Abstract
Introduction
Heart valve disease is an increasingly prevalent and clinically serious condition. There are no clinically effective biological diagnostics or treatment strategies. The only recourse available is replacement with a prosthetic valve, but the inability of these devices to grow or respond biologically to their environments necessitates multiple resizing surgeries and life-long coagulation treatment, especially in children. Tissue engineering has a unique opportunity to impact heart valve disease by providing a living valve conduit, capable of growth and biological integration.
Areas covered
This review will cover current tissue engineering strategies in fabricating heart valves and their progress towards the clinic, including molded scaffolds using naturally-derived or synthetic polymers, decellularization, electrospinning, 3D bioprinting, hybrid techniques, and in vivo engineering.
Expert opinion
While much progress has been made to create functional living heart valves, a clinically viable product is not yet realized. The next leap in engineered living heart valves will require a deeper understanding of how the natural multi-scale structural and biological heterogeneity of the tissue ensures its efficient function. Related, improved fabrication strategies must be developed that can replicate this de novo complexity, which is likely instructive for appropriate cell differentiation and remodeling whether seeded with autologous stem cells in vitro or endogenously recruited cells.
1. Introduction
The heart valves are complex soft tissue structures that are responsible for unidirectional blood flood in the heart. Valvular heart disease (VHD) can be broadly characterized by stenosis—the stiffening of the leaflets such that the valves do not fully open or close—and/or regurgitation—the process of blood flowing back through the valve. VHD is an increasing form of cardiovascular disease, with prevalence increasing with age, affecting more than 5 million adults in the United States.1 A significant portion of newborns (1-2% of all live births) are affected by congenital heart disease, the most common of which affects the valves, attributing to 44,000 cases annually.1 The interplay of genetic, cellular, and microenvironmental contributions to valve disease is not completely understood, but recent evidence supports alteration in developmental morphogenesis signaling pathways, such as Notch1, can lead to valve disease in newborns and adults.2 The number of VHD cases affecting newborns, children, and young adult rises dramatically in the developing world. Rheumatic fever leads to rheumatic valvular disease, with a prevalence of 15.6 million and 233,000 deaths annually.3 The most common treatment option for VHD is heart valve replacement, comprising approximately 300,000 surgeries per year. It is also expected for the number of patients requiring heart valve replacement to triple by 2050.4 Unfortunately, many current treatment options are inadequate for pediatric patients and younger adults because the prosthetics cannot repair, regenerate, nor grow. Therefore, multiple resizing operations are required in children. The Ross procedure is attractive as a means for providing a living valve replacement for the aortic position. Results to date support excellent performance in the adult but also identify a significant risk of pathological dilation and/or stenosis in growing children.5,6 These motivate many researchers to engineer living heart valves that overcomes the shortcomings of the current treatment options.7 This review highlights the structural and functional characteristics of the aortic valve, its cellular heterogeneity, and discusses how current heart valve replacement approaches address these features. We then discuss current techniques to fabricate tissue engineered aortic heart valves, and highlight persistent challenges and potential pathways to overcome them.
2. Functional Anatomy and Composition of the Aortic Heart Valve
2.1 Heterogeneous Structure and Function of the Aortic Valve
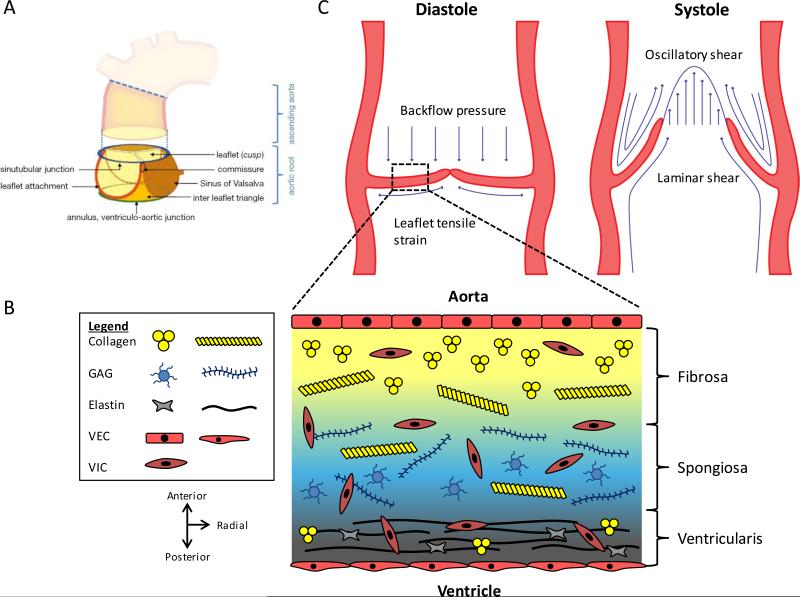
The aortic valve (AV) is composed of three compliant leaflets (cusps) attached to a fibrous annulus wall (root wall), both of which are mechanically anisotropic and structurally heterogeneous (Figure 1A, 1B). The leaflets are the functional structures that act like one-way valves in the heart. The internal heterogeneous structure of the leaflet can be classified in three layers, each composed of different molecules and cells.8 The ventricularis faces towards the left ventricle, and is composed of a laminate of multi-directionally-aligned collagen and radially-aligned elastin. The fibrosa is on the opposite side of the leaflet, and is densely packed with longitudinally-directed collagen fibers, faces outward towards the aorta. The fibrosa is the main load-bearing layer during diastole, but the collagen and elastin in the ventricularis coordinate rapid opening and closing.9 Lastly, the glycosaminoglycan and proteoglycan-rich spongiosa acts as a shear slip buffer zone between the ventricularis and fibrosa during leaflet movement.10
Figure 1.
Functional anatomy and heterogeneous composition of the aortic root. (A) The aortic root is a complex structure consisting of the leaflets and other structures, including the Sinus of Valsalva, commissure, sinutubular junction, ventriculo-aortic junction, and leaflet attachment. Figure from 12 and reprinted with permission. (B) Movat depiction of the ECM componets within the three layers of the aortic leaflet. Collagen is found throughout the valve, but it is packed tightly and aligned circumferentially in the fibrosa. Elastin is radially-aligned and predominately exists in the ventricularis. GAGs are mainly found in the spongiosa. Endothelial cells line the fibrosa and ventricularis sides, and valvular interstitial cells are found throughout the valve. (C) Biomechanical forces acting on the aortic root. During diastole, leaflets are stretched to form the coaptation and prevent backflow. Leaflets experience tensile strain and stress from the aortic pressure. In systole, leaflets are flexed open and experience both oscillatory and laminar shear stresses. Figure adapted from 16.
The aortic root wall, shaped like an inverted bulb, is comprised of the sinuses of Valsalva, leaflet attachments, commissure, inter leaflet fibrous triangle, coronary artery ostia, sinutubular junction, and the ventriculo-arterial junction (Figure 1A).11,12 The root wall is 10-50 fold more stiff than the cusps within physiologically experienced strain ranges.9 The structural composition of the root wall is similar to that of a blood vessel and is composed of three layers: intima (endothelial cells and basal lamina), medial (lamellar organization of smooth muscle cells and elastin fibers), and adventitial (primarily fibroblasts within a dense collagen network).
2.2 Heterogeneous Cellular Composition of the Aortic Valve
There are three major cell types within the aortic heart valve conduit, i.e. valvular endothelial cells (VECs), valve interstitial cells (VICs) and smooth muscle cells (SMCs). VECs are similar to arterial endothelial cells that can maintain a non-thrombogenic surface layer and regulate immune and inflammatory reactions. VICs and SMCs are the major mesenchymal cell populations in valve leaflets and valve root, respectively.
VECs form a monolayer on the ventricularis (squamous morphology) and fibrosa (cuboidal morphology) and align perpendicular to flow.13 VECs are highly sensitive to biomechanical stimuli (Figure 1C). The pulsatile nature of blood flow induces macroscopic deformation dynamics on the valve, which is dependent on the cardiac cycle stage, and such deformations have macro- and micro-scale effects.14,15 During systole when the leaflets are flexed, ventricularis VECs experience laminar shear stress and strain while fibrosa VECs experience oscillatory shear.16 The leaflets coapt at the beginning of diastole and are stretched under the pressure load from the aortic blood. Aortic pressure acts on the whole of the valve leaflet much like the wall of a pressure vessel.17 These cells may also play a role in homeostasis by either providing an intercellular crosstalk with VICs via signal transduction, which can arise from mechanical stimuli9, or by responding to the local mechanical and biochemical stimuli (e.g. coagulation regulation, acute inflammation, oxidative stress).18 In between the endothelial layers, VICs constitute the majority of the valve cells, but they display a relatively continuous spectrum of subphenotypes—spanning embryonic-like progenitor cells, quiescent (qVICs), activated (aVICs), progenitor (pVICs), and osteoblastic (obVICs)—making this cell type distinct from other mesenchymal cell types in other organs.19 Additionally, there is a small percentage of smooth muscle cells (SMCs) primarily residing at the base of the ventricularis.20 The precise roles of each of these sub-populations during valve homeostasis are incompletely elucidated, but evidence to date supports that a number of these cell types participate in aortic valve dysfunction and calcification.21,22 For example, inflammation and/or certain biochemical environments can induce valve quiescent fibroblasts can be activated to myofibroblast-like cells, which then can also differentiate to osteoblastic like phenotypes that calcify the matrix. Alternatively, some VIC can differentiate directly to osteoblast cells without myofibroblast activation, including during dystrophic calcification where apoptotic cells nucleate calcific lesions.23
Similarly, the aortic root wall contains a heterogeneous population of cells, including endothelial cells, fibroblasts, and smooth muscle cells. The aortic root wall endothelial cells, similar to VECs, help regulate the SMCs via endothelium-derived factors such as nitric oxide (for vasodilation) and endothelin-1 (for vasoconstriction).24 Arterial endothelial cells align parallel to flow, whereas VECs align perpendicular to flow. This difference in cell orientation is associated with different signaling pathways and may suggest differences in sensing and responding to biomechanical stimuli between the two cell types.13,25 Although it is not quite understood whether the SMCs found in the root wall behave similar to those in blood vessels, aortic valve SMCs can respond to biomechanical factors directly through cyclic stretching due to the pulsatile blood flow.24
3. Current Treatment Options
When the aortic heart valve becomes diseased, there are few treatment options for the patient.7 Surgical repair of diseased aortic valves can be performed in rare cases, in particular for the very young, but outcomes are poor with significant mortality.26 The vast majority of treatment involves replacement with either a mechanical or bioprosthetic valve. The different types of all the prosthetics have been reviewed extensively before, but a brief summary is discussed below.27
3.1 Mechanical Valves
Since their inception over 50 years ago, mechanical heart valves have remained the most structurally durable valve replacement. There have been numerous iterations of these valves with multiple designs, including single leaflet, bileaflet, and ball-in-cage models. Regardless of design however, there are serious lifestyle and occupational limitations incurred by with living with a mechanical prosthetic valve.28 Patients require life-long anticoagulation medication to manage risks of thromboembolism (due to foreign body response) and hemorrhage (from too much anticoagulation). In addition, resizing of the valve is an important consideration for younger patients, including pediatrics and children.29 However, long term survival (>10 years years) range from 60-70%.28,30 Amongst the newer technologies being explored in mechanical prosthetics are smoother hinge designs to limit blood aggregation and strategies to enable endothelial attachment and survival31,32, but it remains to be seen whether the anticoagulation burden can be reduced sufficiently for more widespread indication.
3.2 Bioprosthetics
Bioprosthetic valves use tissue material derived from either porcine valves or bovine pericardium.27 These materials are generally fixed via a variety of methods, including glutaraldehyde crosslinking to prevent immunogenicity, anti-mineralization to reduce cusp calcification, and high or low pressures.33 Once fixed, the tissue can be attached onto a stent mount, a stentless mount, or an expanding stent. The major advantage for using bioprosthetic valves is that they exhibit more natural opening/closing characteristics and hemodynamic profiles more similar to the native heart valve.33 In addition, bioprosthetics can be delivered via the minimally-invasive transcatheter aortic valve replacement/implantation (TAVI/TAVR) technique. Compared to mechanical valves, bioprosthetics have lower bleeding rates.34,35 However, unlike mechanical valves, bioprosthetics are not nearly as mechanically robust. Their structural degeneration occurs over 10-20 years for elderly patients, attributed to inflammatory/immune response and calcification.36 Younger patients experience much lower survival rates and freedom from explantation when compared to older (> 65 years) patients, with bioprosthetics contraindicated for children37.
3.3 Ross Procedure
Another treatment option is the Ross Procedure, also known as a pulmonary autograft. The patient's healthy pulmonary valve is used to replace the diseased aortic valve, and either a mechanical or bioprosthetic valve will be placed in the hemodynamically weaker pulmonary position. This procedure has shown higher survival rates within the first decade in comparison with mechanical valves, largely due to improved hemodynamic performance and lack of anticoagulation therapy.38 However, the Ross Procedure makes a one-valve surgery into a two-valve operation, and a prosthetic is still needed in the pulmonary position. Recent evidence questions the ability of the child pulmonary valve's ability to grow and remodel into an aortic valve like conduit, and there are notable concerns regarding the performance of the pulmonary replacement, particularly in the youngest patients.39 These are critical questions for the efficacy of this procedure, as it could result in two dysfunctional valves if not performed properly.
4. Tissue Engineered Heart Valves
Clinical need for a living valve replacement is greatest for pediatric populations, where growth and biological integration is essential. Apart from standard requirements for general tissue engineering scaffolds, like biodegradable, biocompatible and non-immunogenic, tissue engineered heart valve (TEHV) scaffolds must meet several other important criteria (Table 1). The engineered valves must be nonthrombogenic, non-obstructive, and able to open and close promptly and completely.40 Ideally, the scaffolds should accommodate somatic growth and remodeling in the recipient, and last the lifetime of the patient.9 At the macroscale, the scaffolds should mimic the anatomical geometry and exhibit mechanical properties appropriate to fulfill the valve function and adapt to the changing physiological conditions. At the microscale, the engineered valves should regulate cellular infiltration, differentiation, and most importantly, phenotypes. These emphasize that not only the choice of biomaterials but also the methods of processing are of key importance in TEHV strategy. A variety of techniques are currently employed to generate TEHV, including decellularization, molding/suturing, electrospinning, and 3D bioprinting. This section will compare these and the hybrid techniques that combine several strategies.
Table 1.
Engineering criteria for heart valve fabrication.
| Crucial | Beneficial | Other design considerations |
|---|---|---|
| • Non-immunogenic • Non-thrombogenic • Non-obstructive • Mechanically robust • Full coaptation • Facilitation of cellular infiltration and differentiation • Biodegradable |
• Anatomically-shaped • Appropriate heterogeneous cell phenotypes and matrix composition • Growth with patient • Combination of durability and ease of replacement |
• Ease of fabrication • Cost • Off-the-shelf product vs. patient-specific • Encapsulated vs. acellular construct • In vitro conditioning • Cell source • Potential for regulatory approval |
4.1 Decellularization
Decellularization is the process of removing cellular content from a tissue or organ while retaining ECM structure and proteins. Specifically for heart valves, human (donor) tissue can also be used41, but the majority of the tissues are animal-derived because of the high availability of the tissue. As such, this section will mainly focus on animal-derived decellularized heart valves. There are many techniques researchers have used to achieve an acellular scaffold, including the use of physical (e.g. agitation, freeze-thaw), chemical (e.g. detergents, hypo/hypertonic buffers), and biological agents (e.g. enzymes, chelators).42 Although there is currently no quantitative metric to measure decellularization, several researchers have established minimum criteria for decellularization to be sufficient: The tissue will contain (1) < 50 ng dsDNA/mg ECM (dry weight), (2) < 200 bp DNA fragment lengths, and (3) no nuclear material in histological samples (e.g. H&E, DAPI).42 Decellularized heart valves, similar to bioprosthetics, provide better hemodynamics than other non-anatomically shaped prosthetics, including mechanical heart valves.43 However, unlike bioprosthetics, decellularized valves are generally not treated with glutaraldehyde, thus enabling cells can repopulate and remodel the tissue.44 Additionally, the decellularization process reduces immune response, which is largely responsible for acute rejection of xenografts.45,46
Much of the current research seek to maintain biomechanical integrity and recellularization potential while reducing immunogenicity, thrombogenicity, and ECM disruption—all of which affect cell repopulation and structural integrity of the valve upon implantation. Many researchers decellularize via immersion47,48 or perfusion49,50 of the valve, with each methodology adequately removing cellular content. Numerous combinations of detergents and biological agents have been used with varying efficacy, including SDS47,48,51–53, trypsin47,54–56, EDTA47,48,53–56, Triton X-10047,50–53, sodium deoxycholate53,54, DNase/RNase47,48, and hypo/hypertonic solutions48,50. Some decellularized valves showed immunogenicity and thrombogenicity54,55 while other valves did not show any immune response52,57,58, the discrepancy of which was dependent on not only the decellularization technique but also postprocessing (e.g. coating the valve with endothelial cells). Mechanical properties are also technique-dependent: the Sacks group found that microstructural alteration caused an increase in extensibility and decrease in effective flexural moduli47, but Jiao et al. found no significant differences in the storage moduli between their decellularized valves and fresh/cryopreserved valves59. In animal and clinical trials, decellularized valves have shown recellularization potential and functional abilities in canine56, ovine53,60, porcine61, and humans41.
Currently, there are two acellular tissue engineered heart valves in the market: Cryolife's SynerGraft® in the United States and Europe and AutoTissue GmbH's Matrix P plus N™ in Europe only. However, the efficacy of each of these products showed varying results. Early SynerGraft® studies proved fatal to three out of four children due to a strong inflammatory response.62 However, a more recent study using the new CryoValve SynerGraft® in the Ross procedure showed favourable results: with an average follow-up time of 4.9 years, there were no signs of early or late death, no conduit reoperation, and no deterioration in conduit valve function.63 Likewise, the AutoTissue's Matrix P™ family of decellularized heart valves showed varying results. In 50 adult Ross operations, 36% of the patients needed additional surgeries although postoperatively, the right ventricular pulmonary artery pressure gradients behaved similarly to native valves in healthy subjects.43 Two other studies reported more failures of the xenograft. Amongst 93 patients, the majority of which were neonatal, infant, and children, 35.5% experienced conduit failure, and 29% experienced conduit dysfunction.64 Conduit stenosis caused many of the failures, followed by pseudoaneurysm, conduit dilatation, and allograft dissection. In total, the two-year freedom from failure and dysfunction was 60.2% and 77.4%, respectively. In another study, 52% of the patients needed replacement due to graft failure, which were related to inflammation and fibrosis. 65
In order to further improve the function and biocompatibility of decellularized valves, Lichtenberg et al reendothelialized the biological matrices.66 Implantation of these valves in an ovine model showed less neointima and thrombotic formation, and a stronger endothelial lining comparing to nonendothelialized counterparts.67,68 Recent study demonstrates that reendothelialization of decellularized pulmonary allografts has the capacity for matrix-guided regeneration even in elderly sheep.69
4.2 Molded or Sutured Scaffolds
A large majority of scaffolds for TEHV applications have been fabricated by molding materials into a tube-like shape or suturing materials to a stent.70,71 Natural biomaterials, especially the components of ECM like collagen and fibrin, have been widely used for developing TEHV. Collagen is major component of the fibrosa layer of native aortic valves. Purified collagen hydrogels have been used to fabricate valve leaflets and whole conduits, each developing preferential cell and matrix fiber alignment.13,72 Fibrin is another important natural biopolymer that is extensively used for TEHV. Fibrinogen can be procured from the patient's own blood and therefore be used to generate autologous fibrin hydrogel by combining with thrombin.73 Flanagan et al. synthesized a completely autologous fibrin-based heart valve structure by molding the fibrin hydrogels and seeding with ovine carotid artery-derived cells.74 Fibrin based valves implanted in the lumen of the pulmonary trunk, or interposed between two sectioned ends of the pulmonary trunk using a sheep model, the explanted valve roots remained intact and showed qualitatively similar matrix organization.75 Recently, Alfonso et al. demonstrated that fibrin in the flex-flow culture of engineered heart valve tissues could promote collagen production of adult human periodontal ligament cells and enhance retention of GAGs within the developing ECM.76 The major problem for the biological protein based TEHV is that leaflets significantly contract because the imposed stress on the leaflets cannot counteract the stress generated by the leaflets, leading to pronounced insufficiency and loss of coaptation.77
Synthetic biopolymers have also been explored because they have the advantage to be tailored to provide precise control of various aspects, such as mechanical properties, chemical properties and degradation rate.78 Some of the most frequently used synthetic biomaterials for TEHV include poly(glycolic acid) (PGA), poly(lactic acid) (PLA), poly(ε-caprolactone) (PCL), and poly(4-hydroxybutyrate) (P4HB).79,80 Using these materials, TEHV are typically engineered by suturing or molding. Scaffolds are then seeded with cells, and culture in vitro under dynamic stimulation before implantation. Mayer and his colleagues pioneered the use of synthetic polymers for TEHV.81,82 They implemented fibrous scaffold composing of a PLA woven mesh sandwiched between two non-woven PGA mesh sheets and seeded autologous myofibroblasts and endothelial cells.83,84 A variety of blends of scaffolds can also be produced with varying mechanical properties and degradation rates via combining, mixing, and altering different polymers at concentrations. For example, bilayer trileaflet heart valve scaffold with a combination of PGA and P4HB or polyhydroxyoctanoate (PHO) were fabricated to increase scaffold flexibility.84,85 Some limitations emerged due to their initial stiffness and inflexibility of the scaffold material made with aliphatic polyesters that led to stenosis. Degradation is an issue when the scaffold degrades faster than cellular ECM production, leading to early structural incompetency.71,86 In contrast, slow and/or incomplete polymer degradation may result in excessive chronic inflammation, possibly leading to fibrosis and hampered valve function.
Recently, the trileaflet heart valves fabricated from PGA-P4HB composite matrices have been integrated into a tubular stainless steel, self-expandable stent and seeded with autologous vascular or stem cells.87 Normally after conditioning in the bioreactor, the constructs were minimally-invasively implanted via the femoral artery (transfemoral approach), or via the left ventricular apex (transapical approach) as pulmonary or aortic valve replacements.88–90 Both in vitro and in vivo results showed the feasibility of merging tissue engineering and minimally-invasive valve replacement technologies. Apart from PGA/P4HB scaffolds, fibrin based TEHV constructs consisting of a tubular construct (fibrin gels with human umbilical vein cells) sewn into a self-expandable nitinol stent at three commissural attachment points were generated.91 The TEHV constructs were conditioned in a bioreactor and underwent crimping to simulate the catheter-based delivery. The crimping process did not affect the valvular functionality in terms of orifice area during systole and complete closure during diastole and no influence on ECM organization and the mechanical properties was observed. Recently, the marrow stromal cell-based TEHV have been successfully implanted into pulmonary92 or orthotopic aortic valve position93 in a sheep model through TAVI/TAVR procedures. Transapical implantation of a TEHV in a one-step intervention in non-human primates was also documented89. Therefore, transcatheter tissue engineered heart valve may be used for the treatment of a lower-risk population that cannot withstand open-heart surgery in the future considering the rapid and substantial improvements in the technology and the increasing experience.94 However, for the transcatheter treatment to be completely viable, it must overcome the challenge of the high incidence of paravalvular leakages (50-85%) in current TAVI/TAVR procedures.95
4.3 Electrospinning
Electrospinning is a technique to fabricate fine fibers with diameters ranging from 5 nm to several microns under a high voltage electrostatic field operated between a metallic capillary of a syringe and a grounded collector. This technique has been implemented to create TEHV with controlled fiber structure (either random or aligned) to mimic the native anisotropy of the whole TEHV to promote cell growth and differentiation.96,97
Many polyesters and elastomers have been used to generate electrospun fibrous scaffolds to accommodate valve cells. Courtney et al. electrospun fibers composed of poly(ester urethane) ureas (PEUU)98 and by optimizing the electrospinning parameters and leaflet shape design, the fibrous PEUU scaffolds can mimic heart valve anisotropic mechanical properties.99,100 Masoumi et al. used directional electrospinning to fabricate aligned poly(glycerol sebacate):poly(caprolactone) (PGS:PCL) fibrous scaffold to mimic the intrinsic anisotropy of native heart valve leaflets.101 Blending PGS with PCL can also improve the degradation properties and cell adhesion properties of the electrospun fibrous scaffolds.102 The VICs were also able to remodel the synthetic scaffold, and deposit new matrix proteins, while maintained the mechanical properties of the scaffolds.103 Hinderer et al. generated an electrospun poly(ethylene glycol) dimethacrylate (PEGdma)-PLA scaffold adapted to the structure and mechanical properties of native valve leaflets. VIC and VECs were seeded on the scaffold, and when cultured under physiological conditions in a bioreactor, the construct performed like a native leaflet (Figure 2A).104
Figure 2.
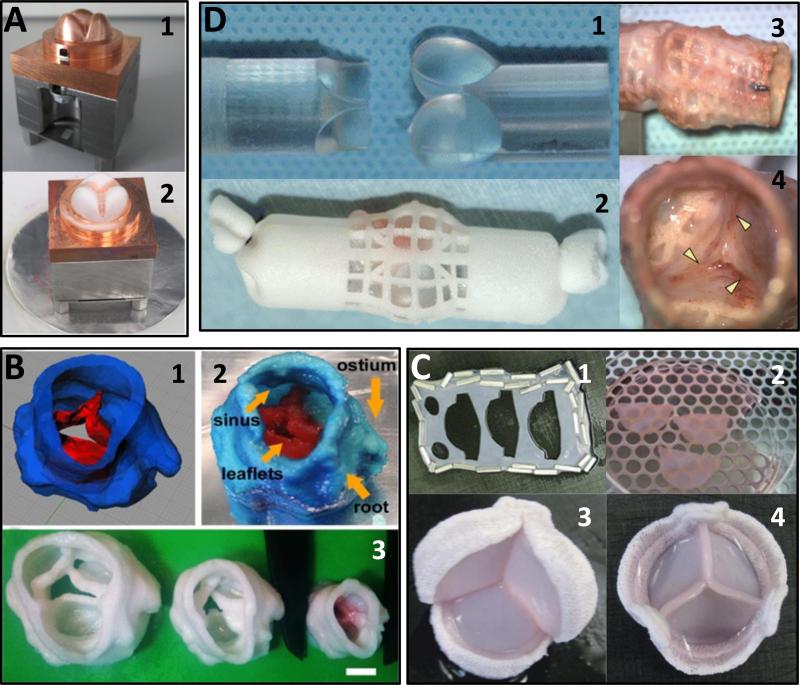
Selected examples of TEHVs. (A) Electrospun PEGdma-PLA fibers onto a valve-shaped target. Figure adapted from 104 with permission from Elsevier. (1) Copper valve mold/target. (2) Mold partially covered with electrospun material (white). (B) 3D bioprinted TEHV using PEGDA. Figure adapted from 115 with permission. (1) 3D model of the scanned porcine heart valve. (2) 3D printed valve showing complex anatomical shapes. (3) Various sizes of the 3D printed heart valves. (C) Self-assembled fibroblast sheets were cut into leaflets and sutured onto a stent. Figure adapted from 124with permission. (1) Cut-outs for the leaflets. (2) Pieces of leaflets cut out from self-assembled fibroblast sheet. (3-4) Bottom and top views of the stented valve. (D) PU-wrapped valve-shaped mold implanted in vivo to form fibrous tissue. Figure adapted from 129 with permission. (1) Silicone valve-shaped rod used as a mold. (2) A sheet of PU was wrapped around the rod and implanted subcutaneously. (3) Fibrous tissue formation after implantation. (4) Close up of the leaflets formed. Arrowheads denote places where the membrane was cut to form the leaflets.
4.4 3D Bioprinting
3D bioprinting, which is also known as rapid prototyping or additive manufacturing, can be used to accurately recapitulate the native anatomy and heterogeneity of the native tissue and to reconstruct engineered tissues in a layer-by-layer manner. In general, a 3D scan of the conduit is taken (e.g. micro-CT or MRI), converted into a stereolithography file for the 3D printer, and printed using bioinks consisting of cell-encapsulated or cell-free biomaterial. The three main technologies used for bioprinting are inkjet, laser-assisted, and extrusion, each with its own advantages and disadvantages as reviewed previously.105
The ability to accurately reconstruct native heart valves has direct clinical impact. Sodian and colleagues have used reconstructed models for numerous surgical planning processes, ranging from congenital heart defects106 to the more common aortic valve replacement operation107–110 and even patients with rare cardiac tumors111. Although the surgical models are nonliving, it was a step towards bioprinting heart valves. In fact, Sodian and his colleagues were amongst the first to use 3D printing to fabricate tissue engineered heart valves. The engineered valve was derived from x-ray computed tomography of human pulmonary and aortic heart valves and fabricated via stereolithography (i.e. laser-assisted printing) with either poly-4-hydroxybutyrate (P4HB) or polyhydroxyoctanoate (PHOH).112,113 Testing in a pulsatile bioreactor demonstrated opening and closing of the leaflets at sub- and supra-physiological flow and pressure conditions but still suffered from mild stenosis and regurgitation.
Duan et al. recently used an extrusion-based 3D printer to fabricate heterogeneous, cell-encapsulated TEHVs.114 An adult porcine valve was scanned using micro-CT and printed using a mixture of gelatin and alginate with porcine aortic smooth muscle cells (PASMCs) or porcine aortic valve interstitial cells (PAVIC) in different syringe barrels. The resulting TEHV was ionically crosslinked using CaCl2. After statically culturing for up to seven days, PAVICs and PASMCs expressed higher levels of vimentin and alpha-smooth muscle actin, respectively, indicating the technical feasibility of bioprinting TEHVs and sustaining native phenotypes of encapsulated cells. Further advancing the technique, Hockaday et al. printed TEHV using poly(ethylene glycol)-diacrylate (PEGDA) supplemented with alginate at clinically-relevant sizes (ID = 12-22 mm, corresponding to infant and adult sizes) (Figure 2B).115 The fabricated valve yielded high shape fidelity and showed key features of the valve, including the coronary ostium and sinuses. The mechanical properties (i.e. modulus) of the PEGDA compositions used were within range of native aortic leaflets in the radial direction but softer than the aortic valve sinus. Duan et al. showed that tuning the ratio of methacrylated hyaluronic acid (Me-HA) and methacrylated gelatin (Me-Gel) hydrogels—thereby altering mechanical properties—influenced VIC behavior, notably facilitating cell spreading, proliferation, migration, and also maintaining VIC fibroblastic phenotype.116 They later used the same material to 3D print a simple, heterogeneous heart valve model encapsulated with human aortic valvular interstitial cells (HAVICs).117 After seven days of static culture, HAVICs maintained their native phenotype and remodeled the ECM, indicated by collagen and GAG deposition, and showed cell spreading morphology similar to cells in the native environment.
More recently, Lueders and colleagues employed a selective laser sintering (SLS) printer to fabricate TEHVs.118 The group is proposing to use a cryomill to process biocompatible polymers (e.g. PGA/PLA co-polymers) to a powder with a particle size of 30–50 μm and use that powder in the SLS device to form the TEHV porous architecture. These studies are ongoing.
4.5 Hybrid Techniques
Although living TEHV can be fabricated using various methods and have shown in vitro and in vivo functionality, the engineered construct with single material and single technique can hardly mimic the whole structure, properties, and function of native valve tissue. Therefore, multiple techniques are implemented and integrated. For example, cell-mediated leaflet retraction is usually observed in TEHV, and thus decellularization of cellular TEHV represents a strategy to reduce retraction of the leaflets. Dijkman et al. fabricated the trileaflet heart valve scaffolds using non-woven PGA meshes coated with P4HB.119 This widely used PGA/P4HB TEHV scaffold was first seeded with ovine vascular derived cells and cultured in dynamic environment and then decellularized and recellularized with MSC. Decellularization of TEHV enabled on-the-shelf storage of the in vitro grown TEHV without altering the collagen structure or tissue strength and strongly reduced cell-mediated retraction, thereby improving valve performance. In vivo studies of similar valves without reseeding in non-human primates and sheep showed cellular infiltration, matrix remodeling, and mild regurgitation.120,121 Similarly, Syedain et al. fabricated tubular fibrin gel with encapsulation of ovine dermal fibroblasts and then decellularized the constructs after mechanical compaction and dynamic condition in a pulsed flow-stretch bioreactor.122 The decellularized engineered tissue tubes displayed compositional and mechanical properties similar to those observed in native ovine heart valve tissue, including a high degree of mechanical anisotropy that is characteristic of the aortic root and valve leaflets.
Recently, living tissue sheets produced by the self-assembly method have been molded as TEHVs. Tremblay et al. fabricated entirely biological stentless aortic valve by stacking several fibroblast sheets produced with the self-assembly technique into thick tissues which were conferred to 3D shape using molding or suturing technique.123 The constructed valve conduit demonstrated opening and closing of the leaflets during bioreactor conditioning and showed uniform cell distribution and dense ECM fabrication. However, the fabrication process took approximately 8 months, which is not clinically tenable unless the process can be expedited. Similarly, Dubé et al. constructed TEHV using self-assembled fibroblast sheets, taking 7 weeks for the sheet to yield the thick construct which were cut and assembled together on a Edwards Lifesciences® stent, based on techniques used for commercially available replacement valves (Figure 2C).124 The valve had similar ultimate tensile strength measurements comparable to native porcine leaflets, but the elastic modulus was at least an order of magnitude lower. This technique generates TEHV containing cells embedded in their own extracellular matrix and has the potential to provide enough strength to support physiological stress.
The electrospinning technique can also integrate with other techniques to form hybrid TEHV scaffolds. For example, most hydrogel scaffolds are similar to heart valve tissue, but they are normally not mechanically suitable for the dynamic stresses of the heart valve microenvironment. Tseng et al. incorporated electrospun chemically-modified PCL fibers into poly(ethylene glycol) (PEG) hydrogels to improve material strength and introduce anisotropic mechanical behavior.125 Similarly, Eslami et al. combined electrospun PGS/PCL microfiber scaffolds with methacrylated hyaluronic acid (Me-HA) and methacrylated gelatin (Me-Gel) hydrogels with encapsulation of sheep mitral VIC.126 Compared to electrospun or hydrogel scaffolds alone, the microfibrous scaffolds preserved their mechanical properties in the presence of the hydrogels, and the hydrogel improved the three-dimensional distribution of mitral VIC. Recently, Masoumia and co-workers fabricated tri-layered scaffolds by assembling microfabricated PGS and fibrous PGS/PCL electrospun sheets to develop elastic scaffolds with tunable anisotropic mechanical properties similar to the mechanical characteristics of the native heart valves.127 The engineered scaffolds supported the growth of VIC and mesenchymal stem cells (MSCs) within the 3D structure and promoted the deposition of heart valve extracellular matrix (ECM).
4.6 In Vivo Tissue Engineering of Heart Valves
Traditional TEHV strategies involving shaping biomaterials into constructs have disadvantages in long-term in vitro culture, risk of infection, and cost-intensive infrastructures. Alternatively, In vivo tissue engineering approach is heavily reliable on the wound healing response and the natural foreign body response to synthesize autologous tissue around an implant material.9 This approach was pioneered by Campbell for blood vessels, showing that bone marrow derived mesothelial and mesenchymal cells were recruited to and remodeled these neo-matrices.128 For TEHV purpose, Yamanami et al. implanted a valve-shaped mold of silicone polyurethane into the dorsal subcutaneous space of a rabbit (Figure 2D).129,130 These valves were able to close and open rapidly in synchrony with the backward and forward pulsatile flow in vitro. The tensile strength of the leaflets was on the same order as native leaflets. Similarly, Kishimoto et al. implanted a sutureless stented biovalve mold subcutaneously into goats to form connective tissue around the mold, and upon implantation orthotopically, the valves showed technical feasibility.131 One advantage of this strategy is that the in vivo engineered valve approach allows for the minimization of cell and tissue culture risks and costs while providing off-the-shelf availability. However, the complexity of the immune response to the foreign biomaterials and scaffolds poses a challenging environment for in vivo tissue engineering of heart valves.132 In addition, the inflammation and the resulted constructs may drive valve calcification.133 It is thus crucial to better understand the inflammatory response towards the foreign biomaterial and the triggers for pathological outcome and to control the fate of implanted biomaterial scaffolds and regulate the inflammatory reactions towards tissue regeneration and remodeling and prevent fibrosis and/or degeneration.134
5. Expert Opinion
Tissue engineering of heart valves has advanced considerably in the past two decades since the first published study that galvanized the research.81,135 The ultimate goal is to fabricate a living valve that can grow and functionally integrate to the patient's cardiovascular system, which is an especially important criteria for children and young adults in developing countries who are affected the most. The first major target for TEHVs, at minimum, must match pulmonary conditions due to a faster clinical pathway via the Ross Procedure, but ultimately, TEHVs should aim to withstand aortic hemodynamic and biomechanical conditions. Some more common and traditional techniques have been improved, including using biopolymers for molding and decellularization, while other newer methods are starting to emerge, such as electrospinning, 3D bioprinting, and in vivo engineering. A selected overview of the current TEHVs are in listed in Table 2.
Table 2.
Selected overview of current TEHVs and scaffolds for TEHVs.
| Technique | Additional detail | Material | Type of construct | Cell seeding | Study type | Reference |
|---|---|---|---|---|---|---|
| Decellularization | Hypotonic, SDS, EDTA, DNase, RNase | Porcine and ovine aortic roots | Whole valve | N/A | In vivo in abdominal aorta of pigs; in vivo in RVOT of juvenile sheep | 48 |
| Decellularization | Sodium deoxycholate, DNase, EDTA | Rat aortic roots | Whole valve | N/A | In vitro architecture characterization and reseeding in vivo implantation | 49 |
| Decellularization | Water lysis, Triton X-100; reseeded with cells or conjugated with CD133 | Porcine pulmonary valves | Whole valve | N/A | In vivo implanation in sheep in pulmonary position | 50 |
| Decellularization | Hypotonic solution, Triton X-100, SLS; treated with PEG; fixed in glutaraldehyde and detoxified | Porcine aortic valves | Stented valve | N/A | Bioreactor | 51 |
| Decellularization | SDS, Triton X-100; coated with fibronectin and stromal cell-derived factor-1α | Ovine aortic valves | Whole valve | N/A | Implanted in sheep pulmonary position | 52 |
| Molding | Fibrinogen with cells casted into mold and polymerized with thrombin | Fibrin | Whole valve | Ovine carotid artery SMCs and fibroblasts; seeded endothelial cells after conditioning | Bioreactor conditioning for 28 days; implantation in ovine pulmonary position for 3 months | 74 |
| Molding | PHO casted onto aluminum valve cast | Polyhydroxyalkanoate (PHO) | Whole valve | Initial seeding with ovine carotid artery vascular cells then with venous endothelial cells | Implanted into pulmonary position of lamb | 85 |
| Molding | Scaffold stitched to inner surface of stent wires | Polyglycolic-acid coated with poly-4-hydroxybutyrate | Self-expandable stented valve | Ovine amniotic fluid cells | Implanted into ovine pulmonary position in-utero | 87 |
| Electrospinning | Poly(ester urethane) urea (PEUU) | Tissue scaffold | N/A | Mechanical characterization | 99 | |
| Electrospinning | Poly(glycerol sebacate):poly(caprolactone) | Tissue scaffold | Human VICs | In vitro characterization of scaffold | 101 | |
| Electrospinning | Poly(ethyleneglycol) dimethacrylate-poly(lactic acid) | Whole valve | Porcine VICs and VECs | Bioreactor conditioning | 104 | |
| 3D bioprinting | Alginate/gelatin | Whole valve | Porcine aortic VICs and SMCs | Static culture | 114 | |
| 3D bioprinting | Poly(ethyleneglycol)-diacrylate | Whole valve | Porcine aortic VICs | Static culture | 115 | |
| 3D bioprinting | Methacrylated hyaluronic acid/methacrylated gelatin | Whole valve | Human aortic VICs | Static culture | 116 | |
| Hybrid | Initially seeded with vascular cells then decellularized and reseeded with MSCs | Polyglycolic-acid coated with poly-4-hydroxybutyrate | Self-expandable stented valve | Ovine vascular cells, ovine MSCs | Bioreactor conditioning | 119 |
| Hybrid | Initially seeded with vascular cells then decellularized | Polyglycolic-acid coated with poly-4-hydroxybutyrate | Self-expandable stented valve | Human/ovine vascular cells | Bioreactor conditioning, in vivo implantation in non-human primate and ovine pulmonary position | 120,121 |
| Hybrid | Seeded construct conditioned in bioreactor first then decellularized | Fibrin | Tube-in-tube stented valve | Ovine determal fibroblasts | Bioreactor conditioning | 122 |
| Hybrid | Molded self-assembled sheets (8 layers) | Self-assembled fibroblast sheets | Whole valve | Human dermal fibroblasts | Bioreactor conditioning | 123 |
| Hybrid | Leaflets formed by self-assembled sheets cut and sutured onto stent | Self-assembled fibroblast sheets | Stented valve | Human dermal fibroblasts | Bioreactor conditioning | 124 |
| Hybrid | Incorporated PCL fibers into PEG hydrogels and seeded cellson top | Poly(caprolactone) and poly(ethyleneglycol) | Tissue scaffold | Porcine aortic VICs | In vitro characterization of scaffold | 125 |
| Hybrid | Immersed PGS/PCL fibers into Me-HA/Me-Gel solution and crosslinked; cells seeded on PGS/PCL and encapsulated in Me-HA/Me-Gel | Poly(glycerol sebacate)-poly(ε-caprolactone) and methacrylated hyaluronic acid/methacrylated gelatin | Tissue scaffold | Ovine mitral VICs | In vitro characterization of scaffold | 126 |
| In vivo engineering | PU mesh wrapped around silicone valve-shaped molds; tissue membrane act as leaflets | Polyurethane mesh, tissue membrane | Whole valve | N/A | In vitro characterization after in vivo synthesis in subcutaneous layer in rabbit | 129 |
| In vivo engineering | Silicone rod, polyurethane scaffold | Self-expandable stented valve | N/A | In vitro characterization after in vivo synthesis in subcutaneous layer in goat | 131 |
Decellularized valve technology is the fastest method clinically, having progressed through animal48,53,56 and human41 studies in addition to two commercially available products43,63. Although some of these in vivo studies showed promising results46, the critical weakness of decellularized TEHVs is a somewhat unpredictable rapid graft failure, which could be driven by immune response but also incomplete recellularization136. Recent advances have focused on maintaining preservation of tissue architecture and improving recellularization in vivo—though some researchers still question the need for in vitro recellularization137. Complete recellularization and remodeling of the acellular scaffold will be essential to limit immune based graft degeneration and structural failure. Such graft remodeling is more important for pediatric patients that are highly active and require significant growth in a short amount of time. Tissue growth will not be possible unless host cells can rapidly reach the full thickness of the root wall and leaflets and resorb local donor matrix. More research is needed to develop technologies that accelerate host cell adhesion, invasion, matrix remodeling, and endothelialization before this can become a long-term viable option. Even if successful, a key challenge that would still remain is the limited availability of allograft tissue, in particular for pediatric sizes. Clinical experience thus far indicates that decellularized xenograft valve tissue retains elevated risks for immunogenic reactions that could be rapid and catastrophic. If only human valve allograft tissue is an acceptable base material, the argument may shift to which approach (crosslinking or decellularization) provides a better bioprosthetic for older adults. If that is the case, then progress in therapy for the younger patients will again be stymied.
Both molding and suturing approaches have shown in vitro feasibility via bioreactor studies and even some in vivo work119, but they also have important limitations. First, the recreation of the anatomical whole valve is difficult due to its complex geometry, including the sinuses, ostia, and the curved leaflets with the triangular coaptation. Factoring in differently sized conduits ranging from infants to adults also add to the complexity. Furthermore, many of these techniques use only one or two material for the entire TEHV without considering the different composition of the leaflet and root wall tissue composition and organization, making it difficult to match native biomechanical properties. The suturing or gluing process is a time-demanding task prone to misplacement and also results in certain critical point susceptible to calcification.
Electrospinning offers the ability to control microarchitecture (e.g. fiber alignment) within TEHV constructs that other techniques cannot, which has an impact on cell differentiation towards native VIC phenotypes and also anisotropic mechanical properties. Electrospun TEHV scaffolds with packed fibrous structure and small pore sizes can hamper cell infiltration and colonization. Sohier et al. used jet-spraying method to create highly porous anisotropic PCL nanofibrillar scaffolds.138 This method implemented compressed airflow, rather than high voltage, to drive the polymer solution from the reservoir to the nozzle, where it is diffracted on the needle and projected onto a collector. The obtained matrices had high porosity and mechanically anisotropic structure. Dynamically seeded human adipose derived stem cells and VIC on scaffolds could penetrate within the matrices and produced new ECM (collagen I, III and elastin) after 20-day condition. Alternatively, Stella et al. were able deliver viable cells during the electrospinning process, which could provide another option of incorporating cells into the scaffold.139
3D bioprinting may arguably be one of the few techniques that can fabricate patient-specific valves while retaining the complex valve anatomy. The current 3D bioprinting techniques can create anatomically-accurate, heterogeneous, and mechanically-tunable TEHVs with fast production rate (< 45 minutes) at clinically relevant and patient-specific sizes.115 However, many of these studies do not provide in vitro studies in a bioreactor to show structural integrity, movement of the leaflets, and most importantly, remodeling of the matrix within this environment. Unfortunately, extrusion-based bioprinting generally do not have high resolution to fabricate constructs with micro- or nano-architecture (e.g. fiber alignment). Stereolithography can provide higher resolution printing by using a laser, but incur difficulty with heterogeneous printing, limited ability to cellularize during printing, and additional material constraints. The ability to construct differently-sized or patient-specific conduits is helpful to ensure a perfectly-fitted valve replacement, but it is not known whether the anatomical geometry is truly needed, which is an attribute that remains to be researched.
As each fabrication method has inherent limitations, researchers will likely need to utilize a variety of methods in a hybrid fashion to create a trileaflet valve with near native macro- and micro-scale architectural complexity. Weber et al. recently generated living tubular valves using modified molding technique.140 The formed tube-in-tube valves had textile polyethylene terephthalate (tPET) or fibrin with tPET as the inert tube sutured to a silicon tube. The design can be easily tailored on the anatomical details of the patient's valve through the production of a rapid prototyped mold. In addition, two components (leaflets and root) can be produced separately and conditioned independently to reproduce the heterogeneity of the native heart valves. Using the same principle but different technique, Syedain et al. created a tubular TEHV mounted on a stent that formed coapting leaflets by collapsing the tube.122 If these valves prove functional and durable in animal models, it may be a strong contender to reach the clinic by vastly improving on current TAVI valves. However, the level of anatomical fidelity of the tube-in-tube design remains to be researched, and the lack of structural heterogeneity may hinder valve performance.
The self-assembly approach by the Auger and Germain groups offers an interesting method to fabricate either a stentless or stented TEHV via living tissue sheets.123,124 Although they fabricated homogeneous TEHVs, the technique has tremendous potential to recapitulate the heterogeneous ECM structure of the native aortic leaflets by superimposing self-assembled sheets with different compositions with potentially higher accuracy than many other techniques. The newest process takes approximately 12 weeks, which may not be clinically feasible, but it has the potential to be scaled-up industrially to decrease construction time and make “off-the-shelf” products. This approach would be best suited with custom molds or tubular valves. Combining the self-assembled sheets with a 3D printed anatomical mold that incorporates the tube-in-tube approach may offer a lucrative solution into the clinic. Additionally, the in vivo engineering approach can also be coupled with 3D printing to generate patient-specific molds or decellularization if the generated tissue contract, similar to natural biopolymer-based molded TEHVs.
Regardless of technique, the constructed valves must overcome persistent problems upon in vivo implantation, including leaflet contraction and calcification. Finite element analysis indicated that the imposed stresses on the leaflets during diastole are equal to the stresses the leaflets generate.77 This suggests that the imposed stresses cannot counteract the generated stresses, which can lead to leaflet retraction and regurgitation. In vitro hemodynamic conditioning will likely remain a critical step for confirming stable tissue geometry pre-implantation. The tendency for these de novo constructs to calcify has yet to be rigorously studied, and it is unclear whether strategies to limit calcification risk are warranted or achievable. Although many researchers are already attempting to reduce calcification via chemical treatments141–143, there is a need for an in vitro TEHV valve model to study these phenomena in well controlled conditions to better understand the mechanism for prevention of calcification in this system.
Living valve replacements are clearly essential for younger patients needing growth, host integration, and controlled biomechanical remodeling. The current advancements in fabricating TEHVs all have potential to be exploited and risks to be managed. Regardless of strategy, rapid cellularization is needed—whether seeded in vitro or recruited in vivo—to sense and respond to local mechanical and biochemical cues. Many TEHVs tested in vivo and in vitro can withstand pulmonary hemodynamic loads, but persistent stenosis has blunted the long-term outlook. The native aortic valves exhibit highly complex multiscale architecture that is likely essential for long-term function—an attribute that has yet to be implemented in macroscopic TEHVs due to limitations in fabrication strategies. Advancement in construction strategies and technologies (e.g. bioprinting, self-assembly, etc.) are essential to overcome the hurdles in incorporating heterogeneity and anatomical fidelity in TEHVs.
We anticipate several of these new strategies will progress to in vivo validation in the coming years, which will greatly help identify what levels of anisotropy and heterogeneity within micro- and macro-scale anatomy are critical for next generation performance. We believe that more hybrid fabrication approaches will also come online to combine the advantages of each respective technique. Although outside of the current review, many of these methods have the capability to incorporate drugs or other soluble factors within the hydrogel to affect inflammation, calcification and/or enhance cell migration, infiltration, and differentiation. There is still much more research to be performed in regards to produce fully functional TEHV in the native aortic position, but it is hopeful that within the next 10 to 20 years, new and improved products will be clinically available to replace the need for mechanical devices and anticoagulant therapy and to lower the global burden of valvular disease.
Highlight.
Valvular heart disease is a rampant global burden, with infants, children, and young adult being the most susceptible in developing countries. Tissue engineered heart valves can provide a structure that can grow with the patient. TEHVs should mimic native valve form and function, including heterogeneity and biomechanical properties.
Decellularized TEHVs have been the most successful technique towards the clinic (two commercial products). However, the pitfalls include valvular deterioration stemming from inflammation.
Current molded TEHVs are generally homogeneous in terms of material composition and cell type. The molds can take on many different shapes, including a tube-in-tube model. Many hybrid techniques utilize molds. Major limitations include leaflet contractions and lack of material and cellular heterogeneity.
Electrospinning offers one of the highest resolution fabrication techniques and can produce fiber alignment similar to those found in native valves. However, few whole valve conduits have been produced using this method, and cellular infiltration through the packed fibers may be difficult.
3D bioprinting can potentially recapitulate native heterogeneity and anatomical fidelity. Current valves have yet to be tested in vitro.
In vivo TEHVs use the body's natural wound-healing response to fabricate tissue on an inert mold. Feasibility studies have been performed in animals, but much more work in understanding inflammation response for tissue regeneration purposes before moving into humans.
Acknowledgments
Funding was received from the National Science Foundation, the National Institute of Health and The Hartwell Foundation.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1*.Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics—2013 Update A Report From the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. doi:10.1161/CIR.0b013e31828124ad. [Annual update by the American Heart Association that provides most recent clinical data on heart valve disease in the US.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.MacGrogan D, Luna-Zurita L, de la Pompa JL. Notch signaling in cardiac valve development and disease. Birt Defects Res A Clin Mol Teratol. 2011;91(6):449–459. doi: 10.1002/bdra.20815. doi:10.1002/bdra.20815. [Review on signaling pathways and mechanisms associated with valve disease.] [DOI] [PubMed] [Google Scholar]
- 3.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–694. doi: 10.1016/S1473-3099(05)70267-X. doi:10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 4.Yacoub MH, Takkenberg JJM. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med. 2005;2(2):60–61. doi: 10.1038/ncpcardio0112. doi:10.1038/ncpcardio0112. [DOI] [PubMed] [Google Scholar]
- 5.El-Hamamsy I, Eryigit Z, Stevens L-M, et al. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet. 2010;376(9740):524–531. doi: 10.1016/S0140-6736(10)60828-8. doi:10.1016/S0140-6736(10)60828-8. [DOI] [PubMed] [Google Scholar]
- 6.Juthier F, Vincentelli A, Pinçon C, et al. Reoperation after the Ross procedure: incidence, management, and survival. Ann Thorac Surg. 2012;93(2):598–604. doi: 10.1016/j.athoracsur.2011.06.083. discussion 605 doi:10.1016/j.athoracsur.2011.06.083. [DOI] [PubMed] [Google Scholar]
- 7*.Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33(19):2451–2496. doi: 10.1093/eurheartj/ehs109. doi:10.1093/eurheartj/ehs109. [European guidelines for intervention benchmarks and tailoring of replacement choice to the patient.] [DOI] [PubMed] [Google Scholar]
- 8.Latif N, Sarathchandra P, Taylor PM, Antoniw J, Yacoub MH. Localization and pattern of expression of extracellular matrix components in human heart valves. J Heart Valve Dis. 2005;14(2):218–227. [PubMed] [Google Scholar]
- 9*.Butcher JT, Mahler GJ, Hockaday LA. Aortic valve disease and treatment: The need for naturally engineered solutions. Adv Drug Deliv Rev. 2011;63(4–5):242–268. doi: 10.1016/j.addr.2011.01.008. doi:10.1016/j.addr.2011.01.008. [Thorough review of aortic valve biology, physiology, and pathogenesis.] [DOI] [PubMed] [Google Scholar]
- 10*.MacGrogan D, Luxán G, Driessen-Mol A, Bouten C, Baaijens F, Pompa JL de la. How to Make a Heart Valve: From Embryonic Development to Bioengineering of Living Valve Substitutes. Cold Spring Harb Perspect Med. 2014;4(11):a013912. doi: 10.1101/cshperspect.a013912. doi:10.1101/cshperspect.a013912. [An excellent review on valve development and using valvular morphogenesis and matrix remodeling as models to direct valve regeneration.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho SY. Structure and anatomy of the aortic root. Eur Heart J - Cardiovasc Imaging. 2009;10(1):i3–i10. doi: 10.1093/ejechocard/jen243. doi:10.1093/ejechocard/jen243. [DOI] [PubMed] [Google Scholar]
- 12.Charitos EI, Sievers H-H. Anatomy of the aortic root: implications for valve-sparing surgery. Ann Cardiothorac Surg. 2013;2(1):53–56. doi: 10.3978/j.issn.2225-319X.2012.11.18. doi:10.3978/j.issn.2225-319X.2012.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butcher JT, Nerem RM. Valvular Endothelial Cells Regulate the Phenotype of Interstitial Cells in Co-culture: Effects of Steady Shear Stress. Tissue Eng. 2006;12(4):905–915. doi: 10.1089/ten.2006.12.905. doi:10.1089/ten.2006.12.905. [DOI] [PubMed] [Google Scholar]
- 14.Dagum P, Green GR, Nistal FJ, et al. Deformational Dynamics of the Aortic Root Modes and Physiologic Determinants. Circulation. 1999;100(suppl 2):II - 54–Ii - 62. doi: 10.1161/01.cir.100.suppl_2.ii-54. doi:10.1161/01.CIR.100.suppl_2.II-54. [DOI] [PubMed] [Google Scholar]
- 15**.Sacks MS, Yoganathan AP. Heart valve function: a biomechanical perspective. Philos Trans R Soc B Biol Sci. 2007;362(1484):1369–1391. doi: 10.1098/rstb.2007.2122. doi:10.1098/rstb.2007.2122. [Excellent collection of data regarding the biomechanical function of the aortic heart valve.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balachandran K, Sucosky P, Yoganathan AP. Hemodynamics and Mechanobiology of Aortic Valve Inflammation and Calcification. Int J Inflamm. 2011;2011:e263870. doi: 10.4061/2011/263870. doi:10.4061/2011/263870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thubrikar MJ, Aouad J, Nolan SP. Comparison of the in vivo and in vitro mechanical properties of aortic valve leaflets. J Thorac Cardiovasc Surg. 1986;92(1):29–36. [PubMed] [Google Scholar]
- 18.Butcher JT, Markwald RR. Valvulogenesis: the moving target. Philos Trans R Soc B Biol Sci. 2007;362(1484):1489–1503. doi: 10.1098/rstb.2007.2130. doi:10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Liu AC, Joag VR, Gotlieb AI. The Emerging Role of Valve Interstitial Cell Phenotypes in Regulating Heart Valve Pathobiology. Am J Pathol. 2007;171(5):1407–1418. doi: 10.2353/ajpath.2007.070251. doi:10.2353/ajpath.2007.070251. [Identification, characterization, and comparison of the various VIC sub-populations.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latif N, Sarathchandra P, Chester AH, Yacoub MH. Expression of smooth muscle cell markers and co-activators in calcified aortic valves. Eur Heart J. 2014:eht547. doi: 10.1093/eurheartj/eht547. doi:10.1093/eurheartj/eht547. [DOI] [PubMed] [Google Scholar]
- 21.Chen JH, Yip CYY, Sone ED, Simmons CA. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. 2009;174:1109–1119. doi: 10.2353/ajpath.2009.080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahler GJ, Farrar EJ, Butcher JT. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(1):121–130. doi: 10.1161/ATVBAHA.112.300504. doi:10.1161/ATVBAHA.112.300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yip CYY, Chen J-H, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29(6):936–942. doi: 10.1161/ATVBAHA.108.182394. doi:10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 24.Bäck M, Gasser TC, Michel J-B, Caligiuri G. Biomechanical factors in the biology of aortic wall and aortic valve diseases. Cardiovasc Res. 2013;99(2):232–241. doi: 10.1093/cvr/cvt040. doi:10.1093/cvr/cvt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95(5):459–470. doi: 10.1161/01.RES.0000141146.95728.da. doi:10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JW, Ruzmetov M, Vijay P, Rodefeld MD, Turrentine MW. Closed transventricular aortic valvotomy for critical aortic stenosis in neonates: outcomes, risk factors, and reoperations. Ann Thorac Surg. 2006;81(1):236–242. doi: 10.1016/j.athoracsur.2005.06.075. doi:10.1016/j.athoracsur.2005.06.075. [DOI] [PubMed] [Google Scholar]
- 27.Pibarot P, Dumesnil JG. Prosthetic Heart Valves Selection of the Optimal Prosthesis and Long-Term Management. Circulation. 2009;119(7):1034–1048. doi: 10.1161/CIRCULATIONAHA.108.778886. doi:10.1161/CIRCULATIONAHA.108.778886. [DOI] [PubMed] [Google Scholar]
- 28.Misawa Y. Valve-related complications after mechanical heart valve implantation. Surg Today. 2014 doi: 10.1007/s00595-014-1104-0. doi:10.1007/s00595-014-1104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masuda M, Kado H, Ando Y, et al. Intermediate-term results after the aortic valve replacement using bileaflet mechanical prosthetic valve in children. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2008;34(1):42–47. doi: 10.1016/j.ejcts.2008.04.005. doi:10.1016/j.ejcts.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Songur CM, Simsek E, Ozen A, Kocabeyoglu S, Donmez TA. Long Term Results Comparing Mechanical and Biological Prostheses in the Tricuspid Valve Position: Which valve types are better - mechanical or biological prostheses? Heart Lung Circ. 2014;23(12):1175–1178. doi: 10.1016/j.hlc.2014.05.015. doi:10.1016/j.hlc.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Frendl CM, Tucker SM, Khan NA, et al. Endothelial retention and phenotype on carbonized cardiovascular implant surfaces. Biomaterials. 2014;35(27):7714–7723. doi: 10.1016/j.biomaterials.2014.05.075. doi:10.1016/j.biomaterials.2014.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fallon AM, Shah N, Marzec UM, Warnock JN, Yoganathan AP, Hanson SR. Flow and thrombosis at orifices simulating mechanical heart valve leakage regions. J Biomech Eng. 2006;128(1):30–39. doi: 10.1115/1.2133768. [DOI] [PubMed] [Google Scholar]
- 33.Singhal P, Luk A, Butany J. Bioprosthetic Heart Valves: Impact of Implantation on Biomaterials. Int Sch Res Not. 2013;2013:e728791. doi:10.5402/2013/728791. [Google Scholar]
- 34**.Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36(4):1152–1158. doi: 10.1016/s0735-1097(00)00834-2. doi:10.1016/S0735-1097(00)00834-2. [Landmark study comparing biological vs. mechanical valve prosthesis for heart valve replacement.] [DOI] [PubMed] [Google Scholar]
- 35.Silberman S, Oren A, Dotan M, et al. Aortic Valve Replacement: Choice Between Mechanical Valves and Bioprostheses. J Card Surg. 2008;23(4):299–306. doi: 10.1111/j.1540-8191.2008.00580.x. doi:10.1111/j.1540-8191.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 36.Gong G, Seifter E, Lyman WD, Factor SM, Blau S, Frater RW. Bioprosthetic cardiac valve degeneration: role of inflammatory and immune reactions. J Heart Valve Dis. 1993;2(6):684–693. [PubMed] [Google Scholar]
- 37.Welke KF, Wu Y, Grunkemeier GL, Ahmad A, Starr A. Long-term results after Carpentier-Edwards pericardial aortic valve implantation, with attention to the impact of age. Heart Surg Forum. 2011;14(3):E160–E165. doi: 10.1532/HSF98.20101140. doi:10.1532/HSF98.20101140. [DOI] [PubMed] [Google Scholar]
- 38.Stelzer P. The Ross Procedure: State of the Art 2011. Semin Thorac Cardiovasc Surg. 2011;23(2):115–123. doi: 10.1053/j.semtcvs.2011.07.003. doi:10.1053/j.semtcvs.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Kallio M, Pihkala J, Sairanen H, Mattila I. Long-term results of the Ross procedure in a population-based follow-up†. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2015 doi: 10.1093/ejcts/ezv004. doi:10.1093/ejcts/ezv004. [DOI] [PubMed] [Google Scholar]
- 40.Sacks MS, Schoen FJ, Mayer JE. Bioengineering Challenges for Heart Valve Tissue Engineering. Annu Rev Biomed Eng. 2009;11(1):289–313. doi: 10.1146/annurev-bioeng-061008-124903. doi:10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- 41*.Neumann A, Sarikouch S, Breymann T, et al. Early Systemic Cellular Immune Response in Children and Young Adults Receiving Decellularized Fresh Allografts for Pulmonary Valve Replacement. Tissue Eng Part A. 2014;20(5-6):1003–1011. doi: 10.1089/ten.tea.2013.0316. doi:10.1089/ten.tea.2013.0316. [Important study to identify risk mechanisms for decellularized valve conduits in younger patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. doi:10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konertz W, Dohmen PM, Liu J, et al. Hemodynamic characteristics of the Matrix P decellularized xenograft for pulmonary valve replacement during the Ross operation. J Heart Valve Dis. 2005;14(1):78–81. [PubMed] [Google Scholar]
- 44.Dohmen PM, da Costa F, Holinski S, et al. Is There a Possibility for a Glutaraldehyde-Free Porcine Heart Valve to Grow? Eur Surg Res. 2006;38(1):54–61. doi: 10.1159/000091597. doi:10.1159/000091597. [DOI] [PubMed] [Google Scholar]
- 45.Kneib C, von Glehn CQC, Costa FDA, Costa MTBA, Susin MF. Evaluation of humoral immune response to donor HLA after implantation of cellularized versus decellularized human heart valve allografts. Tissue Antigens. 2012;80(2):165–174. doi: 10.1111/j.1399-0039.2012.01885.x. doi:10.1111/j.1399-0039.2012.01885.x. [DOI] [PubMed] [Google Scholar]
- 46.Bloch O, Golde P, Dohmen PM, Posner S, Konertz W, Erdbrügger W. Immune response in patients receiving a bioprosthetic heart valve: lack of response with decellularized valves. Tissue Eng Part A. 2011;17(19-20):2399–2405. doi: 10.1089/ten.TEA.2011.0046. doi:10.1089/ten.TEA.2011.0046. [DOI] [PubMed] [Google Scholar]
- 47*.Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29(8):1065–1074. doi: 10.1016/j.biomaterials.2007.11.007. doi:10.1016/j.biomaterials.2007.11.007. [Thorough report of the biomechanical consequences of decellularization of aortic valve leaflets.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paniagua Gutierrez JR, Berry H, Korossis S, et al. Regenerative Potential of Low-Concentration SDS-Decellularized Porcine Aortic Valved Conduits In Vivo. Tissue Eng Part A. 2015;21(1-2):332–342. doi: 10.1089/ten.tea.2014.0003. doi:10.1089/ten.tea.2014.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedrich LH, Jungebluth P, Sjöqvist S, et al. Preservation of aortic root architecture and properties using a detergent-enzymatic perfusion protocol. Biomaterials. 2014;35(6):1907–1913. doi: 10.1016/j.biomaterials.2013.11.053. doi:10.1016/j.biomaterials.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 50.Jordan JE, Williams JK, Lee S-J, Raghavan D, Atala A, Yoo JJ. Bioengineered self-seeding heart valves. J Thorac Cardiovasc Surg. 2012;143(1):201–208. doi: 10.1016/j.jtcvs.2011.10.005. doi:10.1016/j.jtcvs.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Lim H-G, Kim GB, Jeong S, Kim YJ. Development of a next-generation tissue valve using a glutaraldehyde-fixed porcine aortic valve treated with decellularization, α-galactosidase, space filler, organic solvent and detoxification. Eur J Cardiothorac Surg. 2014:ezu385. doi: 10.1093/ejcts/ezu385. doi:10.1093/ejcts/ezu385. [DOI] [PubMed] [Google Scholar]
- 52.Flameng W, De Visscher G, Mesure L, Hermans H, Jashari R, Meuris B. Coating with fibronectin and stromal cell–derived factor-1α of decellularized homografts used for right ventricular outflow tract reconstruction eliminates immune response–related degeneration. J Thorac Cardiovasc Surg. 2014;147(4):1398–1404. e2. doi: 10.1016/j.jtcvs.2013.06.022. doi:10.1016/j.jtcvs.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 53.Akhyari P, Kamiya H, Gwanmesia P, et al. In vivo functional performance and structural maturation of decellularised allogenic aortic valves in the subcoronary position. Eur J Cardiothorac Surg. 2010;38(5):539–546. doi: 10.1016/j.ejcts.2010.03.024. doi:10.1016/j.ejcts.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 54.Zhou J, Fritze O, Schleicher M, et al. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials. 2010;31(9):2549–2554. doi: 10.1016/j.biomaterials.2009.11.088. doi:10.1016/j.biomaterials.2009.11.088. [DOI] [PubMed] [Google Scholar]
- 55**.Bayrak A, Tyralla M, Ladhoff J, et al. Human immune responses to porcine xenogeneic matrices and their extracellular matrix constituents in vitro. Biomaterials. 2010;31(14):3793–3803. doi: 10.1016/j.biomaterials.2010.01.120. doi:10.1016/j.biomaterials.2010.01.120. [Key paper identifying immunogenicity of xenogenic matrix components.] [DOI] [PubMed] [Google Scholar]
- 56.Takagi K, Fukunaga S, Nishi A, et al. In Vivo Recellularization of Plain Decellularized Xenografts With Specific Cell Characterization in the Systemic Circulation: Histological and Immunohistochemical Study. Artif Organs. 2006;30(4):233–241. doi: 10.1111/j.1525-1594.2006.00210.x. doi:10.1111/j.1525-1594.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 57.Arai S, Orton EC. Immunoblot detection of soluble protein antigens from sodium dodecyl sulfate- and sodium deoxycholate-treated candidate bioscaffold tissues. J Heart Valve Dis. 2009;18(4):439–443. [PubMed] [Google Scholar]
- 58.Ota T, Taketani S, Iwai S, et al. Novel Method of Decellularization of Porcine Valves Using Polyethylene Glycol and Gamma Irradiation. Ann Thorac Surg. 2007;83(4):1501–1507. doi: 10.1016/j.athoracsur.2006.11.083. doi:10.1016/j.athoracsur.2006.11.083. [DOI] [PubMed] [Google Scholar]
- 59.Jiao T, Clifton RJ, Converse GL, Hopkins RA. Measurements of the Effects of Decellularization on Viscoelastic Properties of Tissues in Ovine, Baboon, and Human Heart Valves. Tissue Eng Part A. 2012;18(3-4):423–431. doi: 10.1089/ten.tea.2010.0677. doi:10.1089/ten.tea.2010.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lauten A, Laube A, Schubert H, et al. Transcatheter treatment of tricuspid regurgitation by caval valve implantation-experimental evaluation of decellularized tissue valves in central venous position: Decellularized Heterotopic Valves in Severe TR. Catheter Cardiovasc Interv. 2015;85(1):150–160. doi: 10.1002/ccd.25380. doi:10.1002/ccd.25380. [DOI] [PubMed] [Google Scholar]
- 61.Honge JL, Funder J, Hansen E, Dohmen PM, Konertz W, Hasenkam JM. Recellularization of aortic valves in pigs. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2011;39(6):829–834. doi: 10.1016/j.ejcts.2010.08.054. doi:10.1016/j.ejcts.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 62**.Simon P, Kasimir MT, Seebacher G, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT® in pediatric patients. Eur J Cardiothorac Surg. 2003;23(6):1002–1006. doi: 10.1016/s1010-7940(03)00094-0. [First paper to identify risk of decellularized valves implanted in children.] [DOI] [PubMed] [Google Scholar]
- 63.Brown JW, Ruzmetov M, Eltayeb O, Rodefeld MD, Turrentine MW. Performance of SynerGraft decellularized pulmonary homograft in patients undergoing a Ross procedure. Ann Thorac Surg. 2011;91(2):416–422. doi: 10.1016/j.athoracsur.2010.10.069. discussion 422-423 doi:10.1016/j.athoracsur.2010.10.069. [DOI] [PubMed] [Google Scholar]
- 64.Perri G, Polito A, Esposito C, et al. Early and late failure of tissue-engineered pulmonary valve conduits used for right ventricular outflow tract reconstruction in patients with congenital heart disease. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2012;41(6):1320–1325. doi: 10.1093/ejcts/ezr221. doi:10.1093/ejcts/ezr221. [DOI] [PubMed] [Google Scholar]
- 65.Voges I, Bräsen JH, Entenmann A, et al. Adverse results of a decellularized tissue-engineered pulmonary valve in humans assessed with magnetic resonance imaging. Eur J Cardiothorac Surg. 2013;44(4):e272–e279. doi: 10.1093/ejcts/ezt328. doi:10.1093/ejcts/ezt328. [DOI] [PubMed] [Google Scholar]
- 66*.Lichtenberg A, Tudorache I, Cebotari S, et al. In vitro re-endothelialization of detergent decellularized heart valves under simulated physiological dynamic conditions. Biomaterials. 2006;27(23):4221–4229. doi: 10.1016/j.biomaterials.2006.03.047. doi:10.1016/j.biomaterials.2006.03.047. [One of the first papers to assess the functional performance of reseeded decellularized valves.] [DOI] [PubMed] [Google Scholar]
- 67.Lichtenberg A, Tudorache I, Cebotari S, et al. Preclinical testing of tissue-engineered heart valves re-endothelialized under simulated physiological conditions. Circulation. 2006;114(1 Suppl):I559–I565. doi: 10.1161/CIRCULATIONAHA.105.001206. doi:10.1161/CIRCULATIONAHA.105.001206. [DOI] [PubMed] [Google Scholar]
- 68.Tudorache I, Calistru A, Baraki H, et al. Orthotopic replacement of aortic heart valves with tissue-engineered grafts. Tissue Eng Part A. 2013;19(15-16):1686–1694. doi: 10.1089/ten.tea.2012.0074. doi:10.1089/ten.TEA.2012.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Theodoridis K, Tudorache I, Calistru A, et al. Successful matrix guided tissue regeneration of decellularized pulmonary heart valve allografts in elderly sheep. Biomaterials. 2015;52:221–228. doi: 10.1016/j.biomaterials.2015.02.023. doi:10.1016/j.biomaterials.2015.02.023. [Demonstrate the capacity of decellularized allografts for heart valve replacement in older individuals.] [DOI] [PubMed] [Google Scholar]
- 70.Sewell-Loftin MK, Chun YW, Khademhosseini A, Merryman WD. EMT-inducing biomaterials for heart valve engineering: taking cues from developmental biology. J Cardiovasc Transl Res. 2011;4(5):658–671. doi: 10.1007/s12265-011-9300-4. doi:10.1007/s12265-011-9300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Claiborne TE, Slepian MJ, Hossainy S, Bluestein D. Polymeric trileaflet prosthetic heart valves: evolution and path to clinical reality. Expert Rev Med Devices. 2012;9(6):577–594. doi: 10.1586/erd.12.51. doi:10.1586/erd.12.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72*.Neidert MR, Tranquillo RT. Tissue-Engineered Valves with Commissural Alignment. Tissue Eng. 2006;12(4):891–903. doi: 10.1089/ten.2006.12.891. doi:10.1089/ten.2006.12.891. [TEHV fabrication strategy that utilized geometric constraints for achieving defined matrix fiber anisotropy.] [DOI] [PubMed] [Google Scholar]
- 73.Ye Q, Zünd G, Benedikt P, et al. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2000;17(5):587–591. doi: 10.1016/s1010-7940(00)00373-0. doi:10.1016/S1010-7940(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 74.Flanagan TC, Cornelissen C, Koch S, et al. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials. 2007;28(23):3388–3397. doi: 10.1016/j.biomaterials.2007.04.012. doi:10.1016/j.biomaterials.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 75**.Flanagan TC, Sachweh JS, Frese J, et al. In Vivo Remodeling and Structural Characterization of Fibrin-Based Tissue-Engineered Heart Valves in the Adult Sheep Model. Tissue Eng Part A. 2009;15(10):2965–2976. doi: 10.1089/ten.TEA.2009.0018. doi:10.1089/ten.tea.2009.0018. [One of the first papers to identify in vivo tissue contraction problem for TEHV.] [DOI] [PubMed] [Google Scholar]
- 76.Alfonso AR, Rath S, Rafiee P, et al. Glycosaminoglycan entrapment by fibrin in engineered heart valve tissues. Acta Biomater. 2013;9(9):8149–8157. doi: 10.1016/j.actbio.2013.06.009. doi:10.1016/j.actbio.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 77.Van Loosdregt IAEW, Argento G, Driessen-Mol A, Oomens CWJ, Baaijens FPT. Cell-mediated retraction versus hemodynamic loading – A delicate balance in tissue-engineered heart valves. J Biomech. 2013 doi: 10.1016/j.jbiomech.2013.10.049. doi:10.1016/j.jbiomech.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 78.Pérez RA, Won J-E, Knowles JC, Kim H-W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv Drug Deliv Rev. 2013;65(4):471–496. doi: 10.1016/j.addr.2012.03.009. doi:10.1016/j.addr.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Sodian R, Sperling JS, Martin DP, et al. Technical Report: Fabrication of a Trileaflet Heart Valve Scaffold from a Polyhydroxyalkanoate Biopolyester for Use in Tissue Engineering. Tissue Eng. 2000;6(2):183–188. doi: 10.1089/107632700320793. doi:10.1089/107632700320793. [DOI] [PubMed] [Google Scholar]
- 80.Filova E, Straka F, Mirejovsky T, Masin J, Bacakova L. Tissue-engineered heart valves. Physiol Res. 2009;58:S141–S158. doi: 10.33549/physiolres.931919. [DOI] [PubMed] [Google Scholar]
- 81**.Shinoka T, Breuer CK, Tanel RE, et al. Tissue engineering heart valves: valve leaflet replacement study in a lamb model. Ann Thorac Surg. 1995;60(6 Suppl):S513–S516. doi: 10.1016/0003-4975(95)00733-4. [First published study using a de novo aortic valve tissue replacement.] [DOI] [PubMed] [Google Scholar]
- 82.Breuer CK, Shin'oka T, Tanel RE, et al. Tissue engineering lamb heart valve leaflets. Biotechnol Bioeng. 1996;50(5):562–567. doi: 10.1002/(SICI)1097-0290(19960605)50:5<562::AID-BIT11>3.0.CO;2-L. doi:10.1002/(SICI)1097-0290(19960605)50:5<562::AID-BIT11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 83.Sodian R, Sperling JS, Martin DP, Stock U, Mayer JE, Vacanti JP. Tissue engineering of a trileaflet heart valve-early in vitro experiences with a combined polymer. Tissue Eng. 1999;5(5):489–494. doi: 10.1089/ten.1999.5.489. [DOI] [PubMed] [Google Scholar]
- 84.Sodian R, Hoerstrup SP, Sperling JS, et al. Tissue engineering of heart valves: in vitro experiences. Ann Thorac Surg. 2000;70(1):140–144. doi: 10.1016/s0003-4975(00)01255-8. [DOI] [PubMed] [Google Scholar]
- 85.Sodian R, Hoerstrup SP, Sperling JS, et al. Early In Vivo Experience With Tissue-Engineered Trileaflet Heart Valves. Circulation. 2000;102(suppl 3):Iii - 22–Iii - 29. doi: 10.1161/01.cir.102.suppl_3.iii-22. doi:10.1161/01.CIR.102.suppl_3.III-22. [DOI] [PubMed] [Google Scholar]
- 86.Ghanbari H, Viatge H, Kidane AG, Burriesci G, Tavakoli M, Seifalian AM. Polymeric heart valves: new materials, emerging hopes. Trends Biotechnol. 2009;27(6):359–367. doi: 10.1016/j.tibtech.2009.03.002. doi:10.1016/j.tibtech.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 87.Weber B, Emmert MY, Behr L, et al. Prenatally engineered autologous amniotic fluid stem cell-based heart valves in the fetal circulation. Biomaterials. 2012;33:4031–4043. doi: 10.1016/j.biomaterials.2011.11.087. [DOI] [PubMed] [Google Scholar]
- 88.Schmidt D, Dijkman PE, Driessen-Mol A, et al. Minimally-Invasive Implantation of Living Tissue Engineered Heart ValvesA Comprehensive Approach From Autologous Vascular Cells to Stem Cells. J Am Coll Cardiol. 2010;56(6):510–520. doi: 10.1016/j.jacc.2010.04.024. doi:10.1016/j.jacc.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 89**.Weber B, Scherman J, Emmert MY, et al. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur Heart J. 2011;32(22):2830–2840. doi: 10.1093/eurheartj/ehr059. doi:10.1093/eurheartj/ehr059. [Complete study of TEHV fabrication to implantation in primates.] [DOI] [PubMed] [Google Scholar]
- 90.Dijkman PE, Driessen-Mol A, de Heer LM, et al. Trans-apical versus surgical implantation of autologous ovine tissue-engineered heart valves. J Heart Valve Dis. 2012;21(5):670–678. [PubMed] [Google Scholar]
- 91.Moreira R, Velz T, Alves N, et al. Tissue-Engineered Heart Valve with a Tubular Leaflet Design for Minimally Invasive Transcatheter Implantation. Tissue Eng Part C Methods. 2014 doi: 10.1089/ten.tec.2014.0214. doi:10.1089/ten.TEC.2014.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92**.Emmert MY, Weber B, Behr L, et al. Transapical aortic implantation of autologous marrow stromal cell-based tissue-engineered heart valves: first experiences in the systemic circulation. JACC Cardiovasc Interv. 2011;4(7):822–823. doi: 10.1016/j.jcin.2011.02.020. doi:10.1016/j.jcin.2011.02.020. [First time for the transapical delivery of tissue engineered valve into the aortic valve position.] [DOI] [PubMed] [Google Scholar]
- 93.Emmert MY, Weber B, Behr L, et al. Transcatheter aortic valve implantation using anatomically oriented, marrow stromal cell-based, stented, tissue-engineered heart valves: technical considerations and implications for translational cell-based heart valve concepts. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2014;45(1):61–68. doi: 10.1093/ejcts/ezt243. doi:10.1093/ejcts/ezt243. [DOI] [PubMed] [Google Scholar]
- 94.Emmert MY, Weber B, Falk V, Hoerstrup SP. Transcatheter tissue engineered heart valves. Expert Rev Med Devices. 2014;11(1):15–21. doi: 10.1586/17434440.2014.864231. doi:10.1586/17434440.2014.864231. [DOI] [PubMed] [Google Scholar]
- 95**.Lerakis S, Hayek SS, Douglas PS. Paravalvular Aortic Leak After Transcatheter Aortic Valve Replacement Current Knowledge. Circulation. 2013;127(3):397–407. doi: 10.1161/CIRCULATIONAHA.112.142000. doi:10.1161/CIRCULATIONAHA.112.142000. [Important study analysing risk of leak during TAVI/RAVR.] [DOI] [PubMed] [Google Scholar]
- 96.Sun B, Long YZ, Zhang HD, et al. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog Polym Sci. 2014;39(5):862–890. doi:10.1016/j.progpolymsci.2013.06.002. [Google Scholar]
- 97.Fallahiarezoudar E, Ahmadipourroudposht M, Idris A, Mohd Yusof N. A review of: Application of synthetic scaffold in tissue engineering heart valves. Mater Sci Eng C. 2015;48:556–565. doi: 10.1016/j.msec.2014.12.016. doi:10.1016/j.msec.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 98*.Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27(19):3631–3638. doi: 10.1016/j.biomaterials.2006.02.024. doi:10.1016/j.biomaterials.2006.02.024. [Rational design of TEHV materials based on natural valve leaflet elastic biomechanics.] [DOI] [PubMed] [Google Scholar]
- 99.Amoroso NJ, D'Amore A, Hong Y, Rivera CP, Sacks MS, Wagner WR. Microstructural manipulation of electrospun scaffolds for specific bending stiffness for heart valve tissue engineering. Acta Biomater. 2012;8(12):4268–4277. doi: 10.1016/j.actbio.2012.08.002. doi:10.1016/j.actbio.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fan R, Bayoumi AS, Chen P, et al. Optimal elastomeric scaffold leaflet shape for pulmonary heart valve leaflet replacement. J Biomech. 2013;46(4):662–669. doi: 10.1016/j.jbiomech.2012.11.046. doi:10.1016/j.jbiomech.2012.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Masoumi N, Larson BL, Annabi N, et al. Electrospun PGS:PCL Microfibers Align Human Valvular Interstitial Cells and Provide Tunable Scaffold Anisotropy. Adv Healthc Mater. 2014 doi: 10.1002/adhm.201300505. doi:10.1002/adhm.201300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sant S, Hwang CM, Lee S-H, Khademhosseini A. Hybrid PGS-PCL microfibrous scaffolds with improved mechanical and biological properties. J Tissue Eng Regen Med. 2011;5(4):283–291. doi: 10.1002/term.313. doi:10.1002/term.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sant S, Iyer D, Gaharwar AK, Patel A, Khademhosseini A. Effect of biodegradation and de novo matrix synthesis on the mechanical properties of valvular interstitial cell-seeded polyglycerol sebacate-polycaprolactone scaffolds. Acta Biomater. 2013;9(4):5963–5973. doi: 10.1016/j.actbio.2012.11.014. doi:10.1016/j.actbio.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hinderer S, Seifert J, Votteler M, et al. Engineering of a bio-functionalized hybrid off-the-shelf heart valve. Biomaterials. 2014;35(7):2130–2139. doi: 10.1016/j.biomaterials.2013.10.080. doi:10.1016/j.biomaterials.2013.10.080. [DOI] [PubMed] [Google Scholar]
- 105.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. doi:10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 106*.Sodian R, Weber S, Markert M, et al. Stereolithographic Models for Surgical Planning in Congenital Heart Surgery. Ann Thorac Surg. 2007;83(5):1854–1857. doi: 10.1016/j.athoracsur.2006.12.004. doi:10.1016/j.athoracsur.2006.12.004. [First paper to use 3D biofabrication technology to create a complete heart valve.] [DOI] [PubMed] [Google Scholar]
- 107.Sodian R, Schmauss D, Markert M, et al. Three-Dimensional Printing Creates Models for Surgical Planning of Aortic Valve Replacement After Previous Coronary Bypass Grafting. Ann Thorac Surg. 2008;85(6):2105–2108. doi: 10.1016/j.athoracsur.2007.12.033. doi:10.1016/j.athoracsur.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 108.Sodian R, Weber S, Markert M, et al. Pediatric cardiac transplantation: Three-dimensional printing of anatomic models for surgical planning of heart transplantation in patients with univentricular heart. J Thorac Cardiovasc Surg. 2008;136(4):1098–1099. doi: 10.1016/j.jtcvs.2008.03.055. doi:10.1016/j.jtcvs.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 109.Sodian R, Schmauss D, Schmitz C, et al. 3-Dimensional Printing of Models to Create Custom-Made Devices for Coil Embolization of an Anastomotic Leak After Aortic Arch Replacement. Ann Thorac Surg. 2009;88(3):974–978. doi: 10.1016/j.athoracsur.2009.03.014. doi:10.1016/j.athoracsur.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 110.Schmauss D, Schmitz C, Bigdeli AK, et al. Three-Dimensional Printing of Models for Preoperative Planning and Simulation of Transcatheter Valve Replacement. Ann Thorac Surg. 2012;93(2):e31–e33. doi: 10.1016/j.athoracsur.2011.09.031. doi:10.1016/j.athoracsur.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 111.Schmauss D, Gerber N, Sodian R. Three-dimensional printing of models for surgical planning in patients with primary cardiac tumors. J Thorac Cardiovasc Surg. 2013;145(5):1407–1408. doi: 10.1016/j.jtcvs.2012.12.030. doi:10.1016/j.jtcvs.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 112.Sodian R, Loebe M, Hein A, et al. Application of stereolithography for scaffold fabrication for tissue engineered heart valves. ASAIO J Am Soc Artif Intern Organs 1992. 2002;48(1):12–16. doi: 10.1097/00002480-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 113.Schaefermeier PK, Szymanski D, Weiss F, et al. Design and fabrication of three-dimensional scaffolds for tissue engineering of human heart valves. Eur Surg Res Eur Chir Forsch Rech Chir Eur. 2009;42(1):49–53. doi: 10.1159/000168317. doi:10.1159/000168317. [DOI] [PubMed] [Google Scholar]
- 114.Duan B, Hockaday LA, Kang KH, Butcher JT. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101A(5):1255–1264. doi: 10.1002/jbm.a.34420. doi:10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115*.Hockaday LA, Kang KH, Colangelo NW, et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4(3):035005. doi: 10.1088/1758-5082/4/3/035005. doi:10.1088/1758-5082/4/3/035005. [First paper to 3D print an anatomical heart valve using aqueous hydrogels.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116**.Duan B, Hockaday LA, Kapetanovic E, Kang KH, Butcher JT. Stiffness and adhesivity control aortic valve interstitial cell behavior within hyaluronic acid based hydrogels. Acta Biomater. 2013;9(8):7640–7650. doi: 10.1016/j.actbio.2013.04.050. doi:10.1016/j.actbio.2013.04.050. [3D hydrogel scaffold design for controlling local valve cell differentiation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Duan B, Kapetanovic E, Hockaday LA, Butcher JT. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2013 doi: 10.1016/j.actbio.2013.12.005. doi:10.1016/j.actbio.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lueders C, Jastram B, Hetzer R, Schwandt H. Rapid manufacturing techniques for the tissue engineering of human heart valves. Eur J Cardiothorac Surg. 2014;46(4):593–601. doi: 10.1093/ejcts/ezt510. doi:10.1093/ejcts/ezt510. [DOI] [PubMed] [Google Scholar]
- 119.Dijkman PE, Driessen-Mol A, Frese L, Hoerstrup SP, Baaijens FPT. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials. 2012;33(18):4545–4554. doi: 10.1016/j.biomaterials.2012.03.015. doi:10.1016/j.biomaterials.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 120**.Weber B, Dijkman PE, Scherman J, et al. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials. 2013;34(30):7269–7280. doi: 10.1016/j.biomaterials.2013.04.059. doi:10.1016/j.biomaterials.2013.04.059. [Application of a novel strategy to decellularize a de novo TEHV and implant as a scaffold. Evidence of rapid in vivo recellularization, but also leaflet contraction and insufficiency.] [DOI] [PubMed] [Google Scholar]
- 121.Driessen-Mol A, Emmert MY, Dijkman PE, et al. Transcatheter Implantation of Homologous “Off the-Shelf” Tissue-Engineered Heart Valves With Self-Repair Capacity: Long-Term Functionality and Rapid In Vivo Remodeling in Sheep. J Am Coll Cardiol. 2014;63(13):1320–1329. doi: 10.1016/j.jacc.2013.09.082. doi:10.1016/j.jacc.2013.09.082. [DOI] [PubMed] [Google Scholar]
- 122**.Syedain ZH, Meier LA, Reimer JM, Tranquillo RT. Tubular Heart Valves from Decellularized Engineered Tissue. Ann Biomed Eng. 2013;41(12):2645–2654. doi: 10.1007/s10439-013-0872-9. doi:10.1007/s10439-013-0872-9. [Interesting tube in tube approach creates valves with less risk of insufficiency with tissue contraction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tremblay C, Ruel J, Bourget J-M, et al. A New Construction Technique for Tissue-Engineered Heart Valves Using the Self-Assembly Method. Tissue Eng Part C Methods. 2014:140228041418006. doi: 10.1089/ten.TEC.2013.0698. doi:10.1089/ten.TEC.2013.0698. [DOI] [PubMed] [Google Scholar]
- 124*.Dubé J, Bourget J-M, Gauvin R, et al. Progress in developing a living human tissue-engineered trileaflet heart valve assembled from tissue produced by the self-assembly approach. Acta Biomater. doi: 10.1016/j.actbio.2014.04.033. doi:10.1016/j.actbio.2014.04.033. [Combine cell sheet technique and molding/suturing technique to generate living valve conduits.] [DOI] [PubMed] [Google Scholar]
- 125.Tseng H, Puperi DS, Kim EJ, et al. Anisotropic poly(ethylene glycol)/polycaprolactone (PEG/PCL) hydrogel-fiber composites for heart valve tissue engineering. Tissue Eng Part A. 2014 doi: 10.1089/ten.tea.2013.0397. doi:10.1089/ten.TEA.2013.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eslami M, Vrana NE, Zorlutuna P, et al. Fiber-reinforced hydrogel scaffolds for heart valve tissue engineering. J Biomater Appl. 2014;29(3):399–410. doi: 10.1177/0885328214530589. doi:10.1177/0885328214530589. [DOI] [PubMed] [Google Scholar]
- 127.Masoumi N, Annabi N, Assmann A, et al. Tri-layered elastomeric scaffolds for engineering heart valve leaflets. Biomaterials. 2014;35(27):7774–7785. doi: 10.1016/j.biomaterials.2014.04.039. doi:10.1016/j.biomaterials.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Daly CD, Campbell GR, Walker PJ, Campbell JH. In vivo engineering of blood vessels. Front Biosci J Virtual Libr. 2004;9:1915–1924. doi: 10.2741/1384. [DOI] [PubMed] [Google Scholar]
- 129.Yamanami M, Yahata Y, Tajikawa T, et al. Preparation of in-vivo tissue-engineered valved conduit with the sinus of Valsalva (type IV biovalve). J Artif Organs Off J Jpn Soc Artif Organs. 2010;13(2):106–112. doi: 10.1007/s10047-010-0491-2. doi:10.1007/s10047-010-0491-2. [DOI] [PubMed] [Google Scholar]
- 130**.Yamanami M, Yahata Y, Uechi M, et al. Development of a completely autologous valved conduit with the sinus of Valsalva using in-body tissue architecture technology: a pilot study in pulmonary valve replacement in a beagle model. Circulation. 2010;122(11 Suppl):S100–S106. doi: 10.1161/CIRCULATIONAHA.109.922211. doi:10.1161/CIRCULATIONAHA.109.922211. [First paper to demonstrate the potential for in vivo tissue engineering of heart valves.] [DOI] [PubMed] [Google Scholar]
- 131.Kishimoto S, Takewa Y, Nakayama Y, et al. Sutureless aortic valve replacement using a novel autologous tissue heart valve with stent (stent biovalve): proof of concept. J Artif Organs Off J Jpn Soc Artif Organs. 2015 doi: 10.1007/s10047-015-0817-1. doi:10.1007/s10047-015-0817-1. [DOI] [PubMed] [Google Scholar]
- 132.Boehler RM, Graham JG, Shea LD. Tissue engineering tools for modulation of the immune response. BioTechniques. 2011;51(4):239–240, 242, 244 passim. doi: 10.2144/000113754. doi:10.2144/000113754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mathieu P, Bouchareb R, Boulanger M-C. Innate and Adaptive Immunity in Calcific Aortic Valve Disease. [January 16, 2015];J Immunol Res. doi: 10.1155/2015/851945. http://www.hindawi.com/journals/jir/2014/851945/abs/. [DOI] [PMC free article] [PubMed]
- 134.Simionescu A, Schulte JB, Fercana G, Simionescu DT. Inflammation in cardiovascular tissue engineering: the challenge to a promise: a minireview. Int J Inflamm. 2011;2011:958247. doi: 10.4061/2011/958247. doi:10.4061/2011/958247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rippel RA, Ghanbari H, Seifalian AM. Tissue-Engineered Heart Valve: Future of Cardiac Surgery. World J Surg. 2012;36(7):1581–1591. doi: 10.1007/s00268-012-1535-y. doi:10.1007/s00268-012-1535-y. [DOI] [PubMed] [Google Scholar]
- 136.Cicha I, Rüffer A, Cesnjevar R, et al. Early obstruction of decellularized xenogenic valves in pediatric patients: involvement of inflammatory and fibroproliferative processes. Cardiovasc Pathol Off J Soc Cardiovasc Pathol. 2011;20(4):222–231. doi: 10.1016/j.carpath.2010.04.006. doi:10.1016/j.carpath.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 137.Dohmen PM, da Costa F, Yoshi S, et al. Histological evaluation of tissue-engineered heart valves implanted in the juvenile sheep model: is there a need for in-vitro seeding? J Heart Valve Dis. 2006;15(6):823–829. [PubMed] [Google Scholar]
- 138.Sohier J, Carubelli I, Sarathchandra P, Latif N, Chester AH, Yacoub MH. The potential of anisotropic matrices as substrate for heart valve engineering. Biomaterials. 2014;35(6):1833–1844. doi: 10.1016/j.biomaterials.2013.10.061. doi:10.1016/j.biomaterials.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 139.Stella JA, Liao J, Hong Y, David Merryman W, Wagner WR, Sacks MS. Tissue-to-cellular level deformation coupling in cell micro-integrated elastomeric scaffolds. Biomaterials. 2008;29(22):3228–3236. doi: 10.1016/j.biomaterials.2008.04.029. doi:10.1016/j.biomaterials.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weber M, Heta E, Moreira R, et al. Tissue-Engineered Fibrin-Based Heart Valve with a Tubular Leaflet Design. Tissue Eng Part C Methods. 2013:131019071410009. doi: 10.1089/ten.tec.2013.0258. doi:10.1089/ten.tec.2013.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brizard CP, Brink J, Horton SB, Edwards GA, Galati JC, Neethling WML. New engineering treatment of bovine pericardium confers outstanding resistance to calcification in mitral and pulmonary implantations in a juvenile sheep model. J Thorac Cardiovasc Surg. 2014;148(6):3194–3201. doi: 10.1016/j.jtcvs.2014.08.002. doi:10.1016/j.jtcvs.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 142.Tepeköylü C, Lobenwein D, Blunder S, et al. Alteration of inflammatory response by shock wave therapy leads to reduced calcification of decellularized aortic xenografts in mice†. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2015;47(3):e80–e90. doi: 10.1093/ejcts/ezu428. doi:10.1093/ejcts/ezu428. [DOI] [PubMed] [Google Scholar]
- 143.Neethling WML, Glancy R, Hodge AJ. Mitigation of calcification and cytotoxicity of a glutaraldehyde-preserved bovine pericardial matrix: improved biocompatibility after extended implantation in the subcutaneous rat model. J Heart Valve Dis. 2010;19(6):778–785. [PubMed] [Google Scholar]