), IDO-1 (NF-κB activation), CD206 (STAT-6 activation) and CD163 (glucocorticoid receptor activation) (Figures 3B, C). Quantitative analysis of staining showed an NF-κB and IFN signature, with 90% and 70% of immunopositive cells expressing IDO-1 and IRF5 proteins, respectively (Figure 3F). In early and intermediate stages of disease, tendon stromal cells that expressed neither CD68 nor CD206 expressed IRF5 and IDO-1, suggesting that in addition to macrophages, a small proportion of non-macrophage cells such as tendon stromal cells also expressed these proteins (Figures 2B, 3B).
Figure 3.
Inflammation activation pathway signatures in intermediate and advanced stage disease tendons. (A) A mixed inflammation activation gene signature in intermediate stage diseased supraspinatus tendon samples (n=6) compared to healthy subscapularis tendons (n=3). (B, C) Representative immunofluorescence images of the inflammation activation protein signature in intermediate stage diseased tendons. (B) Markers indicate activation of the following pathways: STAT-6 (CD206, green), NF-κB (IDO-1, red), and IFN (IRF5, purple). (C) Markers indicate activation of the glucocorticoid receptor pathway (CD163, red); also shown is the tissue resident macrophage marker Mer tyrosine kinase (MerTK, purple). (D) A STAT-6/glucocorticoid receptor gene signature predominates in advanced stage diseased supraspinatus tendons (n=5) compared to healthy subscapularis tendons (n=3). Gene expression is normalized to β-actin. Statistically significant differences were calculated using pairwise Mann Whitney U tests. Bar represents median, *denotes p<0.05. (E) Representative immunofluorescence images of the inflammation activation protein signature in advanced stage diseased supraspinatus tendon. Shown is staining for the STAT-6 (CD206, green) and glucocorticoid receptor (CD163, red) activation pathways; tissue resident macrophages are stained with Mer tyrosine kinase (MerTK, purple). Cyan represents POPO-1 nuclear counterstain. Scale bar = 20µm. (F) Quantitative analysis showing the percentage of cells staining immunopositive for inflammation pathway proteins. Data are shown as mean and SEM.
A transition in inflammation activation signature was observed in advanced disease tendon samples (Figure 3). A STAT-6/glucocorticoid receptor activation gene signature predominated in these samples compared to healthy subscapularis tendons, with increased expression of CD206 mRNA (p=0.036, mean >500-fold increase), CD1D mRNA (mean 2.7-fold increase), complement component 1 mRNA (CIQA, mean 6.6-fold increase) and CD163 mRNA (mean 6.9-fold increase) (Figure 3D). In support of this, proteins in the STAT-6 pathway (CD206) and glucocorticoid receptor pathway (CD163) were highly expressed in advanced stage disease tendons (Figures 3E, F). The transition in inflammation signatures between intermediate and advanced disease tendons was further supported by immunostaining for phosphorylated NF-κB-p65 and NF-κB-p65. Tendon samples with intermediate stage disease showed increased expression of both phosphorylated and unphosphorylated forms of NF-κB-p65 compared to advanced disease tendon samples (Figure S1A). Dual labeling demonstrated that NFκB-p65 expression predominantly occurred on CD68negative cells (Figure S1B). Low-level expression of phosphorylated STAT-6 (pSTAT6) occurred in both intermediate and advanced stage disease tendons with pSTAT6 expressed by CD68positive macrophages (Figure S2).
Inflammation status of stromal cells derived from healthy and diseased tendon
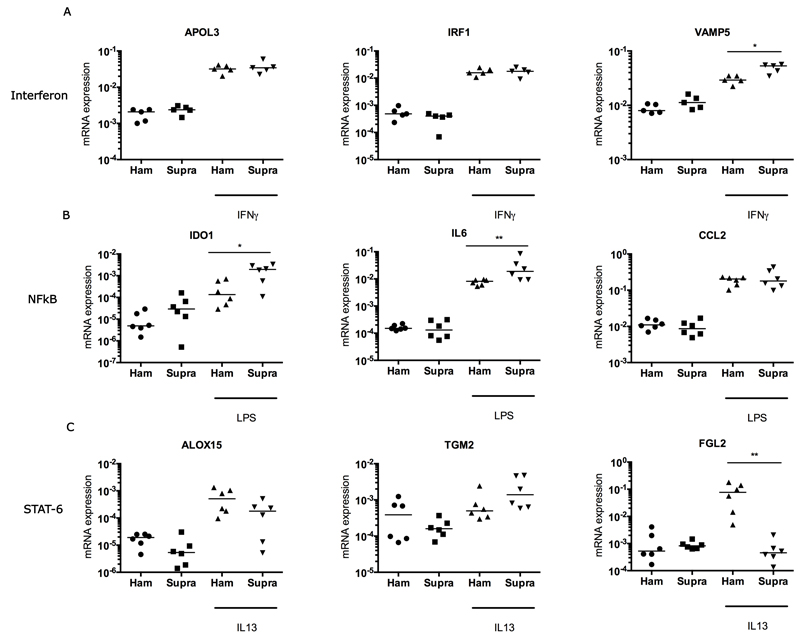
Inflammation activation pathways identified in diseased human tendons were further studied in healthy and diseased stromal cells derived from tendons in vitro to investigate if cytokines could induce a pro-inflammatory phenotype. Treatment of stromal cells derived from healthy human hamstring or torn supraspinatus tendons with IFNγ, IL13 (each 20ngml-1), or lipopolysaccharide (LPS) (100ngml-1) for 96 hours increased expression of inducible target genes compared to untreated controls. Both healthy and diseased tendon-derived stromal cells showed increased expression of IFN target genes including apolipoprotein L3 (APOL3), Interferon regulatory factor 1 (IRF1) and VAMP5 in IFNγ-treated compared to untreated control tendon-derived stromal cells (Figure 1A). IFNγ-treated diseased supraspinatus tendon cells showed increased expression of VAMP5 mRNA (p=0.03) compared to IFNγ-treated healthy hamstring cells (Figure 4A). Treatment of hamstring or supraspinatus tendon-derived stromal cells with LPS boosted expression of NF-κB target genes including IDO1, IL6 and CCL2 compared to untreated control tendon cells (Figure 4B). LPS-treated diseased supraspinatus tendon-derived stromal cells showed increased IDO1 (p=0.04) and IL6 (p=0.009) mRNA expression compared to LPS-treated healthy hamstring-derived stromal cells. IL13 treatment induced increased expression of STAT-6 pathway genes including ALOX15, Transglutaminase-2 (TGM2) and fibrinogen-like protein 2 (FGL2) compared to untreated control tendon-derived stromal cells (Figure 4C). Differences were observed between stromal cells derived from healthy hamstring tendon versus stromal cells derived from diseased supraspinatus tendon. Expression of a potential marker of resolution, ALOX15, was increased in healthy hamstring tendon-derived stromal cells compared to those from diseased supraspinatus tendon (mean 3-fold increase). There was increased expression of a fibrogenic marker TGM2 (mean 2-fold increase) and reduced expression of FGL2 (p=0.002, mean 7-fold decrease) in diseased supraspinatus tendon-derived stromal cells compared to healthy hamstring tendon-derived stromal cells (Figure 4C).
Figure 4.
Gene expression after treatment of cultured tendon-derived stromal cells with IFNγ, LPS or IL13 for 96 hours. Tendon stromal cells were derived from healthy hamstring or diseased supraspinatus tendons (n=6 donors for LPS and IL13 treatment, n=5 donors for IFNγ treatment). (A) Treatment of cells from healthy hamstring (Ham) or diseased supraspinatus tendons (Supra) with 20 ngmL-1 IFNγ. (B) Stimulation of cells from healthy hamstring (Ham) or diseased supraspinatus tendons (Supra) with 100 ngmL-1 LPS. (C) Stimulation of cells from healthy hamstring (Ham) or diseased supraspinatus tendons (Supra) with 20ngmL-1 IL13. Statistically significant differences were calculated using pairwise Mann Whitney U tests. Gene expression is normalized to β-actin; bar represents median. *p<0.05, **p<0.01.
IFNγ, IL13, or LPS treatment of co-cultures between tendon-derived stromal cells (from healthy hamstring or torn supraspinatus tendons) and macrophages for 96 hours also increased expression of respective inducible genes compared to untreated control co-cultures. IFNγ treatment boosted expression of IFN target genes APOL3, IRF1 and VAMP5 compared to untreated controls (Figure S3A). There was a trend for increased expression of VAMP5 mRNA in supraspinatus tendon cell/macrophage co-cultures. LPS treatment induced increased expression of NF-κB target genes CCL2, IL6 and IL8 compared to untreated controls in both hamstring and supraspinatus tendon-derived stromal cell/macrophage co-cultures (Figure S3B). IL13 treatment induced expression of STAT-6 pathway target genes compared to untreated controls, with a trend towards increased expression of Cytokine-inducible SH2-containing protein (CISH), IL17RB and TGM2 genes in healthy hamstring tendon-derived stromal cell/macrophage co-cultures compared to diseased supraspinatus tendon-derived stromal cells (Figure S3C).
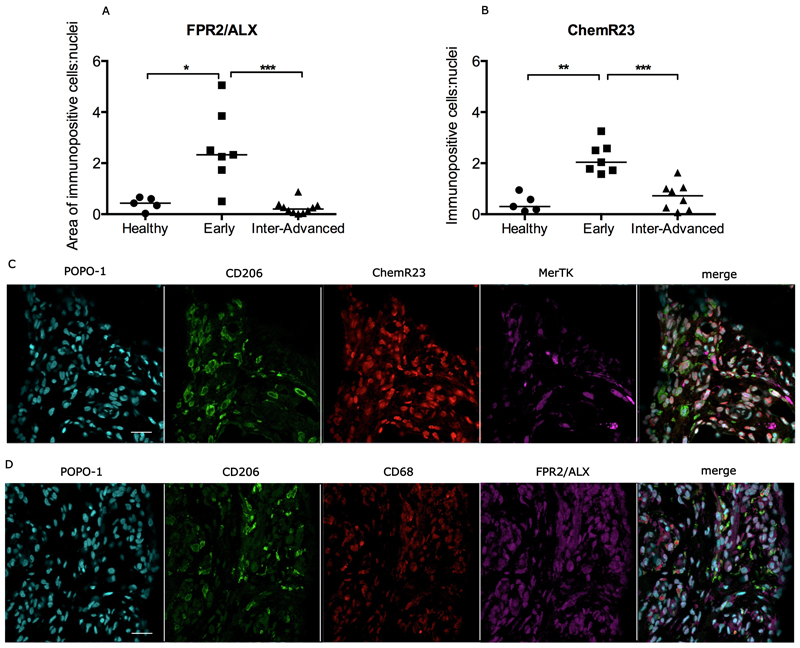
Increased expression of pro-resolving proteins in early stage disease tendons
In addition to characterizing the plasticity of inflammation activation signatures in the different stages of tendon disease, we also investigated if inflamed supraspinatus tendons were capable of mounting a resolution response. We investigated expression of the pro-resolving proteins Formyl peptide receptor 2 (FPR2/ALX) and Chemerin Receptor 23 (ChemR23) in sections of healthy tendon, and in tendons at the early and intermediate-advanced stages of disease. There was increased expression of FPR2/ALX in early disease tendons compared to healthy tendons (p=0.01) and intermediate-advanced disease tendons (p=0.0002) (Figure 5A). There was also increased expression of ChemR23 in early disease tendons compared to healthy tendons (p=0.0025) and intermediate-advanced disease tendons (p=0.0006) (Figure 5B). Immunofluorescence labeling showed low-level expression of FPR2/ALX and ChemR23 by CD68+ and CD206+ macrophages in early stage disease tendon (Figure 5C, D). Tendon-derived stromal cells that expressed neither CD68 nor CD206 also expressed the pro-resolving proteins FPR2/ALX and ChemR23.
Figure 5.
Expression of pro-resolving proteins in healthy and diseased supraspinatus tendons. Quantitative analyses show increased expression of FPR2/ALX (A) and ChemR23 (B) in early stage disease tendons compared to intermediate-advanced stage disease tendons or healthy tendons. Bar represents median. Statistically significant differences were calculated using Kruskal-Wallis with pairwise post-hoc Mann Whitney U tests. *p<0.05, **p<0.01, ***p<0.001. Representative confocal immunofluorescence images showing staining for macrophages (CD206+, CD68+, MerTK+) and the pro-resolving proteins ChemR23 (red, C) and FPR2/ALX (purple, D) in sections of early stage tendon disease. Cyan represents nuclear counterstain. Scale bar = 20µm.
ALOX15 and CD206 pathways are implicated in the resolution of pain after surgical treatment
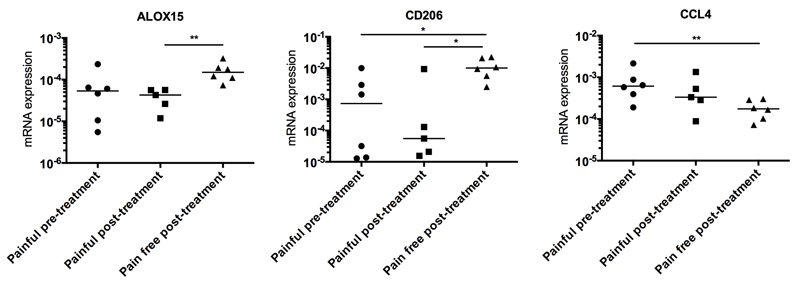
It is not known if inflammation activation pathways in diseased tendons are altered after surgical treatment. To investigate this, biopsies from patients with supraspinatus tendinopathy who remained in pain post-treatment (n=5) were compared with biopsies from patients who became pain-free post-treatment (n=6). The interval between surgical intervention and repeat biopsy was 2 to 4 years in both pain-free and painful post-treatment groups. The IFN activation gene signature seen in early stage tendon disease before treatment persisted in samples from tendinopathy patients from both groups after treatment, with expression of the IFN target genes APOL3, CXCL11, IRF1, VAMP5, WARS and SRRM2 remaining unchanged (Figure S4). However, tendon samples from pain-free post-treatment patients showed increased expression of ALOX15 mRNA (p=0.004, mean 4.3-fold increase) and CD206 mRNA (p=0.017, mean 6.3-fold increase) compared to tendon samples from patients who continued to experience pain post-treatment (Figure 6). CD206 mRNA expression was also increased in the pain-free post-treatment group compared to patients before treatment (p=0.026, mean 4.9-fold increase) (Figure 6). CCL4 mRNA expression was reduced in samples from pain-free post-treatment patients compared to the painful pre-treatment group (p=0.009, mean 4.3-fold decrease) (Figure 6).
Figure 6.
Expression of ALOX15, CD206 and CCL4 mRNA in biopsies from patients with early stage tendon disease before and after surgical sub-acromial decompression treatment. Pre-treatment tendinopathy samples from patients experiencing pain (n=6) were obtained after presentation to a referral clinic and prior to surgery. Biopsies were also collected from patients 2 to 4 years post-treatment. Post-treatment patients either had persistent pain (n=5) or were pain-free (n=6).Statistically significant differences were calculated using pairwise Mann Whitney U tests. Gene expression is normalized to β-actin; bar represents median. * p<0.05, **p<0.01.
15-epi Lipoxin A4 promotes resolution of inflammation in LPS-treated stromal cells derived from diseased tendon
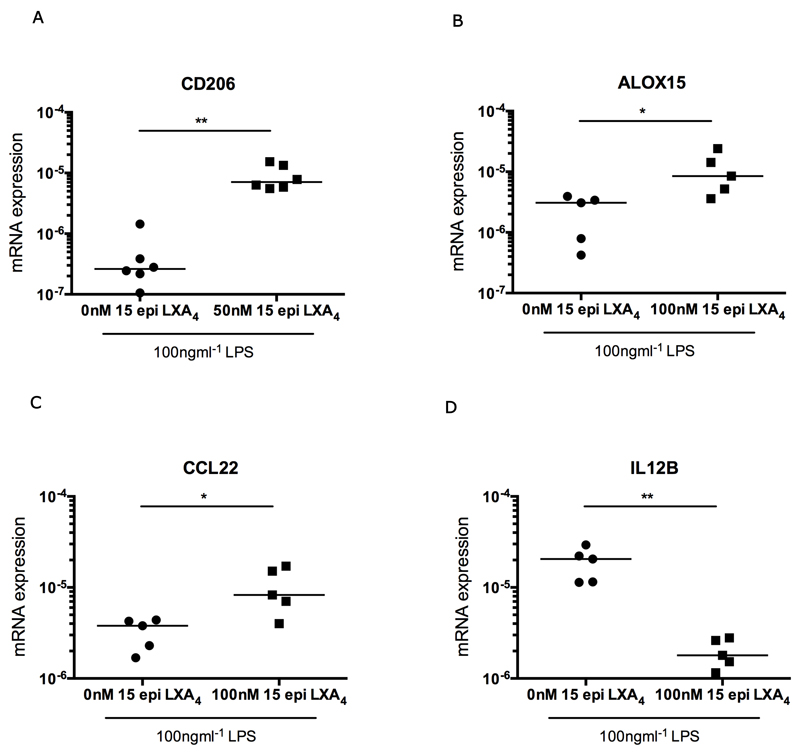
Given that we found increased CD206 and ALOX15 mRNA expression in tendon samples from patients whose clinical symptoms had resolved after treatment, we sought to therapeutically augment these pathways in stromal cells derived from diseased supraspinatus tendons in vitro. Low dose aspirin is known to promote the release of stable aspirin-triggered isoforms of Lipoxin A4, which potentiates resolution of inflammation (33, 43). In light of the importance of promoting timely resolution of tendon inflammation and the pro-inflammatory phenotype exhibited by cells from early and intermediate stage disease tendons, we investigated if a stable lipoxin metabolite derived from aspirin treatment (15-epi LXA4) could potentiate CD206 and ALOX15 mRNA expression in an in vitro model of tendon inflammation. Treatment of stromal cells derived from intermediate stage disease tendons (n=minimum of 5) with 100ngml-1 LPS in the presence of 50nM 15-epi LXA4 increased CD206 mRNA expression after 96 hours compared to tendon stromal cells treated with LPS alone (p=0.002, 20.2-fold increase) (Figure 7A). Treatment of diseased tendon cells with LPS in the presence of 100nM 15-epi LXA4 increased expression of both ALOX15 mRNA (p=0.016, 4.8-fold increase) and CCL22 mRNA (p=0.03, 3.1-fold increase) and reduced expression of IL12B mRNA (p=0.008, 9.6-fold decrease) after 96 hours compared to tendon cells stimulated with LPS alone (Figures 7B-D).
Figure 7.
The aspirin metabolite 15-epi LXA4 modulates inflammation in LPS-treated stromal cells derived from diseased tendons. Tendon cells were derived from intermediate stage disease supraspinatus tendons from a minimum of 5 patients. (A) Tendon-derived cells were treated with 100ngmL-1 LPS in medium containing 50nM 15-epi LXA4 for 96 hours. There was increased expression of CD206 mRNA after treatment with LPS and 15-epi LXA4 compared to LPS-alone (cells derived from tendons from 6 patients). (B,C,D) Tendon cells were treated with 100ngmL-1 LPS in medium containing 100nM 15-epi LXA4 for 96 hours. There was increased expression of ALOX15 mRNA (B) and CCL22 mRNA (C), and reduced expression of IL12B mRNA (D) compared to LPS-alone (cells derived from tendons from 5 patients). Statistically significant differences were calculated using pairwise Mann Whitney U tests. Gene expression is normalized to β-actin. Bar shows median values. * p<0.05, **p<0.01.
Discussion
Monocytes and macrophages exert dichotomous roles during the stages of inflammation, repair and remodeling of tissues (44–46). Diseased tendons from patients with tendinopathy showed an abundance of CD14+ and CD68+ myeloid cells, supporting the concept of an active axis of recruitment of CD14+ monocytes from blood and a process of maturation of these cells into CD68+ tissue resident macrophages. Whilst it is increasingly accepted that these inflammatory cell populations contribute to the initiation of tendon pathology, it is not known how inflammation activation pathways in tendon tissue change during the progression of pathology or after clinical treatment. This study provides new insights into the pathogenesis of diseases affecting musculoskeletal soft tissues. This longitudinal cohort study of phenotyped human tendon tissues collected before and after treatment revealed that gene and protein signatures of inflammation are complex and show a high degree of plasticity, with an activation pattern that parallels that of macrophages. Samples from patients with early stage tendon disease showed a mixed inflammation signature with increased expression of genes and proteins regulated by IFN and NF-κB activation pathways. Intermediate stage disease tendons showed a mixed inflammation signature with increased expression of NF-κB target genes and proteins; advanced stage disease tendons had an inflammation signature where expression of STAT-6 and glucocorticoid receptor pathway components predominated. This transition in inflammation activation signatures suggests that different therapeutic interventions may be applicable at different disease stages. Furthermore, the plasticity of inflammation signatures in diseased tendons has important implications for the surgical repair of tendon tears with biomaterials, as immune cell populations have been shown to influence biomaterial compatibility (47, 48).
Further investigation into the mechanisms by which inflammation becomes persistent during tendinopathy will be essential to drive development of new therapies to target chronic inflammatory diseases of musculoskeletal soft tissues. In the current study, inflammation activation pathways identified in samples of diseased tendons were further studied in vitro to investigate if activation of these inflammatory pathways induced tendon-derived stromal cells to become pro-inflammatory. Treatment of healthy and diseased tendon stromal cells with IFNγ, LPS or IL13 induced expression of respective target genes compared to untreated controls. This effect was observed in experiments where tendon cells were stimulated in isolation and in direct co-cultures with macrophages. Notably, LPS induced increases in IDO1 and IL6 mRNA expression and IFNγ induced increases in VAMP5 mRNA expression in diseased compared to healthy tendon-derived stromal cells. These findings suggest that inflammation alters the activation status of tendon-derived stromal cells. This concept is further supported by the expression of IFN and NF-κB target proteins such as IRF5, IDO1 and phosphorylated NF-κB-p65 by non-myeloid cells in sections of diseased human tendons. We suggest that tendon-derived stromal cells become altered by the inflammatory milieu and adopt a pro-inflammatory phenotype. It is possible that diseased tendon cells that have been exposed to an inflammatory milieu previously may be primed and hyper-responsive on subsequent exposure to pro-inflammatory mediators. In support of this, studies of cells in the tumor microenvironment (29, 30) and of fibroblasts derived from rheumatoid arthritis tissue (27, 28) indicate that resident stromal cell populations contributes to the development of chronic inflammation.
Studies of rheumatoid arthritis and the spondyloarthropathies have revealed interactions between myeloid and stromal cell populations during inflammation, identifying potential disease-stage specific therapeutic targets. To identify factors implicated in the resolution of tendon pain after surgical subacromial decompression treatment, we compared inflammation signatures in tendon samples collected from patients whose symptoms resolved after treatment and compared them with samples from patients who remained symptomatic. Key differences in expression of NF-κB and STAT-6 activation pathways were identified in patients with tendon pathology before and after treatment. Expression of CCL4 mRNA was increased in tendons from pre-treatment patients experiencing pain compared to post-treatment patients who were pain-free. In support of this, increased expression of CCL4 mRNA in injured nerves has been shown to correlate with neuropathic pain in a mouse model of sciatic nerve injury (49). We identified activation of inflammation-resolving pathways in tendon samples from patients two to four years after surgery who were pain-free. These tendon samples showed increased expression of CD206 and ALOX15 mRNA compared to tendons from patients who remained symptomatic after treatment. Macrophages expressing CD206 are frequently associated with the repair and remodeling of healing tissues (50) and have also been identified in healing equine digital flexor tendons (18). ALOX15 is a critical enzyme in the biosynthesis of lipid mediators such as the lipoxins (LXA4 and LXB4), promoting resolution and restoration of homeostasis in inflamed tissues (51, 52). These findings implicate CD206 and ALOX15 pathways in the resolution of inflammatory-associated pain in tendon samples from patients whose clinical symptoms resolved after treatment.
In the current study, expression of pro-resolving proteins FPR2/ALX and ChemR23 was increased in early stage tendon pathology, suggesting tendons mount a counter-resolution response to inflammation. However, this activation of pro-resolving pathways was not sustained in intermediate to advanced disease stage tendons. Samples from patients with advanced stage tendinopathy showed increased numbers of CD14+ and CD68+ macrophages with diminished expression of the pro-resolving proteins FPR2/ALX and ChemR23 and marked disorganization of the extracellular matrix, implying failure of the tissue to return to homeostasis. In support of the findings from the current study, FPR2/ALX has also been shown to increase in the early stages of equine digital flexor tendon healing (18, 53). Experimental murine models of systemic inflammation have illustrated that treatment with COX-2 selective NSAIDs diminishes endogenous resolution responses (37, 54). COX-2 selective NSAIDs are frequently used in the management of musculoskeletal pain and in light of this, may also impede the healing response of inflamed soft tissues such as tendons. Instead of total inflammatory blockade, an alternative approach to moderating inflammation whilst simultaneously potentiating resolution may have therapeutic benefit for patients with tendon pathology. Low dose aspirin is known to promote the release of stable aspirin-triggered isoforms of Lipoxin A4, which potentiate the resolution of inflammation (33, 43). Stable lipid mediators of aspirin-induced eicosanoid metabolism such as 15-epi LXA4 have proven efficacious in other chronic inflammatory diseases including in murine models of pulmonary inflammation (55) and in the treatment of human infantile eczema (56). In the current study, incubation of LPS-treated diseased tendon-derived stromal cells in 50nM or 100nM 15-epi LXA4 increased expression of CD206, ALOX15 and CCL22 mRNA and reduced expression of IL12B mRNA compared to LPS-stimulated tendon cells not exposed to 15-epi LXA4. It is possible that low dose aspirin and its metabolites may act by triggering the expression of anti-inflammatory and pro-resolving mediators (43, 57). These stable aspirin-induced lipid mediators may hold promise for treating patients with tendinopathy by potentiating resolution of tendon inflammation. Limitations of our study include the relatively small number of patients studied and the single post-treatment tendon tissue biopsy. It was not ethically possible to obtain multiple sequential biopsies. Future studies using animal models of tendinopathy and randomized controlled human clinical trials with a placebo arm will be necessary to further investigate the therapeutic potential of inflammation resolving mediators in vivo.
In summary, we found that inflammation activation signatures displayed a high degree of plasticity throughout the stages of tendon disease. We have implicated the ALOX15 and CD206 pathways in the resolution of tendon pain in patients whose symptoms resolved after treatment. We suggest that the aspirin metabolite 15-epi LXA4 may contribute to resolving tendon inflammation.
Materials and Methods
Study Design
The objective of this study was to investigate inflammation activation pathways in samples of early, intermediate and advanced stage disease tendons from patients with tendinopathy. Tissue samples were obtained using high definition ultrasound guidance from well-phenotyped patient cohorts before and after treatment. We compared inflammation signatures in patients whose clinical symptoms resolved after surgical sub-acromial decompression treatment with those that remained persistently painful. Inflammation activation pathways were further studied in stromal cells derived from healthy and diseased human tendons in vitro. Statistical analysis and sample size justification were derived from previous studies (18, 53) that were sufficiently powered to gain insights from studying inflammation in samples of native tendon disease and using an in vitro model of inflammation. For histological studies, a single blinded investigator undertook image capturing. Further details of sample size / replicates are given in the figure legends.
Study Approval
Ethical approval for this study was granted by the local research ethics committee, Oxfordshire REC B refs: 10/H0402/24, 09/H0605/111, 10/H0606/60 and South Central Oxford B ref: 14/SC/0222. Full informed consent according to the Declaration of Helsinki was obtained from all patients.
Tissue collection from patients with shoulder tendinopathy
All patients were recruited from orthopedic referral clinics where the structural integrity of the rotator cuff was determined ultrasonographically. Patients completed the Oxford Shoulder Score (OSS), a validated and widely used clinical outcome measure scoring from 0 (severe pathology) to 48 (normal function) (58). Samples of healthy and early stage (tendinopathic) supraspinatus were collected via percutaneous ultrasound guided biopsy under local anaesthesia by a senior consultant shoulder surgeon. The biopsy specimen was taken using a trucut needle approximately 5-10mm posterior to the anterior edge of the supraspinatus tendon. This validated biopsy technique is described in detail elsewhere (59). Biopsies of healthy supraspinatus tendons (n=9) were collected from male or female patients (aged 20-30, mean age 23 ± 3.8 years) who underwent shoulder surgery for post-traumatic instability. These patients had intact non-degenerative supraspinatus tendons on ultrasound, which was confirmed at surgery. Healthy subscapularis tendons were also collected from 3 male or female patients undergoing shoulder surgery for post-traumatic instability (aged 61-77, mean 66 ± 8 years).
Biopsies of early stage pre-treatment tendinopathic supraspinatus were taken from 21 male and female patients undergoing subacromial decompression surgery (painful pre-treatment group). Biopsies were also taken from patients between 2 to 4 years after undergoing subacromial decompression surgery, in whom pain had resolved completely (pain free post-treatment, n=6) or pain persisted (painful post-treatment, n=5). Patients in the pain free group had significant pain before their surgery was performed as evidenced by a median Oxford Shoulder Score of 24 (range 20-40) before surgery and the biopsy was taken away from the surgical margins. All early stage tendinopathic patients were aged between 38 and 65 (mean 52 ± 8 years).
Intermediate-advanced stage disease tendons (supraspinatus tears) were collected at the time of surgical debridement of the edges of the torn tendons from 11 male and female patients aged between 50 and 78 (mean 61 ± 6.5 years). All patients were symptomatic and had full thickness tendon tears. Tear sizes were classified as follows: small (≤1cm), medium (>1 and ≤3cm), large (>3 and ≤ 5cm) and massive (>5cm in anterior-posterior length) (60). Increasing tear size has been shown to correlate with the progression of pathology with reduced likelihood of repair with chronic disease (61). Torn tendons were collected under research ethics from the Oxford Musculoskeletal Biobank (09/H0606/11) or from patients participating in the United Kingdom Rotator Cuff Trial (UKUFF), a multicenter randomized controlled trial investigating the efficacy of open versus arthroscopic surgical repair for supraspinatus tendon tears (REC reference 10/H0402/24). Exclusion criteria for all patients in this study included previous shoulder surgery, other shoulder pathology, rheumatoid arthritis and systemic inflammatory disease (7).
Tissue collection from patients with healthy hamstring tendons
Healthy hamstring tendons were collected from 10 male and female patients undergoing surgical reconstruction of their anterior cruciate ligament. All patients were aged between 18 and 48 (mean 25.5 ± 11 years). Tissues were collected under research ethics from the Oxford Musculoskeletal Biobank (09/H0606/11). Hamstring tendons were immediately placed in DMEM F12 medium (Lonza) and processed in tissue culture to isolate tendon-derived stromal cells.
Processing of human shoulder tendons
Immunohistochemistry and Immunofluorescence
Samples of healthy and diseased supraspinatus tendons were immersed in 10% buffered formalin and left for approximately 0.5mm/hr. After fixation, tendons were processed using a Leica ASP300S tissue processor and embedded in paraffin wax. Tissues were sectioned to 4µm using a rotary RM2135 microtome (Leica Microsystems Ltd) onto adhesive glass slides and baked at 60 °C for 30 minutes and 37 °C for 60 minutes.
Gene expression
Samples of healthy subscapularis and diseased supraspinatus tendons were immediately snap frozen in liquid nitrogen and stored at -80 °C until RNA extraction.
Assessment of diseased supraspinatus tendons
Histological assessment of tendons collected from the study cohort was performed on Haematoxylin and Eosin stained sections using the Bonar scoring system (0-12) that evaluates tissue structure (62). Median and interquartile ranges of healthy supraspinatus tendons exhibited more normal tissue architecture (2, range 1-2) compared to early (7, range 6-8) and intermediate to advanced disease stages (10, range 8.25-10).
Immunohistochemistry
For antigen retrieval, slides were baked at 60°C for 60 minutes and tissue sections were taken through deparaffinisation and target retrieval steps (high pH heat mediated antigen retrieval) using an automated PT Link (Dako). Antibody staining was performed using the EnVision FLEX visualization system with an Autostainer Link 48 (Dako). Antibody binding was visualized using FLEX 3,3’-Diaminobenzidine (DAB) substrate working solution and haematoxylin counterstain (Dako) as per protocols provided by the manufacturer. Details of antibodies and their working dilutions are shown in Table S3. Positive controls consisted of sections of normal human spleen. For negative controls the primary antibody was substituted for universal isotype control antibodies: cocktail of mouse IgG1, IgG2a, IgG2b, IgG3 and IgM (Dako) and rabbit immunoglobulin fraction of serum from non-immunised rabbits, solid phase absorbed (Dako). Isotype control images are shown in Figures S5A & B. After staining, slides were taken through graded industrial methylated spirit and xylene and mounted in Pertex mounting medium (Histolab).
Image acquisition and quantitative analyses for immunohistochemistry
Images were acquired on an inverted microscope using Axiovision software (Zeiss). Twenty images (or until the tissue section was exhausted) were acquired in a systematic manner at ×100 magnification with oil immersion by a single blinded investigator. Image analysis was conducted using ImageJ (NIH) as previously described (63). For each sample, immunopositive staining was normalized to the number of haematoxylin-counterstained nuclei within the field of view.
Immunofluorescence for co-localization of macrophage markers
After antigen retrieval steps, tissues were blocked in 5% normal goat serum (Sigma) in PBS for 60 minutes in a humid chamber at room temperature. After removal of blocking solution, sections were incubated with the primary antibody cocktail diluted in 5% normal goat serum in PBS for 2 hours at room temperature. Details of primary antibodies used for immunofluorescence are listed in Table S3. After incubation with the primary antibody, sections were washed 3 times with PBST for 5 minutes. Slides were incubated in the secondary antibody cocktail each diluted 1:200 in 5% normal equine serum (Sigma) in PBS for 2 hours and shielded from light. Secondary antibodies were purchased from Alexa Fluor (goat anti-mouse IgG2a, IgG2b or goat anti-rabbit IgG, Life Technologies) and goat anti-mouse IgG1 (Southern Biotech). After washing with PBST, sections were incubated in 2µM POPO-1 nuclear counterstain (Life Technologies) diluted in PBS containing 0.05% Saponin (Sigma) for 20 minutes. After washing with PBST, tissue auto fluorescence was quenched with a solution of 0.1% Sudan Black B (Applichem) in 70% ethanol for 10 minutes (18). After washing, slides were mounted using fluorescent mounting medium (VectaShield), sealed and stored at 4°C until image acquisition. For negative controls the primary antibody was substituted for universal isotype control antibodies: cocktail of mouse IgG1, IgG2a, IgG2b, IgG3 and IgM (Dako) and rabbit immunoglobulin fraction of serum from non-immunised rabbits, solid phase absorbed (Dako). Isotype control images are shown in Figures S5C & D.
Immunofluorescence image acquisition
Immunofluorescence images were acquired on a Zeiss LSM 710 confocal microscope using a ×40 oil immersion objective (NA=0.95). The fluorophores of POPO-1, Alexa Fluor 488, Alexa Fluor 568, and Alexa Fluor 633 were excited using the 405nm, 488nm, 561nm, and 633nm laser lines respectively. To minimize bleed-through, all channels were acquired sequentially. Averaging was set to 2 and the pinhole was set to approximately 1 Airy unit. Two-dimensional image reconstructions were created using ZEN 2009 (Zeiss).
Image analysis
Grayscale tiff files from confocal images were imported and analysed in a custom Matlab (Mathworks) script. Single cells were identified based on the POPO-1 nuclear staining and converted to regions of interests. These were then dilated to encompass the cell to give new regions of interests. From the enlarged region, the average intensity of any surface marker staining in the green, red, and purple channels were measured. The average intensity of the antibody stain was then measured for each cell, with cells expressing low CD68 and/or CD206 staining being gated. The intensities of proteins of interest for these gated cells were then plotted. Isotype controls were used to set a threshold to distinguish between the negatively and positively stained cells. Co-localized cells were identified as having positive staining for two or more markers. To calculate the percentage of immunopositive cells for each surface marker, the number of cells exhibiting average intensities above the threshold were divided by the total number of POPO-1 nuclei.
Isolation of tendon-derived stromal cells from healthy hamstring and diseased supraspinatus tendons
Fresh samples of healthy hamstring or torn (intermediate-advanced stage) supraspinatus tendons were cut into 2mm3 explants and incubated in DMEM F12 media (Lonza) containing 50% fetal calf serum (FCS, Labtech) and 1% Penicillin Streptomycin (Pen-Strep, Lonza). Fresh media were replaced every 4 days and cells allowed to grow out from explants over time in a tissue culture incubator at 37°C and 5% CO2. Once cells were 90% confluent, explants were removed and media replaced with DMEM F12 containing 10% FCS and 1% Pen-Strep. Cells between passages 1-3 were used for all experiments.
Treatment of tendon-derived stromal cell-macrophage co-cultures with cytokines
Tendon-derived stromal cells from healthy hamstring and diseased supraspinatus were seeded at a density of 15,000 cells per well in a 24 well plate. Tendon cells were allowed to reach 70% confluence prior to stimulation with cytokines or addition of monocytes. Human monocytes (98% CD14+, 13% CD16+) were obtained from healthy donor buffy coats by 2-step gradient centrifugation as described in detail elsewhere (64). For co-culture experiments, 150,000 monocytes per well were added and allowed to differentiate into macrophages for 2 days prior to cytokine stimulation. Tendon-derived stromal cells in isolation and tendon-derived stromal cell-macrophage co-cultures were incubated in X-Vivo10 media (Lonza) containing 1% heat inactivated human serum (Sigma). Cells were treated as previously described (23) with either LPS (100ngmL-1, E. coli 055:B5. L2880, Sigma), IFNγ (20ngmL-1, R&D Systems), or IL13 (20ngmL-1, BioLegend), non-treated (vehicle only) cells served as controls for each experiment. After treatment, cells were then incubated at 37°C and 5% CO2 until harvest of the cell lysate for mRNA after 96 hours.
Modulating inflammation with 15-epi Lipoxin A4 in LPS-stimulated tendon-derived stromal cells
Tendon stromal cells were derived from intermediate disease stage supraspinatus tendons from a minimum of 5 patients. Cells were seeded at a density of 15,000 cells per well. Once cells were at 70% confluence, they were pre-incubated in 50nM or 100nM 15-epi Lipoxin A4 (Cayman Chemical) for 24 hours in X-Vivo10 media (Lonza) containing 1% heat inactivated human serum (Sigma). Cells were subsequently treated with 100ngmL-1 LPS as described above in the presence of media containing either 50nM or 100nM 15-epi Lipoxin A4 or vehicle only control media. After LPS treatment, cells were shielded from light and incubated at 37°C and 5% CO2 until harvest of the cell lysate for mRNA after 96 hours.
Extraction of RNA from human tendons
Tissues
Tendon samples (30-100mg) were homogenized in 1mL of RNABee (Ams Biotechnology) using an IKA Ultra Turrax T8 homogenizer (Fischer Scientific). Samples included healthy subscapularis (n=3), early disease stage tendinopathic supraspinatus (painful pre-treatment n=6, painful post-treatment n=5, pain free post-treatment, n=6) and intermediate-advanced disease stage (torn) supraspinatus tendons (small-medium tears, n=6, large-massive tears, n=5). RNA extraction was performed according to the manufacturer’s protocol. RNA clean up was subsequently performed using an RNeasy Mini Kit (74106, Qiagen) with an on column DNA treatment using DNase I (EN0521, Thermo Scientific).
Cells
Cells were lysed in RLT lysis buffer and RNA was isolated using an RNeasy Mini Kit (74106, Qiagen) with an on column DNA treatment using DNase I (EN0521, Thermo Scientific). Quality of extracted and cleaned RNA from tissues and cells was determined using a NanoDrop 1000 spectrophotometer (Thermo Scientific). The ratio of absorbance of 260:280nm was used to determine RNA quality, with all samples achieving a minimum of 1.80.
cDNA synthesis and quantitative PCR
RNA (250ng) was reverse transcribed into cDNA using High Capacity Reverse Transcription Kit (4368813, Applied Biosystems). cDNA was diluted to 2.5ng/µL with RNase free water and 1µL was used in a 10µL qPCR reaction with Fast SYBR Green Master Mix (4385612, Applied Biosystems) and primers diluted to 200nM (Eurofins Genomics) in 384 wells plates (4309849, Life Technologies). Gene signatures consisted of a panel of genes for Interferon, NF-κB, STAT-6 and glucocorticoid receptor activation pathways. Primers for each gene are shown in Table S1. The reaction efficiency was calculated by measuring the Ct values for both sets of genes in a cDNA mix dilution series and applying formula 1: Efficiency = 10(−1/slope) − 1 as previously described (64). Duplicate reactions for each gene were run on a ViiA7 qPCR machine (Applied Biosystems) and the results were calculated using the ddCt method using 3 reference genes for human βactin, GAPDH and beta 2 macroglobulin. Results were consistent using these 3 reference genes and data are shown normalized to βactin.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software). Normality was tested using the Shapiro-Wilk normality test. Kruskal-Wallis followed by pairwise post-hoc Mann Whitney U tests were used to compare expression of markers of monocytes, macrophages and pro-resolving proteins in sections of healthy and diseased human supraspinatus tendons. Pairwise Mann Whitney U tests were used to test for differences in gene expression in tendon samples from pain-free patients and patients experiencing pain before and after treatment. Pairwise Mann Whitney U tests were used to test for differences between expression of NF-κB, IFN and STAT-6 target genes in cytokine treated stromal cells derived from healthy and diseased tendons. Pairwise Mann Whitney U tests were used to test for differences in gene expression between LPS-stimulated tendon-derived stromal cells treated with 15-epi LXA4 compared to LPS-stimulated cells not exposed to 15-epi LXA4. p<0.05 was considered statistically significant.
Supplementary Material
Single sentence summary.
Resolution of inflammatory pathway gene expression signatures in diseased tendon correlates with reduced tendon pain.
Editor’s Summary.
Inflammation activation and resolution pathways remain poorly defined in tendon disease. In new work, Dakin et al. investigate shoulder tendons from patients before and after surgery. Diseased tendons showed different inflammation gene and protein signatures in early stage disease compared to advanced stage disease. The researchers identified pathways implicated in the resolution of tendon pain after treatment. Investigation of inflammation activation pathways in cultured stromal cells derived from human tendons revealed that stromal cells from diseased tendons may be primed for inflammation. The authors also identified stable isoforms of aspirin that may be therapeutically beneficial for the resolution of tendon inflammation.
Acknowledgements
We thank Simon Gordon and Jonathan Sherlock for their review of the manuscript.
Funding: SGD is funded by Arthritis Research UK grant 20506. Research in our laboratories is supported through funding from Arthritis Research UK (program grant number 20522), the NIHR Oxford Biomedical Research Unit and the Rosetrees Trust. The Structural Genomics Consortium is a registered charity (number 1097737) that receives funds from Abbvie, Bayer Healthcare, Boehringer Ingelheim, the Canadian Institutes for Health Research, the Canadian Foundation for Innovation, Eli Lilly and Company, Genome Canada, GlaxoSmithKline, the Ontario Ministry of Economic Development and Innovation, Janssen, the Novartis Research Foundation, Pfizer, Takeda, and the Wellcome Trust.
Footnotes
Author contributions: SGD performed all experiments and wrote the manuscript with input from all co-authors. SGD, AJC and FOM designed the study. FOM, UO and GW provided qPCR reagents and FOM and UO performed array analysis. FOM and GW provided human macrophages for co-culture experiments. CY facilitated confocal image acquisition. BD, KW, BW, LR and AJC facilitated procurement and collection of healthy and diseased shoulder tendons from patients.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: Primer sequences and validation data are shown in Table S1.
References
- 1.Hinsley H, Nicholls A, Daines M, Wallace G, Arden N, Carr A. Classification of rotator cuff tendinopathy using high definition ultrasound. Muscles ligaments and tendons journal. 2014;4:391–397. [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy RJ, Carr AJ. Shoulder pain. BMJ clinical evidence. 2010;2010 [PMC free article] [PubMed] [Google Scholar]
- 3.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- 4.Dakin SG, Smith RK, Heinegard D, Onnerfjord P, Khabut A, Dudhia J. Proteomic analysis of tendon extracellular matrix reveals disease stage-specific fragmentation and differential cleavage of COMP (cartilage oligomeric matrix protein) J Biol Chem. 2014;289:4919–4927. doi: 10.1074/jbc.M113.511972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Judge A, Murphy RJ, Maxwell R, Arden NK, Carr AJ. Temporal trends and geographical variation in the use of subacromial decompression and rotator cuff repair of the shoulder in England. The bone & joint journal. 2014;96-B:70–74. doi: 10.1302/0301-620X.96B1.32556. [DOI] [PubMed] [Google Scholar]
- 6.Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15:290–299. doi: 10.1016/j.jse.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Carr AJ, Rees JL, Ramsay CR, Fitzpatrick R, Gray A, Moser J, Dawson J, Bruhn H, Cooper CD, Beard DJ, Campbell MK. Protocol for the United Kingdom Rotator Cuff Study (UKUFF): a randomised controlled trial of open and arthroscopic rotator cuff repair. Bone & joint research. 2014;3:155–160. doi: 10.1302/2046-3758.35.2000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gwilym SE, Watkins B, Cooper CD, Harvie P, Auplish S, Pollard TC, Rees JL, Carr AJ. Genetic influences in the progression of tears of the rotator cuff. J Bone Joint Surg Br. 2009;91:915–917. doi: 10.1302/0301-620X.91B7.22353. [DOI] [PubMed] [Google Scholar]
- 9.Almekinders LC, Temple JD. Etiology, diagnosis, and treatment of tendonitis: an analysis of the literature. Med Sci Sports Exerc. 1998;30:1183–1190. doi: 10.1097/00005768-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Dudhia J, Scott CM, Draper ER, Heinegard D, Pitsillides AA, Smith RK. Aging enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity. Aging Cell. 2007;6:547–556. doi: 10.1111/j.1474-9726.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhury S, Gwilym SE, Moser J, Carr AJ. Surgical options for patients with shoulder pain. Nature reviews Rheumatology. 2010;6:217–226. doi: 10.1038/nrrheum.2010.25. [DOI] [PubMed] [Google Scholar]
- 12.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 13.Wright C, Edelmann M, diGleria K, Kollnberger S, Kramer H, McGowan S, McHugh K, Taylor S, Kessler B, Bowness P. Ankylosing spondylitis monocytes show upregulation of proteins involved in inflammation and the ubiquitin proteasome pathway. Ann Rheum Dis. 2009;68:1626–1632. doi: 10.1136/ard.2008.097204. [DOI] [PubMed] [Google Scholar]
- 14.Bowness P, Ridley A, Shaw J, Chan AT, Wong-Baeza I, Fleming M, Cummings F, McMichael A, Kollnberger S. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011;186:2672–2680. doi: 10.4049/jimmunol.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, Gorman DM, Bowman EP, McClanahan TK, Yearley JH, Eberl G, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8-entheseal resident T cells. Nat Med. 2012;18:1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 16.Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GA. Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Joint Surg Br. 2009;91:417–424. doi: 10.1302/0301-620X.91B3.21652. [DOI] [PubMed] [Google Scholar]
- 17.Millar NL, Hueber AJ, Reilly JH, Xu Y, Fazzi UG, Murrell GA, McInnes IB. Inflammation is present in early human tendinopathy. Am J Sports Med. 2010;38:2085–2091. doi: 10.1177/0363546510372613. [DOI] [PubMed] [Google Scholar]
- 18.Dakin SG, Werling D, Hibbert A, Abayasekara DR, Young NJ, Smith RK, Dudhia J. Macrophage sub-populations and the lipoxin A4 receptor implicate active inflammation during equine tendon repair. PLoS One. 2012;7:e32333. doi: 10.1371/journal.pone.0032333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rees JD, Stride M, Scott A. Tendons--time to revisit inflammation. Br J Sports Med. 2014;48:1553–1557. doi: 10.1136/bjsports-2012-091957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kragsnaes MS, Fredberg U, Stribolt K, Kjaer SG, Bendix K, Ellingsen T. Stereological quantification of immune-competent cells in baseline biopsy specimens from achilles tendons: results from patients with chronic tendinopathy followed for more than 4 years. Am J Sports Med. 2014;42:2435–2445. doi: 10.1177/0363546514542329. [DOI] [PubMed] [Google Scholar]
- 21.Jelinsky SA, Rodeo SA, Li J, Gulotta LV, Archambault JM, Seeherman HJ. Regulation of gene expression in human tendinopathy. BMC Musculoskelet Disord. 2011;12:86. doi: 10.1186/1471-2474-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsolais D, Cote CH, Frenette J. Neutrophils and macrophages accumulate sequentially following Achilles tendon injury. J Orthop Res. 2001;19:1203–1209. doi: 10.1016/S0736-0266(01)00031-6. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 24.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 25.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douglas MR, Morrison KE, Salmon M, Buckley CD. Why does inflammation persist: a dominant role for the stromal microenvironment? Expert Rev Mol Med. 2002;4:1–18. doi: 10.1017/S1462399402005264. [DOI] [PubMed] [Google Scholar]
- 28.Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 29.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. American journal of cancer research. 2011;1:482–497. [PMC free article] [PubMed] [Google Scholar]
- 30.Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Frontiers in oncology. 2014;4:62. doi: 10.3389/fonc.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 32.Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A. 1984;81:5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serhan CN, Levy BD, Clish CB, Gronert K, Chiang N. Lipoxins, aspirin-triggered 15-epi-lipoxin stable analogs and their receptors in anti-inflammation: a window for therapeutic opportunity. Ernst Schering Res Found Workshop. 2000:143–185. doi: 10.1007/978-3-662-04047-8_8. [DOI] [PubMed] [Google Scholar]
- 34.Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 38.Lawrence T, Gilroy DW. Chronic inflammation: a failure of resolution? Int J Exp Pathol. 2007;88:85–94. doi: 10.1111/j.1365-2613.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–4127. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J. 2004;18:489–498. doi: 10.1096/fj.03-0837com. [DOI] [PubMed] [Google Scholar]
- 41.Rajakariar R, Lawrence T, Bystrom J, Hilliard M, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW. Novel biphasic role for lymphocytes revealed during resolving inflammation. Blood. 2008;111:4184–4192. doi: 10.1182/blood-2007-08-108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stables MJ, Shah S, Camon EB, Lovering RC, Newson J, Bystrom J, Farrow S, Gilroy DW. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118:e192–208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, Newson J, Bellingan G, Gilroy DW. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 44.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 45.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming S. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 46.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem cells. 2013;31:384–396. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- 47.Oliva N, Carcole M, Beckerman M, Seliktar S, Hayward A, Stanley J, Parry NM, Edelman ER, Artzi N. Regulation of dendrimer/dextran material performance by altered tissue microenvironment in inflammation and neoplasia. Science translational medicine. 2015;7:272ra211. doi: 10.1126/scitranslmed.aaa1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratner BD. Healing with medical implants: The body battles back. Science translational medicine. 2015;7:272fs274. doi: 10.1126/scitranslmed.aaa5371. [DOI] [PubMed] [Google Scholar]
- 49.Saika F, Kiguchi N, Kobayashi Y, Fukazawa Y, Kishioka S. CC-chemokine ligand 4/macrophage inflammatory protein-1beta participates in the induction of neuropathic pain after peripheral nerve injury. European journal of pain. 2012;16:1271–1280. doi: 10.1002/j.1532-2149.2012.00146.x. [DOI] [PubMed] [Google Scholar]
- 50.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serhan CN, Chiang N. Lipid-derived mediators in endogenous anti-inflammation and resolution: lipoxins and aspirin-triggered 15-epi-lipoxins. ScientificWorldJournal. 2002;2:169–204. doi: 10.1100/tsw.2002.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dakin SG, Dudhia J, Werling NJ, Werling D, Abayasekara DR, Smith RK. Inflamm-aging and arachadonic Acid metabolite differences with stage of tendon disease. PLoS One. 2012;7:e48978. doi: 10.1371/journal.pone.0048978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 55.Levy BD, Lukacs NW, Berlin AA, Schmidt B, Guilford WJ, Serhan CN, Parkinson JF. Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB J. 2007;21:3877–3884. doi: 10.1096/fj.07-8653com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu SH, Chen XQ, Liu B, Wu HJ, Dong L. Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. Br J Dermatol. 2013;168:172–178. doi: 10.1111/j.1365-2133.2012.11177.x. [DOI] [PubMed] [Google Scholar]
- 57.Gilroy DW. The role of aspirin-triggered lipoxins in the mechanism of action of aspirin. Prostaglandins Leukot Essent Fatty Acids. 2005;73:203–210. doi: 10.1016/j.plefa.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Dawson J, Fitzpatrick R, Carr A. Questionnaire on the perceptions of patients about shoulder surgery. J Bone Joint Surg Br. 1996;78:593–600. [PubMed] [Google Scholar]
- 59.Murphy RJ, Floyd Dean BJ, Wheway K, Watkins B, Morrey ME, Carr AJ. A Novel Minimally Invasive Ultrasound-Guided Technique to Biopsy Supraspinatus Tendon. Oper Tech Orthop. 2013;23:56–62. [Google Scholar]
- 60.Post M, Silver R, Singh M. Rotator cuff tear. Diagnosis and treatment. Clin Orthop Relat Res. 1983:78–91. [PubMed] [Google Scholar]
- 61.Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88:489–495. doi: 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- 62.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes' patellar tendons. J Orthop Res. 2004;22:334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Franklin SL, Dean BJ, Wheway K, Watkins B, Javaid MK, Carr AJ. Up-regulation of Glutamate in Painful Human Supraspinatus Tendon Tears. Am J Sports Med. 2014;42:1955–1962. doi: 10.1177/0363546514532754. [DOI] [PubMed] [Google Scholar]
- 64.Martinez FO. Analysis of gene expression and gene silencing in human macrophages. Current protocols in immunology / edited by John E. Coligan … [et al.] 2012:11–23. doi: 10.1002/0471142735.im1428s96. Chapter 14, Unit 14. 28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.