Graphical Abstract

1. INTRODUCTION
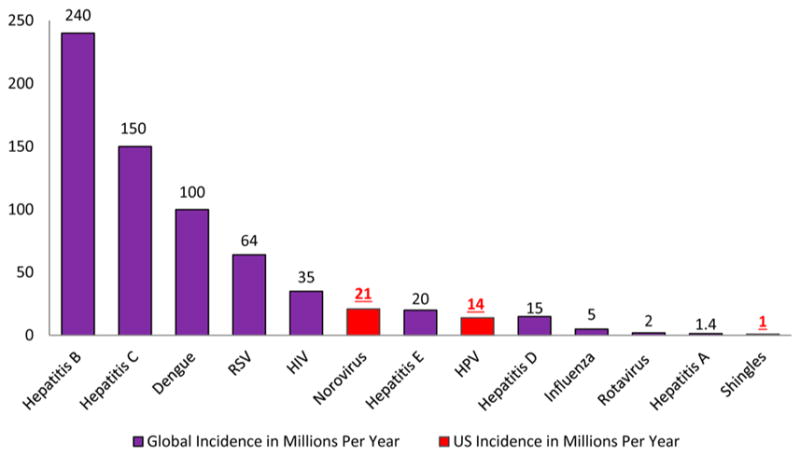
Mammals have complex biological systems and are constantly prone to infections by a wide array of bacteria, fungi, viruses, and parasites, a significant challenge to the constant development of disease-strains resistance to current drugs.1 As a result, there is always a need to identify new anti-infective agents against these organisms. An anti-infective agent is defined by Webster as “an agent capable of acting against an infection, by inhibiting the spread of an infectious agent or by killing the infectious agent outright”.2 Some of the emerging and drug-resistant infectious diseases having research priority are human immunodeficiency virus (HIV) or AIDS, hepatitis B and C viruses, respiratory infections such as influenza and respiratory syncytial virus (RSV), and dengue fever.1 Figures 1 and 2 provide us with the data in regards to the mortality and incidence rates, respectively, of people with viral diseases.3–5
Figure 1.
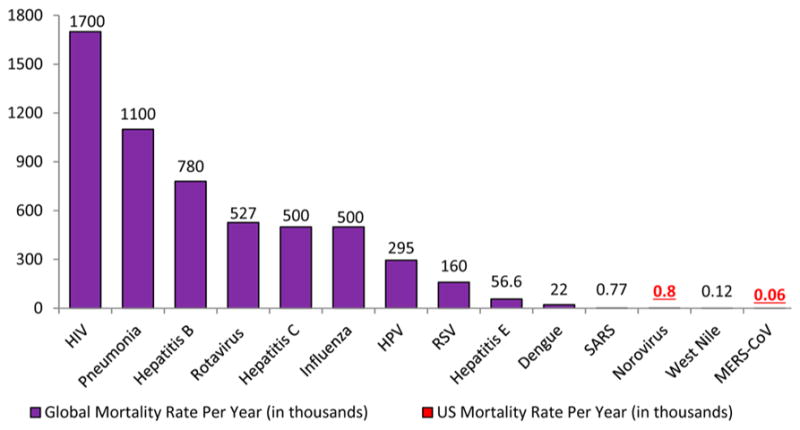
Figure 2.
Search engines utilized to identify the literature reviewed here include Google scholar, Scifinder, Pubmed, government documents from the CDC, NIH, and the World Health Organization (WHO), academic journals, and books.
2. HUMAN IMMUNODEFICIENCY VIRUS DEMOGRAPHICS
HIV-1 and HIV-2 can infect humans and cause severe immunosuppression through depletion of CD4+ cells. HIV-2 was first isolated in West Africa in 1986, and its mode of transmission is similar to HIV-1 except that it is generally less infectious and the disease develops more slowly and is milder. As the disease progresses, there are more infections with shorter durations compared to that of HIV-1. HIV-2 is seen predominately in Africa, but increasing incidences have been documented in the United States since 1987.6
HIV and the resulting AIDS-associated infections have become an international epidemic,7 resulting in over 30 million AIDS-related deaths worldwide.8 In 2011, around 2.5 million people were diagnosed with HIV, and an estimated 1.7 million men, women, and children died from the complications of AIDS. Around 34 million people were living with HIV by the end of 2011, with 69% of those infected in Sub-Saharan Africa. Following Sub-Saharan Africa, the regions most affected with HIV are the Caribbean, Eastern Europe, and Central Asia, where 1% of infected adults were living in 2011.7
In Asia, it is estimated that at least 4.8 million people are currently living with HIV, with China accounting for 780 000 of those infected individuals followed by Thailand and Indonesia. Eastern Europe and Latin America each has around 1.4 million infected people.9
In the United States, more than half a million people have died from AIDS-related complications,10 and it has been estimated that over 1.3 million people are infected with HIV,7 some of whom may not even be aware of their infection status.11 Those at greatest risk for infection include individuals engaged in high-risk behaviors, such as intravenous (IV) drug use.12 During 2007, in the United States, HIV was the fourth leading cause of death for Latinos and Hispanics between the ages of 35–44 and the sixth leading cause of death between the ages of 25–34.13 According to the National HIV/AIDS strategy,14 HIV and AIDS are most commonly seen among African Americans. AIDS was first documented by the United States CDC in 1982, in two females, one Latina and the other African American. The epidemic of AIDS began to spread among the African American population from this point forward.15
2.1. Nomenclature of HIV/AIDS
The first instances of AIDS can be traced back to 1981,6 when a strange illness began occurring in the homosexual communities; however, the pandemic is reported to have started in the late 1970s16 originating in Africa. In 1982, AIDS had different names that included gay cancer, gay-related immune deficiency (GRID),17 gay compromise syndrome,18 and community-acquired immune dysfunction. The term AIDS derived its acronym in July 1982, at a meeting in Washington, DC.19 Initially it was thought to be the disease of the “four H club” that included heroin addicts, hemophiliacs, homosexuals, and Haitians.16
The virus that was known to cause AIDS was initially named as lymphadenopathy-associated virus, or LAV, in May 1983.16 On April 23, it was announced that the virus known to cause AIDS was isolated and was named Human T-cell Leukemia Virus-III (HTLV-III). It was thought that the LAV and HTLV-III could be the same virus.20 In March 1985, the United States Food and Drug Administration (FDA) licensed the first blood test for AIDS21 created by Abbott Laboratories to identify possible antibodies (for HIV).22 The name HIV or Human Immunodeficiency Virus was given by the International Commission on Virological Nomenclature in May 1986.16
2.2. Emergence of Drugs From Marine Sources
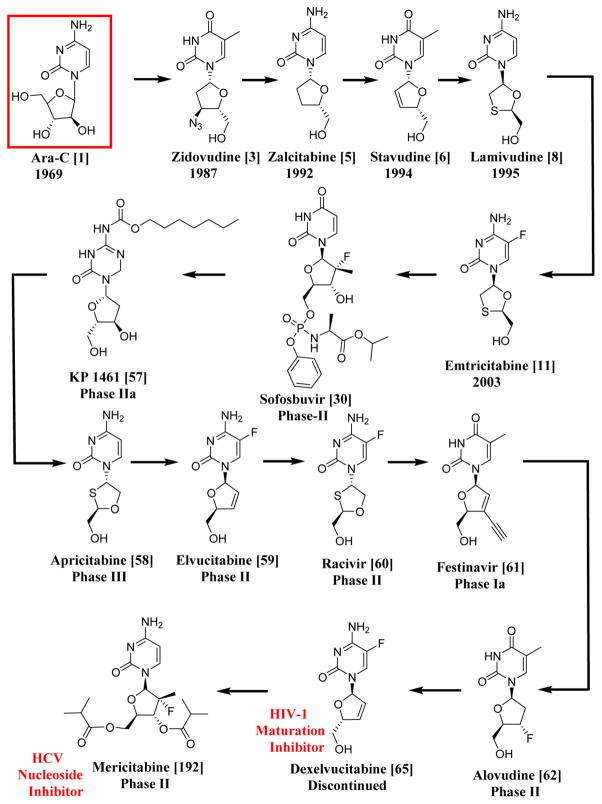
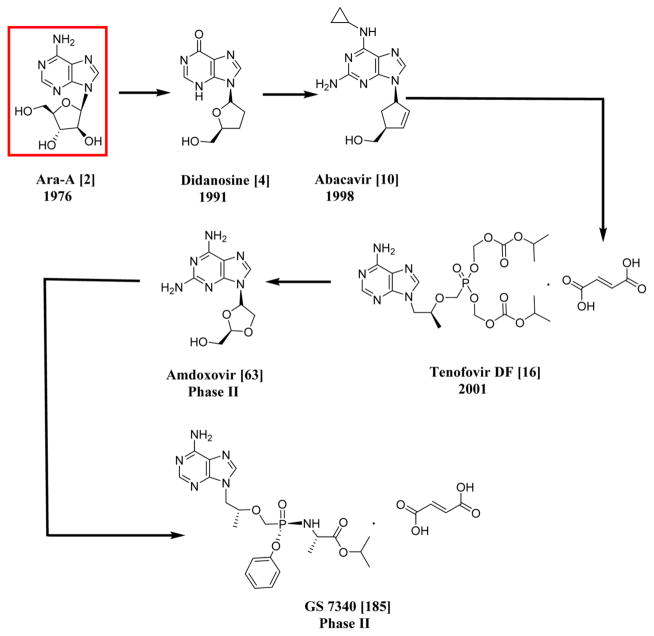
Nature plays an important role in the generation of unique drug prototypes of which about 60% of anticancer agents are derived from natural sources and around 95% of the earth’s biosphere are represented by the marine ecosystem.23 Hence, marine sources can be an invaluable source for the discovery of new compounds for the treatment of diseases like AIDS or cancer. As an example, three compounds, spongosine, spongothymidine,24 and spongouridine,25 were isolated from Cryptotethia crypta, a Caribbean marine sponge. These compounds were some of the first reported bioactive nucleosides isolated from a marine species. The first anticancer lead from marine organisms was cytosine arabinoside or ara-C (1), a synthetic derivative developed from a sponge natural product prototype.25 The syntheses of ara-C were first reported by Walwick in 1959, and Lee first reported the syntheses for ara-A (vidarabine) (2) in 1960.26 Ara-A was used as an antiviral agent for the treatment of herpes simplex virus type 1 and type 2 infections for many years, although acyclovir (83) is more widely prescribed. Ara-C is used for the treatment of acute myelocytic leukaemia and Hodgkin’s lymphoma.26 The above mentioned drugs are the first FDA-approved marine-derived products used for the treatment of disease. They were also the basis for the synthesis and development of zidovudine (3′-azido-3′-deoxythymidine, AZT or ZDV) (3), which was initially tested for cancer as an antibacterial but was later approved for HIV. Since then, extensive investigations have taken place for the treatment of various diseases from marine sources as well as for the further development of nucleosides (Schemes 1 and 2).
Scheme 1.
Systematic Representation of the Chronological Order of HIV-1 and HCV Drugs Having Similar Structures to Ara-C, the First Anticancer Lead from a Sponge; All the Drugs and Compounds Mentioned in This Scheme Are NRTIs except for Dexelvucitabine (65), Which Is an HIV-1 Maturation Inhibitor, and Mericitabine (192), Which Is an HCV Nucleoside Inhibitor
Scheme 2.
Systematic Representation of the Chronological Order of HIV-1 and HCV Drugs Having Similar Nuclei to Ara-A, an Antiviral Agent; All the Drugs and Compounds Mentioned in This Scheme Are NRTIs
2.3. History of AIDS/HIV
Jerome Horwitz synthesized AZT (3) in 1964 as an anticancer drug.16 Samuel Broder and Hiroaki Mitsuya, who were investigators at the National Cancer Institute (NCI), determined its potent anti-HIV activity in February 1985, which led to its clinical development. In 1974, it was shown to inhibit the Friend leukemia virus (murine leukemia virus) replication,27 and in 1986, the clinical trials were conducted on HIV-infected persons.16 The drug was first approved by the FDA in March 198721 under the commercial name Retrovir.16 AZT (3) in its triphosphate form inhibits HIV reverse transcriptase, thereby blocking the expression of p24 gag protein of the virus.28 AZT (3) was the first drug to be used in the treatment of AIDS,21 and the first AIDS campaign coordinated by the nation was launched in 1988.29
In October 1991, FDA approved didanosine or 2′,3′-dideoxyinosine (4).30 In June 1992, zalcitabine, also known as 2′,3′-dideoxycytidine (ddC) (5), a nucleoside reverse transcriptase inhibitor (NRTI), was approved by the FDA;8 it was mainly used in patients who were resistant to AZT.31 During the same year, a combination therapy was developed that became successful in utilizing both AZT (3) and ddC (5).32 In 1993, many cases occurred where people were resistant to AZT (3),33 and in June 1994 the FDA approved another NRTI, stavudine (6).8,34 It was also shown that the transmission of HIV from mother to child could be reduced by up to 66% with the use of AZT (3) during pregnancy.35 A recent example corresponding to this includes the antiretroviral therapy (ART) given 30 h after birth to an infant born with HIV-1 infection. ART was continued with detection of HIV-1 DNA and RNA; after the discontinuation of the therapy when the child reached 18 months of age, the child was found with undetectable HIV-1 antibodies, suggesting that early ART may help alter the long-term persistence of HIV-1 infection.36 However, the child was later found viral positive after two years off ART, suggesting continued challenges in controlling HIV infection.37
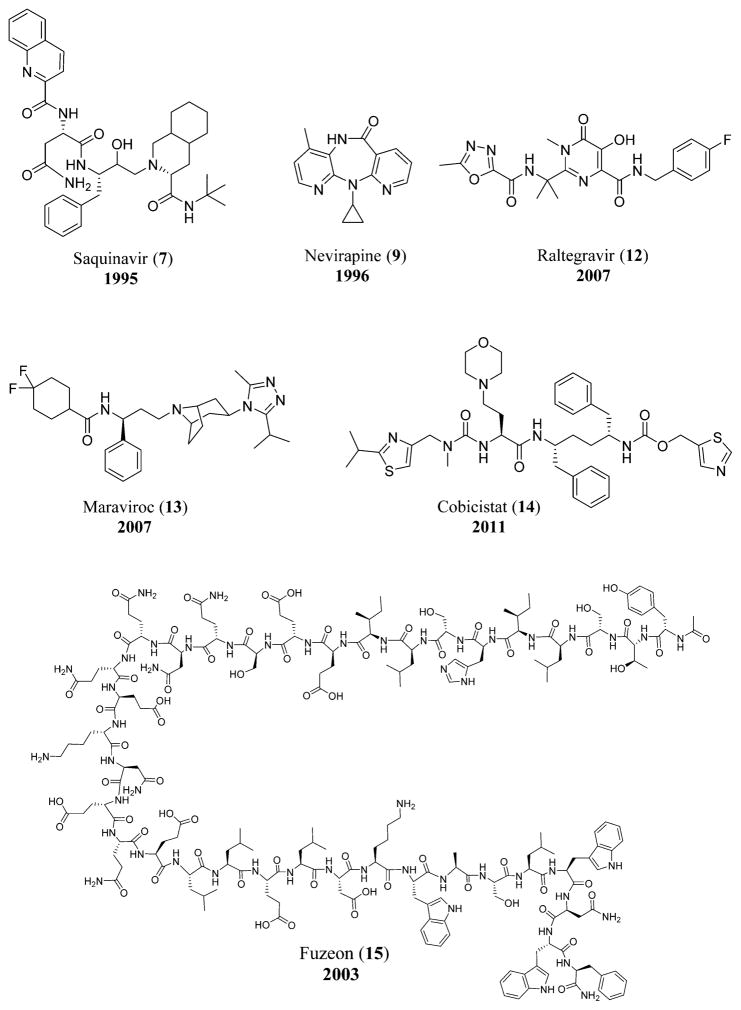
In 1995 the protease inhibitor saquinavir (invirase) (7) was approved by the FDA,38 and during the same year in November, the FDA approved the NRTI lamivudine or 3TC (8).39 In 1996 a new non-nucleoside reverse transcriptase inhibitor (NNRTI) viramune, also known as nevirapine (9), was approved by the FDA,40 and in the same year, the viral load test called Amplicor HIV-1 Monitor Test was introduced. This test gave clinicians the ability to document the progression of the disease.41 In September 1997, the FDA approved an NRTI combivir, which is a combination of lamivudine (8) and zidovudine (3).39 In 1998, two clinical trials proved that the combination of AZT (3) with ddC (5) was much more effective in prolonging life and delaying the disease compared to AZT (3) used alone.42 Abacavir (10),43 another NRTI, was approved by the FDA in December of the same year.8 In 1999, the original source of HIV was found to be from Pan troglodytes, a type of chimpanzee common in West Central Africa. It is hypothesized that the virus entered the human population when hunters were exposed to the infected blood of the chimpanzee.44
Nonoxynol-9, a spermicide, was proven to be an ineffective microbicide in the reduction of transmission of HIV during sex.45 The first HIV vaccine trial took place in Oxford, U.K., in September of 2000.46 In 2001 an Indian company, Cipla, offered AIDS drugs for as low as $1 per day,47 and in 2002 WHO provided guidelines for antiretroviral drugs and also released a list of 12 drugs that could be used for AIDS.48 T-20 (Fuzeon) (15), a fusion inhibitor, also came into existence as an injectable drug,49 bringing forth a new campaign for the control of the spread of HIV that was called ABC, short for “Abstinence, Being faithful, and Condom use”.50
A great mission of rescue was initiated by the former United States president George W. Bush in 2003 to combat AIDS in the Caribbean and in Africa.51 In the same year, Vaxgen made an announcement regarding the failure of the AIDS vaccine to reduce the HIV infection rate,52 and in November the vaccine was found to be a failure in a clinical trial in Thailand.53 On March 15, 2003, the FDA approved a new type of anti-HIV drug for the prevention of HIV entry into human cells. Fuzeon, also known as enfuvirtide or T-20 (15), was the first drug to be classified as a “fusion inhibitor”. Fuzeon was available in the form of an injection, and it could be used as combination therapy in patients resistant to other antiretroviral drugs.54 The unnatural L-nucleosides, lamivudine (8) and emtricitabine (11), became commercially available, and they revolutionized the treatment of HIV (and hepatitis B virus (HBV)), since these drugs are now part of many highly effective fixed-dose combinations.55 In July 2003, the FDA approved emtricitabine (11),8,56 and on December 1, 2003, World AIDS Day, the WHO declared a “three by five” campaign of providing antiretroviral treatment to three million people in resource-poor countries by 2005.57 Another similar policy called the “Four Free and One Care” was declared by the Chinese government that included many services such as providing free antiretroviral agents to the poor and rural communities, free testing and counseling, free drugs for the prevention of the transmission from mother to child, free schools for orphans of AIDS-related deaths, and proper care and economic help for people afflicted with HIV or AIDS.58
In 2004, for the first time in their history, the Global Fund stopped the funding scheme to fight AIDS.59 In March, the first oral fluid rapid test for HIV was approved by the United States FDA,60 and President George W. Bush’s PEPFAR, also known as the “President’s Emergency Plan For AIDS Relief” was fully implemented in June 2004. This was designed to focus on 15 countries in Africa as well as Haiti, Guyana, and Vietnam.61
In January 2005, the FDA approved an antiretroviral agent co-packaged drug regimen (lamivudine (8)/zidovudine (3) and nevirapine (9)) made by Aspen Pharmacare, a South African company. This marked the first HIV drug regimen to be approved by a non-United States based pharmaceutical company, a milestone that represented a huge turning point in Africa for providing cheaper pharmaceutical treatments for HIV-1.62 In September 2005, the patent period for AZT came to an end, allowing many pharmaceutical companies to produce the drug and sell it at greatly reduced prices.62 In 2006, for the first time, a pill that could be taken only once a day for the treatment of HIV-1 infection was approved in United States. Now widely used in first-line treatment, Atripla included a combination of three drugs: Emtriva (emtricitabine (11)), Sustiva (efavirenz (19)), and Viread (tenofovir disoproxil fumarate or TDF-(16)).63–65
In 2007, the FDA approved two new drugs, raltegravir (Isentress) (12)66 and maraviroc (Selzentry) (13),67 that could be used in patients resistant to all other classes of anti-HIV drugs. In October the initial results of a vaccine being developed by Merck pharmaceutical company were found to be ineffective, resulting in terminating the trial being conducted on hundreds of participants.68 In 2008, the American funding program PEPFAR was renewed for the treatment of HIV/AIDS, malaria, and tuberculosis for the years 2009–2013.69
In 2009 President Obama promised to lift the travel ban that had been implemented for 22 years that prevented people infected with HIV/AIDS from entering the United States,70 and finally on January 2010 the ban was lifted.71 The results from a microbicide trial CAPRISA 004 highlighted the biannual International AIDS Conference in July 2010. According to Phase IIb trial results, it was found to be safe and effective to use an antiretroviral-based gel on HIV-negative women that reduced the risk of acquiring the infection by 40%.72 In October 2011, a new drug application for a single-drug regimen also known as the “QUAD” pill was submitted by Gilead Sciences, Inc., and this included elvitegravir (53), cobicistat (14), emtricitabine (11), and TDF (16).73 Truvada, a fixed-dose combination of emtricitabine (11) and tenofovir disoproxil fumarate (16), was approved by the FDA in July 2012 for the pre-exposure prophylaxis (PrEP) of HIV and was manufactured by Gilead.74 Dolutegravir (54) or Tivicay was approved by the FDA in August 2013 and was marketed by ViiV Healthcare and manufactured by GSK for use in treatment-naïve and treatment-experienced patients.75 The history of HIV/AIDS is outlined from its conception until 2014, and all of the HIV/AIDS drugs that are currently available in the market can only be used to prevent further replication of the virus in the body and to extend the lifetime of people who are HIV-positive by a few more years. A cure to eradicate or eliminate HIV completely has yet to be identified (Figure 3).
Figure 3.
Anti-HIV-1 drugs referenced in the history. Compounds include saquinavir (7),410 nevirapine (9),410 raltegravir (12), maraviroc (13), cobicistat (14),411 and fuzeon (15).412
2.4. Description of the Virus
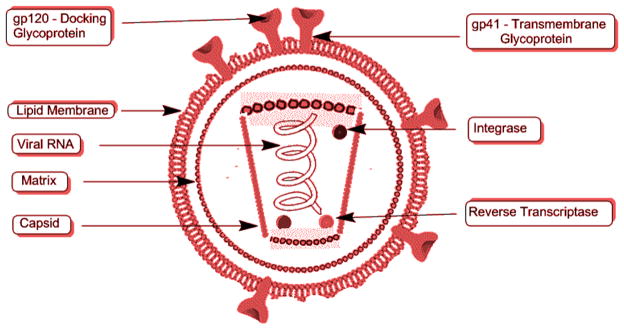
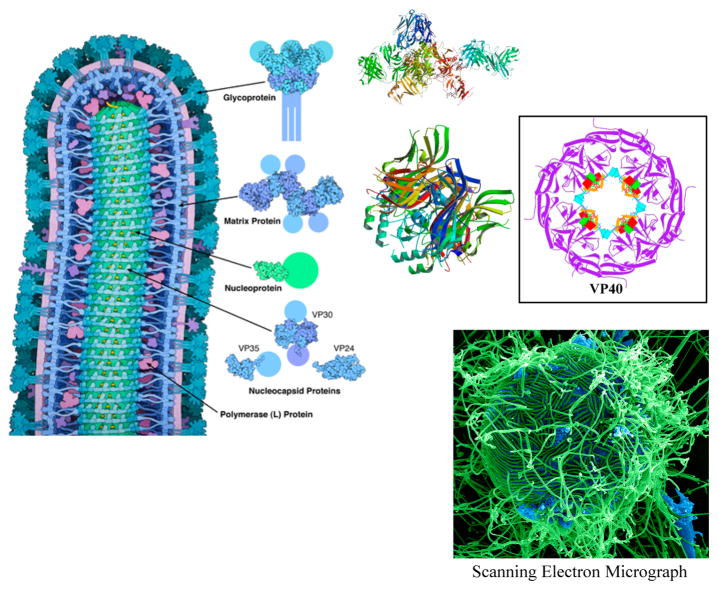
The virus particles are spherical with a diameter of one ten-thousandth of a millimeter. The viral envelope, which is the outer coat, includes two layers of lipids that are taken from the human membrane by the virus when a new virus is formed from the cell. Throughout the viral envelope, proteins including 72 copies of complex HIV-1 protein called env are embedded. The surface of the virus is spiked with these env copies and is called a “virion”. env includes glycoprotein 120 (gp120), which is a cap containing three molecules, and glycoprotein 41 (gp41), which is a stem with three molecules. These two proteins adhere to the structure of the viral envelope, and research concerning an HIV vaccine is mainly focused on these proteins.76
A bullet-shaped capsid or core is present inside the viral envelope that is made of ~2000 copies of p24, the viral protein. Surrounding the capsid are two single-stranded HIV-1 RNA, each including a complete copy of the viral genes. Gag, pol, and env are the three structural genes that carry information necessary for the formation of structural proteins for new viral particles. Similarly tat, nef, vif, vpr, vpu, and rev are the six regulatory genes responsible for the control of the virus in regards to infecting the host and producing the disease. An RNA sequence called the long terminal repeat (LTR) is present at the end of each strand of the viral RNA. These regions control the production of the new viruses. The nucleocapsid protein p7 is present in the core, and the matrix protein p17 is present between the core and the envelope. The virus also requires three enzymes for the completion of its replication cycle: reverse transcriptase, protease, and integrase (Figure 4).76
Figure 4.
Structure of HIV.
2.5. Virus Replication Cycle
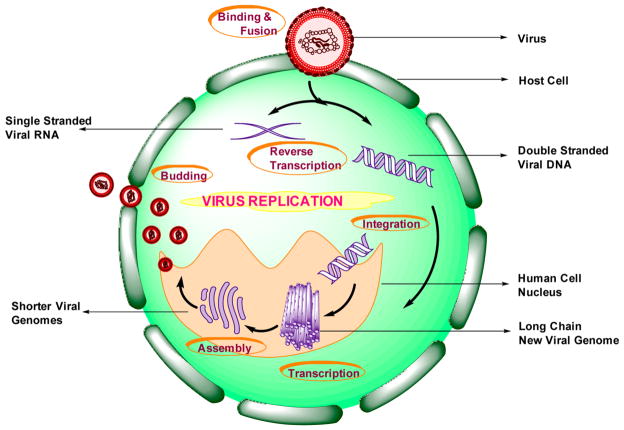
HIV can only reproduce in humans, and its replication cycle occurs in six stages, beginning with its binding to a CD4 receptor along with one of the co-receptors (among the two) on the CD4+ T-lymphocyte surface. This leads to the fusion of the virus (HIV-1) to the host cell, thereby leading to the release of viral RNA into the cell. This is called the Binding and Fusion stage. In stage II, called the Reverse Transcription stage, the reverse transcriptase enzyme present in the virus (HIV-1) converts single-stranded viral RNA to double-stranded viral DNA. Stage III is the Integration stage, where the viral DNA formed in stage II enters into the host cell nucleus and incorporates the viral DNA within the host cell’s own DNA by the viral enzyme integrase. This integrated viral DNA is called a provirus and could reproduce few or no copies or remain inactive for many years.
The fourth stage, Transcription, is where the provirus produces copies of the viral genome as well as messenger RNA or mRNA (shorter strands of RNA) with the help of the host’s RNA polymerase enzymes. This occurs whenever a signal is sent to the host cell to become active. The mRNA produced above is used to further produce long chains of viral proteins. Assembly is the fifth stage, where the long chains are cut into shorter individual proteins by the viral enzyme protease. These proteins along with the copies of viral RNA form a new virus particle. The sixth and the final stage is Budding. The above assembled virus buds out of the host cell. During this stage, the virus takes some of the cell’s outer membrane that contains sugar or protein combinations called HIV glycoproteins. These are required by the virus in order to bind with the CD4 and the co-receptors. After this is complete, these copies are now ready to infect new cells (Figures 5 and 6).77
Figure 5.
Schematic representation of the virus replication cycle.
Figure 6.
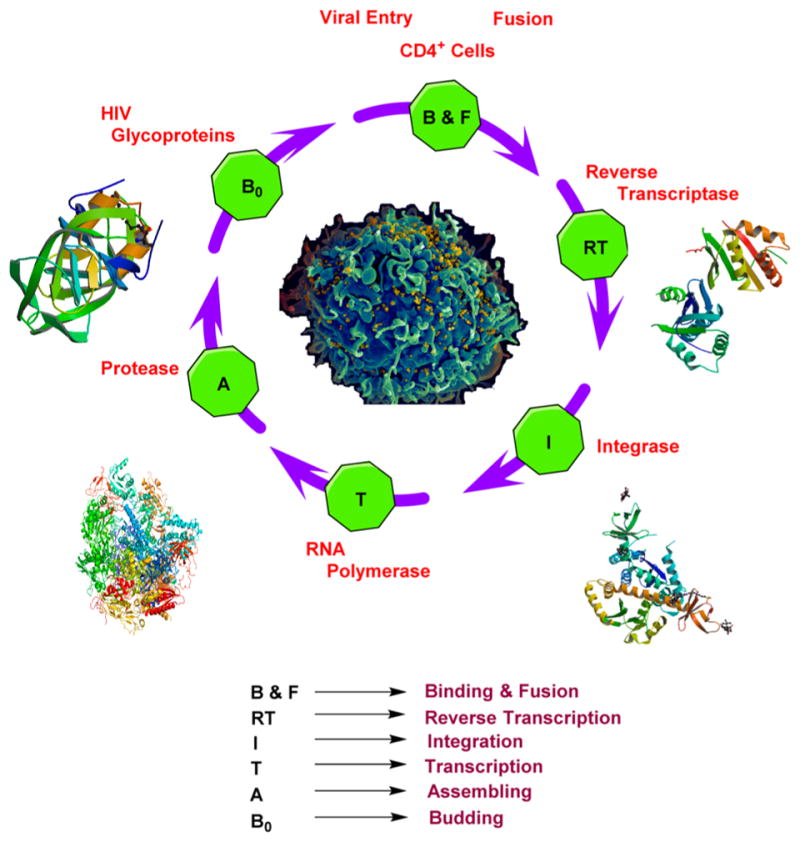
Crystal structures of HIV reverse transcriptase,413 integrase,414 RNA polymerase,415 and protease416 and scanning electron micrograph of HIV.417 Courtesy: National Institute of Allergy and Infectious Diseases, http://www.nih.gov/science/hiv/.
2.6. HIV–HCV Coinfection
HIV coinfection with hepatitis C virus (HCV) is common due to the shared routes of transmission,78 and it is known to affect about one-third of all the people infected with HIV worldwide. The prevalence can vary depending on the factors of transmission.79 In the United States, approximately 25% of people infected with HIV are also infected with HCV. The statistics greatly increase for those that have additional risk factors such as intravenous drug use.80
HIV–HCV coinfection leads to higher concentrations of HCV RNA, thereby increasing the risk of cirrhosis by accelerating the progress of HCV-related liver disease. In HIV-infected persons, the natural progression of HCV is drastically increased as a result of the HCV infection simulating opportunistic diseases.81 Transmission by sexual or vertical factors is more important in cases of HIV than HCV, but coinfection of HIV–HCV increases the risk of both vertical and sexual transmission of HCV.82 As the infection of HIV progresses, it leads to a decrease in cell-mediated immunity that further enhances HCV replication, leading to an increase in infection rate and hepatocyte injury. The immune cells cannot respond to HCV in coinfected persons because they are impaired.83 High CD4 cell counts can result in significant fibrotic progression in coinfected patients.84 HIV–HCV coinfection also leads to hepatocellular carcinoma (HCC), which is known to have a higher incidence compared to those infected with HCV alone.85
Along with liver problems, HIV–HCV coinfected people exhibit symptoms of kidney disease and have a bleaker renal prognosis.86,87 There is an increased risk of significant kidney function deterioration in HIV–HCV coinfected women.88 Other coinfected persons developed membranous nephropathy, immunotactoid glomerulopathy, mesangial proliferative glomer-ulonephritis, and immune-deposited collapsing glomerulopathy.89 In summary, these coinfections lead to complicated diagnoses, clinical progression of the disease, monitoring, treatment, and the basic immunology.78
The treatment for the coinfection of HIV–HCV usually includes dual combination therapy of interferon (IFN)–ribavirin (RBV) and highly active antiretroviral therapy (HAART). However, the coinfection increases the complexity of the treatment. Problems arise with the safety and efficacy of the drugs in these individuals.90 There are viral genome replication inhibitors that are used for the treatment of HIV–HCV coinfection.
The clinical symptoms and pathogenesis of HIV and HCV are similar. The polymerases of HIV include RNA-dependent DNA polymerase, also known as reverse transcriptase, and that of HCV includes RNA-dependent RNA polymerase, referred to as RNA replicase. Whereas HIV protease is an aspartyl protease, HCV’s protease is a serine protease. Some of the drugs belonging to different categories used in the treatment of HIV and HCV are mentioned below.
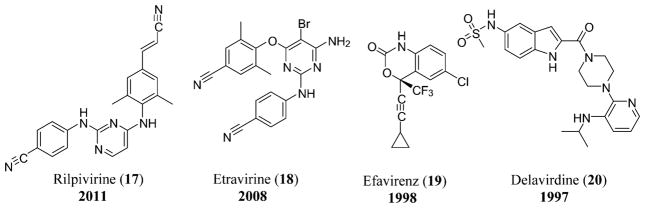
As of 2015, the only nucleotide reverse transcriptase inhibitor (NtRTI) approved for the treatment of HIV is TDF or Viread (16), although all the currently approved nucleoside analogues require phosphorylation for inhibition of HIV polymerase.91 The non-nucleoside reverse transcriptase inhibitors (NNRTIs) used in the treatment of HIV include nevirapine (9), rilpivirine or edurant (17), etravirine (18),8 efavirenz (19), and delavirdine (20) (Figure 7).92
Figure 7.
NNRTIs used for the treatment of HIV that include rilpivirine (17),410 etravirine (18),410 efavirenz (19),410 and delavirdine (20).410
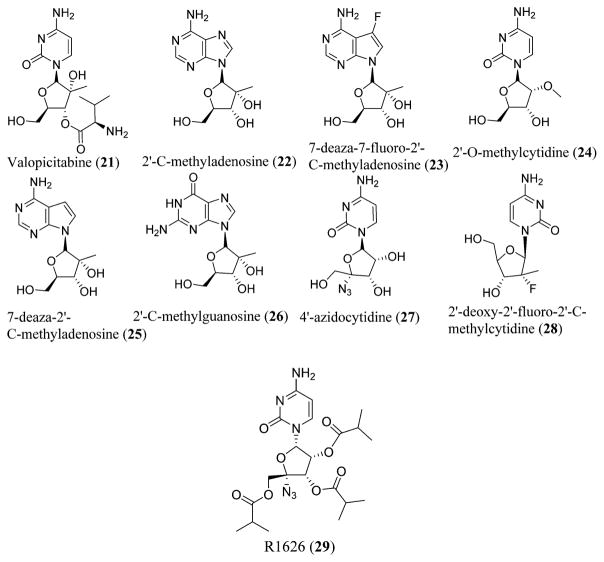
The known nucleoside RNA replicase inhibitors (NRRIs) possessing anti-HCV activity in vitro known to date include valopicitabine (21),93 2′-C-methyladenosine (22),94,95 7-deaza-7-fluoro-2′-C-methyladenosine (23),94,96 2′-O-methylcytidine (24),94 7-deaza-2′-C-methyladenosine (25),97 2′-C-methylguanosine (26),98,99 4′-azidocytidine (27),100 2′-deoxy-2′-fluoro-2′-C-methylcytidine (28),101 oral prodrugs like R1626 (29),102,103 and novel analogues of 4′-azido-2′-deoxynucleoside (Figure 8).104
Figure 8.
Various NRRIs possessing anti-HCV activity. NRRIs include valopicitabine (21), 2′-C-methyladenosine (22), 7-deaza-7-fluoro-2′-C-methyladenosine (23), 2′-O-methylcytidine (24), 7-deaza-2′-C-methyladenosine (25), 2′-C-methylguanosine (26), 4′-azidocytidine (27), 2′-deoxy-2′-fluoro-2′-C-methylcytidine (28), and R1626 (29).
To possess anti-HCV activity, the presence of a methyl group or a fluorine group at the 2′-C position or an azido group at the 4′-C position is essential. Any new nucleoside derivatives containing both substitutions in a single molecule would be an area of exploration for anti-HCV activity (Figure 9).92
Figure 9.
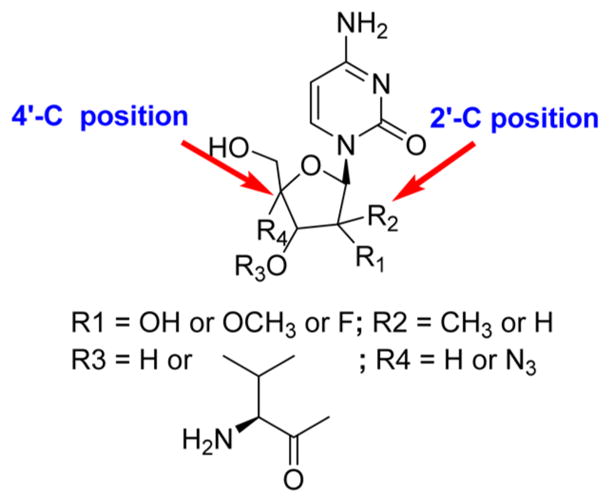
Structure–activity relationship (SAR) of anti-HCV NRRIs.
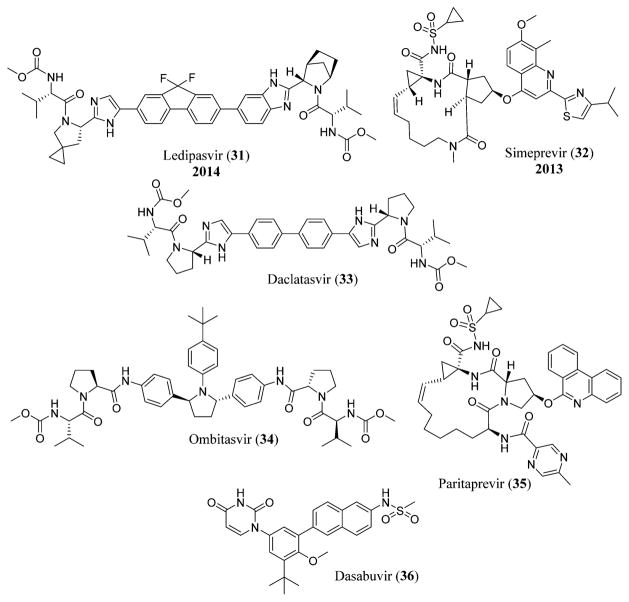
Sofosbuvir or GS-7977 (30), previously named as PSI-7977,105 is a uridine nucleotide analogue currently in Phase 2 trial for the treatment of HCV infection.106 It is a selective inhibitor of HCV NS5B polymerase. Combination with pegylated interferon and ribavirin is also being tried for the efficacy of sofosbuvir (30) in treating HCV,107 although interferon-free treatments are now more popular (e.g., the use of sofosbuvir (30) with the NS5A inhibitor ledipasvir (31), a combination called Harvoni that has been approved by the FDA in October 2014).108 Simeprevir (32), a second-generation macrocyclic compound, is a NS3/4A HCV protease inhibitor that binds non-covalently to the HCV protease and was approved by the FDA in November 2013.109 Daclatasvir (33), a NS5A replication complex inhibitor manufactured by Bristol-Myers Squibb, was in Phase III clinical trials in combination with sofosbuvir (30) for the treatment of HCV,110 and its use in combination with other antiviral drugs for the treatment of HCV was declined by the FDA in November 2014.111 The FDA approved Viekira Pak in December 2014, which is a combination of ombitasvir (34), paritaprevir (ABT-450) (35), and ritonavir (46) tablets that are co-packed with dasabuvir (36) tablets for the treatment of chronic HCV infection (Figure 10).112
Figure 10.
Structures of ledipasvir (31),418 simeprevir (32),419 daclatasvir (33),418 ombitasvir (34),420 paritaprevir (35),421 and dasabuvir (36).422
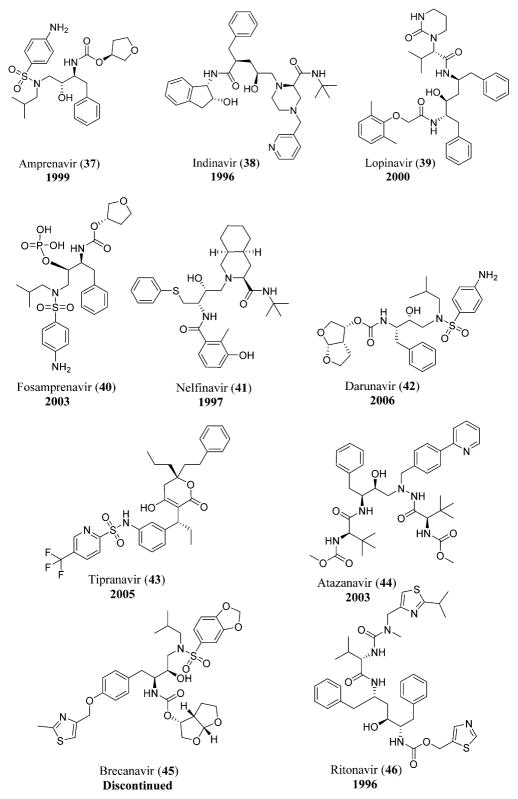
The proteases of HIV and HCV are attractive drug targets. The viral protease inhibitors used for the treatment of HIV include amprenavir (37), indinavir (38), lopinavir (39), fosamprenavir (40), nelfinavir (41),8 darunavir (42), tipranavir (43), and atazanavir (44).92 Another protease inhibitor, brecanavir (45), could be used in combination with ritonavir (46) for the treatment of HIV-1-infected persons (Figure 11).113,114
Figure 11.
HIV protease inhibitors including amprenavir (37),410 indinavir (38),410 lopinavir (39),410 fosamprenavir (40),410 nelfinavir (41),410 darunavir (42),423 tipranavir (43),410 atazanavir (44),410 brecanavir (45),424 and ritonavir (46).410
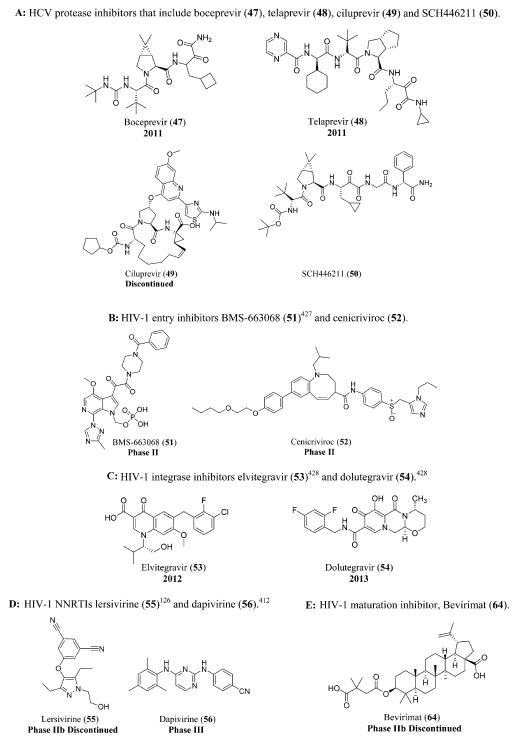
The HCV serine protease inhibitors include boceprevir (47),115 telaprevir (48),116 ciluprevir (49),117 and SCH446211 (50) (Figure 12A).118
Figure 12.
(A) HCV protease inhibitors that include boceprevir (47), telaprevir (48), ciluprevir (49), and SCH446211 (50). (B) HIV-1 entry inhibitors BMS-663068 (51)425 and cenicriviroc (52). (C) HIV-1 integrase inhibitors elvitegravir (53)426 and dolutegravir (54). (D) HIV-1 NNRTIs lersivirine (55)126 and dapivirine (56).410 (E) HIV-1 maturation inhibitor, Bevirimat (64).
Other anti-HIV-1 drugs that are still in clinical trials include entry inhibitors such as PRO 140,119 TNX-355 or ibalizumab,120 BMS-663068 (51)121 and cenicriviroc (52),122 integrase inhibitors such as elvitegravir (53)123 and dolutegravir (54),124 maturation inhibitor vivecon,125 NNRTIs like lersivirine (55)126 and dapivirine (56),127 and NRTIs like KP 1461 (57),128 apricitabine (58),129 elvucitabine (59),130 racivir (60),131 festinavir (61),132 alovudine (62),133 and amdoxovir (63) (Figure 12B, C, and D and Schemes 1 and 2).134
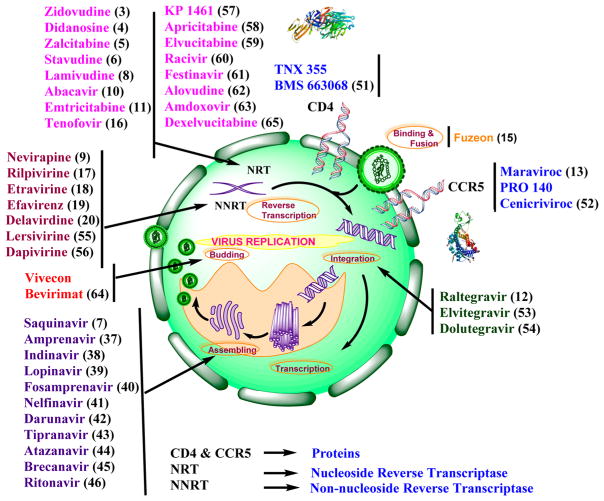
Bevirimat (64), a maturation inhibitor,135 and dexelvucitabine or reverset (65), a NRTI,136 are examples of discontinued anti-HIV-1 drugs that have undergone clinical trials (Figure 12E and Scheme 1). A schematic representation of all the anti-HIV-1 drugs that act at various stages of the viral replication cycle is shown (Figure 13).
Figure 13.
Schematic representation of all the anti-HIV-1 drugs that act at various stages of viral replication cycle including the crystal structures of CD4427 and CCR5.428
3. PNEUMONIA
Pneumonia is the second leading cause of death globally, with around 3.3 million cases annually in the United States. The proper medications to completely treat the disease state do not exist.137 Pneumonia can occur in continuum to the acute influenza syndrome when it is caused by the virus alone, termed as “primary infection”, or it could be caused as a mixed infection of viruses and bacteria that is termed as “secondary infection”,138 which often is difficult to identify the etiological pathogen with many different bacterial strains such as Streptococcus pneumonia, Mycoplasma pneumoniae, Chlamydia pneumoniae, Haemophilus influenzae, and Legionella pneumophila. The common cause of death due to pneumonia is mainly from the secondary bacterial infections due to one or several of the above mentioned strains leading to a combined bacterial/viral or post-influenza pneumonia.137
Influenza viruses belong to the family of Orthomyxoviridae and are enveloped with lipids, negative sense, single-stranded, segmented RNA viruses that exist in three forms: influenza A, B, and C. Influenza A virus is known to infect mammals, including horses and pigs, and birds, whereas influenza B and C are known to infect only humans.139
3.1. Past Pandemics
There are three known pandemics during the 20th century, which include the 1918 H1N1, Spanish flu; the 1957 H2N2, Asian flu; and the 1968 H3N2, Hong Kong flu. The Spanish flu pandemic resulted in higher morbidity and mortality compared to the 1957 or 1968 pandemics that are thought to have their origins in Asia. The 2009 A(H1N1) influenza virus was the first influenza pandemic in the 21st century.139
3.2. Transmission and Pathogenesis
The cause of transmission could be through the spread of droplets via small-sized aerosols produced from talking, coughing, or sneezing. Patients exposed to mechanical ventilation or intubations are infected through airborne transmission. The incubation period is about 24–48 h, and the viral shedding begins in 24 h in the absence of antiviral environment.138
Following inhalation, the virus deposits on the epithelium of the respiratory tract and becomes attached to the ciliated epithelial cells through the surface hemagglutinin. Some of the viral particles will be eliminated by the secretion of the IgA antibodies or mucociliary clearance. The viral particles then invade the respiratory epithelial cells and continue the viral replication. The newly formed viruses then infect the epithelial cells in large numbers and eliminate the synthesis of the critical proteins and lead to the death of the host cells.139
3.3. Treatment
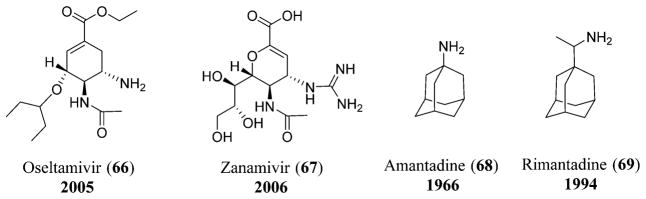
Various antiviral drugs used for the treatment of influenza include the neuraminidase inhibitors that include oseltamivir (66) and zanamivir (67)140 and adamantane drugs that include amantadine (68) and rimantadine (69).141 These antiviral drugs do not reduce the risk of complications, and vaccination is the only means of controlling or preventing influenza (Figure 14).142
Figure 14.
Neuraminidase inhibitors: oseltamivir (66) and zanamivir (67); adamantane drugs: amantadine (68) and rimantadine (69).
4. HEPATITIS B
Hepatitis B is the third leading cause of death globally and also the first major viral disease with which most people are chronically infected. Currently, more than 240 million3 people are carriers of hepatitis B and are at increased risk of developing hepatic decompensation, hepatocellular carcinoma, and cirrhosis. Hepatitis B is a chronic necro-inflammatory liver disease caused by hepatitis B virus (HBV) that could be further divided into HBeAg positive and negative chronic hepatitis B.143
4.1. Description of the Virus
HBV is the smallest known DNA virus (hepadnavirus) possessing only 3200 bases in its genome. The genome consists of circular DNA that is partly double-stranded where one strand is termed “minus”, which is almost completely circular and includes overlapping genes encoding the replicative proteins like polymerase, X, and structural proteins like surface, core, and pre-S, while the other is termed “plus”, which is short and varied in length (Figure 15).144
Figure 15.
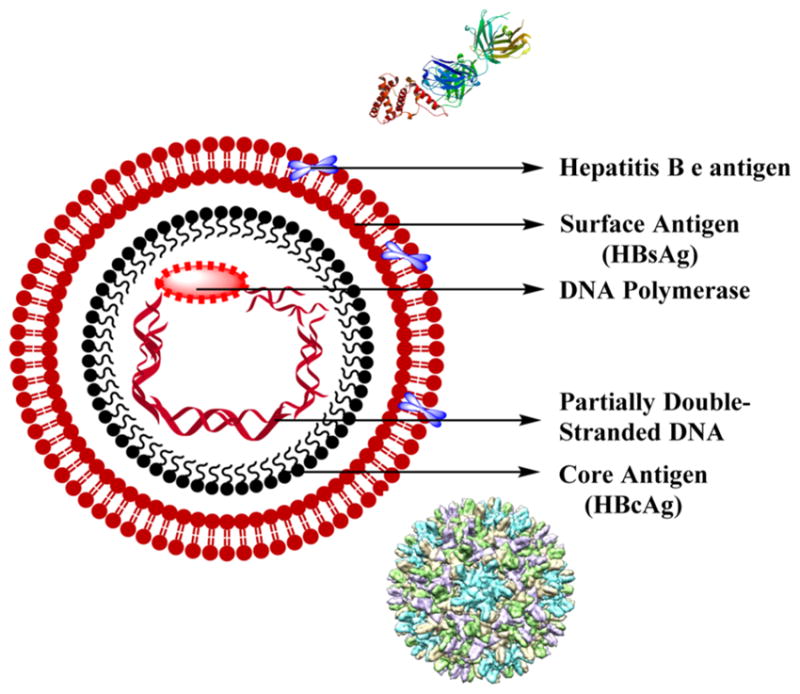
Structure of hepatitis B virus with crystal structure of HBV e antigen429 and cryo-electron microscopy (CryoEM) structure of HBV core antigen.430
ENH I and ENH II are two enhancer elements where ENH I functions competently in the hepatocytes only and is tissue-specific while ENH II acts on the surface gene promoters stimulating the transcriptional activity.144 HBV has a nucleocapsid that is a 27 nm sphere bearing a core antigen along with HBV e antigen, the viral DNA, and the DNA polymerase. The nucleocapsid is enveloped with a HBV surface antigen, HBsAg, which has the determinant a. In addition to the determinant a, the nucleocapsid also carries one of the two pairs of the subtypes w and r or d and y. This results in four subtypes of HBsAg that include ayr, ayw, adr, and adw, which represent the phenotypic expression of the HBV genotypes.145
The viral particle has four mRNA transcripts whose functions are known. The longest transcript is 3.5 kb that templates both for expression of polymerase and pre-core/core proteins and also for genome replication, while the second longest transcript that is 2.4 kb codes for HBsAg, pre-S1, and pre-S2. The third transcript is 2.1 kb that codes for HBsAg and pre-S2, while the smallest transcript is 0.7 kb encoding the X protein.144
The core gene possessing the pre-core region codes both the core antigen, HBcAg, and the cleavage product, HBeAg, which is an e antigen. Transcription of pre-core leads in cleavage by targeting the HBcAg to the endoplasmic reticulum (ER) and further secretion of HBeAg. HBcAg is the integral part of the core-particle and is essential for viral package. The pre-S gene on the surface codes the viral envelope and is essential for HBV attachment to hepatocytes.144
4.2. HBV Viral Replication
HBV viral replication is known to proceed in three stages where in the first phase the DNA strands are synthesized with the completion of the minus strand prior to the synthesis of the other strand. During the second phase, the virus polymerase acts as a reverse transcriptase, and in the final phase, the minus strand is primed at the 5′ end with a terminal protein while the plus strand is primed by oligoribonucleotide resulting from the genomic viral RNA.144
HBV binds to the surface of the cell followed by penetration into the cell. The viral core then transports into the nucleus where the circular viral DNA is further converted to covalently closed circular DNA, cccDNA, that acts as a template for the synthesis of viral RNA. HBV does not undergo integration during normal replication as seen with retroviruses. The minus strand DNA synthesis is initiated at the 3′ DR1 (short direct repeats) with polymerase as primer while the plus strand DNA synthesis is initiated at the 3′ DR2 and continues until the passage of the 5′ end of the minus strand. This leads to the production of an open circular DNA similar to the matured HBV. The matured core particles will then be packed into HBsAg/pre-S in the ER and exported out of the cell. The nucleus maintains a stable pool of cccDNA for the transport of freshly synthesized DNA back to the nucleus (Figure 16).144
Figure 16.
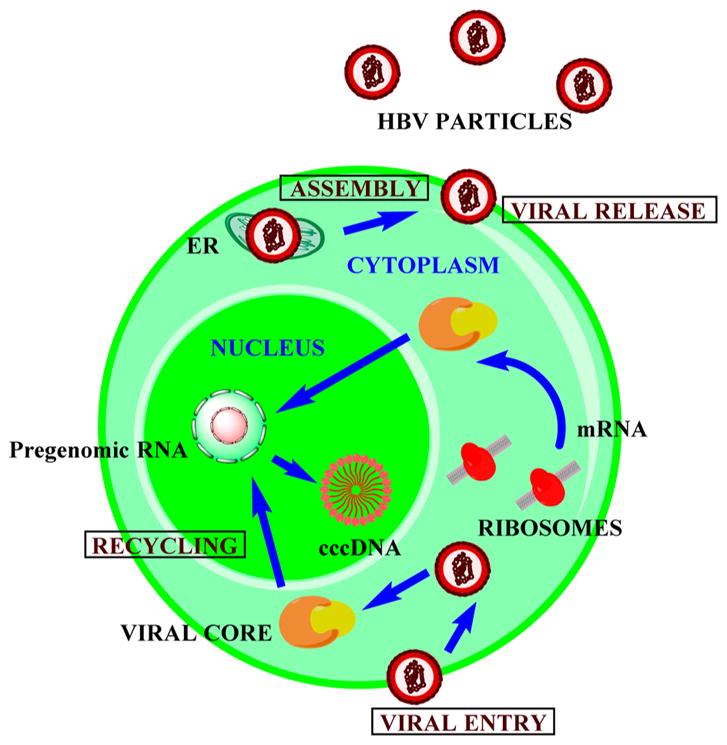
Viral replication of Hepatitis B virus.
4.3. Treatment
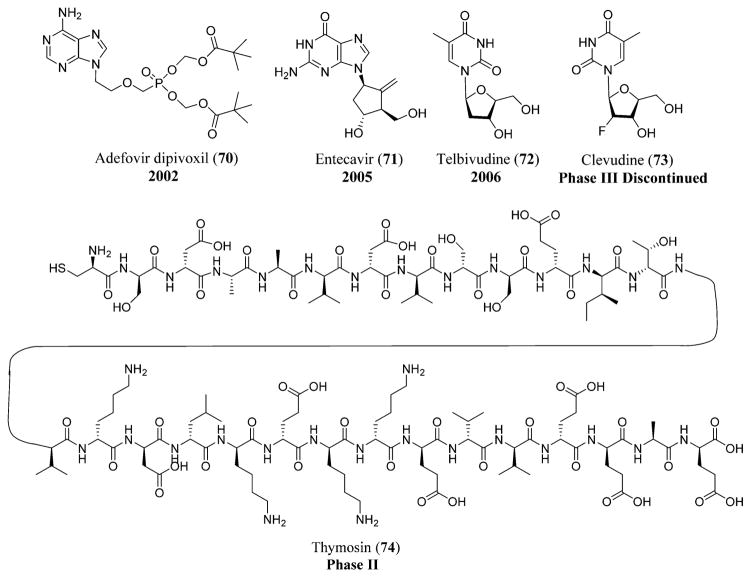
Lamivudine (8) is used in the treatment of HBV by inhibiting the viral DNA synthesis from becoming incorporated into the growing DNA and resulting in premature termination of the chain. Adefovir dipivoxil (70),146 a nucleotide analogue of AMP (adenosine monophosphate), is the prodrug of adefovir used in the treatment of HBV by inhibiting both the DNA polymerase and also reverse transcriptase activities by incorporating into the viral DNA and resulting in chain termination. Entecavir (71)147 acts by inhibiting the HBV replication at three different stages: DNA polymerase priming, negative-strand DNA reverse transcription, and positive-strand DNA synthesis.143 Entecavir (71) is also being used in persons suffering from chronic hepatitis B with decompensated liver disease.148 Telbivudine (72),149 a L-nucleoside analogue reverse transcriptase inhibitor,150 is another drug with selective potent antiviral activity against hepatitis B virus (Figure 17).143
Figure 17.
HBV drugs: adefovir dipivoxil (70), entecavir (71), telbivudine (72), clevudine (73), and thymosin (74).
Emtricitabine (11), a potent HIV inhibitor, is also used in the treatment of HBV by inhibiting the viral replication. Tenofovir DF (16), a nucleotide analogue also used in the treatment of HIV, is used in persons with hepatitis B,143 especially those who are resistant to the treatment of lamivudine (8).151 Clevudine (73),152 a pyrimidine nucleoside, is also effective in inhibiting the viral replication, but it is only approved in South Korea. Thymosin (74),153 a thymic-derived peptide, has the potency to stimulate the function of T-cells and is still under clinical trials (Figure 17 and Schemes 1 and 2).143
5. HUMAN PAPILLOMA VIRUS
Papillomaviruses were first discovered as viral particles in 1949 with around 73 more genotypes identified later. Papillomaviruses are found in mammals, birds, and reptiles like turtles. The mode of transmission is not clear, but the basal layer infections seem to play the major role. Anogenital human papilloma virus (HPV) infection is known to be transmitted through sexual contact.154
HPVs are non-enveloped, double-stranded DNA viruses belonging to the family Papillomaviridae. They are known to infect the skin mucosal surfaces and epithelial cells. The virus has a circular genome that is 8.0 kb and is encircled in a protein shell made of major, L1 and minor, L2 capsid proteins. There are seven ORFs (open reading frames) that encode the viral proteins with six “early” proteins, E1–E6. The early proteins are encoded by the transcripts present in the suprabasal and basal epithelial cells that allow viral transcription and replication. The E6 and E7 proteins play a major role in the cell transformation and immortalization. The L1 ORF encrypts the viral protein shell and its surface, while the L2 ORF encrypts the capsid mass, playing a major role in the viral genome encapsulation. The L1 protein also helps in assembling the structures to virus-like particles, VLPs.155
HPV results in various cancers and genital warts, especially cervical cancer, with more than 100 types of HPV;156 the first FDA-approved test for its identification was the cobas HPV test in 2011, which is a follow-up to the Pap test.157 The treatment of HPV does not include specific antiviral therapy except for the presence of lesions, and two vaccines have been licensed in the United States against HPV 16 and 18 types causing cervical cancer.156
6. RESPIRATORY SYNCYTIAL VIRUS
Respiratory syncytial virus (RSV) is considered the major pediatric respiratory pathogen,158 which was first isolated from a chimpanzee,159 causing life threatening illness during the first few months. RSV has been placed in the genus Pneumovirus belonging to the family Paramyxoviridae.158
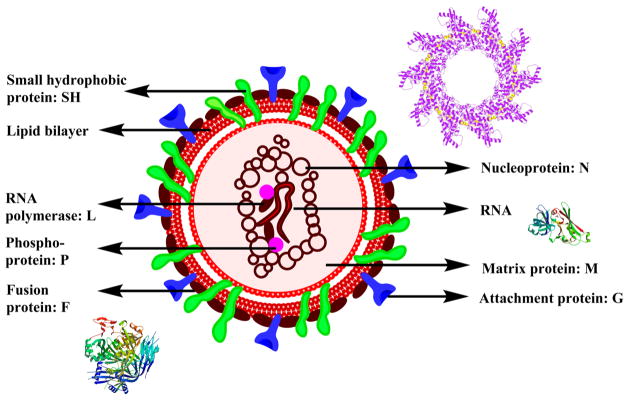
RSV is a medium-sized, linear, enveloped RNA virus with 10 viral polypeptides of which 8 are structural proteins with 7 largest that include SH, L, F, P, M, N, and G and two NS proteins, NS1 and NS2. The polymerase (L), nucleoprotein (N), and phosphoprotein (P) are the viral capsid proteins linked with the mRNA genome. The non-glycosylated membrane proteins M and M2 are the two matrix proteins. The attachment protein (G), the small non-glycosylated hydrophobic protein (SH), and glycosylated fusion protein (F) are all part of the transmembrane surface proteins. The G and F proteins play a major role in immunity (Figure 18).158
Figure 18.
Structure of RSV along with crystal structures of nucleoprotein,431 matrix protein,432 and fusion protein.433
6.1. Treatment
RSV is extremely contagious; at least half of the infants acquire RSV during their first year, and 40% of these result in lower respiratory tract diseases leading to pneumonia and/or bronchiolitis. Ribavirin (75), a synthetic nucleoside, has been approved for RSV infection, which is known to interfere with mRNA expression.158 Palivizumab (synagis), a humanized monoclonal antibody, IgG1κ,160 is another drug that was approved by the FDA in June 1998.161 Synagis is known to possess fusion and neutralizing inhibitory activity against RSV.160 The first vaccine for the treatment of RSV was developed in the 1960s, which was the alum-precipitated, formalin-inactivated vaccine (Figure 19).158
Figure 19.
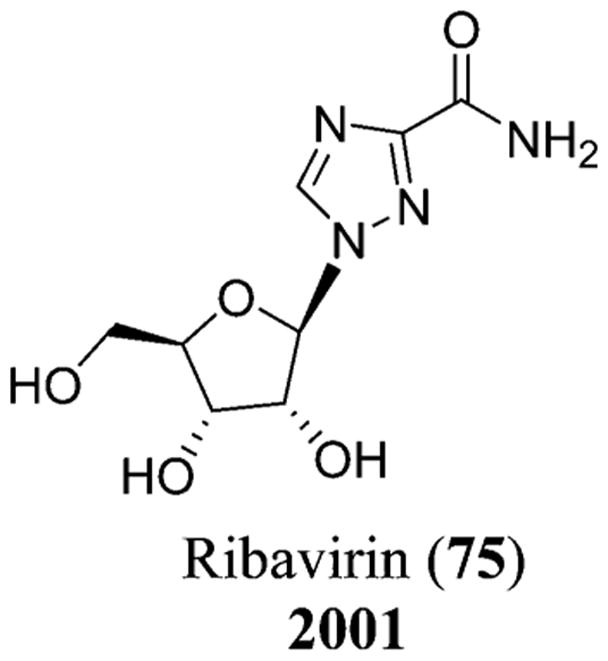
Chemical structure of ribavirin (75).
7. HEPATITIS E
Hepatitis E virus (HEV) is the causative organism of hepatitis E and was initially termed as non-A, non-B hepatitis.162 HEV was recognized in the year 2004 as the major cause of acute hepatitis worldwide with four genotypes.163 HEV spread to other developing countries apart from Asia and is a major concern in pregnant women, leading to liver disease.164
HEV has been categorized in the genus Hepevirus belonging to the family Hepeviridae. Hepatitis E viral genome is 7.2 kb in size with three ORFs and 3′ and 5′ cis elements that play a major role in HEV transcription and replication. ORF1 encodes for replicase, methyl transferase, protease, and helicase; ORF2 encodes for the protein capsid, and ORF3 encodes for a protein of non-defined function.164 The natural host for HEV is humans, with animals acting as a possible reservoir in the amplification of the virus.164
The mode of transmission for HEV could be through transfusion. There is no specific treatment for HEV infection, but ribavirin therapy has been effective in some persons, although it is contraindicated with pregnant women. Combination of ribavirin (75) and interferon-α has also been used in chronically infected patients.163 Sofosbuvir (30) was reported at the EASL (European Association for the Study of the Liver) 2015 meeting to be a modest inhibitor of HEV in culture (Figure 19 and Scheme 1).165
8. DENGUE
Dengue and dengue hemorrhagic fever, DHF, are arthropod-borne viral infections166 known to be caused by the four virus serotypes, DEN 1–4, belonging to the genus Flavivirus. People in dengue endemic areas could have all four types of infections in their lifetime as cross-protective immunity is not provided when infected with one of the serotypes. Dengue is considered an urban disease, and the virus completes its cycle in humans via the day-biting mosquito, Aedes aegypti.167
8.1. Pandemics of Dengue
The first pandemic of dengue occurred in 1779–1780 in North America, Asia, and Africa, where simultaneous outbreaks were seen followed by a global pandemic after World War II in Southeast Asia. The geographical distribution of the multiple viral serotypes expanded to the Americas, and the Pacific region and Southeast Asia had their epidemic in the 1950s, leading to multiple hospitalizations and deaths by 1975 in many countries. The Maldives, India, and Sri Lanka had their first epidemics in the 1980s, whereas Pakistan had an epidemic in 1994. Although dengue was eradicated for a few years in the American region, it slowly migrated into the United States by 1995.167 Dengue started to emerge worldwide, and currently an estimated 100 million people are at risk annually with this viral disease.168
8.2. Dengue Virus
Dengue virus (DENV) belongs to the genus Flavivirus comprising over 70 viruses, most of which are arthropod-borne infections. The virus has a lipid envelope with an inner nucleocapsid comprising single-stranded RNA and capsid protein. The viral RNA is transformed into a polyprotein during infection, which is further cleaved into structural and non-structural (NS) proteins. The components of the matured viral particles include the envelope (E), the structural capsid (C), and the membrane (M), which are all thought to be involved in the viral replication (Figure 20).
Figure 20.
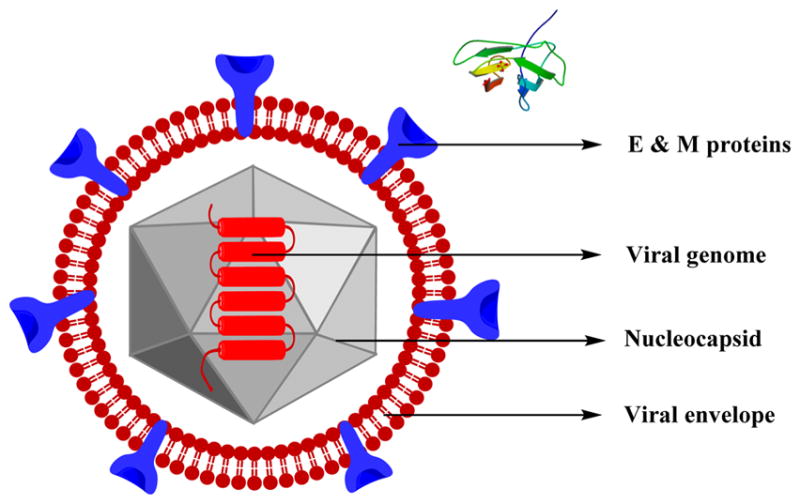
Structure of dengue virus along with the crystal structure of the envelope protein (E)434
The NS proteins include NS1, NS2A–2B, NS3, NS4A–4B, and NS5, which are only expressed in the host cell and thought to play a role in viral replication. The function of NS1 is not completely understood. NS2B, NS3, and its cofactor are known to be involved in the process of the viral polyprotein, while NS3 also shows nucleotide triphosphatase and RNA helicase activities. NS2A and NS4A–4B are hydrophobic proteins, and their functions are not completely understood but are thought to be involved in anchoring viral replicase proteins to the cell membranes and contributing to the assembly of the virions; NS5 plays a role in the capping of the viral RNA progenies. NS4A also has a role in membrane alterations and helps in the complex formation of viral replicase.169
8.3. Transmission, Characteristics, and Treatment
The primary vector of dengue is the Aedes aegypti mosquito, and transmission to humans occurs from the bites of the infected female mosquitoes. The incubation period is 4–10 days, and the infected mosquito has the ability to transmit the virus for the rest of the insect’s life. The infected humans serve as the source of the virus for the uninfected mosquitoes and could transmit the infection in 4–5 days during which the appearance of the first symptoms occur. A secondary vector for the spread of dengue is Aedes albopictus.168
Dengue fever (DENF) is a severe, flu-like infection affecting adults, infants, and young children, leading to occasional deaths. The fever is usually accompanied by vomiting, rash, severe headache, joint and muscle pains, pain behind the eyes, and swollen glands. The symptoms last for about 2–7 days following the incubation period. Dengue becomes deadly when one or more of the following symptoms occur: organ impairment, plasma leaking, respiratory stress, fluid accumulation, or severe bleeding.168 Currently, there is no proper treatment or vaccination for DENF. In cases of severe dengue, the medical practitioners and nurses give the necessary medical care and maintain the volumes of the patient’s body fluids.168
9. SEVERE ACUTE RESPIRATORY SYNDROME
Severe acute respiratory syndrome (SARS) is caused by a coronavirus170 and could be termed as atypical pneumonia, first identified in China in Guangdong Province, that later resulted in the spread to many countries.171 Coronaviruses belong to the family that includes enveloped viruses where the replication occurs in the host-cell cytoplasm. They include a plus sense, single-strand RNA with a 3′polyadenylation tract and a 5′cap structure. Following the infection, the 5′ORF of the virus is transformed to a polyprotein that is further cleaved by the proteases, releasing many nonstructural proteins that include the ATPase helicase, Hel, and the RNA-dependent RNA polymerase, which are responsible for the viral replication and protein synthesis (Figure 21).171
Figure 21.
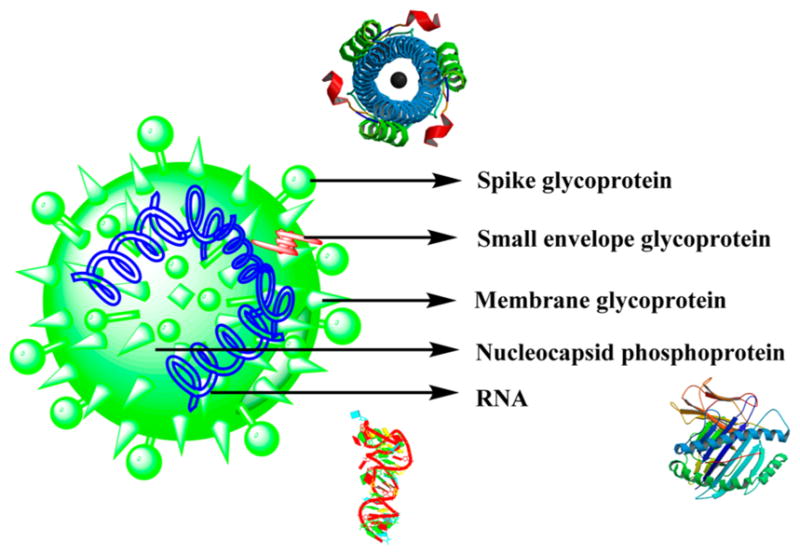
Structure of the SARS virus along with the crystal structures of spike glycoprotein,435 nucleocapsid phosphoprotein,436 and RNA.437
The viral membrane includes the major proteins spike, S, and membrane, M, which insert into the ER of the Golgi compartment while the RNA plus strands accumulate in the nucleocapsid protein. The protein–RNA complex is then associated with the membrane protein of the ER, and the formed viral particles bud into the ER lumen. The viral particles then migrate into the Golgi complex, exiting the cell by means of exocytosis.171
There are no FDA-approved drugs for the treatment of SARS, although a few drugs like ribavirin have been considered but proven to be ineffective in preventing SARS viral growth inhibition.172 The literature also shows the combination therapy with lopinavir (39)–ritonavir (46) for the treatment of SARS, which is thought to reduce the viral load.173
10. NOROVIRUS
Noroviruses belonging to the family Caliciviridae (derived from calyx, meaning “cup” in Greek)174 and the genus Norovirus were discovered in the year 1972 and were previously called Norwalk-like viruses. Like other viruses, this virus also has a single-strand, plus sense RNA of 7.5 kb including three ORFs. ORF1 is known to encode the non-structural polyprotein that could be cleaved by the viral protease into 6 proteins, while ORF2 and 3 encode the major and minor capsid proteins, VP1 and VP2, respectively. The VP1 protein is involved in the formation of two domains: shell, S, and protruding, P (P1 and P2). The P2 subdomain is further involved in immune recognition and cellular interactions.175 The virus-encoded 3C-like cysteine protease [3CLpro] processes the mature polyprotein for the generation of the six non-structural proteins: p48 [NS1 and NS2], NTPase/RNA helicase [NS3], p22 [NS4], VPg [NS5], protease [NS6], and a RNA-dependent RNA polymerase [RdRp] [NS7].176
Five genotypes of noroviruses are known from the molecular characterization where GI, GII, and GIV are found in humans while GIII and GV strains are seen in cattle and mice, respectively.175 Noroviruses are the major causative organisms of acute gastroenteritis.177 The common genotype responsible for many of the outbreaks worldwide is GII.174
10.1. Transmission and Treatment
The primary mode of transmission includes either the oral or fecal routes, and the other common routes include water- or foodborne and person-to-person contacts. Humans are thought to be the only hosts for human noroviruses. The virus can sustain a wide range of temperatures and exists in various food items including fruits, vegetables, and raw oysters along with drinking water. Noroviruses also result in repeated infections and undergo mutations, resulting in the evolution of novel strains infecting the hosts. The disease results in fever, watery diarrhea, and vomiting along with other symptoms such as myalgias, headaches, and chills.174
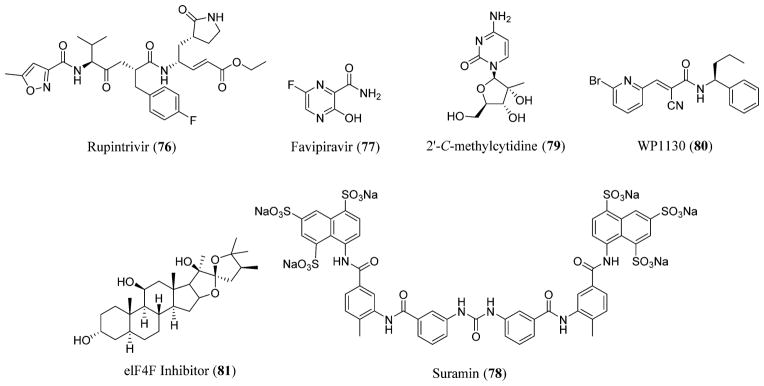
There are no known FDA-approved antiviral agents for the treatment of norovirus gastroenteritis except for oral rehydration with electrolytes and fluids. Antisecretory and antimotility drugs are used in adults suffering from diarrhea.174 Ribavirin (75) and interferons are known to inhibit the Norwalk viral replication whose therapeutic efficacy needs further evaluation.178 Rupintrivir (76), formerly known as AG7088, an irreversible inhibitor of 3CLpro that possessed in vitro antiviral activity against picornaviruses,179 is known to display anti-norovirus activity.180 Favipiravir (77), also known as T-705, currently in advanced clinical developments for influenza virus, is considered to be an RdRp inhibitor of norovirus, inhibiting the viral replication.181 Suramin (78), a naphthalene sulfonate derivative, is another RdRp inhibitor that is known to inhibit the genome replication and prevent the synthesis of viral sub-genomic RNA.182 2′-C-Methylcytidine (79), a nucleoside analogue, is considered to be a potent inhibitor of norovirus-induced diarrhea and mortality in vitro in a mouse model.183,184 Certain deubiquitinase and elF4F inhibitors (80–81) are considered promising candidates in the development of norovirus therapeutics (Figures 19 and 22).185,186
Figure 22.
Norovirus inhibitors: rupintrivir (76),438 favipiravir (77), suramin (78),439 2′-C-methylcytidine (79), deubiquitinase [WP1130] (80), and elF4F inhibitors (81).
11. MIDDLE EAST RESPIRATORY SYNDROME CORONAVIRUS
Middle East Respiratory Syndrome Coronavirus (MERS-CoV), a coronavirus that could lead to severe pulmonary disease in humans,187 was first identified in Jeddah, Saudi Arabia, in September 2012,188 from a patient suffering from renal failure and pneumonia. The infection is known to be linked geographically to the Middle Eastern countries like Saudi Arabia, the United Arab Emirates, Jordan, and Qatar.189
MERS-CoV belongs to the subfamily Coronavirinae that represents a new species in the genus Betacoronavirus that currently includes Pipistrellus bat coronavirus HKU5 and Tylonycteris bat coronavirus HKU4. This novel virus seem to relate to the viruses belonging to the families Nycteridae and Vespertilionidae that include insectivorous African and European bats, respectively. The infection is thought to be zoonotic primarily with limited transmission from human to human.189
To date, there are no effective antivirals against MERS-CoV and emphasis is placed on organ support for renal and respiratory failures. IFN-α has shown inhibition of in vitro MERS-CoV replication while its action in vivo is unknown.190
12. WEST NILE
West Nile fever is a mosquito-borne viral disease that led to sporadic outbreaks of equine and human diseases in Europe. The largest outbreak occurred in 1996–1997 in Romania, near Bucharest, which was considered the major arboviral illness in Europe.191
West Nile virus (WNV), a member of the Japanese encephalitis, belongs to the genus Flavivirus of the family Flaviviridae. This virus was initially isolated from the blood of a febrile female in Uganda in the year 1937 from the West Nile district. The primary vectors of the virus include mosquitoes, predominantly belonging to the genus Culex, and the primary hosts are wild birds.191
Currently, there are no FDA-approved drugs or licensed vaccines available for the treatment of WNV.192 An adjuvant therapy used in the treatment of WNV encephalitis is intravenous immunoglobulin (IVIG).193
13. HEPATITIS D AND A
Hepatitis D is caused by the hepatitis D virus (HDV) and leads to severe liver disease. Hepatitis D is uncommon in the United States, and it generally occurs as a coinfection with hepatitis B virus.194 HDV is a hepatotropic defective virus that is dependent on the HBV for its envelope provision. HDV includes a hepatitis B surface antigen, HBsAg, and the RNA genome, which is a rodlike, circular structure possessing self-ligation and autocatalytic cleavage properties. RNA polymerase II effects the RNA replication, and the only protein that is encoded by the HDV-RNA is the hepatitis D antigen, HDAg, whose short and long forms play a role in the morphogenesis and replication of the virus.195
The only treatment available for chronic hepatitis D is alpha interferon, although inhibiting HBV results in a decrease in hepatitis D virus replication.195 Hepatitis D complications are preventable with hepatitis B vaccine.196
Hepatitis A is caused by the hepatitis A virus (HAV), an enterovirus, belonging to the family Picornaviridae.197 The large epidemics of hepatitis A occurred in the years 1954, 1961, and 1971.198 HAV has a single-molecule RNA surrounded by a small protein capsid of 27 nm diameter and has an incubation period of 10–50 days.197
Chronic infection is not seen with hepatitis A, and the common mode of transmission is through person-to-person contact with oral ingestion as the major route. The clinical illness could be protected by giving immune globulin during the incubation period or before the exposure to the HAV, and certain hepatitis A vaccines are also effective against the disease.198
14. ROTAVIRUS
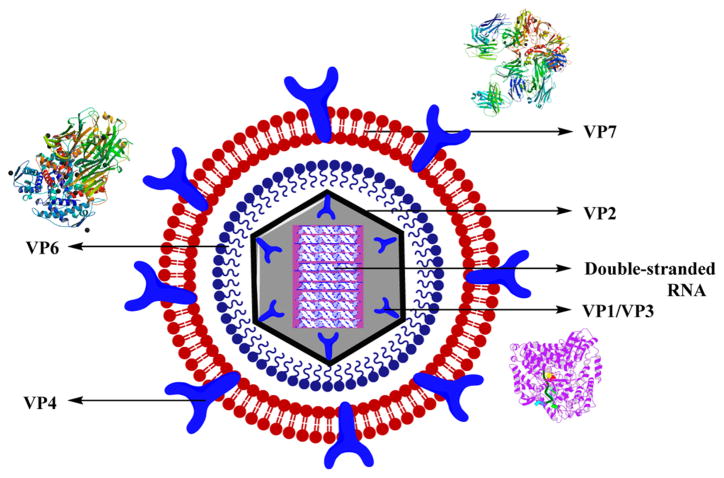
Rotavirus is the leading cause of diarrhea among children worldwide.199 The viral genome includes an 11-segmented double-stranded RNA that is enclosed in a three-layered viral capsid with four major capsid proteins–VP2, VP4, VP6, and VP7–and two minor proteins–VP1 and VP3. The co-expression of the major capsid proteins as different combinations resulted in the production of stable virus-like particles (VLPs) that are responsible for the maintenance of the functional and structural characteristics of the matured viral particles. The outer layer is composed of the glycoprotein, VP7, and dimeric spikes of VP4 responsible for inducing neutralizing antibodies, with VP4 as the viral hemagglutinin. The inner capsid has VP6 as the major protein, which constitutes more than 80% that is known to possess RNA polymerase activity. The core part includes VP1–VP3, and the 11 double-stranded RNA segments with VP2 covering >90% of the core-protein mass (Figure 23).200
Figure 23.
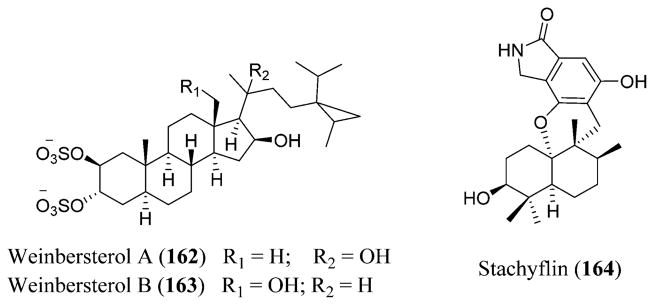
Structure of rotavirus along with the crystal structures of VP7,440 VP1,441 and VP6.442
Rotavirus is known to infect the intestine, leading to diarrhea that could last for about 8 days. There is no treatment for rotavirus infection, and this disease could be prevented through vaccination. The severity of the disease lies in dehydration that can be treated through fluids.201
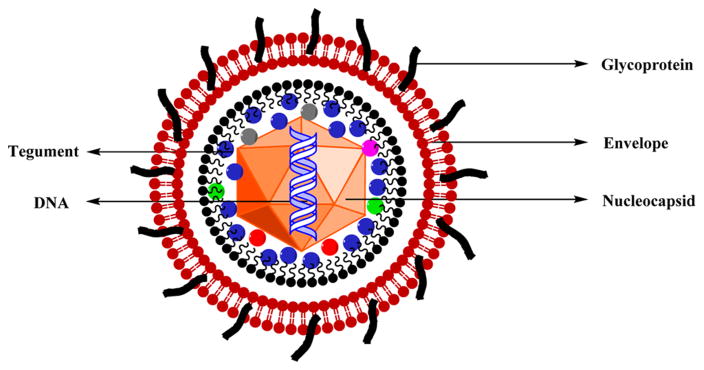
15. SHINGLES
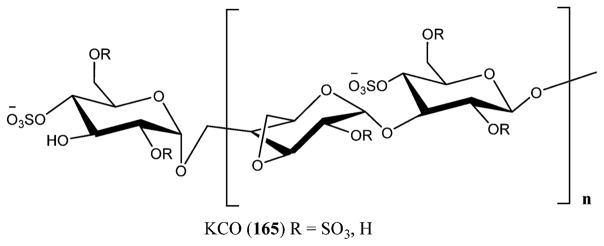
Varicella-zoster virus (VZV) is a herpesvirus leading to both chicken pox (varicella) and shingles (herpes zoster). The virus includes a nucleocapsid encapsulating the core with a linear double-stranded DNA whose arrangement is done in short and long unique segments with 69 ORFs with about 125 000 bp and a tegument made of protein that separates the capsid from the lipid envelope. The lipid envelope incorporates the main viral glycoproteins. VZV is the smallest known human herpesvirus (Figure 24).202
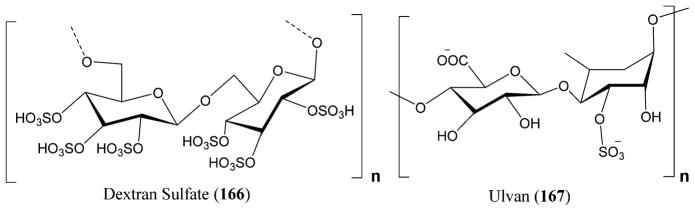
Figure 24.
Structure of varicella-zoster virus (VZV).
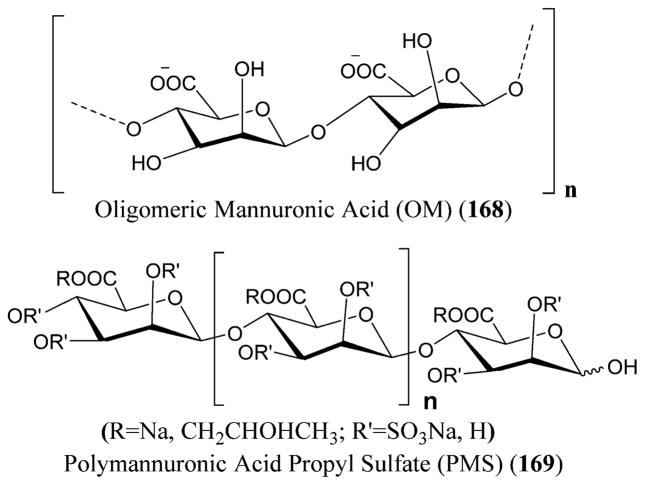
People infected with chicken pox are prone to shingles that can occur in all ages with the risk increasing with growing age. Chicken pox develops into blisters or rash, meaning that the virus is dormant in the nerve cells and can reactivate by producing shingles203 and travel through the nerves to the skin. Inflammation is seen in the nerves leading to after-pain, termed as post-herpetic neuralgia (PHN) that could be chronic and severe.204
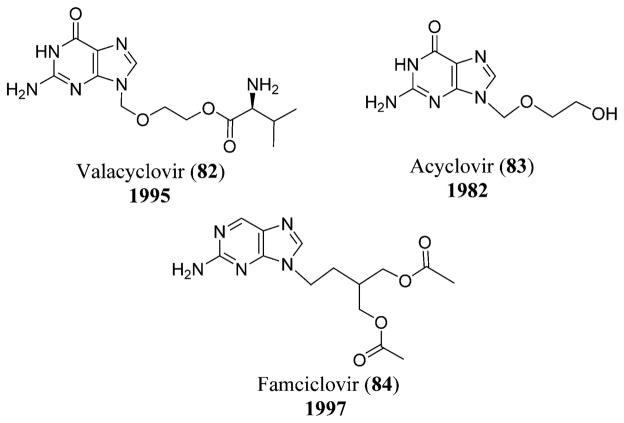
VZV transmission occurs through respiratory route, and zostavax is the vaccine used against VZV that has been approved by the FDA and is the only U.S.-licensed vaccine known to date.204 The antiviral drugs that have been approved for the treatment of VZV infections include valacyclovir (82),205 acyclovir (83),206 and famciclovir (84),207 which replaced the nucleoside analogues IFN-α and vidarabine or Ara-A (2). Acyclovir (83) and valacyclovir (82) (a valine ester derivative of acyclovir) act as competitive inhibitors and result in the chain termination of the viral DNA polymerase.202 Varizig (varicella zoster immune globulin) was approved by the U.S. FDA in December 2012 as an orphan drug that could be given after exposure to VZV (Figure 25 and Scheme 2).208
Figure 25.
Chemical structures of valacyclovir (82), acyclovir (83), and famciclovir (84).
16. HERPES SIMPLEX VIRUS
Genital herpes, a sexually transmitted disease (STD), is caused by two types of viruses, herpes simplex virus type 1 and type 2. It is highly common in the United States, and its transmission occurs through anal, oral, or vaginal sex with any person infected with the disease.209
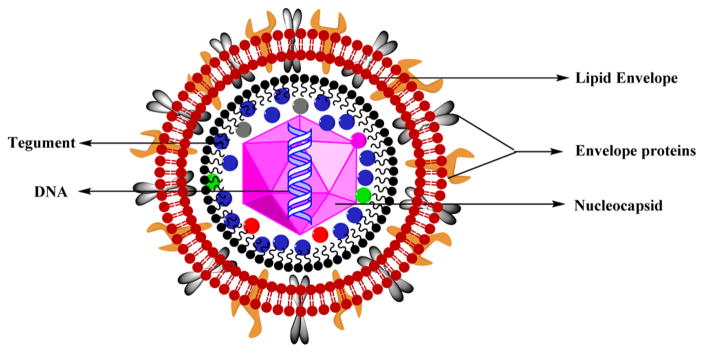
Herpes simplex virus (HSV) is the largest herpes virus belonging to the family Herpesviridae. The viruses belonging to this family are enveloped viruses including a tegument, a capsid, and a genome. The viral envelope is fragile, and the space between the capsid and the viral envelope is called a tegument, which usually contains the glycoproteins and the enzymes necessary for the viral replication. The nucleocapsid is icosahedral with about 150 hexameric and 12 pentameric capsomeres that are doughnut-shaped. The viral genome is a linear, double-stranded DNA wrapped in a core (Figure 26).210
Figure 26.
Structure of HSV.
HSV-1 and -2 have similar genome structures with 83% homology in the protein-coding and 40% homology in the sequencing regions. HSV-1 is associated with oral disease whereas HSV-2 is associated with genital disease in certain parts of the world such as Sub-Saharan Africa, where HSV-1 is known to occur in childhood and HSV-2 is sexually transmitted. In contrast, based on the anatomical site, HSV-2 is responsible for genital herpes in developed countries.211
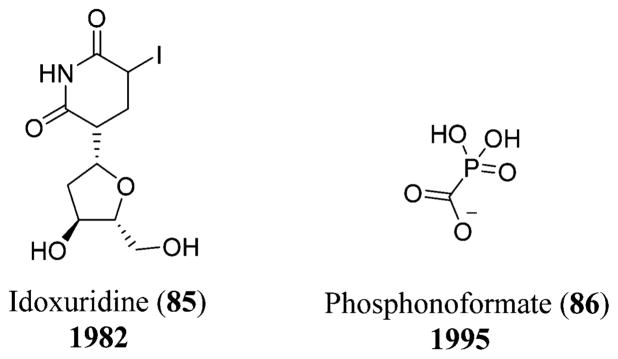
Current medications for the treatment of HSV infection include antiviral agents like acyclovir (83), vidarabine (2), idoxuridine (85),212 ribavirin (75), and phosphonoformate (86),213 of which acyclovir (83) or its valyl prodrug form known as valacyclovir are widely used. Acyclovir (83), a purine analogue, acts as a substrate for the viral thymidine kinase, thereby inhibiting the viral DNA polymerase selectively. Because of the viral resistance to acyclovir (83), new antivirals have evolved for the treatment of HSV. Phosphonoformate (86), which is a derivative of phosphonoacetic acid, is a potent inhibitor of the HSV DNA polymerase (Scheme 2 and Figures 19, 25, and 27).214
Figure 27.
Chemical structures of idoxuridine (85) and phosphonoformate (86).
17. EBOLA VIRUS
Ebola hemorrhagic fever (Ebola HF) is a viral disease caused by the Ebola virus (EBOV) that gains importance in this review due to the current, ongoing outbreak in West Africa along with Guinea, Liberia, and Uganda since March 2014.215 Reports showed around 21 deaths and 37 cases that have been reported from Guinea, 13 cases from Sierra Leone, and 1 suspected case in Liberia between May 29 and June 1 of 2014.216 The number of ebola deaths has been raised since then to 7 857 between December 24 and 27 of 2014, with 3 413 deaths in Liberia, 2 732 deaths in Sierra Leone, 1 697 deaths in Guinea, 8 deaths in Nigeria, 6 deaths in Mali, and 1 death in the United States.217 The first human outbreak of Ebola virus that has been recorded was in 1976 followed by major outbreaks in 2001 and 2003 in Gabon and the Republic of Congo.218
17.1. Description of EBOV
The EBOV is a non-segmented, enveloped, negative strand RNA virus belonging to the family Filoviridae. Four species of EBOV (Sudan, Côte d’Ivoire, Reston, and Zaire) are known to cause disease in humans, with Zaire being associated with the highest human lethality. The genome consists of seven genes responsible for the synthesis of eight proteins.219
Ebola virus is enclosed with a membrane of the infected cell enveloped with ebola glycoproteins. The inner membrane is supported with a layer of matrix proteins and possesses a central cylindrical nucleocapsid that is necessary for the storage and delivery of the RNA genome. The ebola glycoprotein binds to the cell-surface receptors to get the genome inside. The EBOV shares many features similar to the HIV envelope glycoprotein and influenza hemagglutinin covered with carbohydrate chains that would help the virus hide from the immune system. The virus could transform into a different shape when bound to the cell surface, dragging the cell and the virus close enough to cause membrane fusion.220
The matrix protein, also called VP40, helps in the shape and budding of the virus. The proteins present on the membrane help make the connection between the nucleocapsid and the membrane. The nucleocapsid, present at the center of the virus, helps protect the viral genome; however, the nucleoprotein subunits are not rigid as in other viruses, showing a wavy structure.220
Transcription of the fourth gene leads to the expression of glycoprotein that is transmembrane-linked (GP) and a secreted glycoprotein (sGP). GP remains the key target for the design of entry inhibitors and vaccines. GP is cleaved by furin post-translationally yielding GP1 and GP2 subunits, which are disulfide-linked. GP1 is known to effect the attachment to the host cells while GP2 facilitates the fusion of the host and viral membranes (Figure 28).219
Figure 28.
Structure of Ebola virus.220 Reprinted with permission from ref 210. Copyright 1989 American Chemical Society. Image from the RCSB PDB October 2014 Molecule of the Month featured by David Goodsell (DOI: 10.2210/rcsb_pdb/mom_2014_10)], crystal structures of glycoprotein,219 matrix proteins,443,444 and electron scanning microscopic image445 (Courtesy: National Institute of Allergy and Infectious Diseases, http://www.niaid.nih.gov/news/newsreleases/2014/Pages/EbolaDisparities.aspx).
There is no standard treatment for EBOV HF, but it is limited to the supportive therapy that includes balancing the electrolytes and fluids of the patients, maintaining blood pressure and oxygen supply, and treating for further complicated infections. The early symptoms of EBOV HF include fever and headache, which are difficult to diagnose for EBOV. No known treatments are yet available for humans for the treatment of Ebola virus,221 but recently favipiravir (77), a pyrazinecarboxamide derivative, showed successful suppression of the replication of Zaire EBOV both in vitro and in vivo at an IC90 of 110 μM.222
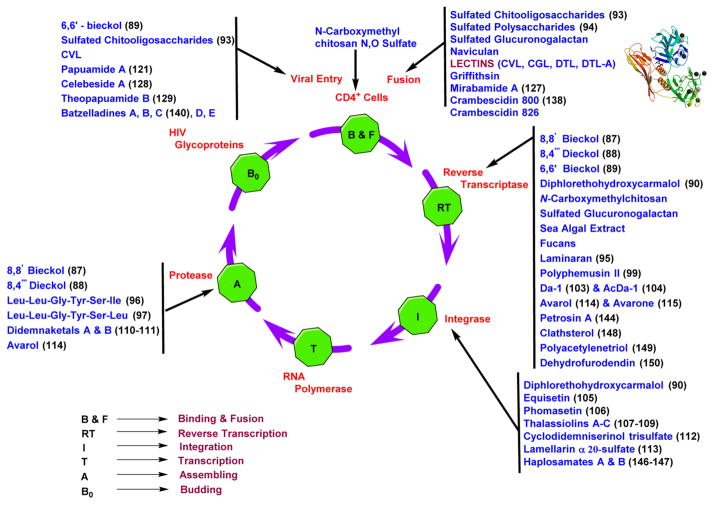
18. MARINE DRUGS FOR THE TREATMENT OF HIV/AIDS
Although there are many drugs available commercially from synthetic sources, limitations including drug resistance, side effects, cell toxicity, and long-term drug treatment are all possible explanations for the failure of the previously mentioned anti-HIV drugs. In addition, the evolution and development of nucleoside antivirals reveal the tremendous potential marine products have for the identification of novel prototypes and also reveals the necessity of developing drugs from natural resources such as the marine environment.223 Marine species cover over two-thirds of the planet, making them a significant source for the production of novel compounds with possibly fewer adverse effects and higher inhibition activity.224 The compounds obtained from marine sources that are discussed in the following sections were found to possess anti-HIV-1 activity (Figure 29).
Figure 29.
Schematic representation of the marine metabolites that are active at various stages of the viral replication cycle including the crystal structure446 of lectin.
18.1. Phlorotannins
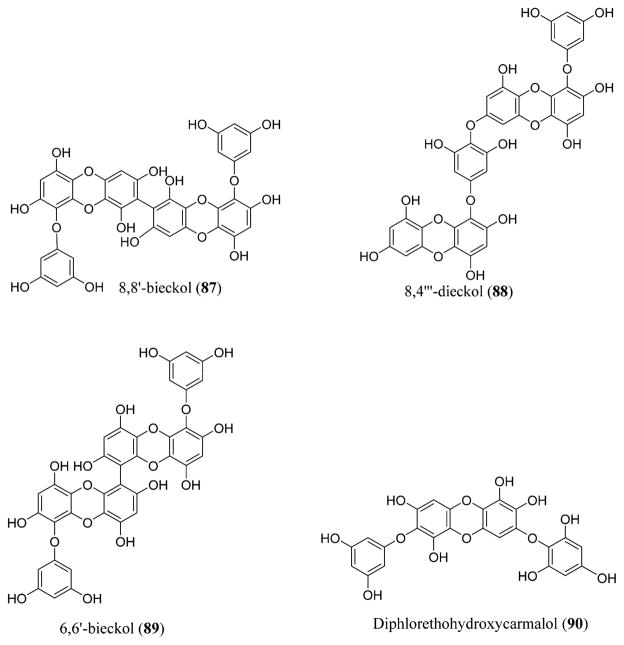
Phlorotannins are tannin derivatives that were isolated from brown algae and are biosynthesized by the polymerization of the phloroglucinol monomer units acquired from the pathway of acetatemalonate. These are highly water-soluble compounds.225,226 They can be classified into four different categories: phlorotannins containing an ether linkage (fuhalols and phlorethols), phenyl linkage (fucols), phenyl and ether linkage (fucophloroethols), and dibenzodioxin linkage (eckols).227 8,4‴-Dieckol (88) and 8,8′-bieckol (87) are isolated from Ecklonia cava, a brown algae, and show inhibitory activity on HIV-1 reverse transcriptase and protease at inhibitory concentration (IC50) values of 5.3 and 0.5 μM, respectively.228 This is due to the inhibition of the gp41 six-helix bundle formation.229,230 6,6′-Bieckol (89), a natural derivate in Ecklonia cava, possesses lytic effects, causes p24 antigen production, and has inhibitory activity against HIV-1-induced syncytia formation. It showed selective inhibition against the HIV-1 entry and the activity of HIV-1 reverse transcriptase enzyme at an IC50 of 1.07 μM.231 Diphlorethohydroxycarmalol (90), derived from Ishige okamurae, also possessed inhibitory activity against HIV-1. It inhibits HIV-1 reverse transcriptase and integrase at IC50 values of 9.1 and 25.2 μM, respectively (Figure 30).232
Figure 30.
Phlorotannins 8,8′-bieckol (87), 8,4‴-dieckol (88), and 6,6′-bieckol (89) isolated from a brown algae, Ecklonia cava, and diphlorethohydroxycarmalol (90) isolated from Ishige okamurae.
ADMET predictor shows that 8,8′-bieckol (87), 8,4‴-dieckol (88), 6,6′-bieckol (89), and diphlorethohydroxycarmalol (90) violate three criteria of the Lipinski guidelines. These compounds also show low permeability and tend to be poor at permeating the cell membranes based on the polar surface area.
6,6′-Bieckol (89), a phloroglucinol derivative, did not exhibit any cytotoxicity at concentrations that inhibited HIV-1 replication almost entirely.231 The phloroglucinol derivatives exhibited HIV-1 inhibition similar to that of flavonoids where they block the interaction between reverse transcriptase (RT) and the RNA template.233 6,6′-Bieckol (89) is a viral entry inhibitor, and so the above-mentioned factors like permeability may not be a problem; this could be considered as an important lead as this compound could inhibit the viral entry.
Most of the currently synthesized and FDA-approved drugs could only inhibit the virus at various replication stages. Hence, the above-mentioned limitations for various compounds could be overcome by changing the route of administration (using intravenous or subcutaneous (SC) instead of oral) (Table S1).
18.2. Chitin, Chitosan, and Chitooligosaccharide Derivatives
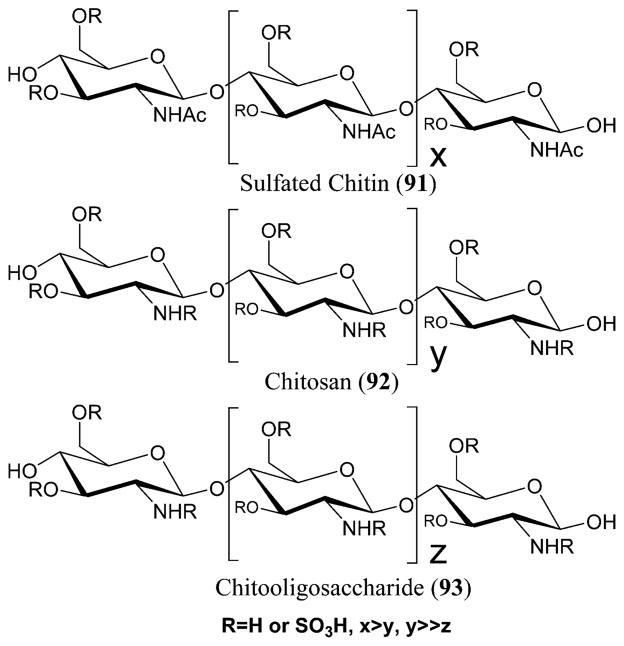
Chitin is widely found in crustaceans, fungi, invertebrates, and insects.234 It is a long-chain polymer of N-acetylglucosamine that is most abundantly seen in the shells of shrimp and crabs.235 Chitosan is formed by deacetylating chitin. The sulfated derivatives of chitin (91) and chitosan (92) possess activities including anti-HIV-1, antimicrobial, antioxidant, and others.223 N-carboxymethylchitosan N,O-sulfate (NCMCS), a derivative of N-carboxymethyl chitosan, is known to inhibit the transmission of HIV-1 in human CD4+ cells. This inhibition is due to the blockade of the interactions between the glycoprotein receptors present on the viral coat and the target proteins present on the lymphocytes, thereby inhibiting the HIV-1 reverse transcriptase.236 The sulfation at the 2 and 3 positions led to the complete inhibition of HIV-1 infection to T-lymphocytes at 0.02 μM concentrations without any cytotoxicity. These results show that the biological activity of the sulfated chitins can be controlled by changing the sulfate group position.237 Chitosan is converted to chitooligosaccharides to improve its water solubility and, therefore, its biological activity.223
Low molecular weight sulfated chitooligosaccharides (SCOSs) (93) are known to possess anti-HIV activity.238 They show lytic effects and inhibit HIV-1-induced syncytia formation at median effective concentration (EC50) values of 1.43 and 2.19 μg/mL, respectively. The p24 antigen production could be suppressed at EC50 values of 7.76 and 4.33 μg/mL for HIV-1Ba-L and HIV-1RF, respectively.238 They also inhibited viral entry and cell fusion by preventing the bond between gp120 of the HIV and CD4 surface receptor (Figure 31).223
Figure 31.
Sulfated chitin (91), chitosan (92), and chitooligosaccharide (93) derivatives.
ADMET predictor shows that sulfated chitins violate three criteria of the Lipinski guidelines whereas the chitosans violate all four criteria. In addition, they also show low permeability, low flexibility, and a tendency to not permeate the cell membranes. However, as per the mechanism of action, these are known to inhibit the viral entry and fusion and so permeability could not be considered as a limiting factor.
SCOSs inhibit HIV-1 replication by binding to the V3 loop of gp120 and thereby interfering with the gp120–CD4 binding.238 They possess a high rate of intestinal absorption, which is a crucial property for a drug candidate.239 The main drawback with chitosan and its sulfated oligosaccharide derivatives is its high anti-coagulant activity that limits SCOS to be clinically tested and approved on infected HIV subjects.240 The above limitation could be overcome by SAR (structure–activity relationship) studies and by trying various medicinal chemistry alterations to alleviate the anticoagulant activity (Table S1).
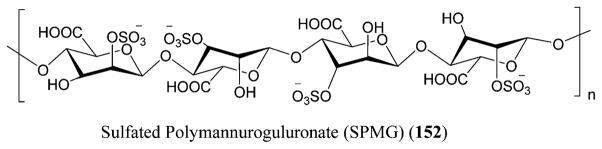
18.3. Sulfated Polysaccharides
Sulfated polysaccharides (94) are macromolecules that are chemically anionic and present in marine algae along with mammals and invertebrates, although marine algae are the major source.241,242 They also possess anti-HIV-1, along with anticancer and anticoagulant activities (Figure 32).223
Figure 32.
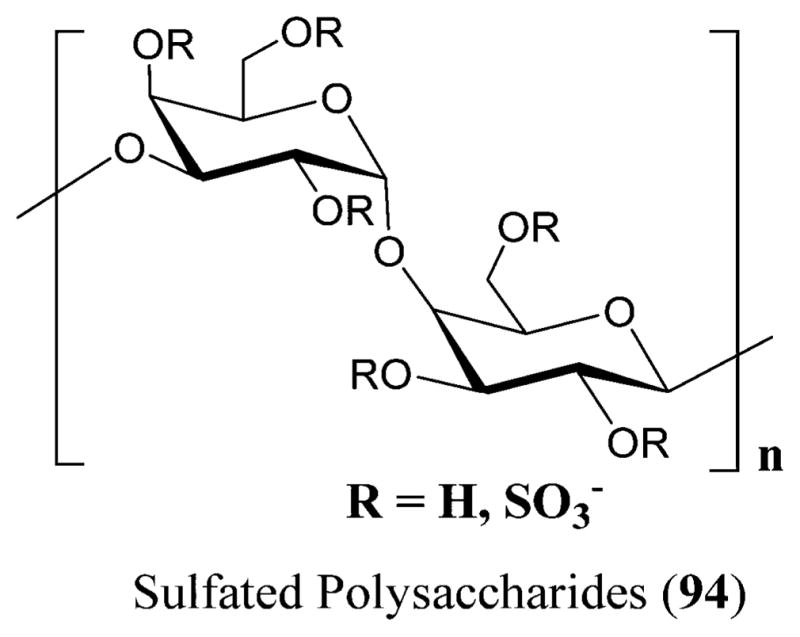
Sulfated polysaccharides (94) isolated from red seaweeds.447
The SPs (sulfate polysaccharides) prevent the virus from attaching to the target molecules on the cell surface. The SPs possess a binding site on the CD4 that is relatively similar to the HIV–gp120 binding region.243 Hence, by binding to this lymphocyte, the SPs inhibit the binding of the monoclonal antibodies to the initial two domains of the CD4,244 thereby disrupting the CD4–gp120 interaction. The anti-HIV activity of the SPs is by shielding off the positively charged sites on the V3 loop of the gp120 protein, thereby preventing the virus attachment to the cell surface.245
The SPs from red algae are also known to possess anti-HIV-1 activity.246 Schizymenia dubyi, a red algae, produces a sulfated glucuronogalactan that is known to possess anti-HIV-1 activity. This polysaccharide suppressed the syncytial formation completely at a concentration of 5 μg/mL and also inhibited the HIV-1 reverse transcriptase at the same low concentration without any cytotoxicity to the MT4 cells.247 Its mechanism of action involves inhibiting the attachment of virus to the host cell. Nakashima et al. prepared an extract of citrate buffer, sea algal extract (SAE) from the marine red alga Schizymenia pacif ica, that showed inhibition against HIV replication and HIV reverse transcriptase. SAE is a sulfated polysaccharide with a molecular weight of ~2 000 000 belonging to the family of λ-carrageenan that includes 3,6-anhydrogalactose (0.65%), sulfonate (20%), and galactose (73%), with the sulfate residues being responsible for the inhibition of the HIV reverse transcriptase at an inhibitory dose of 9.5 × 103 IU/mL.248,249
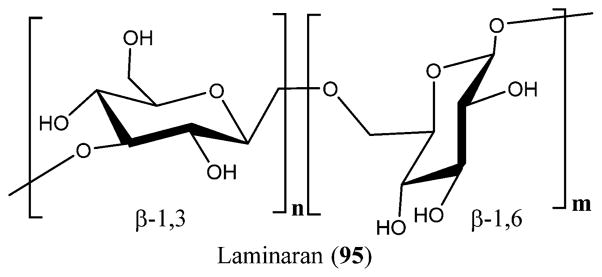
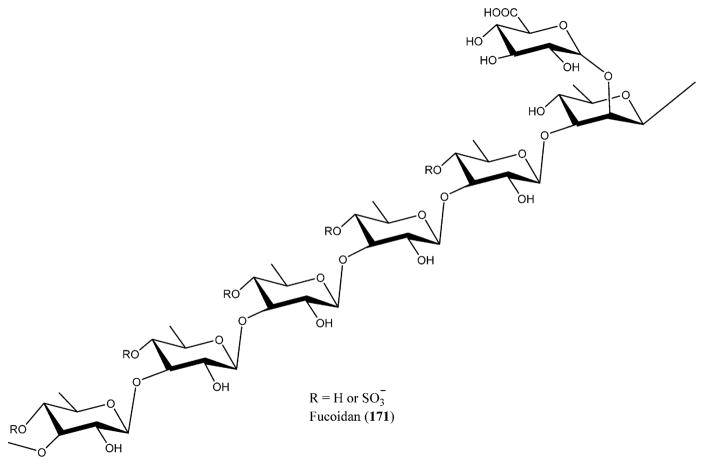
Brown algae are also known to produce SPs with anti-HIV-1 activity via a different mechanism of action.223 Fucans are present mainly in brown algae, and they have high molecular weights and possess a repeated chain of sulfated fucose. Fucans derived from the seaweed species of Lobophora variegate, Spatoglossum schroederi, Fucus vesiculosus,250 and Dictyota mertensii inhibit the reverse transcriptase of HIV-1. The galactofucan fractions of L. variegate showed reverse transcriptase inhibition at 1.0 μg/mL concentration with 94% synthetic polynucleotides inhibition. Fucan A from D. mertensii and S. schroederi displayed inhibition activity against the reverse transcriptase enzyme at 1.0 mg/mL with 99.3% and 99.03% inhibition, respectively. Fucan B from S. schroederi showed inhibition activity of 53.9% at the same concentration. The fucan fraction from F. vesiculosus showed high inhibition activity of 98.1% on the reverse transcriptase enzyme of HIV-1 at 0.5 μg/mL concentration.251 Similarly, fractions of galactofucan from Adenocystis utricularis also presented anti-HIV-1 activity by blocking the prior events of virus replication. EA1-20 and EC2-20 displayed strong inhibition activity on HIV-1 replication at low IC50 values of 0.6 and 0.9 μg/mL, respectively.252 SPs are also produced by the microalgae. Naviculan is one such example that is isolated from Navicula directa that possesses anti-HIV-1 activity. It demonstrated inhibitory effect against the formation of cell–cell fusion between CD4-expressing HeLa cells and HIV gp160 at an IC50 value of 0.24 μM.253 A new type of D-galactan sulfate was isolated from Meretrix petechialis (clam) that possessed anti-HIV-1 activity by inhibiting syncytia formation at 200 μg/mL concentration with 56% inhibition.254 Laminaran (95), also termed laminarin, is a water-soluble polysaccharide including 20–25 glucose units possessing (1,3)-β-D-glucan with β(1,6) branching isolated from the brown algae. Laminaran (95) along with the laminaran polysaccharides prepared from kelp are known to inhibit the HIV reverse transcriptase and adsorption at a concentration of 50 μg/mL, suggesting good inhibition against HIV replication (Figure 33).255
Figure 33.
Structure of laminaran (95).
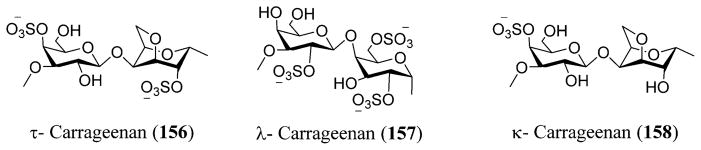
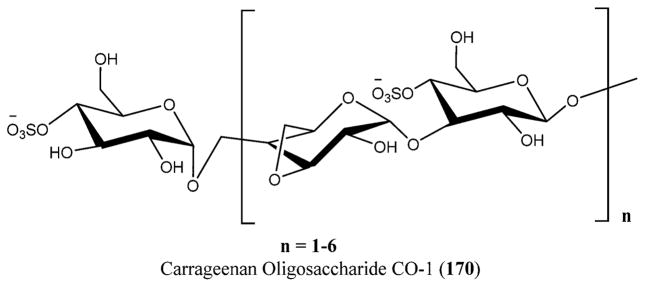
Galactan sulfate (GS), a polysaccharide isolated from Agardhiella tenera, a red seaweed, showed activity against HIV-1 and HIV-2 at IC50 values of 0.5 and 0.05 μg/L, respectively. GS prevents the binding of HIV-1 to cells along with the binding of anti-gp120 mAb to HIV-1 gp120. It is also known to be active against enveloped viruses including togaviruses, arenaviruses, herpesviruses, and others.256 Carrageenans are extracted mainly from certain genera of red seaweeds that include Hypnea, Eucheuma, Gigartina, and Chondrus. Yamada and co-workers reported anti-HIV activity for O-acylated carrageenan polysaccharides with various molecular weights by means of sulfation and depolymerization.257,258
Calcium spirulan (Ca-SP), a sulfated polysaccharide isolated from the marine blue–green alga Arthrospira platensis, showed potent anti-HIV-1 activity at an IC50 value of 2900 μg/mL and reduced viral replication at ED50 values of 11.4 and 2.3 μg/mL. Ca-SP is also known to inhibit the viral replication of other viruses that include HCMV, measles, polio, and coxsackie virus by preventing the penetration of the virus into the host cells.259 Xin et al. reported 911, a marine polysaccharide derived from alginate that inhibited the HIV replication both in vivo and in vitro, attributing to the inhibition of the viral reverse transcriptase, interfering with the viral adsorption, and enhancing immune function.260,261 Woo et al. reported the inhibitory effects of the marine shellfish polysaccharides from seven different shellfish, including Meretrix lusoria, Meretrix petechialis, Ruditapes philippinarum, Sinonovacula constricta Lamark, Scapharca subcrenata, Scapharca broughtonii, and Mytilus coruscus, against HIV-1 in vitro by inhibiting the viral fusion gp120/gp41 with the CD4 protein on the T-lymphocyte surface.262
SPs effectively inhibit the cell–cell adhesion.245 Studies proved that SPs could be used as antiviral vaginal formulations because they do not disturb the functions of the epithelial cells in the vagina or the normal bacterial flora.223,245
18.4. Lectins
Lectins are proteins that bind to carbohydrates present in prokaryotes, algae, fungi, plants, corals, vertebrates, and invertebrates.223 They have specificity for different glycan structures. Hence, they bind to the glycans present on the gp120 molecule of the HIV envelope, resulting in inhibition of viral cell fusion,263 HIV infectivity,264,265 and syncytium formation.266 Several different lectins like griffithsin (GRFT), obtained from the red algae Grif fithsia sp., have been reported to possess anti-HIV-1 activity. This lectin possesses about 120 common amino acids along with an unusual one at position 31. The cytopathic effects produced by the laboratory strains and the HIV-1 clinical primary isolates on the T-lymphoblastic cells are inhibited at concentrations as low as 0.000043 μM. GRFT blocks the cell-to-cell fusion of the uninfected and infected cells. It also prevents the binding of gp120, which is a CD4-dependent glycoprotein, with 2G12 and 48d monoclonal antibody.267
In recent years, marine invertebrates have become new sources for unusual lectins. One such example is CVL, a β-galactose specific lectin isolated from Chaetopterus variopedatus, a marine worm. It inhibited the cytopathic effects caused by HIV-1 and the viral p24 antigen production at EC50 values of 0.0043 and 0.057 μM, respectively. At an EC50 value of 0.073 μM, CVL caused blockade of HIV-1 infected and uninfected cell-to-cell fusion process. At concentrations of 0.07 and 0.33 μM, CVL exhibited 21% and 86% inhibition, respectively, of HIV-1 entry into the host cells.268 Another lectin, CGL, isolated from the C. grayanus mussel, presented a high affinity to the mucin type glycoproteins. Two other lectins, DTL and DTL-A, were isolated from ascidium D. ternatanum.269 Similarly, SVL-1 and SVL-2, isolated from the marine worm Serpula vermicularis, are calcium (Ca2+)-independent lectins shown to possess anti-HIV-1 activity.223 CGL, DTL, DTL-A, and SVL-2 displayed inhibition of HIV-1 IIIB induced syncytium formation at EC50 values of 27.9, 0.002, 0.36, and 0.15 μg/mL, respectively. Among them, the CGL, DTL, and DTL-A showed activity against the cell fusion between the HIV-1/H9 cells that are infected chronically and the uninfected C8166 cells at EC50 values of 35.12, 1.37, and 6.97 μg/mL, respectively.269
18.5. Bioactive Peptides
Bioactive peptides are widely isolated from marine organisms by means of enzymatic hydrolysis. It is reported that many marine bioactive peptides have anti-HIV-1 activity.223 Some oysters possess antiviral and anti-bacterial substances that prevent infectious diseases. Hence, a large isolation effort has been made to discover HIV-1 protease inhibiting substances from the oyster Crassostrea gigas, yielding two peptides Leu-Leu-Glu-Tyr-Ser-Ile (96) and Leu-Leu-Glu-Tyr-Ser-Leu (97). They exhibited strong inhibition of the HIV-1 protease at IC50 values of 0.02 and 0.015 μM, respectively. The inhibitory activity was found to be related to the length of the amino acid sequence and the presence of C-,N-terminal hydrophobic amino acid (Figure 34).270
Figure 34.
Structures of Leu-Leu-Glu-Tyr-Ser-Ile (96) and Leu-Leu-Glu-Tyr-Ser-Leu (97).
Cyanovirin-N (CV-N) isolated from the cyanobacterium Nostoc ellipsosporum is a 101-amino-acid antiviral peptide with a molecular weight of 11 kDa with inhibitory effects against HIV-1 and HIV-2 at low nanomolar concentrations. CV-N prevents the transmission of HIV from infected to uninfected cells by preventing cell-to-cell fusion.271 The peptides polyphemusins I (98) and II (99) and tachyplesins I-II (100–101) isolated from the hemocyte debris of the horseshoe crabs Limulus polyphemus and Tachypleus tridentatus were found to inhibit HIV cell fusion.272,273 Many synthetic peptide analogues have been prepared of which the synthetic peptide T22 analogue of polyphemusin II (99) showed the strongest anti-HIV activity with low in vitro cytotoxicity at EC50 value of 2.6 μM, which is comparatively less than that of azidothymidine (3) (5.2 μM) (Figure 35).273
Figure 35.
Structures of polyphemusin I (98)–II (99) and tachyplesins I (100)–II (101).
18.6. Miscellaneous Antivirals Possessing Anti-HIV Activity
Macrolactin-A (102), an antiviral compound isolated from the marine bacteria, included 24-membered ring lactones, open-chain acids, and related glucose β-pyranosides that inhibited HIV replication at a concentration of 10 μg/mL.274 The diterpenes Da-1 (103) and AcDa-1 (104) isolated from Dictyota menstrualis, the marine alga, inhibited the replication of HIV-1 virus at EC50 values of 40 and 70 μM, respectively. The above diterpenes did not affect the viral internalization or viral attachment but instead inhibited the HIV reverse transcriptase at IC50 values of 10 and 35 μM, respectively (Figure 36).275
Figure 36.
Structures of macrolactin-A (102), Da-1 (103), and AcDa-1 (104).
The antivirals equisetin (105) and phomasetin (106) isolated from the marine fungi Fusarium heterosporum and a Phoma sp. showed inhibition against HIV-1 integrase at IC50 ranging between 7 and 20 μM (Figure 37).276
Figure 37.
Structures of equisetin (105) and phomasetin (106).
Thalassiolins A–C (107–109) isolated from the Caribbean sea grass Thalassia testudinum are sulfated flavone glycosides that inhibited HIV replication at IC50 30 μM, targeting the integrase-catalyzed strand transfer at IC50 0.4 μM. Thalassiolins A–C (107–109) showed potency against HIV integrase due to the presence of sulfated glucose functionality (Figure 38).277
Figure 38.
Structures of thalassiolins A (107), B (108), and C (109).
Didemnaketals A (110) and B (111), isolated from the ascidian Didemnum sp., were shown to inhibit the HIV-protease at IC50 values of 2 and 10 μM, respectively.278 Cyclo-didemniserinol trisulfate (112), isolated from the extracts of Palauan ascidian Didemnum guttatum, showed inhibition of the HIV-integrase at IC50 60 μg/mL (Figure 39).279
Figure 39.
Structures of didemnaketals A (110) and B (111) and cyclodidemniserinol trisulfate (112).
Lamellarins were first isolated from the prosobranch mollusks belonging to the genus Lamellaria,280 and lamellarin α 20-sulfate (113), isolated from the Arabian Sea ascidian, was shown to inhibit the HIV-integrase. Lamellarin α 20-sulfate (113) inhibited the strand-transfer activity at an IC50 of 22 μM and the integrase terminal cleavage activity at IC50 of 16 μM (Figure 40).281
Figure 40.
Structure of lamellarin α 20-sulfate (113).
19. ANTIVIRALS FROM PORIFERA
Sponges have historically produced promising drug leads. Most of the drugs obtained from sponges are being used for the treatment of HIV and also HSV.282 Some examples of the compounds isolated from sponges that are now being used for the treatment of HIV-1 are discussed in detail in the following sections.
19.1. Sesquiterpene Hydroquinones
Avarol (114) was first isolated from the marine sponge Disidea avara, and avarone (115) is synthesized by the silver oxide oxidation of avarol.283 These structures have a rearranged drimane skeleton.284 They showed an inhibitory effect on the replication of AIDS and HTLV IIIB-infected H9 human cells at 0.3 μM.283 Its mechanism involves the blockade of p17 and p24 gag proteins after viral infection and, thereby, prevents replication of the virus. After further exploration of the mechanism of action, avarol was shown to block the tRNA, required for glutamine synthesis, which plays a vital role in the synthesis of viral proteases necessary for its replication (Figure 41).282
Figure 41.
Avarol (114) and avarone (115) possessing anti-HIV-1 activity.
ADMET predictor shows that avarol (114) violates only one criterion of the Lipinski guidelines and avarone (115) does not violate any. The carbonyl group of the quinone ring includes a hydroxyl group at the ortho position, which is known to be responsible for the blockade of the reverse transcriptase activity.285 Hence, both of them may likely be good candidates to be considered for further in vivo studies because the limitations are minor (Table S1).
Curcuphenol (116), a sesquiterpene phenol, was isolated from the sponges Didiscus oxeata, Didiscus flavus, Myrmekioderma styx, and Epipolasis sp. Semi-synthetic analogues a (117), c (118), j (119), and r (120) had anti-HIV-1 activity at EC50 values of 31.2, 29.2, 18.4, and 18.2 μM, respectively.286 Isoquinoline derivatives have been previously patented for the treatment of anti-HIV-1 activity by inhibiting the reverse transcriptase enzyme287 or protease enzyme288 or by viral entry inhibition,289 whereas indole alkaloids act as reverse transcriptase inhibitors.290 The anti-HIV-1 activity is known to increase with indole substitution.291 Hence, the analogues showed activity due to the presence of one of the above-mentioned moieties. The activity was shown to improve with the alkylation of the indole nitrogen as seen in analogue j (119) (Figure 42).286
Figure 42.
Curcuphenol (116) and its analogues a (117), c (118), j (119), and r (120) isolated and modified from the sponges Didiscus oxeata, Didiscus flavus, Myrmekioderma styx, and Epipolasis sp.
ADMET predictor shows that curcuphenol (116) and its analogues violate only one criterion of the Lipinski guidelines. Hence, they may be considered as potential candidates for the syntheses because as compared to some FDA-approved drugs like saquinavir (7) or fosamprenavir (40), which violate more than one criterion, the limitations are less in this case (Table S1).
19.2. Cyclic Depsipeptides
Papuamides, isolated from the marine sponges Theonella swinhoei and Theonella mirabilis, are known to possess anti-HIV-1 and cytotoxic effects.292 Papuamides A (121) and B (122) were isolated from the sponge T. mirabilis and exhibited anti-HIV-1 activity at a concentration of 0.003 μM.293 Papuamides A (121), B (122), C (123), and D (124) were isolated from T. swinhoei (Figure 43).282
Figure 43.
Papuamides A (121), B (122), C (123), and D (124) isolated from the marine sponges Theonella swinhoei and T. mirabilis.
Papuamide A (121) has a membrane-targeting mechanism, and this mechanism of action has been shown to be responsible for its virucidal activity.294 Its mechanism of action involves inhibition of HIV pseudo-type viruses that express glycoprotein envelope from the amphotropic murine leukemia virus or vesicular stomatitis virus, suggesting that the entry inhibition is not HIV-1 envelope glycoprotein specific. It inhibits the virus only at the initial stage of the viral replication cycle, and it targets the virus and not the cell. Hence, it exhibits a direct virucidal mechanism of HIV-1. Papuamide B (122) targets the phospholipid, phosphatidylserine (PS), present on the virus. Papuamides C (123) and D (124) also inhibit the viral entry (Figure 43).294
ADMET predictor shows that papuamides A (121), B (122), C (123), and D (124) violate three criteria of the Lipinski guidelines. They also show low flexibility and do not possess the tendency to permeate the cell membranes. In addition, most peptide-derived drugs must be administered IV. This could overcome the permeability limitation, and they also represent potentially challenging molecules (Table S1).
Callipeltin A (125), isolated from a sponge belonging to the genus Callipelta, also possesses anti-HIV-1 activity. The HIV-1-induced cytopathic effects were inhibited at a median effective dose (ED50) value of 0.007 μM.295 Similarly, neamphamide A (126), isolated from Neamphius huxleyi, possesses cytoprotective activity against HIV-1 infection at an EC50 value of 0.028 μM.296 Certain mirabamides that inhibit HIV-1 fusion were isolated from the sponge Siliquariaspongia mirabilis. Among them, mirabamide A (127) was shown to be active against HIV-1 fusion assays and neutralization at IC50 values between 0.04 and 0.14 μM, respectively. The mirabamides were shown to be more selective for HIV-1 by inhibiting them at the membrane fusion level.297 Celebeside A (128) and theopapuamide B (129) were also isolated from the same sponge, S. mirabilis. Celebeside A (128) showed HIV-1 entry inhibition at an IC50 value of 2.13 μM, whereas theopapuamide B (129) showed inhibition at an IC50 value of 0.99 μM. Additionally, celebeside A (128) possesses anti-HIV-1 activity due to the presence of a phosphoserine residue that is absent in theopapuamide B (129) (Figure 44).298
Figure 44.
Callipeltin A (125) isolated from Callipelta, neamphamide A (126) isolated from Neamphius huxleyi, mirabamide A (127), celebeside A (128),448 and theopapuamide B (129)448 isolated from Siliquariaspongia mirabilis.
ADMET predictor shows that callipeltin A (125), mirabamide A (127), and celebeside A (128) violate three criteria of Lipinski guidelines whereas neamphamide A (126) and theopapuamide B (129) violate all four criteria. In addition, they also show low permeability, low flexibility, and no tendency to permeate the cell membranes. All of the above-mentioned compounds belong to the class of depsipeptides, and their mechanism of action is similar to that of papuamides. The limitations could be overcome similar to that of any other peptide. The route of administration should be intravenous to limit the permeation factor (Table S1).
Microspinosamide (130) was isolated from the Indonesian sponge Sidonops microspinosa. The peptide contains 13 amino acid residues. Both organic and aqueous extracts showed anti-HIV-1 activity. It showed anti-HIV-1 activity at a concentration of 0.12 μM.299 Homophymine A, a new anti-HIV-1 compound, was isolated from the Homophymia sp. It possessed cytoprotective activity against the infection of HIV-1 at an IC50 value of 0.075 μM (Figure 45).300
Figure 45.
Microspinosamide (130) isolated from the Indonesian sponge Sidonops microspinosa.
ADMET predictor shows that microspinosamide (130) violates three criteria of the Lipinski guidelines. In addition, it also shows low permeability, low flexibility, and a low tendency to permeate the cell membranes. However, the interesting fact about microspinosamide (130) is that its structure could incorporate 13 amino acid residues along with numerous uncommon amino acids, and this is the first natural peptide that includes a β-hydroxy-p-bromophenylalanine residue.299 Hence, it is always a challenge to develop such molecules. Different techniques can be adopted to synthesize such compounds and to reduce their limitations (Table S1).
19.3. Alkaloids
Dragmacidin F (131), a new bromoindole alkaloid isolated from the sponge belonging to the genus Halicortex from the southern coast of Ustica Island in Italy, showed in vitro activity against HIV-1 and HSV-1 at EC50 values of 0.9 and 96 μM, respectively.301 This compound is known to inhibit the syncytia formation later to infecting the HIV-MT4, without causing any direct toxicity to the cell (Figure 46).301–303
Figure 46.
Dragmacidin F (131) isolated from the marine sponge belonging to the Halicortex genus from the southern coast of Ustica Island in Italy.
ADMET predictor shows that dragmacidin F (131) violates two criteria of the Lipinski guidelines. In addition, it also possesses a low tendency to permeate the cell membranes. Because there is no direct toxicity involved to the cells using this compound, the side effects could be reduced to some extent. Fosamprenavir (40), an FDA-approved drug (Table S4), also violates more than one criterion of the Lipinski guideline. Hence, this should not be a limiting factor for Dragmacidin F (131) to avoid considering it for further development and bringing it to clinical trials (Table S1).
Manzamine A (132) was isolated from the Haliclona sp.304 It has a unique complex polycyclic ring system that is coupled with a β-carboline moiety and is known to possess anti-HIV-1, antiparasitic, antimicrobial, and other biological activities.282 Manzamine A (132), 6-deoxymanzamine X (133),305 neokauluamine, and 8-hydroxymanzamine (134) also showed anti-HIV-1 activity at EC50 values of 4.2, 1.6, 2.3, and 0.6 μM, respectively (Figure 47).306
Figure 47.
Manzamine A (132), 6-deoxymanzamine X (133), and 8-hydroxymanzamine (134) isolated from Haliclona sp.
ADMET predictor indicates that manzamine A (132) violates two criteria of the Lipinski guidelines. This compound showed low metabolic clearance, good oral bioavailability (20.6%), and long half-life from the oral and IV pharmacokinetic studies conducted in rats. Manzamine A (132) has a good acid solubility, which suggests that it readily dissolves in the stomach.306 Hence, this could be considered as a potentially good candidate for clinical assessments and further development (Table S1).
Manadomanzamines A (135) and B (136) were isolated from the Indonesian sponge Acanthostrongylophora sp. Manadomanzamines A (135) and B (136) and xestomanzamine A (137) showed activity against HIV-1 at EC50 values of 11.5, 27.0, and 40.5 μM, respectively. These also exhibited activities against AIDS opportunistic fungal infections and HIV-1 (Figure 48).307
Figure 48.
Manadomanzamines A (135) and B (136) and xestomanzamine A (137) isolated from the Indonesian sponge Acanthostrongylophora sp.
ADMET predictor shows that manadomanzamines A (135) and B (136) violate two criteria of the Lipinski guidelines whereas the xestomanzamine A (137) does not violate any of the criteria. The structures of manadomanzamines A (135) and B (136) are the unprecedented rearrangement of the skeleton of manzamine.307 Hence, these compounds could be considered similar to manzamine and could be dealt with accordingly (Table S1).
Batzelladines A–E, which are also known to possess anti-HIV-1308 activity, were isolated from the sponges belonging to the Batzella and Monanchora genera. These are known to be the first small-molecular-weight (between 400 and 800) HIV-1 entry inhibitors. They were shown to inhibit gp120–CD4 interaction.308 Crambescidins 800 (138) and 826, isolated from starfishes and the sponges of the genus groups Batzella and Monanchora showed similar anti-HIV-1 activity by inhibiting HIV-1 envelope-mediated fusion along with fromiamycalin, isolated from starfishes and some synthetic batzelladine analogues. Crambescidine 800 (138) had activity against HIV-1 with an EC50 value of 0.04 μM. Similarly, ptilomycalin A (139), isolated from the sponge Monanchora unguifera and starfishes, is also known to exhibit anti-HIV-1 activity at an EC50 value of 0.011 μM; meanwhile, batzelladine C (140) showed inhibition at an EC50 of 7.7 μM. Comparatively, batzelladine and ptilomycalin show potent activity against AIDS opportunistic infections and HIV-1 (Figure 49).309
Figure 49.
Ptilomycalin A (139), isolated from the sponge Monanchora unguifera, and crambescidine 800 (138) and batzelladine C (140) isolated from the Batzella and Monanchora genera.
ADMET predictor shows that ptilomycalin A (139) and crambescidin 800 (138) violate three criteria of the Lipinski guidelines whereas batzelladine C (140) does not violate any. In addition, ptilomycalin A (139) shows low flexibility and a low tendency to permeate into cell membranes whereas crambescidin 800 (138) exhibits low permeability, low flexibility, and less tendency to permeate the cell membranes. Batzelladine C (140) shows low permeability and less flexibility. Because these compounds are HIV-1 entry inhibitors, limitation of permeability should not be considered as a serious issue, and one should rather concentrate on improving the limitations for further development (Table S1).
Isoaaptamine (141) and aaptamine (142) were isolated from various sponges belonging to the Suberites, Hymenlacidon, and Aaptos generas. They are known to possess anti-HIV-1 activity at concentrations of 0.6 and 1.30 μM, respectively. The potency of this activity has been further explored through a SAR of fluorine substitution on an indole heterocyclic system (Figure 50).310
Figure 50.
Isoaaptamine (141) and aaptamine (142) isolated from the sponge Aaptos aaptos.
ADMET predictor predicts that isoaaptamine (141) and aaptamine (142) do not violate any of the Lipinski guidelines. This could be a good indicator that they might not be involved in any potential side effects or problems, and perhaps they could be considered as potential candidates for further development (Table S1).
Petrosin (143) and petrosin A (144) are bisquinolizidine alkaloids isolated from the Indian marine sponge Petrosia similis, which showed inhibition against HIV-1 replication at IC50 values of 41.3 and 53 μM, the recombinant reverse transcriptase at IC50 values of 10.6 and 14.8 μM, and the formation of giant cells at IC50 21.2 and 36.1 μM, respectively (Figure 51).311
Figure 51.
Structures of petrosin (143) and petrosin A (144).
19.4. Diterpenes
In 1992, four cyanthiwigins were isolated from Epipolasis reiswigi, a Jamaican sponge. These included cyanthiwigins A, B (145), C, and D. Among these, only cyanthiwigin (145) B is known to possess moderate anti-HIV-1 activity at an EC50 value of 42.1 μM. This may be due to the C-8 ketone group, which is only present in cyanthiwigin B and is absent in the other three compounds (Figure 52).312
Figure 52.
Cyanthiwigin B (145) isolated from the Jamaican sponge Epipolasis reiswigi.
ADMET predictor shows that cyanthiwigin B (145) does not violate any of the Lipinski guidelines. The structure looks simple to prepare, and because it does not have any limitations, this could be another likely candidate to be considered for preclinical trials and further development (Table S1).
19.5. Sulfated Sterols
Two sulfated sterols isolated from the species Xestospongia and the other from an unidentified haplosclerid sponge are known to possess the unusual steroidal sulfamate esters–haplosamates A (146) and B (147), which showed inhibition towards HIV-1 integrase at IC50 values of 50 and 15 μg/mL, respectively. The sulfamate group present in the haplosamates is responsible for the inhibition of HIV-1 integrase (Figure 53).313
Figure 53.
Structures of haplosamates A (146) and B (147).
Clathsterol (148) is an active and novel sulfated sterol isolated from the Red Sea sponge Clathria sp. that showed inhibition against HIV-1 reverse transcriptase at 10 μM (Figure 54).314
Figure 54.
Structure of clathsterol (148).
19.6. Miscellaneous Antivirals Possessing Anti-HIV Activity
Polyacetylenetriol (PAT) (149), isolated from the marine sponge Petrosia sp., showed selective inhibition against DNA-and RNA-dependent DNA polymerase activities of the retroviral reverse transcriptase at an IC50 value of 0.95 μM. PAT is considered to be a reversible, noncompetitive inhibitor and is known to interfere with the catalytic steps of the viral DNA polymerization (Figure 55).315
Figure 55.
Structure of polyacetylenetriol (149).
A new C22 furanoterpene isolated from the Madagascan sponge, Lendenfeldia, is designated as dehydrofurodendin (150), which showed inhibition against HIV-1 reverse transcriptase associated DNA- and RNA-directed DNA polymerase at IC50 values ranging between 3.2 and 5.6 μM (Figure 56).316
Figure 56.
Structure of dehydrofurodendin (150).
Cimino et al. reported a new anti-HIV polysaccharide named rosacelose (151) that comprises glucose and fucose sulfate isolated from the aqueous extract of the marine sponge Mixylla rosacea, which possessed a linear polysaccharide structure comprising 4,6-disulfated 3-O-glycosylated α-D-glucopyranosyl and 2,4-disulfated 3-O-glycosylated α-L-fucopyranosyl residues in a molar ratio of 3:1 (Figure 57).317
Figure 57.
Structure of the repeating unit of rosacelose (151).
Table 1 shows the summary of all the marine antivirals possessing anti-HIV-1 activity.
Table 1.
Summary of the Marine Organisms Showing Anti-HIV-1 Activity
| compound | type | source | cellular target | activity | ref |
|---|---|---|---|---|---|
| 8,4‴-dieckol | phlorotannin | Ecklonia cava | reverse transcriptase | HIV-1 IC50 5.3 μM |
227 and 228 |
| 8,8′-bieckol | phlorotannin | Ecklonia cava | reverse transcriptase | HIV-1 IC50 0.5 μM |
227 and 228 |
| 6,6′-bieckol | phlorotannin | Ecklonia cava | entry and reverse transcriptase | HIV-1 IC50 1.07 μM | 231 |
| diphlorethohydroxycarmalol | phlorotannin | Ishige okamurae | reverse transcriptase | IC50 9.1 and25.2 μM | 232 |
| N-carboxymethyl chitosan | chitin polysaccharide | shells of shrimps & crabs | reverse transcriptase | HIV-1 IC50 0.02 μM | 236 and 237 |
| sulfated glucuronogalactan | sulfated polysaccharide | Schizymenia dubyi | syncytial formation and reverse transcriptase | HIV-1 5 μg/mL | 247 |
| sea algal extract (SAE) | citrate buffer extract | Schizymenia pacifica | reverse transcriptase | 9.5 × 103 IU/mL | 248 and 249 |
| fucan A | fucan | Dictyota mertensii and Lobophora variegate | reverse transcriptase | HIV-1 1.0 mg/mL | 251 |
| fucan B | fucan | Fucus vesiculosus | reverse transcriptase | HIV-1 0.5 μg/mL | 251 |
| naviculan | sulfated polysaccharide | Navicula directa | cell–cell fusion | IC50 0.24 μM | 253 |
| laminaran | polysaccharide | Kelp | reverse transcriptase | HIV-1 50 μg/mL | 255 |
| galactan sulfate | polysaccharide | Agardhiella tenera | cell binding | HIV-1 and HIV-2 IC50 0.5 and 0.05 μg/L | 256 |
| calcium spirulan (Ca-SP) | sulfated polysaccharide | Arthrospira platensis | viral replication | HIV-1 ED50 11.4 and 2.3 μg/mL | 259 |
| griffithsin (GRFT) | lectin | Griffithsia sps. | cell–cell fusion | HIV-1 EC50 0.000043 μM | 267 |
| CVL | β-galactose specific lectin | Chaetopterus variopedatus | cytopathic effects cell–cell fusion |
EC50 0.0043–0.057 μM EC50 0.073 μM |
268 |
| CGL | lectin | Crenomytilus grayanus | syncytium formation | EC50 27.9 μg/mL | 269 |
| DTL and DTL-A | lectins | Didemnum ternatanum | syncytium formation | EC50 0.002 and 0.36 μg/mL | 269 |
| SVL-2 | GlcNAc-specific lectin | Serpula vermicularis | syncytium formation | EC50 0.15 μg/mL | 223 and 269 |
| Leu-Leu-Glu-Tyr-Ser-Ile | peptide | Crassostrea gigas | protease | IC50 0.02 μM | 270 |
| Leu-Leu-Glu-Tyr-Ser-Leu | peptide | Crassostrea gigas | protease | IC50 0.015 μM | 270 |
| polyphemusin II | peptide | Limulus polyphemus and Tachypleus tridentatus | viral adsorption preceding reverse transcription | HIV-1 EC50 2.6 μM | 272 and 273 |
| macrolactin-A | lactone | marine bacteria | replication | 10 μg/mL | 274 |
| Da-1 | diterpene | Dictyota menstrualis | replication reverse transcriptase | HIV-1 EC50 40 μM HIV-1 IC50 10 μM | 275 |
| AcDa-1 | diterpene | Dictyota menstrualis | replication reverse transcriptase | HIV-1 EC50 70 μM HIV-1 IC50 35 μM | 275 |
| equisetin | acyl tetrameric acid | Fusarium heterosporum | integrase | IC50 7–20 μM | 276 |
| phomasetin | acyl tetrameric acid | Fusarium heterosporum | integrase | IC50 7–20 μM | 276 |
| thalassiolins A–C | sulfated flavone glycosides | Thalassia testudinum | integrase | IC50 30 μM | 277 |
| didemnaketals A and B | spiroketals | Didemnum sp. | protease | IC50 2 and 10 μM | 278 |
| cyclodidemniseriol trisulfate | sulfated serinolipid | Didemnum guttatum | integrase | IC50 60 μg/mL | 279 |
| lamellarin α 20-sulfate avarol | alkaloid | Arabian Sea ascidian | integrase | IC50 16 μM | 281 |
| sesquiterpene hydroquinone | Disidea avara | replication | HIV-1 IC50 0.3 μM | 283 | |
| curcuphenols a, c, j, and r | sesquiterpene phenols | Didiscus oxeata, D. flavus, Myrmekioderma styx and Epipolasis sps. | reverse transcriptase, protease, and viral entry | EC50 31.2, 29.2, 18.4, and 18.2 μM | 286 |
| papuamides A and B | cyclic depsipeptides | Theonella swinhoei and T. mirabilis | viral entry and phospholipids | HIV-1 EC50 0.003 μM | 293 |
| callipeltin A | cyclic depsipeptide | Callipelta genus | cytotoxicity | ED50 0.007 μM | 295 |
| neamphamide A | cyclic depsipeptide | Neamphius huxleyi | cytoprotective | EC50 0.028 μM | 296 |
| mirabamide A | cyclic depsipeptide | Siliquariaspongia mirabilis | fusion | IC50 0.04 and 0.14 μM | 297 |
| celebeside A | cyclic depsipeptide | S. mirabilis | entry | IC50 2.13 μM | 298 |
| theopapuamide B | cyclic depsipeptide | S. mirabilis | entry | IC50 0.99 μM | 298 |
| microspinosamide | cyclic depsipeptide | Sidonops microspinosa | cytopathic effect | HIV-1 EC50 0.12 μM | 299 |
| homophymine A | cyclic depsipeptide | Homophymia sps. | cytoprotective effect | IC50 0.075 μM | 300 |
| dragmacidin F | alkaloid | Halicortex genus | syncytia formation | EC50 0.9 μM | 301 |
| manzamine A | alkaloid | Haliclona sps. | unknown | EC50 4.2 μM | 304 and 306 |
| manadomanzamines A and B | alkaloids | Acanthostrongylophora sps. | unknown | EC50 11.5 and 27.0 μM | 307 |
| xestomanzamine A | alkaloid | Acanthostrongylophora sps. | unknown | EC50 40.5 μM | 307 |
| batzelladine C | alkaloid | Batzella and Monanchora genera | gp120-CD4 interaction | EC50 7.7 μM | 309 |
| crambescidin 800 | alkaloid | Batzella and Monanchora genera | fusion | EC50 7.7 μM | 309 |
| ptilomycalin A | alkaloid | Monanchora unguifera | unknown | EC50 0.011 μM | 309 |
| isoaaptamine | alkaloid | Aaptos aaptos | unknown | EC50 0.6 μM | 310 |
| aaptamine | alkaloid | Aaptos aaptos | unknown | EC50 1.3 μM | 310 |
| petrosin | bisquinolizidine alkaloid | Petrosia similis | replication and reverse transcriptase | IC50 41.3 and 10.6 μM | 311 |
| petrosin A | bisquinolizidine alkaloid | Petrosia similis | replication and reverse transcriptase | IC50 53 and 14.8 μM | 311 |
| cyanthiwigin B | diterpene | Epipolasis reiswigi | unknown | EC50 42.1 μM | 312 |
| haplosamates A and B | sulfated sterols | Xestospongia sp. | integrase | HIV-1 IC 50 50 and 15 μg/mL | 313 |
| clathsterol | sulfated sterol | Clathria sp. | reverse transcriptase | 10 μM | 314 |
| polyacetylenetriol | polymer | Petrosia sp. | reverse transcriptase | IC50 0.95 μM | 315 |
| dehydrofurodendin | C22 furanoterpene | Lendenfeldia | reverse transcriptase | IC50 3.2–5.6 μM | 316 |
20. MARINE DRUGS FOR THE TREATMENT OF OTHER VIRAL DISEASES
20.1. Hepatitis B
The marine polysaccharide 911 that is isolated from alginate is known to prevent the replication of hepatitis B virus (HBV) by inhibiting the activity of HBV DNA-polymerase, improving the host-cell immune function.318 Wu et al. reported that the DNA replication of the duck HBV could be inhibited by the oyster polysaccharides by reducing the duck HBV–DNA content from the serum, thus showing anti-HBV in vivo effects.319 Guan identified that the polymeric mannuronic acid sulfate could effectively reduce the antigen levels and the DNA levels of the HBV in duck blood, thus improving the cellular and humoral immune function.320 The replication of HBV is also known to be indirectly inhibited by the sulfated polymannuroguluronate (SPMG) (152) isolated from the brown algae by improving the cellular and humoral immune functions (Figure 58).261,318
Figure 58.
Structure of sulfated polymannuroguluronate (SPMG) (152).
20.2. HCV
Discorhabdins A (153) and C (154) and dihydrodiscorhabdin C (155) isolated from the Latrunculia genus of the Alaskan sponge species exhibited anti-HCV activity at EC90 concentrations of <10 μM (Figure 59).321,322
Figure 59.
Structures of discorhabdins A (153) and C (154) and dihydrodiscorhabdin C (155).
20.3. HPV
Buck et al. established that carrageenans could inhibit the HPV infection process, and the antiviral effect is known to be better in the ι-carrageenan (156) isolated from Eucheuma denticulatum compared to λ- (157) and κ-carrageenans (158).323 Carrageenans bind to the HPV capsid, inhibiting the viral adsorption process along with the viral-entry and viral-uncoating processes. The mechanism involved in the inhibition of HPV is known to be independent of heparin sulfate after the viral adsorption (Figure 60).324
Figure 60.
Structures of ι- (156), λ- (157), and κ-carrageenans (158).
20.4. Influenza Virus
Extracellular sulfated polysaccharides isolated from the marine microalgae Cochlodinium polykrikoides, A1 and A2, are known to inhibit the cytopathic effects of the influenza virus (IAV) types A and B.325 The active sulfated calyceramides A–C (159–161) isolated from the marine sponge Discodermia calyx are known to inhibit the IAV neuraminidase inhibitors. Calyceramides A–C (159–161) are known to inhibit the neuraminidase from Clostridium perf ringens at IC50 values of 0.4, 0.2, and 0.8 μg/mL, respectively (Figure 61).326
Figure 61.
Structures of calyceramides A (159), B (160), and C (161).
Weinbersterols A (162) and B (163),326 isolated from the sponge Petrosia weinbergi, are known to inhibit the mouse influenza virus.327 A novel terpenoid stachyflin (164), isolated from the fungus Stachybotrys sp. RF-7260, is known to inhibit influenza A virus (H1N1) at an inhibitory concentration of 0.003 μM, which is known to be better than the other anti-H1N1 drugs such as zanamivir (67) and amantadine (68). Stachyflin (164)328 possesses a novel cis-fused decalin along with a pentacyclic moiety and is known to exhibit antiviral activity by inhibiting the viral host-cell membrane and envelope (Figure 62).329,330
Figure 62.
Structures of weinbersterols A (162) and B (163) and stachyflin (164).
Tang et al. showed that the low-molecular-weight (MW) carrageenans along with their derivatives exhibited significant inhibition activity against influenza virus in mice (FM1-induced pulmonary edema).331 Wang and co-workers reported the inhibition of IAV replication both in vitro and in vivo by the low MW carrageenan oligosaccharide KCO (κ-carrageenan oligosaccharide) (165) along with their sulfated derivatives. The sulfate content, specific sugar linkage, and certain sugar length of KCO (165) are known to be essential for the anti-IAV activity of the κ-carrageenan (165) oligosaccharide. KCO (165) inhibits the replication phase of the IAV life cycle following viral internalization and prior to the release of the virus. κ-Carrageenan (165) saccharide, being the most active, has a molecular weight of 1–3 kDa with sulfate content 0.8–1.0 mol/mol of disaccharide (Figures 60 and 63).332,333
Figure 63.
Structure of KCO (165).
Lüscher-Mattli and Glück reported the anti-IAV activity of dextran sulfate (166) through the inhibition of the IAV fusion with cell membranes and suppression of the viral replication in vivo.334 Ivanova et al. reported the inhibitory effect on IAV by ulvan polysaccharides (167) isolated from the green algae whose effect is known to be strain-specific and dose-dependent.335 Akamatsu et al. reported a new type of fucose polysaccharide, MC26, isolated from the brown seaweed that is known to possess excellent anti-IAV effects in vitro and in vivo.336 Zhang et al. reported the inhibition activity of marine polysaccharides isolated from Perna viridis against the replication of IAV in MDCK cells and its additive effect on the ribavirin anti-IAV actions, suggesting further investigation on these marine polysaccharides as anti-IAV agents (Figure 64).337
Figure 64.
Structures of dextran sulfate (166) and ulvan (167).
A low-molecular-weight (<5 kDa) oligomeric mannuronic acid (OM) (168,) which is an oligosaccharide derivative of polymannuronic acid polysaccharide, showed good activity against H1N1 influenza A virus both in vitro and in vivo.338 Guan reported another polymannuronic acid derivative, polymannuronic acid propyl sulfate (PMS) (169), prepared by the propyl modification and sulfation of polymannuronic acid polysaccharide,339 which showed an inhibitory effect on neuraminidase activity of IAV along with alleviating pneumonia symptoms effectively caused by influenza A viral infection, reducing the mortality rate in mice (Figure 65).340
Figure 65.
Structures of oligomeric mannuronic acid (OM) (168) and polymannuronic acid propyl sulfate (PMS) (169).
Kim et al. reported p-KG03, a sulfated polysaccharide isolated from the marine microalga Gyrodinium impudium, which showed maximum inhibition activity against IAV infection targeting the viral internalization and adsorption, preventing the viral replication.341 Wang et al. reported a low-molecular-weight κ-carrageenan (165) oligosaccharide that is known to effectively inhibit the IAV replication both in vitro and in vivo.332,333 It was also noted that the carrageenan oligosaccharide CO-1 (170) could enter into the host cells, inhibiting the protein translation and mRNA transcription after the internalization, thereby preventing the viral replication (Figures 60 and 66).332
Figure 66.
Structure of the carrageenan oligosaccharide CO-1 (170).
20.5. Respiratory Syncytial Virus
The isolation of two extracellular sulfated polysaccharides, A1 and A2 from Cochlodinium polykrikoides, was reported by Hasui et al. and showed inhibition on the cytopathic effects against RSV types A and B growing on Hep-2 cells.325
20.6. Dengue
A fucan polysaccharide, fucoidan (171)342 isolated from Cladosiphon okamuranus comprising the sulfated fucose units and glucuronic acid, showed inhibition of BHK-21 cells in DENV-2 with little effect on the other three types, DENV-1, -3, and -4.343 Talarico and co-workers reported ι- (156) and λ-carrageenans (157) that interfere with the internalization and adsorption of the DENV-2 host cells.344 The mechanism that is involved in inhibition is thought to be due to the interference of carrageenans on the subsequent uncoating and release of DENV from the endosomes. ι-Carrageenan (156) exhibited inhibition through direct interaction with the viral membrane glycoprotein E (gE).344–346 Talarico et al. found that the ι-carrageenan (156) exhibited inhibition of DENV replication in both mammalian and mosquito cells,344 affecting the potential targets within the DENV host cells (Figures 60 and 67).347
Figure 67.
Structure of fucoidan (171).
20.7. Herpes Simplex Virus
Two novel exopolysaccharides EPS-1 and EPS-2, isolated from the marine bacteria Geobacillus thermodenitrif icans and Bacillus licheniformis, respectively, are known to inhibit HSV-2 replication at 200 and 300 μg/mL.348,349 A1, an extracellular sulfated polysaccharide isolated from a marine microalgae, Cochlodinium polykrikoides, showed activity against HSV-1.325 Fucoidan (171), a sulfated polysaccharide isolated from Fucus vesiculosus, a brown seaweed, is known to inhibit the replication of HSV-1 and HSV-2 viruses.350,351 Calcium-spirulan (Ca-SP), a sulfated polysaccharide isolated from Arthrospira platensis, a marine blue–green alga, is also known to inhibit the replication of HSV-1 and influenza-A.259
Naviculan, isolated from Navicula directa, is a sulfated polysaccharide that inhibited the early viral replication stages at IC50 values ranging between 7 and 14 μg/mL.253 Halovirs A (172), B (173), C (174), D(175), and E (176) isolated from the marine fungus Scytidium species showed potent antiviral activity against HSV-1 and HSV-2 at ED50 values 1.1, 3.5, 2.2, 2.0, and 3.1 μM, respectively. Halovir A (172) also inhibited the replication of HSV-1 and HSV-2 at an ED50 of 280 nm. The mechanism of action is not yet known but is thought to be due to membrane destabilization (Figures 67 and 68).352
Figure 68.
Structures of halovirs A (172), B (173), C (174), D (175), and E (176).
Eudistomins (177)353 and their related β-carboline alkaloids isolated from the tunicate Eudistoma olivaceum showed inhibition against HSV-1 and HSV-2.354 Didemnins (178)355 isolated from Trididemnum sp. of tunicates are cyclic depsipeptides that showed in vivo and in vitro antiviral activities against HSV-1 (Figure 69).356
Figure 69.
Structures of eudistomins (177) and didemnins (178).
Studies indicated the use of heparin polysaccharides and low-molecular-weight heparin (179) as natural inhibitors of HSV-1 that require a unique sulfation moiety.357,358 The marine heparinoid polysaccharides are considered to be structurally similar to heparin (179), possessing glycosaminoglycan (GAG)-like biological properties, and include ulvans (167), alginates, and their sulfated derivatives along with chitosan sulfate and dextran sulfate (166). Recent studies indicated the cell-surface heparinoid sulfate proteoglycans as the initial receptors of HSV-1 and HSV-2.359–361 Heparinoid polysaccharides are known to prevent the viral binding to the cell surface by interacting with the positively charged regions of the cell-surface glycoproteins (Figures 64 and 70).359
Figure 70.
Structure of heparin (179).
Yu et al. studied the inhibitory actions of the scallop skirt GAG (SS-GAG) on HSV-1 at various concentrations with significant in vitro effects, and the antiviral effect of SS-GAG is known to increase gradually with prolonged duration of action.362 Carlucci et al. identified the firm binding of λ-carrageenan (157) to HSV, leading to the virion inactivation, thereby inhibiting the HSV replication.363 The studies also suggested the structural changes of HSV-glycoproteins gB and gC by carrageenan.363,364 Further it was also noted that cyclized μ (180)-/ι-carrageenan (156) isolated from Gigartina skottsbergii also showed potent antiviral effects against HSV-1 and HSV-2 during the viral-adsorption stage (Figures 60 and 71).365
Figure 71.
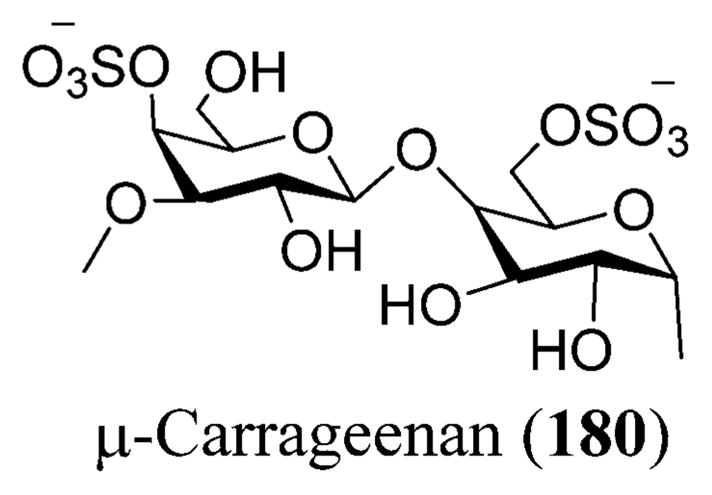
Structure of μ-carrageenan (180).
Harden et al. also reported the direct inactivation of HSV-2 by carrageenan polysaccharides isolated from red algae at low concentrations. The virucidal activities of the carrageenan polysaccharides are known to increase with increasing molecular weight until 100 kDa.366 The direct virucidal activity of carrageenans is known to be due to the formation of stable carrageenan–virion complex, making the binding irreversible and filling the viral envelope sites necessary for viral attachment to the host cells with sulfated polysaccharide preventing the completion of the viral infection process.367 Kanekiyo et al. reported that nostoflan, an acidic polysaccharide isolated from Nostoc flagelliforme, an edible blue–green alga, showed good inhibitory activity against HSV-1, preventing the binding of virus to the host cells.368
Perry et al. reported the isolation of mycalamide A (181) and B (182) from the New Zealand sponge Mycale in the year 1988 that inhibited herpes simplex virus type I at a concentration of 5 ng/disc. Of the two, mycalamide B (182) was found to be more potent at a concentration of 1–2 ng/disc. 4-Methylaaptamine (183) isolated from the marine sponge Aaptos sp. showed anti-HSV-1 activity at an EC50 of 2.4 μM and was found to be more potent than acyclovir (83) [EC50 8.6 μM]. Dragmacidin F (131), a bromoindole alkaloid isolated from the marine sponge Halicortex, showed antiviral activity against HSV-1 at an EC50 of 96 μM. Hamigeran B (184) isolated from the marine sponge Hamigera tarangaensis also showed inhibition against herpes virus (Figures 25, 46, and 72).282
Figure 72.
Structures of mycalamide A (181), mycalamide B (182), 4-methylaaptamine (183), and hamigeran B (184).
21. IN THE PIPELINE: HIV AND HCV DRUGS UNDER DEVELOPMENT
21.1. HIV
GS-7340 (185) also known as tenofovir alafenamide fumarate (TAF)369 is an anti-HIV-1 prodrug that has been patented by Gilead (no. WO2002008241). GS-7340 belongs to the NRTIs and is currently under Phase III clinical trials.370 It is known to inhibit the reverse transcriptase enzyme, thereby preventing viral replication, and is also known as a prodrug of tenofovir.371
SPI-452 is another anti-HIV-1 drug currently in Phase II clinical trials that has been patented by Sequoia Pharmaceuticals (no. WO2008022345).370 The drug is a boosting agent that has no antiviral properties but enhances the levels of the second drug taken.372
There are investigational NNRTIs for the treatment of HIV-1, namely, DPC 083 (186).373 DPC 083 (186)374 is a quinazolinone derivative of efavirenz (19), but Bristol-Myers Squibb never developed this potent molecule.375,376 Some of the entry inhibitors that are in investigation include PRO 542, Schering C (187), and T-1249 (189).373 PRO 542 could be classified under CD4-attachment inhibitors that show activity against viral isolates by binding to viral gp120.377 Schering C or SCH-C (187) belongs to the class of chemokine receptor (CCR5) inhibitors and has oral bioavailability.378 T-1249 (189) is a synthetic 39-amino-acid peptide belonging to the class of HIV fusion inhibitors.379,380 S-1360 (188), a 1,3-diketone derivative, is an investigational HIV integrase inhibitor and shows anti-retroviral activity when the amino acids are substituted at the active site of the integrase.381 T-1249 (189) has oral bioavailability and is currently undergoing Phase I/II clinical studies.373 MK-1439 (190),382 an investigational oral HIV NNRTI, is currently undergoing Phase II clinical trials by Merck & Co., Inc.383 S/GSK1265744 (191),384 also known as cabotegravir, a HIV integrase inhibitor, is in ongoing Phase II clinical trials under GlaxoSmithKline (Figure 73).385
Figure 73.
HIV drugs in late stage development: GS 7340 (185),449 DPC 083 (186), SCH-C (187), S-1360 (188), T-1249 (189), MK-1439 (190),450 and S/GSK1265744 (191).451
21.2. HCV
Mericitabine (192), also known as MCB or RG7128 once bioconverted to its 5′-NTP (nucleotide triphosphate), is a HCV nucleoside inhibitor of NS5B RNA-dependent RNA polymerase.386 It was studied up to Phase II clinical trials, but it was not found to be as potent as sofosbuvir (30) and is not being developed by Roche in the U.S. or Europe.387 Danoprevir388 (193) is a macrocyclic peptidomimetic compound that inhibits HCV NS3/4A protease and is currently in Phase II clinical trials managed by Roche.387 Setrobuvir389 (194) is another HCV non-nucleoside inhibitor that inhibits NS5b RNA polymerase.387
BI 201335390 (195) is an oral HCV NS3/4A protease inhibitor that was stopped at Phase III clinical trials managed by Boehringer-Ingelheim. Similarly BI 207127 or deleobuvir (196),390,391 an oral HCV NS5B RNA-dependent polymerase inhibitor, was undergoing Phase II clinical trials.392
Merck Co. has two HCV drugs: MK-5172 (198),393 an oral NS3/4A protease inhibitor, and MK-8742 (197),394 an oral NS5A protease inhibitor undergoing Phase II clinical trials. MK-7009395 or vaniprevir396 (199) is an investigational oral NS3/4A protease inhibitor that is currently undergoing Phase III clinical trials.383 GSK2336805 (200)397 is a HCV inhibitor that is undergoing Phase II clinical trials under GlaxoSmithKline (Figure 74).385
Figure 74.
Novel HCV drugs: mericitabine (192),452 danoprevir (193),453 setrobuvir (194),454 BI 201335 (195),455 BI 207127 (196), MK-8742 (197),456 MK-5172 (198), MK-7009 or vaniprevir (199), and GSK 2336805 (200).457
21.3. Pneumonia
Merck Co. developed a pneumoconjugate vaccine V114 that is currently under Phase II clinical trials. V114 is an investigational vaccine that showed protection against pneumococcal disease that is caused by serotypes present in the vaccine.398
21.4. HBV
Gilead Sciences developed three drugs for the treatment of chronic HBV infection—tenofovir alafenamide (TAF), also GS-7340 (185), a nucleotide reverse transcriptase inhibitor399 that is currently under Phase III clinical trials;400 GS-4774, a therapeutic vaccine that is a tarmogen T cell immunity stimulator;401 and GS-9620 (201), a toll-like receptor 7 (TLR-7) agonist402—which are under Phase II clinical trials (Figures 73 and 75).400
Figure 75.
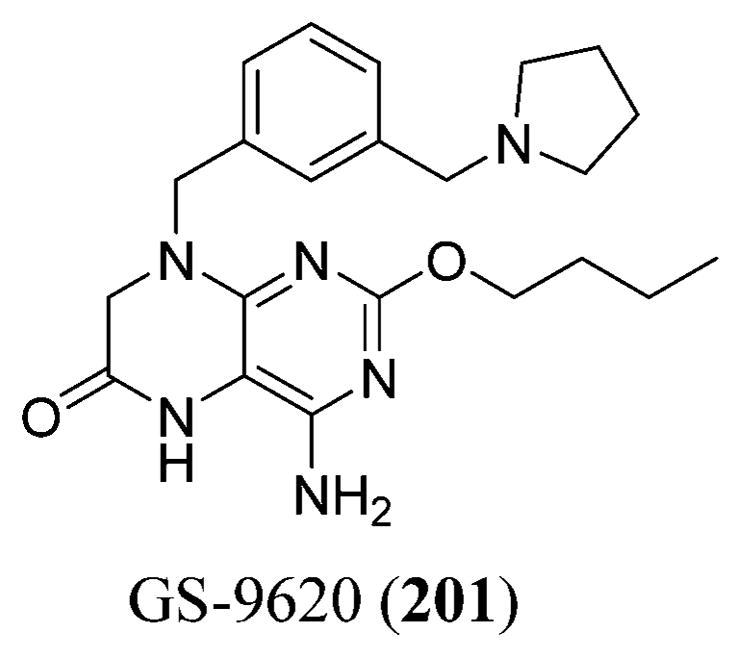
Structure of GS-9620 (201).458
21.5. HPV
Merck developed HPV vaccine V503 against HPV-related cancers; it is an investigational human papillomavirus (HPV) vaccine that targets nine subtypes—6, 11, 16, 18, 31, 33, 45, 52, and 58. V503 is being evaluated for the prevention of HPV, which is currently under review.398
21.6. Norovirus
Romark Laboratories developed nitazoxanide (202), a thiazolide agent for the treatment of viral gastroenteritis, and conducted a double-blind placebo-controlled clinical trial for the evaluation of its activity against norovirus, adenovirus, and rotavirus. Nitazoxanide (202) is known to interfere with the enzyme-dependent transfer reactions of pyruvate ferredoxin oxidoreductase [PFOR] necessary for the anaerobic energy metabolism, preventing the synthesis of the viral proteins (Figure 76).403
Figure 76.
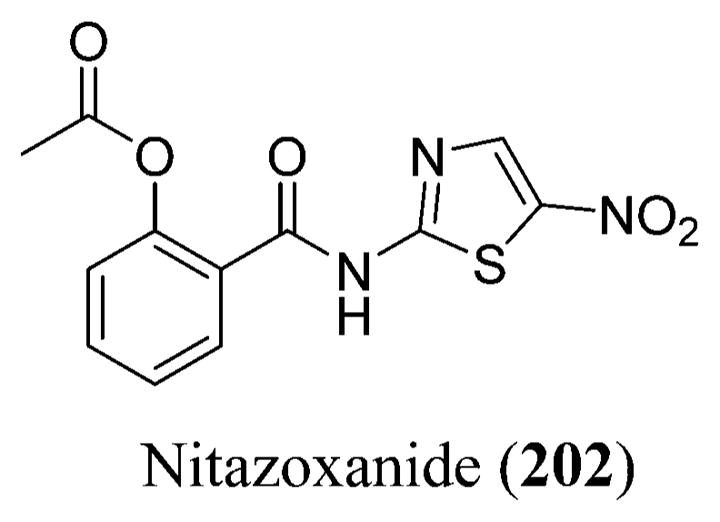
Structure of nitazoxanide (202).459
21.7. Influenza
Roche developed RG7745, a human monoclonal antibody that is designed to neutralize the influenza A virus against a wide range of strains. RG7745 binds to the epitope on the influenza A hemagglutinin stalk region that is highly conserved and is currently under Phase II clinical trials.387 GlaxoSmithKline developed relenza (zanamivir) (67), a neuraminidase inhibitor for the treatment of influenza that is currently under Phase III clinical trials.385 Nitazoxanide (202), a broad-spectrum antiviral, is in Phase III clinical trials for the treatment of influenza.404
21.8. RSV
Gilead developed GS-5806, a fusion inhibitor for the treatment of respiratory syncytial virus (RSV) that is currently under Phase II clinical trials. GS-5806 blocks RSV replication by inhibiting the RSV F-mediated fusion of the viral RNA.400 GlaxoSmithKline developed a pediatric recombinant viral vector vaccine for the treatment of RSV that is currently in Phase I clinical trials.385
21.9. Shingles
Merck Co. developed a herpes zoster inactivated VZV vaccine, V212, that is being evaluated for the prevention of herpes zoster (HZ) and HZ-related complications in immunocompromised subjects, currently under Phase III clinical trials.398
22. CONCLUSION
Natural products continue to provide an excellent source of new leads for the development of anti-HIV agents. A summary of the marine organisms possessing anti-HIV-1 activity and other antiviral activities are shown in Tables 1 and 2. As per the Lipinski guidelines, the “Lipinski rule of five” states that the compound should have a molecular weight <500, a log P value not more than 5, hydrogen bond donors of <5, and hydrogen bond acceptors of <10 for it to have oral bioavailability and better absorption.405 However, most oral drug formulations available in the market fail in at least one Lipinski guideline that, for example, includes didanosine (4), which exceeds the log P value and yet is available as chewable tablets.406 Zalcitabine (5) (which is now rarely used for the treatment of HIV) also fails in one Lipinski guideline by exceeding the log P value and is available in tablet form.407 There are many such examples (see Table S4).
Table 2.
Summary of the Marine Organisms Possessing Antiviral Activity
| compound | type | source | target | activity | ref |
|---|---|---|---|---|---|
| discorhabdins A and C | alkaloids | Latrunculia | HCV | EC90 < 10 μM | 321 and 322 |
| dihydrodiscorhabin C | alkaloid | Latrunculia | HCV | EC90 < 10 μM | 321 and 322 |
| ι-carrageenan | linear sulfated galactan | Eucheuma denticulatum | HPV capsid | unknown | 323 and 324 |
| A1 and A2 | sulfated polysaccharides | Cochlodinium polykrikoides | influenza virus and RSV | unknown | 325 |
| calyceramides A–C | sulfated ceramides | Discodermia calyx | influenza neuraminidase | IC50 0.4, 0.2, and 0.8 μg/mL | 326 |
| weinbersterols A and B | sulfated tetrahydroxy steroids | Petrosia weinbergi | influenza virus | unknown | 326 and 327 |
| stachyflin | terpenoid | Stachybotrys | influenza A virus | 0.003 μM | 328, 329 and 330 |
| ulvan polysaccharides | polysaccharides | green algae | influenza virus | unknown | 335 |
| MC26 | fucose polysaccharide | brown seaweed | influenza virus | unknown | 336 |
| p-KG03 | sulfated polysaccharide | Gyrodinium impudium | influenza virus | unknown | 341 |
| fucoidan | sulfated (fucan) polysaccharide | Cladosiphon okamuranus | DENV | unknown | 342 and 343 |
| EPS-1 and EPS-2 | exopolysaccharides | Geobacillus thermodenitrificans and Bacillus licheniformis | HSV | 200 and 300 μg/mL | 348 and 349 |
| A1 | sulfated polysaccharide | Cochlodinium polykrikoides | HSV-1 | unknown | 325 |
| fucoidan | sulfated (fucan) polysaccharide | Fucus vesiculosus | HSV-1 and HSV-2 replication | unknown | 350 and 351 |
| calcium spirulan | sulfated polysaccharide | Arthrospira platensis | HSV-1 and influenza | unknown | 259 |
| naviculan | sulfated polysaccharide | Navicula directa | HSV replication stages | IC50 7–14 μg/mL | 253 |
| halovirs A–E | peptides | Scytidium | HSV-1 and HSV-2 | ED50 1.1, 3.5, 2.2, 2.0, and 3.1 μM | 352 |
| eudistomins | β-carboline alkaloids | Eudistoma olivaceum | HSV-1 and HSV-2 | unknown | 354 |
| didemnins | cyclic depsipeptides | Trididemnum | HSV-1 | unknown | 355 and 356 |
| μ- and ι-carrageenan | linear sulfated galactans | Gigartina skottsbergii | HSV-1 and HSV-2 | unknown | 365 |
| nostoflan | acidic polysaccharide | Nostoc flagelliforme | HSV-1 | unknown | 368 |
| mycalamide A and B | tetrahydropyran trioxadecalins | Mycale | HSV-1 | 5 ng/disc | 282 |
| 4-methylaaptamine | alkaloid | Aaptos | HSV-1 | EC50 2.4 μM | 282 |
| dragmacidin F | bromoindole alkaloid | Halicortex | HSV-1 | EC50 96 μM | 282 |
| hamigeran B | tricyclic benzo-fused hydrindane | Hamigeran tarangaensis | HSV | unknown | 282 |
All the absorption criteria mentioned in the Supporting Information are not followed by many of the FDA-approved drugs. Most drugs fail in at least two to three criteria and yet are considered as drugs favored to treat HIV-1. Similarly there are compounds isolated from the marine sources that are not different to these kinds of drugs. These compounds could be synthesized as oral formulations and are inexpensive to formulate based on their structures. Some include avarol (114), curcuphenol (116) and its analogues a (117), c (118), j (119), and r (120), manzamine A (132), manadomanzamines A (135) and B (136), xestomanzamine A (137), crambescidin 800 (138), ptilomycalin A (139), batzelladine C (140), isoaaptamine (141), aaptamine (142), and cyanthiwigin B (145) that could be considered for syntheses in the treatment of HIV-1. The same could be said in the case of distribution, metabolism, and toxicity criteria (see Supporting Information).
All the information discussed above is only a prediction (from ADMET predictor), and hence, the drugs isolated from the marine sources need to undergo in vivo assays, advanced toxicological evaluation, and clinical trials to further strengthen the results and validate safety and efficacy profiles. Although some of the drugs fail in many criteria, they could still be adjusted or altered to better treat the disease. Any further progress could be made only when the compounds from the marine sources can undergo further tests and assays.
The major challenges involved in natural product drug development are drug resistance, long drug-development processes, and sometimes toxic side effects. Many of the sponge-derived compounds have shown toxic effects. This could potentially be solved by applying biochemical technologies such as combinatorial biosynthesis, synthetic biology, metabolic engineering, medicinal chemistry, and post-genomic techniques.223,408
When the virus becomes resistant to one drug, then it is not possible to treat that virus with any other naturally occurring derivative with similar activities against the virus.409 So for the discovery of new drugs, different derivatives belonging to a common class synthesized by multiple organisms could be a solution.
Identifying, cloning, and modifying gene expression using synthetic biology are some of the techniques used to combat the large-scale production problems.223 Therefore, understanding the biology and the natural living conditions that affect the growth and metabolite production of sponges is necessary.282
Supplementary Material
Acknowledgments
We are grateful to Simulations Plus (Lancaster, CA, U.S.A.) for providing us with the reference site license to use the software ADMET Predictor. We also thank the Virtual Computational Chemistry Laboratory that provided the ALOGPS 2.1 software necessary to generate the log S values for the determination of solubility. Drs. Prabhakar Reddy Polepally, Daneel Ferreira, Ziaeddin Shariat-Madar, and Manal Nael, a graduate student (all from the University of Mississippi), are warmly acknowledged for their help with the stereochemistry and pharmacology sections. Pankaj Pandey, a graduate student (University of Mississippi), is being acknowledged for introducing us to the ADMET predictor. M.T.H. is supported in part by NIH NCCAM Grant 1R01AT007318, Kraft Foods, and the Department of Bio-Molecular Sciences, Division of Pharmacognosy. R.F.S. is supported in part by the NIH CFAR Grant 5P30-AI-50409, 1RO1-MH-100999, and the Department of Veterans Affairs.
Biographies

Vedanjali Gogineni received her M.S. in Pharmaceutical Chemistry from Fairleigh Dickinson University, New Jersey, and her Bachelor’s degree from Jawaharlal Nehru Technological University, India. In 2011, she joined as a Visiting Scholar in the Department of Pharmacognosy, University of Mississippi, with Professor Mark Hamann. In 2013, she joined the graduate program in the Department of Biomolecular Sciences, Division of Medicinal Chemistry, University of Mississippi, where she is currently completing her Ph.D. degree with Professor Stephen J. Cutler. Her research interests are focused on isolation and characterization of psychoactive medicinal plants.

Dr. Raymond F. Schinazi, Ph.D., D.Sc., is the Frances Winship Walters Professor of Pediatrics and Director of the Laboratory of Biochemical Pharmacology at Emory University. He serves as Senior Research Career Scientist at the Atlanta Department of Veterans Affairs and as Director of the Scientific Working Group on Viral Eradication for the NIH-sponsored Emory University Center for AIDS Research (CFAR). Dr. Schinazi received his B.Sc. (1972) and Ph.D. (1976) in chemistry from the University of Bath, England. He has authored over 500 peer-reviewed papers and 7 books and holds 92 issued U.S. patents and over 120 non-U.S. national stage patents and patent applications, which have resulted in 13 New Drug Applications (NDAs). A world leader in nucleoside chemistry, Dr. Schinazi is best known for his pioneering work on HIV and HCV drugs d4T (stavudine), 3TC (lamivudine), FTC (emtricitabine/Emtriva), LdT (telbivudine), and most recently sofosbuvir (Sovaldi), which are now approved by the FDA. He is the founder of five biotechnology companies including Pharmasset, Inc. More than 94% of HIV-infected individuals in the US on combination therapy take at least one of the drugs he invented, and it is estimated that his work has saved more than 6.5 million lives worldwide. His contributions related to HCV are expected to have a profound positive impact on over 80 million people worldwide suffering from chronic infection.

Dr. Mark T. Hamann is a Professor of Pharmacognosy, Pharmacology and Chemistry & Biochemistry as well as a Research Professor with the National Center for Natural Products Research at the University of Mississippi and is an Associate Member of the UMMC Cancer Center.
Dr. Hamann has several years experience in GMP pharmaceutical manufacturing at Solvay Pharmaceuticals in Baudette, Minnesota, and completed a Ph.D. degree in Organic/Natural Product Chemistry in 1992 at the University of Hawaii, Chemistry Department, under the guidance of the late Professor Paul Scheuer, a pioneer in the discovery and development of marine products. Dr. Hamann then completed Postdoctoral Studies with Prof. Bill Baker doing field studies in Antarctica. During his research career, Dr. Hamann has published over 190 scientific papers, reviews, and book chapters and currently serves as an Associate Editor for Biochimica et Biophysica Acta, General Subjects. His group is actively involved in the isolation, structure determination, and synthetic optimization of marine/terrestrial natural products and toxins with a focus on plant and invertebrate microbiomes. His group is currently working on the preclinical development of a small pipeline of natural products with activity against infectious diseases, cancer, and neuropsychiatric disorders.
Footnotes
Notes
The authors declare no competing financial interest.
This material is available free of charge via the internet at The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/cr4006318.
Parameters necessary to analyze the pharmacokinetic and toxic properties of the reported marine drugs and the FDA-approved drugs (PDF)
References
- 1.Becker K, Hu Y, Biller-Andorno N. Infectious Diseases - A Global Challenge. Int J Med Microbiol. 2006;296:179–185. doi: 10.1016/j.ijmm.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MedicineNet.com. [accessed November 25, 2011];Definition of Agent, Anti-Infective. http://www.medicinenet.com/script/main/art.asp?articlekey=2178.
- 3.World Health Organization. [accessed May 15, 2014];Media Centre: Fact Sheets. http://www.who.int/mediacentre/factsheets/en/#A.
- 4.National Institute of Allergy and Infectious Diseases. [accessed May 15, 2014];Health & Research Topics. http://www.niaid.nih.gov/topics/Pages/default.aspx.
- 5.Centers for Disease Control and Prevention. [accessed May 15, 2014];Morbidity and Mortality Weekly Report (MMWR) http://www.cdc.gov/mmwr/indss_2014.html.
- 6.Centers for Disease Control and Prevention. [accessed March 10, 2012];Morbidity and Mortality Weekly Report (MMWR) http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6029a3.htm.
- 7.World Health Organization. [accessed March 17, 2013];Global Report: UNAIDS Report on the Global AIDS Epidemic. 2012 http://www.unaids.org/sites/default/files/media_asset/20121120_UNAIDS_Global_Report_2012_with_annexes_en_1.pdf.
- 8.United Nations. [accessed November 27, 2011];Political Declaration on HIV and AIDS: Intensifying Our Efforts to Eliminate HIV/AIDS. http://www.unaids.org/sites/default/files/sub_landing/files/20110610_UN_A-RES-65-277_en.pdf.
- 9.UNAIDS 2012 Global Report. [accessed Mar 17, 2013];UNAIDS World AIDS Day Report. 2012 http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/JC2434_WorldAIDSday_results_en.pdf.
- 10.Centers for Disease Control and Prevention. [accessed November 25, 2011];HIV Surveillance Report. 2009 21:1–79. http://www.cdc.gov/hiv/pdf/statistics_2009_HIV_Surveillance_Report_vol_21.pdf. [Google Scholar]
- 11.Centers for Disease Control and Prevention. [accessed November 27, 2011];HIV Prevalence Estimates—United States, 2006. 2008 57:1073–1076. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5739a2.htm. [Google Scholar]
- 12.Centers for Disease Control and Prevention. [accessed November 27, 2011];New CDC Analysis Reveals Strong Link Between Poverty and HIV Infection. 2010 http://www.cdc.gov/nchhstp/newsroom/2010/povertyandhivpressrelease.html.
- 13.Centers for Disease Control and Prevention. [accessed November 25, 2011];HIV among Latinos. http://www.cdc.gov/hiv/risk/racialethnic/hispaniclatinos/index.html.
- 14.The White House Office of National AIDS Policy. [accessed November 25, 2011];National HIV/AIDS Strategy For The United States. http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf.
- 15.Centers for Disease Control and Prevention. [accessed November 25, 2011];Epidemiologic Notes and Reports Immunodeficiency among Female Sexual Partners of Males with Acquired Immune Deficiency Syndrome (AIDS)—New York. 1983 31:697–698. http://www.cdc.gov/mmwr/preview/mmwrhtml/00001221.htm. [PubMed] [Google Scholar]
- 16.Grmek MD. History of AIDS: Emergence and Origin of a Modern Pandemic. Princeton; Princeton, NJ: 1990. pp. 3–186. [Google Scholar]
- 17.Altman LK. New Homosexual Disorder Worries Health Officials. [accessed November 25, 2011];The New York Times, [Online] 1982 May 11; http://www.nytimes.com/1982/05/11/science/new-homosexual-disorder-worries-health-officials.html?pagewanted=all.
- 18.Oswald GA, Theodossi A, Gazzard BG, Byrom NA, Fisher-Hoch SP. Attempted Immune Stimulation in the “Gay Compromise Syndrome”. Br Med J. 1982;285:1082. doi: 10.1136/bmj.285.6348.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kher U. A Name for the Plague. [accessed January 30, 2012];TIME, [Online] 1982 Jul 27; http://content.time.com/time/specials/packages/article/0,28804,1977881_1977895_1978703,00.html.
- 20.Culliton BJ. Crash Development of AIDS Test Nears Goal. Science. 1984;225:1128–1130. doi: 10.1126/science.6089342. [DOI] [PubMed] [Google Scholar]
- 21.AIDS.gov. [accessed March 19, 2010];A Timeline of AIDS. http://www.aids.gov/hiv-aids-basics/hiv-aids-101/aids-timeline/
- 22.Altman LK. Blood Supply Called Free of AIDS. [accessed November 25, 2011];The New York Times, [Online] 1985 Aug 1; http://www.nytimes.com/1985/08/01/world/blood-supply-called-free-of-aids.html?pagewanted=1.
- 23.Amador ML, Jimeno J, Paz-Ares L, Cortes-Funes H, Hidalgo M. Progress in the Development and Acquisition of Anticancer Agents from Marine Sources. Ann Oncol. 2003;14:1607–1615. doi: 10.1093/annonc/mdg443. [DOI] [PubMed] [Google Scholar]
- 24.Bergmann W, Feeney RJ. Contributions to the Study of Marine Products. XXXII. The Nucleosides of Sponges. J Org Chem. 1951;16:981–987. [Google Scholar]
- 25.Carroll J, Crews P. In: Natural Product Chemistry for Drug Discovery. Buss AD, Butler MS, editors. Royal Society of Cambridge; Cambridge, U.K: 2009. pp. 174–195. [Google Scholar]
- 26.North TW, Cohen SS. Aranucleosides and Aranucleotides in Viral Chemotherapy. Pharmacol Ther. 1979;4:81–108. doi: 10.1016/0163-7258(79)90016-0. [DOI] [PubMed] [Google Scholar]
- 27.Ostertag W, Roesler G, Krieg CJ, Kind J, Cole T, Crozier T, Gaedicke G, Steinheider G, Kluge N, Dube S. Induction of Endogenous Virus and of Thymidine Kinase by Bromodeoxyuridine in Cell Cultures Transformed by Friend Virus. Proc Natl Acad Sci U S A. 1974;71:4980–4985. doi: 10.1073/pnas.71.12.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrman SN, Gallo RC, Bolognesi D, Barry DW, Broder S. 3′-Azido-3′-deoxythymidine (BW A509U): An Antiviral Agent that Inhibits the Infectivity and Cytopathic Effect of Human T-lymphotropic Virus Type III/lymphadenopathy-associated Virus In vitro. Proc Natl Acad Sci U S A. 1985;82:7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan T. Mainstream Strategy for AIDS Group. [accessed February 18, 2012];The New York Times, [Online] 1988 Jul 22; http://www.nytimes.com/1988/07/22/nyregion/mainstream-strategy-for-aids-group.html?pagewanted=2&src=pm.
- 30.Yarchoan R, Mitsuya H, Thomas RV, Pluda JM, Hartman NR, Perno CF, Marczyk KS, Allain JP, Johns DG, Broder S. In vivo Activity Against HIV and Favorable Toxicity Profile of 2′,3′-dideoxyinosine. Science. 1989;245:412–415. doi: 10.1126/science.2502840. [DOI] [PubMed] [Google Scholar]
- 31.Balzarini J, Cooney DA, Dalal M, Kang GJ, Cupp JE, DeClercq E, Broder S, Johns DG. 2′, 3′-Dideoxycytidine: Regulation of its Metabolism and Anti-retroviral Potency by Natural Pyrimidine Nucleosides and by Inhibitors of Pyrimidine Nucleotide Synthesis. Mol Pharmacol. 1987;32:798–806. [PubMed] [Google Scholar]
- 32.U.S. Food and Drug Administration. [accessed February 18, 2012];HIV/AIDS Historical Time Line 1991–1994. http://www.fda.gov/downloads/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/drugandbiologicapprovalreports/ucm278506.pdf.
- 33.New H.I.V. Strains Resist AIDS Drug. [accessed February 18, 2012];The New York Times, [Online] 1993 Jan 1; http://partners.nytimes.com/library/national/science/aids/010193sci-aids.html.
- 34.Riddler SA, Anderson RE, Mellors JW. Antiretroviral Activity of Stavudine (2′, 3′-didehydro-3′-deoxythymidine, d4T) Antiviral Res. 1995;27:189–203. doi: 10.1016/0166-3542(95)00016-f. [DOI] [PubMed] [Google Scholar]
- 35.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, VanDyke R, Bey M, Shearer W, Jacobson RL, Jimenez E, O’Neill E, Bazin B, Delfraissy JF, Culnane M, Coombs R, Elkins M, Moye J, Stratton P, Balsley J. Reduction of Maternal-Infant Transmission of Human Immunodeficiency Virus Type 1 with Zidovudine Treatment. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 36.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M, Jr, Chun TW, Strain M, Richman D, Luzuriaga K. Absence of Detectable HIV-1 Viremia after Treatment Cessation in an Infant. N Engl J Med. 2013;369:1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szabo L. HIV Infection Returns in Mississippi Girl Thought Cured. [accessed August 18, 2014];USA Today, [Online] 2014 Jul 14; http://www.usatoday.com/story/news/nation/2014/07/10/baby-hiv-returns-mississippi/12480595/
- 38.U.S. Food and Drug Administration. [accessed February 16, 2012];FDA Announces Possible Safety Concern for HIV Drug Combination. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm201552.htm.
- 39.U.S. Food and Drug Administration. [accessed March 18, 2013];Antiretroviral Drugs Used in the Treatment of HIV Infection. http://www.fda.gov/forpatients/illness/hivaids/treatment/ucm118915.htm.
- 40.U.S. Food and Drug Administration. [accessed February 16, 2012];Highlights of Prescribing Information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020933s022,020636s032lbl.pdf.
- 41.U.S. Food and Drug Administration. [accessed February 18, 2012];HIV/AIDS Historical Time Line 1995–1999. http://www.fda.gov/forpatients/illness/hivaids/history/ucm151079.htm.
- 42.U.S. Department of Health & Human Services. [accessed February 18, 2012];Providing Access to Promising Therapies for Seriously Ill and Dying Patients. http://www.fda.gov/NewsEvents/Testimony/ucm115120.htm.
- 43.Staszewski S, Katlama C, Harrer T, Massip P, Yeni P, Cutrell A, Tortell SM, Harrigan RP, Steel H, Lanier RE, Pearce G. A Dose-Ranging Study to Evaluate the Safety and Efficacy of Abacavir Alone or in Combination with Zidovudine and Lamivudine in Antiretroviral Treatment-Naïve Subjects. AIDS. 1998;12:F197–F202. doi: 10.1097/00002030-199816000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Weiss RA, Wrangham RW. From Pan to Pandemic. Nature. 1999;397:385–386. doi: 10.1038/17008. [DOI] [PubMed] [Google Scholar]
- 45.Morbidity and Mortality Weekly Report (MMWR) [accessed February 17, 2012];Nonoxynol-9 Spermicide Contraception Use—United States, 1999. 2002 51:389–392. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5118a1.htm. [PubMed] [Google Scholar]
- 46.Fazal A. HIV Vaccine Trials Begin in Oxford. Br Med J. 2000;321:591. [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S. Indian Company Offers Low Cost AIDS Drugs. Lancet. 2001;357:616. doi: 10.1016/S0140-6736(05)71411-2. [DOI] [PubMed] [Google Scholar]
- 48.McNeil DG, Jr W.H.O. Moves to Make AIDS Drugs More Accessible to Poor Worldwide. [accessed November 27, 2011];The New York Times, [Online] 2002 Apr 23; http://www.nytimes.com/2002/04/23/health/who-moves-to-make-aids-drugs-more-accessible-to-poor-worldwide.html?pagewanted=all&src=pm.
- 49.Vass A. Hopes Rise for Patients with Drug Resistant HIV. Br Med J. 2002;325:62. doi: 10.1136/bmj.325.7355.62/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green EC. Rethinking AIDS Prevention: Learning from Successes in Developing Countries. Praeger; Westport, CT: 2003. pp. 1–374. [Google Scholar]
- 51.Gottlieb S. Bush Legislates for $15bn to be Spent on AIDS. Br Med J. 2003;326:1233. doi: 10.1136/bmj.326.7401.1233-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eaton L. AIDS Vaccine may Offer Hope Only for Some Ethnic Groups. Br Med J. 2003;326:463. doi: 10.1136/bmj.326.7387.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCarthy M. HIV Vaccine Fails in Phase 3 Trial. Lancet. 2003;361:755–756. doi: 10.1016/S0140-6736(03)12669-4. [DOI] [PubMed] [Google Scholar]
- 54.U.S. Food and Drug Administration. [accessed November 27, 2011];Fuzeon (Enfuvirtide) for Injection. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021481s020lbl.pdf.
- 55.Hurwitz SJ, Schinazi RF. Practical Considerations For Developing Nucleoside Reverse Transcriptase Inhibitors. Drug Discovery Today: Technol. 2012;9:e183–e193. doi: 10.1016/j.ddtec.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darque A, Valette G, Rousseau F, Wang LH, Sommadossi JP, Zhou XJ. Quantitation of Intracellular Triphosphate of Emtricitabine in Peripheral Blood Mononuclear Cells from Human Immunodeficiency Virus-Infected Patients. Antimicrob Agents Chemother. 1999;43:2245–2250. doi: 10.1128/aac.43.9.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapp C. AIDS Campaign Signals New WHO Priorities and Approach. Lee Promises to Focus on Real Targets and Improving WHO’s Effectiveness. Lancet. 2003;362:1900–1901. doi: 10.1016/S0140-6736(03)15001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Z, Sullivan SG, Wang Y, Rotheram-Borus MJ, Detels R. Evolution of China’s Response to HIV/AIDS. Lancet. 2007;369:679–690. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parfitt T. Global Fund Suspends Payments to Ukraine. Lancet. 2004;363:540. doi: 10.1016/S0140-6736(04)15580-3. [DOI] [PubMed] [Google Scholar]
- 60.U.S. Department of Health & Human Services. [accessed February 16, 2012];FDA Approves First Oral Fluid Based Rapid HIV Test Kit. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108272.htm.
- 61.U.S. Department of State. [accessed November 27, 2011];Bringing Hope and Saving Lives: Building Sustainable HIV/AIDS Treatment. http://www.state.gov/documents/organization/36287.pdf.
- 62.U.S. Department of Health & Human Services. [accessed November 27, 2011];FDA Grants Tentative Approval to Generic AIDS Drug Regimen for Potential Purchase Under the President’s Emergency Plan for AIDS Relief. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108407.htm.
- 63.Pollack A. New Medicine for AIDS Is One Pill, Once a Day. [accessed November 27, 2011];The New York Times, [Online] 2006 Jul 9; http://www.nytimes.com/2006/07/09/health/09aids.html?pagewanted=1&_r=0.
- 64.U.S. Department of Health & Human Services. [accessed May 29, 2012];FDA Approves the First Once-a-Day Three-Drug Combination Tablet for Treatment of HIV-1. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108689.htm?utm_campaign=Google2&utm_source=fdaSearch&utm_medium=website&utm_term=2006%20HIV%20pill&utm_content=1.
- 65.Tuske S, Sarafianos SG, Clark AD, Jr, Ding J, Naeger LK, White KL, Miller MD, Gibbs CS, Boyer PL, Clark P, Wang G, Gaffney BL, Jones RA, Jerina DM, Hughes SH, Arnold E. Structures of HIV-1 RT-DNA Complexes Before and After Incorporation of the Anti-AIDS Drug Tenofovir. Nat Struct Mol Biol. 2004;11:469–474. doi: 10.1038/nsmb760. [DOI] [PubMed] [Google Scholar]
- 66.U.S. Food and Drug Administration. [accessed February 16, 2012];Isentress (Raltegravir) Tablets. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022145s004lbl.pdf.
- 67.U.S. Food and Drug Administration. [accessed February 16, 2012];Selzentry (Maraviroc) Tablets. http://www.fda.gov/downloads/drugs/drugsafety/ucm089122.pdf.
- 68.Desmon S. AIDS Vaccine’s Failure Deals Big Blow. [accessed November 27, 2011];The Baltimore Sun, [Online] 2007 Nov 14; http://articles.baltimoresun.com/2007-11-14/news/0711140127_1_vaccine-merck-hiv-infection.
- 69.The United States President’s Emergency Plan For AIDS Relief. [accessed November 27, 2011];PEPFAR: A Commitment Renewal. http://www.pepfar.gov/press/107735.htm.
- 70.Ban congratulates US leader for lifting entry restriction based on HIV status. [accessed February 17, 2012];UN News Centre, [Online] 2009 Oct 31; http://www.un.org/apps/news/story.asp?NewsID=32799&Cr=hiv&Crl=aids#.VHucSjHF9W4.
- 71.Carter M. US HIV Travel Ban Has Now Ended. [accessed February 17, 2012];Aidsmap, [Online] 2010 Jan 04; http://www.aidsmap.com/page/1437294/
- 72.Treanor JJ. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6. Elsevier Churchill Livingston; Philadelphia, PA: 2005. Influenza virus; pp. 2060–2085. [Google Scholar]
- 73.U.S. Food and Drug Administration. [accessed October 15, 2012];Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Disoproxil Fumarate: Antiviral Drugs Advisory Committee Meeting Briefing Document. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM303397.pdf.
- 74.U.S. Food and Drug Administration. [accessed December 26, 2014];TRUVADA (Emtricitabine/Tenofovir Disoproxil Fumarate) http://www.fda.gov/downloads/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm312304.pdf.
- 75.U.S. Food and Drug Administration. [accessed October 15, 2012];Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Disoproxil Fumarate: Antiviral Drugs Advisory Committee Meeting Briefing Document. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM303397.pdf.
- 76.National Institute of Allergy and Infectious Diseases. [accessed February 14, 2012];Structure of AIDS. http://www.niaid.nih.gov/topics/HIVAIDS/Understanding/howHIVCausesAIDS/Pages/howhiv.aspx.
- 77.AIDSinfo. [accessed November 27, 2011];HIV Life Cycle. http://aidsinfo.nih.gov/education-materials/fact-sheets/19/73/the-hiv-life-cycle.
- 78.Barrett L, Grant M. Brothers in Harm: Immunological and Clinical Implications of Coinfection with Hepatitis C Virus and Human Immunodeficiency Virus. Clin Appl Immunol Rev. 2002;2:93–114. [Google Scholar]
- 79.Staples CT, Jr, Rimland D, Dudas D. Hepatitis C in the HIV (Human Immunodeficiency Virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): The Effect of Coinfection on Survival. Clin Infect Dis. 1999;29:150–154. doi: 10.1086/520144. [DOI] [PubMed] [Google Scholar]
- 80.Centers for Disease Control and Prevention. [accessed April 17, 2012];HIV/AIDS and Viral Hepatitis. http://www.cdc.gov/hepatitis/Populations/hiv.htm.
- 81.Sulkowski MS, Mast EE, Seeff LB, Thomas DL. Hepatitis C Virus Infection as an Opportunistic Disease in Persons Infected with Human Immunodeficiency Virus. Clin Infect Dis. 2000;30:S77–S84. doi: 10.1086/313842. [DOI] [PubMed] [Google Scholar]
- 82.Thomas DL, Villano SA, Riester KA, Hershow R, Mofenson LM, Landesman SH, Hollinger FB, Davenny K, Riley L, Diaz C, Tang HB, Quinn TC. Perinatal Transmission of Hepatitis C Virus from Human Immunodeficiency Virus Type 1-Infected Mothers. J Infect Dis. 1998;177:1480–1488. doi: 10.1086/515315. [DOI] [PubMed] [Google Scholar]
- 83.Woitas RP, Rockstroh JK, Beier I, Jung G, Kochan B, Matz B, Brackmann HH, Sauerbruch T, Spengler U. Antigen-Specific Cytokine Response to Hepatitis C Virus Core Epitopes in HIV/Hepatitis C Virus-Coinfected Patients. AIDS. 1999;13:1313–1322. doi: 10.1097/00002030-199907300-00007. [DOI] [PubMed] [Google Scholar]
- 84.Bonnard P, Lescure FX, Amiel C, Guiard-Schmid JB, Callard P, Gharakhanian S, Pialoux G. Documented Rapid Course of Hepatic Fibrosis Between Two Biopsies in Patients Coinfected by HIV and HCV Despite High CD4 Cell Count. J Viral Hepat. 2007;14:806–811. doi: 10.1111/j.1365-2893.2007.00874.x. [DOI] [PubMed] [Google Scholar]
- 85.McGinnis KA, Fultz SL, Skanderson M, Conigliaro J, Bryant K, Justice AC. Hepatocellular Carcinoma and Non-Hodgkin’s Lymphoma: The Roles of HIV, Hepatitis C Infection, and Alcohol Abuse. J Clin Oncol. 2006;24:5005–5009. doi: 10.1200/JCO.2006.05.7984. [DOI] [PubMed] [Google Scholar]
- 86.Cohen SD, Kimmel PL. Immune Complex Renal Disease and Human Immunodeficiency Virus Infection. Semin Nephrol. 2008;28:535–544. doi: 10.1016/j.semnephrol.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 87.Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, Appel R, Fields TA, Svetkey LP, Flanagan KH, Klotman PE, Winston JA. The Clinical Epidemiology and Course of the Spectrum of Renal Diseases Associated with HIV Infection. Kidney Int. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 88.Tsui J, Vittinghoff E, Anastos K, Augenbraun M, Young M, Nowicki M, Cohen MH, Peters MG, Golub ET, Szczech L. Hepatitis C Seropositivity and Kidney Function Decline Among Women With HIV: Data From the Women’s Interagency HIV Study. Am J Kidney Dis. 2009;54:43–50. doi: 10.1053/j.ajkd.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng JT, Anderson HL, Jr, Markowitz GS, Appel GB, Pogue VA, D’Agati VD. Hepatitis C Virus-Associated Glomerular Disease in Patients with Human Immunodeficiency Virus Coinfection. J Am Soc Nephrol. 1999;10:1566–1574. doi: 10.1681/ASN.V1071566. [DOI] [PubMed] [Google Scholar]
- 90.Bruno R, Sacchi P, Puoti M, Soriano V, Filice G. HCV Chronic Hepatitis in Patients with HIV: Clinical Management Issues. Am J Gastroenterol. 2002;97:1598–1606. doi: 10.1111/j.1572-0241.2002.05817.x. [DOI] [PubMed] [Google Scholar]
- 91.Palmer S, Margot N, Gilbert H, Shaw N, Buckheit R, Jr, Miller M. Tenofovir, Adefovir, and Zidovudine Susceptibilities of Primary Human Immunodeficiency Virus Type 1 Isolates with Non-B Subtypes or Nucleoside Resistance. AIDS Res Hum Retroviruses. 2001;17:1167–1173. doi: 10.1089/088922201316912772. [DOI] [PubMed] [Google Scholar]
- 92.De Clercq E. The Design of Drugs for HIV and HCV. Nat Rev Drug Discovery. 2007;6:1001–1018. doi: 10.1038/nrd2424. [DOI] [PubMed] [Google Scholar]
- 93.De Francesco R, Migliaccio G. Challenges and Successes in Developing New Therapies for Hepatitis C. Nature. 2005;436:953–960. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- 94.Carroll SS, Tomassini JE, Bosserman M, Getty K, Stahlhut MW, Eldrup AB, Bhat B, Hall D, Simcoe AL, LaFemina R, Rutkowski CA, Wolanski B, Yang Z, Migliaccio G, De Francesco R, Kuo LC, MacCoss M, Olsen DB. Inhibition of Hepatitis C Virus RNA Replication by 2′-Modified Nucleoside Analogs. J Biol Chem. 2003;278:11979–11984. doi: 10.1074/jbc.M210914200. [DOI] [PubMed] [Google Scholar]
- 95.Tomassini JE, Getty K, Stahlhut MW, Shim S, Bhat B, Eldrup AB, Prakash TP, Carroll SS, Flores O, MacCoss M, McMasters DR, Migliaccio G, Olsen DB. Inhibitory Effect of 2′-Substituted Nucleosides on Hepatitis C Virus Replication Correlates with Metabolic Properties in Replicon Cells. Antimicrob Agents Chemother. 2005;49:2050–2058. doi: 10.1128/AAC.49.5.2050-2058.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Clercq E, Neyts J. In: Antiviral Strategies. Kräusslich H-G, Bartenschlager R, editors. Springer Berlin; Heidelberg: 2009. pp. 53–84. [Google Scholar]
- 97.Olsen DB, Eldrup AB, Bartholomew L, Bhat B, Bosserman MR, Ceccacci A, Colwell LF, Fay JF, Flores OA, Getty KL, Grobler JA, LaFemina RL, Markel EJ, Migliaccio G, Prhavc M, Stahlhut MW, Tomassini JE, MacCoss M, Hazuda DJ, Carroll SS. A 7-Deaza-Adenosine Analog Is a Potent and Selective Inhibitor of Hepatitis C Virus Replication with Excellent Pharmacokinetic Properties. Antimicrob Agents Chemother. 2004;48:3944–3953. doi: 10.1128/AAC.48.10.3944-3953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Migliaccio G, Tomassini JE, Carroll SS, Tomei L, Altamura S, Bhat B, Bartholomew L, Bosserman MR, Ceccacci A, Colwell LF, Cortese R, De Francesco R, Eldrup AB, Getty KL, Hou XS, LaFemina RL, Ludmerer SW, MacCoss M, McMasters DR, Stahlhut MW, Olsen DB, Hazuda DJ, Flores OA. Characterization of Resistance to Non-Obligate Chain-Terminating Ribonucleoside Analogs That Inhibit Hepatitis C Virus Replication In Vitro. J Biol Chem. 2003;278:49164–49170. doi: 10.1074/jbc.M305041200. [DOI] [PubMed] [Google Scholar]
- 99.Eldrup AB, Allerson CR, Bennett CF, Bera S, Bhat B, Bhat N, Bosserman MR, Brooks J, Burlein C, Carroll SS, Cook PD, Getty KL, MacCoss M, McMasters DR, Olsen DB, Prakash TP, Prhavc M, Song Q, Tomassini JE, Xia J. Structure–Activity Relationship of Purine Ribonucleosides for Inhibition of Hepatitis C Virus RNA-Dependent RNA Polymerase. J Med Chem. 2004;47:2283–2295. doi: 10.1021/jm030424e. [DOI] [PubMed] [Google Scholar]
- 100.Klumpp K, Lévêque V, Le Pogam S, Ma H, Jiang WR, Kang H, Granycome C, Singer M, Laxton C, Qi Hang J, Sarma K, Smith DB, Heindl D, Hobbs CJ, Merrett JH, Symons J, Cammack N, Martin JA, Devos R, Nájera I. The Novel Nucleoside Analog R1479 (4′-Azidocytidine) Is a Potent Inhibitor of NS5B-dependent RNA Synthesis and Hepatitis C Virus Replication in Cell Culture. J Biol Chem. 2006;281:3793–3799. doi: 10.1074/jbc.M510195200. [DOI] [PubMed] [Google Scholar]
- 101.Stuyver LJ, McBrayer TR, Tharnish PM, Clark J, Hollecker L, Lostia S, Nachman T, Grier J, Bennett MA, Xie MY, Schinazi RF, Morrey JD, Julander JL, Furman PA, Otto MJ. Inhibition of Hepatitis C Replicon RNA Synthesis by β-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine: A Specific Inhibitor of Hepatitis C Virus Replication. Antiviral Chem Chemother. 2006;17:79–87. doi: 10.1177/095632020601700203. [DOI] [PubMed] [Google Scholar]
- 102.Klumpp K, Smith D, Brandl M, Alfredson T, Sarma K, Smith M, Najera I, Jiang WR, Le Pogam S, Leveque V, et al. Design and Characterization of R1626, A Prodrug of the HCV Replication Inhibitor R1479 (4′-Azidocytidine) With Enhanced Oral Bioavailability. Antiviral Res. 2007;74:A35. [Google Scholar]
- 103.Brandl M, Wu X, Holper M, Hong L, Jia Z, Birudaraj R, Reddy M, Alfredson T, Tran T, Larrabee S, Hadig X, Sarma K, Washington C, Hill G, Smith DB. Physicochemical Properties of the Nucleoside Prodrug R1626 Leading to High Oral Bioavailability. Drug Dev Ind Pharm. 2008;34:683–691. doi: 10.1080/03639040701836636. [DOI] [PubMed] [Google Scholar]
- 104.Smith D, Ma H, Le Pogam S, Leveque V, Brown C, Johansson NG, Kalayanov G, Eriksson S, Usova E, Sund C, Winqist A, Maltseva T, Smith M, Martin J, Najera I, Klumpp K. Novel 4′-Azido-2′-Deoxy-Nucleoside Analogs are Potent Inhibitors of NS5B-Dependent HCV Replication. Antiviral Res. 2007;74:A36. [Google Scholar]
- 105.Murakami E, Tolstykh T, Bao H, Niu C, Steuer HMM, Bao D, Chang W, Espiritu C, Bansal S, Lam AM, Otto MJ, Sofia MJ, Furman PA. Mechanism of Activation of PSI-7851 and Its Diastereoisomer PSI-7977. J Biol Chem. 2010;285:34337–34347. doi: 10.1074/jbc.M110.161802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, Afdhal NH, Bernstein DE, DeJesus E, Freilich B, Nelson DR, Dieterich DT, Jacobson IM, Jensen D, Abrams GA, Darling JM, Rodriguez-Torres M, Reddy KR, Sulkowski MS, Bzowej NH, Hyland RH, Mo H, Lin M, Mader M, Hindes R, Albanis E, Symonds WT, Berrey MM, Muir A. Sofosbuvir in Combination with Peginterferon Alfa-2a and Ribavirin for Non-Cirrhotic, Treatment-Naive Patients with Genotypes 1, 2, and 3 Hepatitis C Infection: A Randomised, Double-Blind, Phase 2 Trial. Lancet Infect Dis. 2013;13:401–408. doi: 10.1016/S1473-3099(13)70033-1. [DOI] [PubMed] [Google Scholar]
- 107.Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, Bernstein DE, Afdhal N, Vierling JM, Gordon SC, Anderson JK, Hyland RH, Dvory-Sobol H, An D, Hindes RG, Albanis E, Symonds WT, Berrey MM, Nelson DR, Jacobson IM. Sofosbuvir with Pegylated Interferon Alfa-2a and Ribavirin for Treatment-Naive Patients with Hepatitis C Genotype-1 Infection (ATOMIC): An Open-Label, Randomised, Multicentre Phase 2 Trial. Lancet. 2013;381:2100–2107. doi: 10.1016/S0140-6736(13)60247-0. [DOI] [PubMed] [Google Scholar]
- 108.U.S. Food and Drug Administration. [accessed December 27, 2014];FDA Approves First Combination Pills to Treat Hepatitis C. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm418365.htm.
- 109.U.S. Food and Drug Administration. [accessed December 26, 2014];OLYSIO (Simeprevir) Capsules, for Oral Use. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/205123s001lbl.pdf.
- 110.Schinazi R, Halfon P, Marcellin P, Asselah T. HCV Direct-Acting Antiviral Agents: The Best Interferon-Free Combinations. Liver Int. 2014;34:69–78. doi: 10.1111/liv.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pierson R. FDA Declines to Approve Bristol-Myers Hepatitis Drug. [accessed December 27, 2014];Reuters, [Online] 2014 Nov 26; http://www.reuters.com/article/2014/11/26/us-bristol-myers-hepatitis-idUSKCN0JA1QL20141126.
- 112.U.S. Food and Drug Administration. [accessed December 26, 2014];FDA Approves Viekira Pak to Treat Hepatitis C. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm427530.htm.
- 113.Cahn P, Villacian J, Lazzarin A, Katlama C, Grinsztejn B, Arasteh K, López P, Clumeck N, Gerstoft J, Stavrianeas N, Moreno S, Antunes F, Neubacher D, Mayers D. Ritonavir-Boosted Tipranavir Demonstrates Superior Efficacy to Ritonavir-Boosted Protease Inhibitors in Treatment-Experienced HIV-Infected Patients: 24-Week Results of the RESIST-2 Trial. Clin Infect Dis. 2006;43:1347–1356. doi: 10.1086/508352. [DOI] [PubMed] [Google Scholar]
- 114.Gathe J, Cooper DA, Farthing C, Jayaweera D, Norris D, Pierone G, Jr, Steinhart CR, Trottier B, Walmsley SL, Workman C, Mukwaya G, Kohlbrenner V, Dohnanyi C, McCallister S, Mayers D. Efficacy of the Protease Inhibitors Tipranavir plus Ritonavir in Treatment-Experienced Patients: 24-Week Analysis from the RESIST-1 Trial. Clin Infect Dis. 2006;43:1337–1346. doi: 10.1086/508353. [DOI] [PubMed] [Google Scholar]
- 115.Malcolm BA, Liu R, Lahser F, Agrawal S, Belanger B, Butkiewicz N, Chase R, Gheyas F, Hart A, Hesk D, Ingravallo P, Jiang C, Kong R, Lu J, Pichardo J, Prongay A, Skelton A, Tong X, Venkatraman S, Xia E, Girijavallabhan V, Njoroge FG. SCH 503034, a Mechanism-Based Inhibitor of Hepatitis C Virus NS3 Protease, Suppresses Polyprotein Maturation and Enhances the Antiviral Activity of Alpha Interferon in Replicon Cells. Antimicrob Agents Chemother. 2006;50:1013–1020. doi: 10.1128/AAC.50.3.1013-1020.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin C, Lin K, Luong YP, Rao BG, Wei YY, Brennan DL, Fulghum JR, Hsiao HM, Ma S, Maxwell JP, Cottrell KM, Perni RB, Gates CA, Kwong AD. In Vitro Resistance Studies of Hepatitis C Virus Serine Protease Inhibitors, VX-950 and BILN 2061: Structural Analysis Indicates Different Resistant Mechanisms. J Biol Chem. 2004;279:17508–17514. doi: 10.1074/jbc.M313020200. [DOI] [PubMed] [Google Scholar]
- 117.Lamarre D, Anderson PC, Bailey M, Beaulieu P, Bolger G, Bonneau P, Bös M, Cameron DR, Cartier M, Cordingley MG, Faucher AM, Goudreau N, Kawai SH, Kukolj G, Lagacé L, LaPlante SR, Narjes H, Poupart MA, Rancourt J, Sentjens RE, St George R, Simoneau B, Steinmann G, Thibeault D, Tsantrizos YS, Weldon SM, Yong CL, Llinàs-Brunet M. An NS3 Protease Inhibitor with Antiviral Effects in Humans Infected with Hepatitis C Virus. Nature. 2003;426:186–189. doi: 10.1038/nature02099. [DOI] [PubMed] [Google Scholar]
- 118.Bogen SL, Arasappan A, Bennett F, Chen K, Jao E, Liu YT, Lovey RG, Venkatraman S, Pan W, Parekh T, Pike RE, Ruan S, Liu R, Baroudy B, Agrawal S, Chase R, Ingravallo P, Pichardo J, Prongay A, Brisson JM, Hsieh TY, Cheng KC, Kemp SJ, Levy OE, Lim-Wilby M, Tamura SY, Saksena AK, Girijavallabhan V, Njoroge FG. Discovery of SCH446211 (SCH6): A New Ketoamide Inhibitor of the HCV NS3 Serine Protease and HCV Subgenomic RNA Replication. J Med Chem. 2006;49:2750–2757. doi: 10.1021/jm060077j. [DOI] [PubMed] [Google Scholar]
- 119.Jacobson JM, Lalezari JP, Thompson MA, Fichtenbaum CJ, Saag MS, Zingman BS, D’Ambrosio P, Stambler N, Rotshteyn Y, Marozsan AJ, Maddon PJ, Morris SA, Olson WC. Phase 2a Study of the CCR5Monoclonal Antibody PRO 140 Administered Intravenously to HIV-Infected Adults. Antimicrob Agents Chemother. 2010;54:4137–4142. doi: 10.1128/AAC.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kuritzkes DR, Jacobson J, Powderly WG, Godofsky E, DeJesus E, Haas F, Reimann KA, Larson JL, Yarbough PO, Curt V, Shanahan WR., Jr Antiretroviral Activity of the Anti-CD4Monoclonal Antibody TNX-355 in Patients Infected with HIV Type 1. J Infect Dis. 2004;189:286–291. doi: 10.1086/380802. [DOI] [PubMed] [Google Scholar]
- 121.Nowicka-Sans B, Gong YF, McAuliffe B, Dicker I, Ho HT, Zhou N, Eggers B, Lin PF, Ray N, Wind-Rotolo M, Zhu L, Majumdar A, Stock D, Lataillade M, Hanna GJ, Matiskella JD, Ueda Y, Wang T, Kadow JF, Meanwell NA, Krystal M. In Vitro Antiviral Characteristics of HIV-1 Attachment Inhibitor BMS-626529, the Active Component of the Prodrug BMS-663068. Antimicrob Agents Chemother. 2012;56:3498–3507. doi: 10.1128/AAC.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wilkin TJ, Gulick RM. CCR5 Antagonism in HIV Infection: Current Concepts and Future Opportunities. Annu Rev Med. 2012;63:81–93. doi: 10.1146/annurev-med-052010-145454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shimura K, Kodama E, Sakagami Y, Matsuzaki Y, Watanabe W, Yamataka K, Watanabe Y, Ohata Y, Doi S, Sato M, Kano M, Ikeda S, Matsuoka M. Broad Antiretroviral Activity and Resistance Profile of the Novel Human Immunodeficiency Virus Integrase Inhibitor Elvitegravir (JTK-303/GS-9137) J Virol. 2008;82:764–774. doi: 10.1128/JVI.01534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garrido C, Soriano V, Geretti AM, Zahonero N, Garcia S, Booth C, Gutierrez F, Viciana I, de Mendoza C. Resistance Associated Mutations to Dolutegravir (S/GSK1349572) in HIV-Infected Patients – Impact of HIV Subtypes and Prior Raltegravir Experience. Antiviral Res. 2011;90:164–167. doi: 10.1016/j.antiviral.2011.03.178. [DOI] [PubMed] [Google Scholar]
- 125.Papastamopoulos V, Skoutelis A Hellenic Center for Disease Control & Prevention. [accessed June 15, 2012];HIV treatment: What’s in the Pipeline. 2011 Dec 13; http://www2.keelpno.gr/blog/?p=970&lang=en.
- 126.Corbau R, Mori J, Phillips C, Fishburn L, Martin A, Mowbray C, Panton W, Smith-Burchnell C, Thornberry A, Ringrose H, Knöchel T, Irving S, Westby M, Wood A, Perros M. Lersivirine, a Nonnucleoside Reverse Transcriptase Inhibitor with Activity Against Drug-Resistant Human Immunodeficiency Virus Type 1. Antimicrob Agents Chemother. 2010;54:4451–4463. doi: 10.1128/AAC.01455-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gupta KM, Pearce SM, Poursaid AE, Aliyar HA, Tresco PA, Mitchnik MA, Kiser PF. Polyurethane Intravaginal Ring for Controlled Delivery of Dapivirine, A Nonnucleoside Reverse Transcriptase Inhibitor of HIV-1. J Pharm Sci. 2008;97:4228–4239. doi: 10.1002/jps.21331. [DOI] [PubMed] [Google Scholar]
- 128.Clay PG, McRae MP, Laurent JP. Safety, Tolerability, and Pharmacokinetics of KP-1461 in Phase I Clinical Studies. J Int Assoc Provid AIDS Care. 2011;10:232–238. doi: 10.1177/1545109711406442. [DOI] [PubMed] [Google Scholar]
- 129.Holdich T, Shiveley LA, Sawyer J. Pharmacokinetics of Single Oral Doses of Apricitabine, A Novel Deoxycytidine Analogue Reverse Transcriptase Inhibitor, in Healthy Volunteers. Clin Drug Invest. 2006;26:279–286. doi: 10.2165/00044011-200626050-00005. [DOI] [PubMed] [Google Scholar]
- 130.Colucci P, Pottage JC, Robison H, Turgeon J, Ducharme MP. Effect of a Single Dose of Ritonavir on the Pharmacokinetic Behavior of Elvucitabine, A Nucleoside Reverse Transcriptase Inhibitor, Administered in Healthy Volunteers. Antimicrob Agents Chemother. 2009;53:646–650. doi: 10.1128/AAC.00905-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hurwitz SJ, Otto MJ, Schinazi RF. Comparative Pharmacokinetics of Racivir,(+/−)-beta-2′, 3′-dideoxy-5-fluoro-3′-thiacytidine in Rats, Rabbits, Dogs, Monkeys and HIV-Infected Humans. Antiviral Chem Chemother. 2005;16:117–127. doi: 10.1177/095632020501600204. [DOI] [PubMed] [Google Scholar]
- 132.Herman BD, Sluis-Cremer N. In: Pharmacology. Gallelli L, editor. InTech; Rijeka, Croatia: 2012. pp. 63–80. [Google Scholar]
- 133.Ghosn J, Quinson AM, Sabo ND, Cotte L, Piketty C, Dorléacq N, Bravo ML, Mayers D, Harmenberg J, Mårdh G, Valdez H, Katlama C. Antiviral Activity of Low-Dose Alovudine in Antiretroviral-Experienced Patients: Results from a 4-week Randomized, Double-Blind, Placebo-Controlled Dose-Ranging Trial*. HIV Med. 2007;8:142–147. doi: 10.1111/j.1468-1293.2007.00444.x. [DOI] [PubMed] [Google Scholar]
- 134.Thompson MA, Kessler HA, Eron JJ, Jr, Jacobson JM, Adda N, Shen G, Zong J, Harris J, Moxham C, Rousseau FS. Short-Term Safety Pharmacodynamics of Amdoxovir in HIV-Infected Patients. AIDS. 2005;19:1607–1615. doi: 10.1097/01.aids.0000186822.68606.05. [DOI] [PubMed] [Google Scholar]
- 135.Martin DE, Blum R, Wilton J, Doto J, Galbraith H, Burgess GL, Smith PC, Ballow C. Safety and Pharmacokinetics of Bevirimat (PA-457), A Novel Inhibitor of Human Immunodeficiency Virus Maturation, in Healthy Volunteers. Antimicrob Agents Chemother. 2007;51:3063–3066. doi: 10.1128/AAC.01391-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schinazi RF, Massud I, Rapp KL, Cristiano M, Detorio MA, Stanton RA, Bennett MA, Kierlin-Duncan M, Lennerstrand J, Nettles JH. Selection and Characterization of HIV-1 with a Novel S68 Deletion in Reverse Transcriptase. Antimicrob Agents Chemother. 2011;55:2054–2060. doi: 10.1128/AAC.01700-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mandell LA, Niederman M. Antimicrobial Treatment of Community Acquired Pneumonia in Adults: A Conference Report. Can J Infect Dis. 1993;4:25–28. doi: 10.1155/1993/308589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rello J, Pop-Vicas A. Clinical Review: Primary Influenza Viral Pneumonia. Crit Care. 2009;13:235–240. doi: 10.1186/cc8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tang JW, Shetty N, Lam TTY. In: Principles and Practice of Infectious Diseases. Mandell, Bennett, Dolin, editors. Antimicrobe; Pennsylvania: 2000. pp. 2060–2085. [Google Scholar]
- 140.Gubareva LV, Webster RG, Hayden FG. Comparison of the Activities of Zanamivir, Oseltamivir, and RWJ-270201 against Clinical Isolates of Influenza Virus and Neuraminidase Inhibitor-Resistant Variants. Antimicrob Agents Chemother. 2001;45:3403–3408. doi: 10.1128/AAC.45.12.3403-3408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.De Clercq E. Antiviral Drugs in Current Clinical Use. J Clin Virol. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 142.U.S. Food and Drug Administration. [accessed May 25, 2014];Influenza (Flu) Antiviral Drugs and Related Information. http://www.fda.gov/drugs/drugsafety/informationbydrugclass/ucm100228.htm.
- 143.Lok ASF, McMahon BJ. Chronic Hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 144.Lau JYN, Wright TL. Molecular Virology and Pathogenesis of Hepatitis B. Lancet. 1993;342:1311–1340. doi: 10.1016/0140-6736(93)92249-s. [DOI] [PubMed] [Google Scholar]
- 145.Okamoto H, Tsuda F, Sakugawa H, Sastrosoewignjo RI, Imai M, Miyakawa Y, Mayumi M. Typing Hepatitis B Virus by Homology in Nucleotide Sequence: Comparison of Surface Antigen Subtypes. J Gen Virol. 1988;69:2575–2583. doi: 10.1099/0022-1317-69-10-2575. [DOI] [PubMed] [Google Scholar]
- 146.Peters MG, Singer G, Howard T, Jacobsmeyer S, Xiong X, Gibbs CS, Lamy P, Murray A. Fulminant Hepatic Failure Resulting from Lamivudine-Resistant Hepatitis B Virus in a Renal Transplant Recipient: Durable Response After Orthotopic Liver Transplantation on Adefovir Dipivoxil and Hepatitis B Immune Globulin. Transplantation. 1999;68:1912–1914. doi: 10.1097/00007890-199912270-00017. [DOI] [PubMed] [Google Scholar]
- 147.Zoulim F. Entecavir: A New Treatment Option for Chronic Hepatitis B. J Clin Virol. 2006;36:8–12. doi: 10.1016/j.jcv.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 148.U.S. Food and Drug Administration. [accessed May 26, 2014];Baraclude (Entecavir) Tablets. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021797s005,021798s006lbl.pdf.
- 149.Matthews SJ. Telbivudine for the Management of Chronic Hepatitis B Virus Infection. Clin Ther. 2007;29:2635–2653. doi: 10.1016/j.clinthera.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 150.U.S. Food and Drug Administration. [accessed May 27, 2014];Tyzeka (Telbivudine) Label Revisions. http://www.fda.gov/forpatients/illness/hepatitisbc/ucm337306.htm.
- 151.U.S. Food and Drug Administration. [accessed May 27, 2014];Changes to VIREAD (Tenofovir DF) Labeling for Hepatitis B. Services. http://www.fda.gov/forpatients/illness/hepatitisbc/ucm362732.htm.
- 152.Chong Y, Chu CK. Understanding the Unique Mechanism of L-FMAU (Clevudine) against Hepatitis B Virus: Molecular Dynamics Studies. Bioorg Med Chem Lett. 2002;12:3459–3462. doi: 10.1016/s0960-894x(02)00747-3. [DOI] [PubMed] [Google Scholar]
- 153.Low TL, Goldstein AL. Thymosins: Structure, Function and Therapeutic Applications. Thymus. 1984;6:27–42. [PubMed] [Google Scholar]
- 154.zur Hausen H, De Villiers EM. Human Papilloma Viruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]
- 155.World Health Organization. [accessed June 01, 2014];Human Papillomavirus Laboratory Manual. http://whqlibdoc.who.int/hq/2010/WHO_IVB_10.12_eng.pdf.
- 156.Centers for Disease Control and Prevention. [accessed June 01, 2014];Human Papillomavirus (HPV) Infection. http://www.cdc.gov/std/treatment/2010/hpv.htm.
- 157.U.S. Food and Drug Administration. [accessed June 01, 2014];FDA Approves First Human Papillomavirus Test for Primary Cervical Cancer Screening. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm.
- 158.Hall CB. In: Principles and Practice of Clinical Virology. Zuckerman AJ, Banatvala JE, Pattison JR, editors. John Wiley & Sons; Chichester, U.K: 1998. pp. 293–306. [Google Scholar]
- 159.Mclntosh K. In: Viral Infections of Humans. Evans AS, Kaslow RA, editors. Springer; New York: 1997. pp. 691–711. [Google Scholar]
- 160.U.S. Food and Drug Administration. [accessed June 01, 2014];Synagis (Palivizumab) for Intramuscular Administration. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/103770s5113lbl.pdf.
- 161.U.S. Department of Health & Human Services. [accessed June 01, 2014];Palivizumab Product Approval Information—Licensing Action. http://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/ucm093366.htm.
- 162.Huang CC, Nguyen D, Fernandez J, Yun KY, Fry KE, Bradley DW, Tam AW, Reyes GR. Molecular Cloning and Sequencing of the Mexico Isolate of Hepatitis E Virus (HEV) Virology. 1992;191:550–558. doi: 10.1016/0042-6822(92)90230-m. [DOI] [PubMed] [Google Scholar]
- 163.U.S. Food and Drug Administration. [accessed June 02, 2014];Hepatitis E Virus (HEV) and Blood Transfusion Safety. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/bloodvaccinesandotherbiologics/bloodproductsadvisorycommittee/ucm319542.pdf.
- 164.Panda SK, Thakral D, Rehman S. Hepatitis E Virus. Rev Med Virol. 2007;17:151–180. doi: 10.1002/rmv.522. [DOI] [PubMed] [Google Scholar]
- 165.Gouttenoire J. Sofosbuvir Inhibits Hepatitis E Virus Replication in vitro and Results in an Additive Effect when Combined with Ribavirin. Presented at 50th The International Liver Congress 2015; Vienna, Austria. April 22–26, 2015. [Google Scholar]
- 166.World Health Organization. [accessed May 30, 2014];Dengue Control. http://www.who.int/denguecontrol/en/
- 167.Gubler DJ, Clark GG. Dengue/Dengue Hemorrhagic Fever: The Emergence of a Global Health Problem. Emerging Infect Dis. 1995;1:55–57. doi: 10.3201/eid0102.952004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.World Health Organization. [accessed May 30, 2014];Dengue and Severe Dengue. http://www.who.int/mediacentre/factsheets/fs117/en/
- 169.Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CKE, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. Composition and Three-Dimensional Architecture of the Dengue Virus Replication and Assembly Sites. Cell Host Microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fouchier RAM, Kuiken T, Schutten M, van Amerongen G, van Doornum GJJ, van den Hoogen BG, Peiris M, Lim W, Stöhr K, Osterhaus ADME. Aetiology: Koch’s Postulates Fulfilled for SARS Virus. Nature. 2003;423:240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Marra MA, Jones SJM, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YSN, Khattra J, Asano JK, Barber SA, Chan SY, Cloutier A, Coughlin SM, Freeman D, Girn N, Griffith OL, Leach SR, Mayo M, McDonald H, Montgomery SB, Pandoh PK, Petrescu AS, Robertson AG, Schein JE, Siddiqui A, Smailus DE, Stott JM, Yang GS, Plummer F, Andonov A, Artsob H, Bastien N, Bernard K, Booth TF, Bowness D, Czub M, Drebot M, Fernando L, Flick R, Garbutt M, Gray M, Grolla A, Jones S, Feldmann H, Meyers A, Kabani A, Li Y, Normand S, Stroher U, Tipples GA, Tyler S, Vogrig R, Ward D, Watson B, Brunham RC, Krajden M, Petric M, Skowronski DM, Upton C, Roper RL. The Genome Sequence of the SARS-Associated Coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 172.U.S. Department of Health & Human Services. [accessed May 30, 2014];FDA Efforts to Combat the SARS Outbreak. http://www.fda.gov/newsevents/testimony/ucm161401.htm.
- 173.Chu CM, Cheng VCC, Hung IFN, Wong MML, Chan KH, Chan KS, Kao RYT, Poon LLM, Wong CLP, Guan Y, Peiris JSM, Yuen KY. Role of Lopinavir/Ritonavir in the Treatment of SARS: Initial Virological and Clinical Findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Glass RI, Parashar UD, Estes MK. Norovirus Gastroenteritis. N Engl J Med. 2009;361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus Classification and Proposed Strain Nomenclature. Virology. 2006;346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 176.Hardy ME. Norovirus Protein Structure and Function. FEMS Microbiol Lett. 2005;253:1–8. doi: 10.1016/j.femsle.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 177.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW., IV Replication of Norovirus in Cell Culture Reveals a Tropism for Dendritic Cells and Macrophages. PLoS Biol. 2004;2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chang KO, George DW. Interferons and Ribavirin Effectively Inhibit Norwalk Virus Replication in Replicon-Bearing Cells. J Virol. 2007;81:12111–12118. doi: 10.1128/JVI.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Binford SL, Maldonado F, Brothers MA, Weady PT, Zalman LS, Meador JW, III, Matthews DA, Patick AK. Conservation of Amino Acids in Human Rhinovirus 3C Protease Correlates with Broad-Spectrum Antiviral Activity of Rupintrivir a Novel Human Rhinovirus 3C Protease Inhibitor. Antimicrob Agents Chemother. 2005;49:619–626. doi: 10.1128/AAC.49.2.619-626.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rocha-Pereira J, Nascimento MSJ, Ma Q, Hilgenfeld R, Neyts J, Jochmans D. The Enterovirus Protease Inhibitor Rupintrivir Exerts Cross-Genotypic Anti-Norovirus Activity and Clears Cells from the Norovirus Replicon. Antimicrob Agents Chemother. 2014;58:4675–4681. doi: 10.1128/AAC.02546-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rocha-Pereira J, Jochmans D, Dallmeier K, Leyssen P, Nascimento MSJ, Neyts J. Favipiravir (T-705) Inhibits in vitro Norovirus Replication. Biochem Biophys Res Commun. 2012;424:777–780. doi: 10.1016/j.bbrc.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 182.Tarantino D, Pezzullo M, Mastrangelo E, Croci R, Rohayem J, Robel I, Bolognesi M, Milani M. Naphthalene-sulfonate Inhibitors of Human Norovirus RNA-dependent RNA-polymerase. Antiviral Res. 2014;102:23–28. doi: 10.1016/j.antiviral.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 183.Rocha-Pereira J, Jochmans D, Debing Y, Verbeken E, Nascimento MSJ, Neyts J. The Viral Polymerase Inhibitor 2′-C-Methylcytidine Inhibits Norwalk Virus Replication and Protects against Norovirus-Induced Diarrhea and Mortality in a Mouse Model. J Virol. 2013;87:11798–11805. doi: 10.1128/JVI.02064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rocha-Pereira J, Jochmans D, Neyts J. Prophylactic Treatment with the Nucleoside Analogue 2′-C-Methylcytidine Completely Prevents Transmission of Norovirus. J Antimicrob Chemother. 2015;70:190. doi: 10.1093/jac/dku363. [DOI] [PubMed] [Google Scholar]
- 185.Gonzalez-Hernandez MJ, Pal A, Gyan KE, Charbonneau ME, Showalter HD, Donato NJ, O’Riordan M, Wobus CE. Chemical Derivatives of a Small Molecule Deubiquitinase Inhibitor have Antiviral Activity against Several RNA Viruses. PLoS One. 2014;9:e94491. doi: 10.1371/journal.pone.0094491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chaudhry Y, Nayak A, Bordeleau ME, Tanaka J, Pelletier J, Belsham GJ, Roberts LO, Goodfellow IG. Caliciviruses Differ in Their Functional Requirements for eIF4F Components. J Biol Chem. 2006;281:25315–25325. doi: 10.1074/jbc.M602230200. [DOI] [PubMed] [Google Scholar]
- 187.Lu G, Hu Y, Wang Q, Qi J, Gao F, Li Y, Zhang Y, Zhang W, Yuan Y, Bao J, Zhang B, Shi Y, Yan J, Gao GF. Molecular Basis of Binding Between Novel Human Coronavirus MERS-CoV and its Receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mailles A, Blanckaert K, Chaud P, van der Werf S, Lina B, Caro V, Campese C, Guéry B, Prouvost H, Lemaire X, Paty MC, Haeghebaert S, Antoine D, Ettahar N, Noel H, Behillil S, Hendricx S, Manuguerra JC, Enouf V, La Ruche G, Semaille C, Coignard B, Lévy-Bruhl D, Weber F, Saura C, Che D. Euro Surveill; First cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Infections in France, Investigations and Implications for the Prevention of Human-to-Human Transmission; France. May 2013; 2013. [PubMed] [Google Scholar]
- 189.de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RAM, Galiano M, Gorbalenya AE, Memish ZA, Perlman S, Poon LLM, Snijder EJ, Stephens GM, Woo PCY, Zaki AM, Zambon M, Ziebuhr J. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lu L, Liu Q, Du L, Jiang S. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Challenges in Identifying its Source and Controlling its Spread. Microbes Infect. 2013;15:625–629. doi: 10.1016/j.micinf.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hubálek Z, Halouzka J. West Nile Fever–A Reemerging Mosquito-Borne Viral Disease in Europe. Emerging Infect Dis. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.U.S. Department of Health & Human Services. [accessed May 31, 2014];Response to the Emerging Threat of West Nile Virus (WNV) http://www.fda.gov/newsevents/testimony/ucm115165.htm.
- 193.Haley M, Retter AS, Fowler D, Gea-Banacloche J, O’Grady NP. The Role for Intravenous Immunoglobulin in the Treatment of West Nile Virus Encephalitis. Clin Infect Dis. 2003;37:e88–e90. doi: 10.1086/377172. [DOI] [PubMed] [Google Scholar]
- 194.Centers for Disease Control and Prevention. [accessed June 02, 2014];Hepatitis D Information for the Public. http://www.cdc.gov/hepatitis/hdv/index.htm.
- 195.Karayiannis P. Hepatitis D Virus. Rev Med Virol. 1998;8:13–24. doi: 10.1002/(sici)1099-1654(199801/03)8:1<13::aid-rmv208>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 196.Lavanchy D. Worldwide Epidemiology of HBV Infection, Disease Burden, and Vaccine Prevention. J Clin Virol. 2005;34:S1–S3. doi: 10.1016/s1386-6532(05)00384-7. [DOI] [PubMed] [Google Scholar]
- 197.U.S. Food and Drug Administration. [accessed June 03, 2014];BBB - Hepatitis A Virus. http://www.fda.gov/food/foodborneillnesscontaminants/causesofillnessbadbugbook/ucm071294.htm.
- 198.Alter MJ, Mast EE. The Epidemiology of Viral Hepatitis in the United States. Gastroenterol Clin North Am. 1994;23:437–455. [PubMed] [Google Scholar]
- 199.Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and Severe Childhood Diarrhea. Emerging Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Crawford SE, Labbé M, Cohen J, Burroughs MH, Zhou YJ, Estes MK. Characterization of Virus-like Particles Produced by the Expression of Rotavirus Capsid Proteins in Insect Cells. J Virol. 1994;68:5945–5952. doi: 10.1128/jvi.68.9.5945-5952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.U.S. Department of Health & Human Services. [accessed June 05, 2014];Background on Rotavirus. http://www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm205542.htm.
- 202.Arvin AM. Varicella-Zoster Virus. Clin Microbiol Rev. 1996;9:361–381. doi: 10.1128/cmr.9.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Meier JL, Straus SE. Comparative Biology of Latent Varicella-Zoster Virus and Herpes Simplex Virus Infections. J Infect Dis. 1992;166:S13–S23. doi: 10.1093/infdis/166.supplement_1.s13. [DOI] [PubMed] [Google Scholar]
- 204.U.S. Food and Drug Administration. [accessed June 07, 2014];Zostavax (Herpes Zoster Vaccine) Questions and Answers. http://www.fda.gov/biologicsbloodvaccines/vaccines/questionsaboutvaccines/ucm070418.htm.
- 205.Jadhav AS, Pathare DB, Shingare MS. Development and Validation of Enantioselective High Performance Liquid Chromatographic Method for Valacyclovir, an Antiviral Drug in Drug Substance. J Pharm Biomed Anal. 2007;43:1568–1572. doi: 10.1016/j.jpba.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 206.dos Santos DM, Canduri F, Pereira JH, Vinicius Bertacine Dias M, Silva RG, Mendes MA, Palma MS, Basso LA, de Azevedo WF, Jr, Santos DS. Crystal Structure of Human Purine Nucleoside Phosphorylase Complexed with Acyclovir. Biochem Biophys Res Commun. 2003;308:553–559. doi: 10.1016/s0006-291x(03)01433-5. [DOI] [PubMed] [Google Scholar]
- 207.Nesalin JAJ, Babu CJG, Kumar GV, Mani TT. Validated Spectrophotometric Estimation of Famciclovir in Tablet Dosage Form. J Chem. 2009;6:780–784. [Google Scholar]
- 208.U.S. Food and Drug Administration. [accessed June 07, 2014];FDA Approves Varizig for Reducing Chickenpox Symptoms. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm333233.htm.
- 209.Centers for Disease Control and Prevention. [accessed June 08, 2014];Genital Herpes -CDC Fact Sheet. http://www.cdc.gov/std/Herpes/STDFact-Herpes.htm.
- 210.Roizman B. In: The herpesviruses. Roizman B, editor. Springer; New York: 1982. pp. 1–23. [Google Scholar]
- 211.World Health Organization. [accessed June 09, 2014];Laboratory Diagnosis of Sexually Transmitted Infections, Including Human Immunodeficiency Virus. http://apps.who.int/iris/bitstream/10665/85343/1/9789241505840_eng.pdf?ua=1.
- 212.Yu H, Chen J, Xu X, Li Y, Zhao H, Fang Y, Li X, Zhou W, Wang W, Wang Y. A Systematic Prediction of Multiple Drug-Target Interactions from Chemical, Genomic, and Pharmacological Data. PLoS One. 2012;7:e37608. doi: 10.1371/journal.pone.0037608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sundquist B, Öberg B. Phosphonoformate Inhibits Reverse Transcriptase. J Gen Virol. 1979;45:273–281. doi: 10.1099/0022-1317-45-2-273. [DOI] [PubMed] [Google Scholar]
- 214.Lawrence C, Holmes KK. Genital Herpes Simplex Virus Infections: Current Concepts in Diagnosis, Therapy and Prevention. Ann Intern Med. 1983;98:973–983. doi: 10.7326/0003-4819-98-6-973. [DOI] [PubMed] [Google Scholar]
- 215.World Health Organization. [accessed June 09, 2014];Ebola Virus Disease. http://www.who.int/csr/don/archive/disease/ebola/en/
- 216.World Health Organization. [accessed June 09, 2014];Ebola Virus Disease, West Africa -Update. http://www.who.int/csr/don/2014_06_04_ebola/en/
- 217.BBC News Africa. Ebola: Mapping the Outbreak [Online] [accessed December 30, 2014];BBC News. 2014 Available from: http://www.bbc.com/news/world-africa-28755033.
- 218.Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Délicat A, Paweska JT, Gonzalez JP, Swanepoel R. Fruit Bats as Reservoirs of Ebola Virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 219.PDB ID: 3CSY: Lee JE, Fusco ML, Hessell AJ, Oswald WB, Burton DR, Saphire EO. Structure of the Ebola Virus Glycoprotein Bound to an Antibody from a Human Survivor. Nature. 2008;454:177–182. doi: 10.1038/nature07082.
- 220.Protein Data Bank. [accessed October 23, 2014];Ebola Virus Proteins. http://www.rcsb.org/pdb/101/motm.do?momID=178.
- 221.Centers for Disease Control and Prevention. [accessed June 09, 2014];Ebola (Ebola Virus Disease) http://www.cdc.gov/vhf/ebola/treatment/index.html.
- 222.Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful Treatment of Advanced Ebola Virus Infection with T-705 (Favipiravir) in a Small Animal Model. Antiviral Res. 2014;105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 223.Vo TS, Kim SK. Potential Anti-HIV Agents from Marine Resources: An Overview. Mar Drugs. 2010;8:2871–2892. doi: 10.3390/md8122871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Aneiros A, Garateix A. Bioactive Peptides from Marine Sources: Pharmacological Properties and Isolation Procedures. J Chromatogr B: Anal Technol Biomed Life Sci. 2004;803:41–53. doi: 10.1016/j.jchromb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 225.Targett NM, Arnold TM. Minireview—Predicting the Effects of Brown Algal Phlorotannins on Marine Herbivores in Tropical and Temperate Oceans. J Phycol. 1998;34:195–205. [Google Scholar]
- 226.Shibata T, Kawaguchi S, Hama Y, Inagaki M, Yamaguchi K, Nakamura T. Local and Chemical Distribution of Phlorotannins in Brown Algae. J Appl Phycol. 2004;16:291–296. [Google Scholar]
- 227.La Barre S, Potin P, Leblanc C, Delage L. The Halogenated Metabolism of Brown Algae (Phaeophyta), Its Biological Importance and Its Environmental Significance. Mar Drugs. 2010;8:988–1010. doi: 10.3390/md8040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ahn MJ, Yoon KD, Min SY, Lee JS, Kim JH, Kim TG, Kim SH, Kim NG, Huh H, Kim J. Inhibition of HIV-1 Reverse Transcriptase and Protease by Phlorotannins from the Brown Alga Ecklonia cava. Biol Pharm Bull. 2004;27:544–547. doi: 10.1248/bpb.27.544. [DOI] [PubMed] [Google Scholar]
- 229.Lü L, Liu SW, Jiang SB, Wu SG. Tannin Inhibits HIV-1 Entry by Targeting gp41. Acta Pharmacol Sin. 2004;25:213–218. [PubMed] [Google Scholar]
- 230.Cai L, Jiang S. Development of Peptide and Small-Molecule HIV-1 Fusion Inhibitors that Target gp41. ChemMedChem. 2010;5:1813–1824. doi: 10.1002/cmdc.201000289. [DOI] [PubMed] [Google Scholar]
- 231.Artan M, Li Y, Karadeniz F, Lee SH, Kim MM, Kim SK. Anti-HIV-1 Activity of Phloroglucinol Derivative, 6,6′-bieckol, from Ecklonia cava. Bioorg Med Chem. 2008;16:7921–7926. doi: 10.1016/j.bmc.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 232.Ahn MJ, Yoon KD, Kim CY, Kim JH, Shin CG, Kim J. Inhibitory Activity on HIV-1 Reverse Transcriptase and Integrase of a Carmalol Derivative from a Brown Alga. Phytother Res. 2006;20:711–713. doi: 10.1002/ptr.1939. [DOI] [PubMed] [Google Scholar]
- 233.Nakane H, Arisawa M, Fujita A, Koshimura S, Ono K. Inhibition of HIV-Reverse Transcriptase Activity by Some Phloroglucinol Derivatives. FEBS Lett. 1991;286:83–85. doi: 10.1016/0014-5793(91)80946-z. [DOI] [PubMed] [Google Scholar]
- 234.Ngo DN, Kim MM, Kim SK. Chitin Oligosaccharides Inhibit Oxidative Stress in Live Cells. Carbohydr Polym. 2008;74:228–234. [Google Scholar]
- 235.Shahidi F, Arachchi JKV, Jeon YJ. Food Applications of Chitin and Chitosans. Trends Food Sci Technol. 1999;10:37–51. [Google Scholar]
- 236.Sosa MAG, Fazely F, Koch JA, Vercellotti SV, Ruprecht RM. Carboxymethylchitosan-N,O-sulfate as an Anti-HIV-1 Agent. Biochem Biophys Res Commun. 1991;174:489–496. doi: 10.1016/0006-291x(91)91443-g. [DOI] [PubMed] [Google Scholar]
- 237.Nishimura SI, Kai H, Shinada K, Yoshida T, Tokura S, Kurita K, Nakashima H, Yamamoto N, Uryu T. Regioselective Syntheses of Sulfated Polysaccharides: Specific Anti-HIV-1 Activity of Novel Chitin Sulfates. Carbohydr Res. 1998;306:427–433. doi: 10.1016/s0008-6215(97)10081-7. [DOI] [PubMed] [Google Scholar]
- 238.Artan M, Karadeniz F, Karagozlu MZ, Kim MM, Kim SK. Anti-HIV-1 Activity of Low Molecular Weight Sulfated Chitooligosaccharides. Carbohydr Res. 2010;345:656–662. doi: 10.1016/j.carres.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 239.Kim SK, Rajapakse N. Enzymatic Production and Biological Activities of Chitosan Oligosaccharides (COS): A Review. Carbohydr Polym. 2005;62:357–368. [Google Scholar]
- 240.Park PJ, Je JY, Jung WK, Ahn CB, Kim SK. Anticoagulant Activity of Heterochitosans and Their Oligosaccharide Sulfates. Eur Food Res Technol. 2004;219:529–533. [Google Scholar]
- 241.Vijayavel K, Anbuselvam C, Balasubramanian MP. Free Radical Scavenging Activity of the Marine Mangrove Rhizophora apiculata Bark Extract with Reference to Naphthalene Induced Mitochondrial Dysfunction. Chem-Biol Interact. 2006;163:170–175. doi: 10.1016/j.cbi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 242.Alban S, Franz G. Partial Synthetic Glucan Sulfates as Potential New Antithrombotics: A Review. Biomacromolecules. 2001;2:354–361. doi: 10.1021/bm010032u. [DOI] [PubMed] [Google Scholar]
- 243.Parish CR, Low L, Warren HS, Cunningham AL. A Polyanion Binding Site on the CD4 Molecule. Proximity to the HIV-gp120 Binding Region. J Immunol. 1990;145:1188–1195. [PubMed] [Google Scholar]
- 244.Lynch G, Low L, Li S, Sloane A, Adams S, Parish C, Kemp B, Cunningham AL. Sulfated Polyanions Prevent HIV Infection of Lymphocytes by Disruption of the CD4-gp120 Interaction, but do not Inhibit Monocyte Infection. J Leukoc Biol. 1994;56:266–272. doi: 10.1002/jlb.56.3.266. [DOI] [PubMed] [Google Scholar]
- 245.Witvrouw M, De Clercq E. Sulfated Polysaccharides Extracted from Sea Algae as Potential Antiviral Drugs. Gen Pharmacol. 1997;29:497–511. doi: 10.1016/s0306-3623(96)00563-0. [DOI] [PubMed] [Google Scholar]
- 246.Adhikari U, Mateu CG, Chattopadhyay K, Pujol CA, Damonte EB, Ray B. Structure and Antiviral Activity of Sulfated Fucans from Stoechospermum marginatum. Phytochemistry. 2006;67:2474–2482. doi: 10.1016/j.phytochem.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 247.Bourgougnon N, Lahaye M, Quemener B, Chermann JC, Rimbert M, Cormaci M, Furnari G, Kornprobst JM. Annual Variation in Composition and In Vitro Anti-HIV-1 Activity of the Sulfated Glucuronogalactan from Schizymenia dubyi (Rhodophyta, Gigartinales) J Appl Phycol. 1996;8:155–161. [Google Scholar]
- 248.Nakashima H, Kido Y, Kobayashi N, Motoki Y, Neushul M, Yamamoto N. Purification and Characterization of an Avian Myeloblastosis and Human Immunodeficiency Virus Reverse Tran-scriptase Inhibitor, Sulfated Polysaccharides Extracted from Sea Algae. Antimicrob Agents Chemother. 1987;31:1524–1528. doi: 10.1128/aac.31.10.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nakashima H, Kido Y, Kobayashi N, Motoki Y, Neushul M, Yamamoto N. Antiretroviral Activity in a Marine Red Alga: Reverse Transcriptase Inhibition by an Aqueous Extract of Schizymenia pacifica. J Cancer Res Clin Oncol. 1987;113:413–416. doi: 10.1007/BF00390034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Béress A, Wassermann O, Bruhn T, Béress L, Kraiselburd EN, Gonzalez LV, de Motta GE, Chavez PI. A New Procedure for the Isolation of Anti-HIV Compounds (Polysaccharides and Polyphenols) from the Marine Alga Fucus vesiculosus. J Nat Prod. 1993;56:478–488. doi: 10.1021/np50094a005. [DOI] [PubMed] [Google Scholar]
- 251.Queiroz KCS, Medeiros VP, Queiroz LS, Abreu LRD, Rocha HAO, Ferreira CV, Jucá MB, Aoyama H, Leite EL. Inhibition of Reverse Transcriptase Activity of HIV by Polysaccharides of Brown Algae. Biomed Pharmacother. 2008;62:303–307. doi: 10.1016/j.biopha.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 252.Trinchero J, Ponce NMA, Córdoba OL, Flores ML, Pampuro S, Stortz CA, Salomón H, Turk G. Antiretroviral Activity of Fucoidans Extracted from the Brown Seaweed Adenocystis utricularis. Phytother Res. 2009;23:707–712. doi: 10.1002/ptr.2723. [DOI] [PubMed] [Google Scholar]
- 253.Lee JB, Hayashi K, Hirata M, Kuroda E, Suzuki E, Kubo Y, Hayashi T. Antiviral Sulfated Polysaccharide from Navicula directa, a Diatom Collected from Deep-Sea Water in Toyama Bay. Biol Pharm Bull. 2006;29:2135–2139. doi: 10.1248/bpb.29.2135. [DOI] [PubMed] [Google Scholar]
- 254.Amornrut C, Toida T, Imanari T, Woo ER, Park H, Linhardt R, Wu SJ, Kim YS. A New Sulfated β-galactan from Clams with Anti-HIV Activity. Carbohydr Res. 1999;321:121–127. doi: 10.1016/s0008-6215(99)00188-3. [DOI] [PubMed] [Google Scholar]
- 255.Wang W, Wang SX, Guan HS. The Antiviral Activities and Mechanisms of Marine Polysaccharides. Mar Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Witvrouw M, Este JA, Mateu MQ, Reymen D, Andrei G, Snoeck R, Ikeda S, Pauwels R, Bianchini NV, Desmyter J, De Clercq E. Activity of a Sulfated Polysaccharide Extracted from the Red Seaweed Aghardhiella tenera against Human Immunodeficiency Virus and Other Enveloped Viruses. Antiviral Chem Chemother. 1994;5:297–303. [Google Scholar]
- 257.Yamada T, Ogamo A, Saito T, Uchiyama H, Nakagawa Y. Preparation of O-acylated Low-Molecular-Weight Carrageenans with Potent Anti-HIV Activity and Low Anticoagulant Effect. Carbohydr Polym. 2000;41:115–120. [Google Scholar]
- 258.Yamada T, Ogamo A, Saito T, Watanabe J, Uchiyama H, Nakagawa Y. Preparation and Anti-HIV Activity of Low-Molecular-Weight Carrageenans and Their Sulfated Derivatives. Carbohydr Polym. 1997;32:51–55. [Google Scholar]
- 259.Hayashi T, Hayashi K, Maeda M, Kojima I. Calcium Spirulan, an Inhibitor of Enveloped Virus Replication, from a Blue-Green Alga Spirulina platensis. J Nat Prod. 1996;59:83–87. doi: 10.1021/np960017o. [DOI] [PubMed] [Google Scholar]
- 260.Xianliang X, Meiyu G, Huashi G, Zelin L. Effects of Marine Polysaccharide 911 on HIV-1 Proliferation In Vitro. Chin J Mar Drugs. 2000;19:8–11. [Google Scholar]
- 261.Xianliang X, Hua D, Meiyu G, Pingfang L, Yingxia L, Huashi G. Studies of the Anti-AIDS Effects of Marine Polysaccharide Drug 911 and its Related Mechanisms of Action. Chin J Mar Drugs. 2000;19:4–8. [Google Scholar]
- 262.Woo ER, Kim WS, Kim YS. Virus-cell Fusion Inhibitory Activity for the Polysaccharides from Various Korean Edible Clams. Arch Pharmacal Res. 2001;24:514–517. doi: 10.1007/BF02975155. [DOI] [PubMed] [Google Scholar]
- 263.Sato T, Hori K. Cloning, Expression, and Characterization of a Novel Anti-HIV Lectin from the Cultured Cyanobacterium. Fish Sci. 2009;75:743–753. [Google Scholar]
- 264.Lifson J, Coutré S, Huang E, Engleman E. Role of Envelope Glycoprotein Carbohydrate in Human Immunodeficiency Virus (HIV) Infectivity and Virus-Induced Cell Fusion. J Exp Med. 1986;164:2101–2106. doi: 10.1084/jem.164.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hansen JES, Nielsen C, Heegaard P, Mathiesen LR, Nielsen JO. Correlation Between Carbohydrate Structures on the Envelope Glycoprotein gp120 of HIV-1 and HIV-2 and Syncytium Inhibition with Lectins. AIDS. 1989;3:635–642. doi: 10.1097/00002030-198910000-00003. [DOI] [PubMed] [Google Scholar]
- 266.Balzarini J, Neyts J, Schols D, Hosoya M, Van Damme E, Peumans W, De Clercq E. The Mannose-specific Plant Lectins from Cymbidium Hybrid and Epipactis helleborine and the (N-acetylglucosamine)n -specific Plant Lectin from Urtica dioica are Potent and Selective Inhibitors of Human Immunodeficiency Virus and Cytomegalovirus Replication In Vitro. Antiviral Res. 1992;18:191–207. doi: 10.1016/0166-3542(92)90038-7. [DOI] [PubMed] [Google Scholar]
- 267.Mori T, O’Keefe BR, Sowder RC, II, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW, Jr, McMahon JB, Boyd MR. Isolation and Characterization of Griffithsin a Novel HIV-inactivating Protein from the Red Alga Griffithsia sp. J Biol Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 268.Wang JH, Kong J, Li W, Molchanova V, Chikalovets I, Belogortseva N, Luk’yanov P, Zheng YT. A Beta-galactose-specific Lectin Isolated from the Marine Worm Chaetopterus variopedatus Possesses Anti-HIV-1 Activity. Comp Biochem Physiol, Part C: Toxicol Pharmacol. 2006;142:111–117. doi: 10.1016/j.cbpc.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 269.Luk’yanov PA, Chernikov OV, Kobelev SS, Chikalovets IV, Molchanova VI, Li W. Carbohydrate-binding Proteins of Marine Invertebrates. Russ J Bioorg Chem. 2007;33:161–169. [PubMed] [Google Scholar]
- 270.Lee TG, Maruyama S. Isolation of HIV-1 Protease-Inhibiting Peptides from Thermolysin Hydrolysate of Oyster Proteins. Biochem Biophys Res Commun. 1998;253:604–608. doi: 10.1006/bbrc.1998.9824. [DOI] [PubMed] [Google Scholar]
- 271.Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O’Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, Currens MJ, Cardellina JH, 2nd, Buckheit RW, Jr, Nara PL, Sowder RC, 2nd, Henderson LE. Discovery of Cyanovirin-N a Novel Human Immunodeficiency Virus-Inactivating Protein that Binds Viral Surface Envelope Glycoprotein gp120: Potential Applications to Microbicide Development. Antimicrob Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Miyata T, Tokunaga F, Yoneya T, Yoshikawa K, Iwanaga S, Niwa M, Takao T, Shimonishi Y. Antimicrobial Peptides, Isolated from Horseshoe Crab Hemocytes, Tachyplesin II, and Polyphemusins I and II: Chemical Structures and Biological Activity. J Biochem. 1989;106:663–668. doi: 10.1093/oxfordjournals.jbchem.a122913. [DOI] [PubMed] [Google Scholar]
- 273.Morimoto M, Mori H, Otake T, Ueba N, Kunita N, Niwa M, Murakami T, Iwanaga S. Inhibitory Effect of Tachyplesin I on the Proliferation of Human Immunodeficiency Virus In Vitro. Chemotherapy. 1991;37:206–211. doi: 10.1159/000238855. [DOI] [PubMed] [Google Scholar]
- 274.Gustafson K, Roman M, Fenical W. The Macrolactins, a Novel Class of Antiviral and Cytotoxic Macrolides from a Deep-Sea Marine Bacterium. J Am Chem Soc. 1989;111:7519–7524. [Google Scholar]
- 275.Pereira HS, Leão-Ferreira LR, Moussatché N, Teixeira VL, Cavalcanti DN, Costa LJ, Diaz R, Frugulhetti ICPP. Antiviral Activity of Diterpenes Isolated from the Brazilian Marine Alga Dictyota menstrualis Against Human Immunodeficiency Virus Type 1 (HIV-1) Antiviral Res. 2004;64:69–76. doi: 10.1016/j.antiviral.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 276.Singh SB, Zink DL, Goetz MA, Dombrowski AW, Polishook JD, Hazuda DJ. Equisetin and a Novel Opposite Stereochemical Homolog Phomasetin, Two Fungal Metabolites as Inhibitors of HIV-1 Integrase. Tetrahedron Lett. 1998;39:2243–2246. [Google Scholar]
- 277.Rowley DC, Hansen MST, Rhodes D, Sotriffer CA, Ni H, McCammon JA, Bushman FD, Fenical W. Thalassiolins A-C: New Marine-Derived Inhibitors of HIV cDNA Integrase. Bioorg Med Chem. 2002;10:3619–3625. doi: 10.1016/s0968-0896(02)00241-9. [DOI] [PubMed] [Google Scholar]
- 278.Potts BCM, Faulkner DJ, Chan JA, Simolike GC, Offen P, Hemling ME, Francis TA. Didemnaketals A and B, HIV-1 Protease Inhibitors from the Ascidian Didemnum sp. J Am Chem Soc. 1991;113:6321–6322. [Google Scholar]
- 279.Mitchell SS, Rhodes D, Bushman FD, Faulkner DJ. Cyclodidemniserinol Trisulfate, a Sulfated Serinolipid from the Palauan Ascidian Didemnum guttatum that Inhibits HIV-1 Integrase. Org Lett. 2000;2:1605–1607. doi: 10.1021/ol005866o. [DOI] [PubMed] [Google Scholar]
- 280.Andersen RJ, Faulkner DJ, He CH, Van Duyne GD, Clardy J. Metabolites of the Marine Prosobranch Mollusk Lamellaria sp. J Am Chem Soc. 1985;107:5492–5495. [Google Scholar]
- 281.Reddy MVR, Rao MR, Rhodes D, Hansen MST, Rubins K, Bushman FD, Venkateswarlu Y, Faulkner DJ. Lamellarin α 20-Sulfate, an Inhibitor of HIV-1 Integrase Active against HIV-1 Virus in Cell Culture. J Med Chem. 1999;42:1901–1907. doi: 10.1021/jm9806650. [DOI] [PubMed] [Google Scholar]
- 282.Sagar S, Kaur M, Minneman KP. Antiviral Lead Compounds from Marine Sponges. Mar Drugs. 2010;8:2619–2638. doi: 10.3390/md8102619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Sarin PS, Sun D, Thornton A, Müller WEG. Inhibition of Replication of the Etiologic Agent of Acquired Immune Deficiency Syndrome (Human T-lymphotropic Retrovirus/Lymphadenopathy-associated Virus) by Avarol and Avarone. J Natl Cancer Inst. 1987;78:663–666. [PubMed] [Google Scholar]
- 284.Minale L, Riccio R, Sodano G. Avarol, a Novel Sesquiterpenoid Hydroquinone with a Rearranged Drimane Skeleton from the Sponge. Tetrahedron Lett. 1974;15:3401–3404. [Google Scholar]
- 285.Loya S, Hizi A. The Inhibition of Human Immunodeficiency Virus Type 1 Reverse Transcriptase by Avarol and Avarone Derivatives. FEBS Lett. 1990;269:131–134. doi: 10.1016/0014-5793(90)81137-d. [DOI] [PubMed] [Google Scholar]
- 286.Gul W, Hammond NL, Yousaf M, Peng J, Holley A, Hamann MT. Chemical Transformation and Biological Studies of Marine Sesquiterpene (S)-(+)-Curcuphenol and its Analogs. Biochim Biophys Acta, Gen Subj. 2007;1770:1513–1519. doi: 10.1016/j.bbagen.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Althaus IW, Reusser F, Tarpley WG, Skaletzky LL. Preparation of Phenanthrolinedicarboxylate Esters, 4-Aminoquinoline and Isoquinoline Derivatives as Inhibitors of HIV Reverse Tran-scriptase. WO9005523A2. International Patent. 1990 May 31;
- 288.Inaba T, Yamada Y, Shanley J, Deason M. Process for Producing Amide Derivatives as HIV Protease Inhibitors. WO9711938A1. International Patent. 1997 Apr 3;
- 289.Kawano Y, Fujii N, Kanzaki N, Iizawa Y. Preparation of Furo[2,3-h]isoquinoline Derivatives as Viral Entry Inhibitors against HIV. WO03035650A1. International Patent. 2003 May 1;
- 290.Kadow JF, Xue QM, Wang T, Zhang Z, Meanwell NA. Preparation of Indole, Azaindole and Related Heterocyclic Pyrrolidine Derivatives as Antiviral Agents. WO068221A1. International Patent. 2003 Aug 21;
- 291.Romero DL, Morge RA, Genin MJ, Biles C, Busso M, Resnick L, Althaus IW, Reusser F, Thomas RC, Tarpley WG. Bis (heteroaryl) Piperazine (BHAP) Reverse Transcriptase Inhibitors: Structure-Activity Relationships of Novel Substituted Indole Analogs and the Identification of 1-[(5-methanesulfonamido-1H-indol-2-yl) carbonyl]-4-[3-[(1-methylethyl) amino] pyridinyl] piperazinemono-methanesulfonate (U-90152S), a Second-Generation Clinical Candidate. J Med Chem. 1993;36:1505–1508. doi: 10.1021/jm00062a027. [DOI] [PubMed] [Google Scholar]
- 292.Ford PW, Gustafson KR, McKee TC, Shigematsu N, Maurizi LK, Pannell LK, Williams DE, de Silva ED, Lassota P, Allen TM, Van Soest R, Andersen RJ, Boyd MR. Papuamides AD, HIV-Inhibitory and Cytotoxic Depsipeptides from the Sponges Theonella mirabilis and Theonella swinhoei Collected in Papua New Guinea. J Am Chem Soc. 1999;121:5899–5909. [Google Scholar]
- 293.Xie W, Ding D, Zi W, Li G, Ma D. Total Synthesis and Structure Assignment of Papuamide B, a Potent Marine Cyclodepsipeptide with Anti-HIV Properties. Angew Chem, Int Ed. 2008;47:2844–2848. doi: 10.1002/anie.200705557. [DOI] [PubMed] [Google Scholar]
- 294.Andjelic CD, Planelles V, Barrows LR. Characterizing the Anti-HIV Activity of Papuamide A. Mar Drugs. 2008;6:528–549. doi: 10.3390/md20080027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zampella A, D’Auria MV, Paloma LG, Casapullo A, Minale L, Debitus C, Henin Y. Callipeltin A, an Anti-HIV Cyclic Depsipeptide from the New Caledonian Lithistida Sponge Callipelta sp. J Am Chem Soc. 1996;118:6202–6209. [Google Scholar]
- 296.Oku N, Gustafson KR, Cartner LK, Wilson JA, Shigematsu N, Hess S, Pannell LK, Boyd MR, McMahon JB. Neamphamide A, a New HIV-Inhibitory Depsipeptide from the Papua New Guinea Marine Sponge Neamphius huxleyi. J Nat Prod. 2004;67:1407–1411. doi: 10.1021/np040003f. [DOI] [PubMed] [Google Scholar]
- 297.Plaza A, Gustchina E, Baker HL, Kelly M, Bewley CA. Mirabamides A-D, Depsipeptides from the sponge Siliquariaspongia mirabilis That Inhibit HIV-1 Fusion. J Nat Prod. 2007;70:1753–1760. doi: 10.1021/np070306k. [DOI] [PubMed] [Google Scholar]
- 298.Plaza A, Bifulco G, Keffer JL, Lloyd JR, Baker HL, Bewley CA. Celebesides A-C and Theopapuamides B-D, Depsipep-tides from an Indonesian Sponge that Inhibit HIV-Entry. J Org Chem. 2009;74:504–512. doi: 10.1021/jo802232u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Rashid MA, Gustafson KR, Cartner LK, Shigematsu N, Pannell LK, Boyd MR. Microspinosamide, a New HIV-inhibitory Cyclic Depsipeptide from the Marine Sponge Sidonops microspinosa. J Nat Prod. 2001;64:117–121. doi: 10.1021/np0002379. [DOI] [PubMed] [Google Scholar]
- 300.Zampella A, Sepe V, Luciano P, Bellotta F, Monti MC, D’Auria MV, Jepsen T, Petek S, Adeline MT, Laprévôte O, Aubertin AM, Debitus C, Poupat C, Ahond A. Homophymine A, an Anti-HIV Cyclodepsipeptide from the Sponge Homophymia sp. J Org Chem. 2008;73:5319–5327. doi: 10.1021/jo800583b. [DOI] [PubMed] [Google Scholar]
- 301.Cutignano A, Bifulco G, Bruno I, Casapullo A, Gomez-Paloma L, Riccio R, Dragmacidin F. A New Antiviral Bromoindole Alkaloid from the Mediterranean Sponge Halicortex sp. Tetrahedron. 2000;56:3743–3748. [Google Scholar]
- 302.Rey F, Barré-Sinoussi F, Schmidtmayerova H, Chermann JC. Detection and Titration of Neutralizing Antibodies to HIV Using an Inhibition of the Cytopathic Effect of the Virus on MT4 Cells. J Virol Methods. 1987;16:239–249. doi: 10.1016/0166-0934(87)90008-5. [DOI] [PubMed] [Google Scholar]
- 303.Rey F, Dower G, Hirsch I, Chermann JC. Productive Infection of CD4+ Cells by Selected HIV Strains is not Inhibited by Anti-CD4Monoclonal Antibodies. Virology. 1991;181:165–171. doi: 10.1016/0042-6822(91)90481-p. [DOI] [PubMed] [Google Scholar]
- 304.Sakai R, Higa T, Jefford CW, Bernardinelli G. Manzamine A, a Novel Antitumor Alkaloid from a Sponge. J Am Chem Soc. 1986;108:6404–6405. [Google Scholar]
- 305.Edrada RA, Proksch P, Wray V, Witte L, Müller WEG, Van Soest RWM. Four New Bioactive Manzamine-Type Alkaloids from the Philippine Marine Sponge Xestospongia ashmorica. J Nat Prod. 1996;59:1056–1060. doi: 10.1021/np9604083. [DOI] [PubMed] [Google Scholar]
- 306.Yousaf M, Hammond NL, Peng J, Wahyuono S, McIntosh KA, Charman WN, Mayer AMS, Hamann MT. New Manzamine Alkaloids from an Indo-Pacific Sponge. Pharmacokinetics, Oral Availability, and the Significant Activity of Several Manzamines against HIV-I, AIDS Opportunistic Infections, and Inflammatory Diseases. J Med Chem. 2004;47:3512–3517. doi: 10.1021/jm030475b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Peng J, Hu JF, Kazi AB, Li Z, Avery M, Peraud O, Hill RT, Franzblau SG, Zhang F, Schinazi RF, Wirtz SS, Tharnish P, Kelly M, Wahyuono S, Hamann MT. Manadomanzamines A and B: A Novel Alkaloid Ring System with Potent Activity against Mycobacteria and HIV-1. J Am Chem Soc. 2003;125:13382–13386. doi: 10.1021/ja030087z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Patil AD, Kumar NV, Kokke WC, Bean MF, Freyer AJ, De Brosse C, Mai S, Truneh A, Carte B. Novel Alkaloids from the Sponge Batzella sp.: Inhibitors of HIV gp120-Human CD4 Binding. J Org Chem. 1995;60:1182–1188. [Google Scholar]
- 309.Hua HM, Peng J, Dunbar DC, Schinazi RF, de Castro Andrews AG, Cuevas C, Garcia-Fernandez LF, Kelly M, Hamann MT. Batzelladine Alkaloids from the Caribbean Sponge Monanchora unguifera and the Significant Activities against HIV-1 and AIDS Opportunistic Infectious Pathogens. Tetrahedron. 2007;63:11179–11188. [Google Scholar]
- 310.Gul W, Hammond NL, Yousaf M, Bowling JJ, Schinazi RF, Wirtz SS, de Castro Andrews G, Cuevas C, Hamann MT. Modification at the C9 Position of the Marine Natural Product Isoaaptamine and the Impact on HIV-1, Mycobacterial, and Tumor Cell Activity. Bioorg Med Chem. 2006;14:8495–8505. doi: 10.1016/j.bmc.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Venkateshwar Goud T, Srinivasa Reddy N, Raghavendra Swamy N, Siva Ram T, Venkateswarlu Y. Anti-HIV Active Petrosins from the Marine Sponge Petrosia similis. Biol Pharm Bull. 2003;26:1498–1501. doi: 10.1248/bpb.26.1498. [DOI] [PubMed] [Google Scholar]
- 312.Peng J, Walsh K, Weedman V, Bergthold JD, Lynch J, Lieu KL, Braude IA, Kelly M, Hamann MT. The New Bioactive Diterpenes Cyanthiwigins E–AA from the Jamaican Sponge Myrmekioderma styx. Tetrahedron. 2002;58:7809–7819. [Google Scholar]
- 313.Qureshi A, Faulkner DJ. Haplosamates A and B: New Steroidal Sulfamate Esters from Two Haplosclerid Sponges. Tetrahedron. 1999;55:8323–8330. [Google Scholar]
- 314.Rudi A, Yosief T, Loya S, Hizi A, Schleyer M, Kashman Y. Clathsterol, a Novel Anti-HIV-1 RT Sulfated Sterol from the Sponge Clathria Species. J Nat Prod. 2001;64:1451–1453. doi: 10.1021/np010121s. [DOI] [PubMed] [Google Scholar]
- 315.Loya S, Rudi A, Kashman Y, Hizi A. Mode of Inhibition of HIV-1 Reverse Transcriptase by Polyacetylenetriol, a Novel Inhibitor of RNA- and DNA-directed DNA Polymerases. Biochem J. 2002;362:685–692. doi: 10.1042/0264-6021:3620685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chill L, Rudi A, Aknin M, Loya S, Hizi A, Kashman Y. New Sesterterpenes from Madagascan Lendenfeldia Sponges. Tetrahedron. 2004;60:10619–10626. [Google Scholar]
- 317.Cimino P, Bifulco G, Casapullo A, Bruno I, Gomez-Paloma L, Riccio R. Isolation and NMR Characterization of Rosacelose, a Novel Sulfated Polysaccharide from the Sponge Mixylla rosacea. Carbohydr Res. 2001;334:39–47. doi: 10.1016/s0008-6215(01)00141-0. [DOI] [PubMed] [Google Scholar]
- 318.Jiang BF, Xu XF, Li L, Yuan W. Study on “911” Anti-HBV Effect in HepG2.2.15 Cells Culture. Mod Prev Med. 2003;30:517–518. [Google Scholar]
- 319.Wu S-M, Xu W-M, Xu Z. Antiviral Effect of Oyster Extract Compound Capsule in Ducks Infected by DHBV. Chin Pharm J. 1996;31:304–307. [Google Scholar]
- 320.Guan HS. Polymeric Mannuronic Acid Sulfate for Treatment of Hepatitis B. CN1132209A. 1996 Oct 2;
- 321.Na M, Ding Y, Wang B, Tekwani BL, Schinazi RF, Franzblau S, Kelly M, Stone R, Li XC, Ferreira D, Hamann MT. Anti-infective Discorhabdins from a Deep-Water Alaskan Sponge of the Genus Latrunculia. J Nat Prod. 2010;73:383–387. doi: 10.1021/np900281r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Abbas S, Kelly M, Bowling J, Sims J, Waters A, Hamann MT. Advancement into the Arctic Region for Bioactive Sponge Secondary Metabolites. Mar Drugs. 2011;9:2423–2437. doi: 10.3390/md9112423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Campo VL, Kawano DF, da Silva DB, Jr, Carvalho I. Carrageenans: Biological Properties Chemical Modifications and Structural Analysis - A Review. Carbohydr Polym. 2009;77:167–180. [Google Scholar]
- 324.Buck CB, Thompson CD, Roberts JN, Müller M, Lowy DR, Schiller JT. Carrageenan is a Potent Inhibitor of Papillomavirus Infection. PLoS Pathog. 2006;2:e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Hasui M, Matsuda M, Okutani K, Shigeta S. In Vitro Antiviral Activities of Sulfated Polysaccharides from a Marine Microalga (Cochlodinium polykrikoides) against Human Immunodeficiency Virus and Other Enveloped Viruses. Int J Biol Macromol. 1995;17:293–297. doi: 10.1016/0141-8130(95)98157-t. [DOI] [PubMed] [Google Scholar]
- 326.Nakao Y, Takada K, Matsunaga S, Fusetani N. Calyceramides A-C: Neuraminidase Inhibitory Sulfated Ceramides from the Marine Sponge Discodermia calyx. Tetrahedron. 2001;57:3013–3017. [Google Scholar]
- 327.Sun HH, Gross SS, Gunasekera M, Koehn FE. Weinbersterol Disulfates A and B, Antiviral Steroid Sulfates from the Sponge Petrosia weinbergi. Tetrahedron. 1991;47:1185–1190. [Google Scholar]
- 328.Nakatani M, Nakamura M, Suzuki A, Inoue M, Katoh T. A New Strategy toward the Total Synthesis of Stachyflin, a Potent Anti-Influenza A Virus Agent: Concise Route to the Tetracyclic Core Structure. Org Lett. 2002;4:4483–4486. doi: 10.1021/ol0271032. [DOI] [PubMed] [Google Scholar]
- 329.Minagawa K, Kouzuki S, Yoshimoto J, Kawamura Y, Tani H, Iwata T, Terui Y, Nakai H, Yagi S, Hattori N, et al. Stachyflin and Acetylstachyflin, Novel Anti-Influenza A Virus Substances, Produced by Stachybotrys sp. RF-7260. I. Isolation, Structure Elucidation and Biological Activities. J Antibiot. 2002;55:155–164. doi: 10.7164/antibiotics.55.155. [DOI] [PubMed] [Google Scholar]
- 330.Minagawa K, Kouzuki S, Kamigauchi T. Stachyflin and Acetylstachyflin, Novel Anti-Influenza A Virus Substances, Produced by Stachybotrys sp. RF-7260. II. Synthesis and Preliminary Structure-Activity Relationships of Stachyflin Derivatives. J Antibiot. 2002;55:165–171. doi: 10.7164/antibiotics.55.165. [DOI] [PubMed] [Google Scholar]
- 331.Tang F, Chen F, Li F. Preparation and Potential In Vivo Anti-influenza Virus Activity of Low Molecular-weight κ-carrageenans and their Derivatives. J Appl Polym Sci. 2013;127:2110–2115. [Google Scholar]
- 332.Wang W, Zhang P, Hao C, Zhang XE, Cui ZQ, Guan HS. In Vitro Inhibitory Effect of Carrageenan Oligosaccharide on Influenza A H1N1 Virus. Antiviral Res. 2011;92:237–246. doi: 10.1016/j.antiviral.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 333.Wang W, Zhang P, Yu GL, Li CX, Hao C, Qi X, Zhang LJ, Guan HS. Preparation and Anti-Influenza A Virus Activity of κ-carrageenan Oligosaccharide and its Sulphated Derivatives. Food Chem. 2012;133:880–888. [Google Scholar]
- 334.Lüscher-Mattli M, Glück R. Dextran Sulfate Inhibits the Fusion of Influenza Virus with Model Membranes, and Suppresses Influenza Virus Replication In Vivo. Antiviral Res. 1990;14:39–50. doi: 10.1016/0166-3542(90)90064-e. [DOI] [PubMed] [Google Scholar]
- 335.Ivanova V, Rouseva R, Kolarova M, Serkedjieva J, Rachev R, Manolova N. Isolation of a Polysaccharide with Antiviral Effect from Ulva lactuca. Prep Biochem. 1994;24:83–97. doi: 10.1080/10826069408010084. [DOI] [PubMed] [Google Scholar]
- 336.Akamatsu E, Shimanaga M, Kamei Y. Isolation of an Anti-Influenza Virus Substance, MC26 from a Marine Brown Alga, Sargassum piluliferum and its Antiviral Activity Against Influenza Virus. Coastal Bioenvironment. 2003;1:29–34. [Google Scholar]
- 337.Haifeng Z, Jiangbin L, Gan H. Study on the Inhibition Effects of Perna Viridis Polysaccharides on Influenza Virus Reproduction in MDCK Cell Cultures. Mod Med J Chin. 2008;5:4–7. [Google Scholar]
- 338.Wang W, Yu GL, Hao C, Guan HS. Application of Oligomer Mannuronic Acid in Treating H1N1 of Influenza A Virus. CN102488697A. 2012 Jun 13;
- 339.Guan HS. Characteristics and Therapeutic Effects of Propyl Mannuronate Sodium Sulfate. CN1088937A. 1994 Jul 6;
- 340.Wang W, Li CX, Guan HS, Yu GL, Wang SX. Application of Polymannuronic Acid Propyl Ester Sulfate in Preparation of Anti-H1N1 Influenza A (H1N1) Virus Medicines. CN102743409A. 2012 Oct 24;
- 341.Kim M, Yim JH, Kim SY, Kim HS, Lee WG, Kim SJ, Kang PS, Lee CK. In Vitro Inhibition of Influenza A Virus Infection by Marine Microalga-derived Sulfated Polysaccharide p-KG03. Antiviral Res. 2012;93:253–259. doi: 10.1016/j.antiviral.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 342.Nagaoka M, Shibata H, Kimura-Takagi I, Hashimoto S, Kimura K, Makino T, Aiyama R, Ueyama S, Yokokura T. Structural Study of Fucoidan from Cladosiphon okamuranus Tokida. Glycoconjugate J. 1999;16:19–26. doi: 10.1023/a:1006945618657. [DOI] [PubMed] [Google Scholar]
- 343.Hidari KIPJ, Takahashi N, Arihara M, Nagaoka M, Morita K, Suzuki T. Structure and Anti-dengue Virus Activity of Sulfated Polysaccharide from a Marine Alga. Biochem Biophys Res Commun. 2008;376:91–95. doi: 10.1016/j.bbrc.2008.08.100. [DOI] [PubMed] [Google Scholar]
- 344.Talarico LB, Damonte EB. Interference in Dengue Virus Adsorption and Uncoating by Carrageenans. Virology. 2007;363:473–485. doi: 10.1016/j.virol.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 345.Talarico LB, Pujol CA, Zibetti RGM, Faria PCS, Noseda MD, Duarte MER, Damonte EB. The Antiviral Activity of Sulfated Polysaccharides against Dengue Virus is Dependent on Virus Serotype and Host Cell. Antiviral Res. 2005;66:103–110. doi: 10.1016/j.antiviral.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 346.Talarico LB, Duarte MER, Zibetti RGM, Noseda MD, Damonte EB. An Algal-Derived DL-Galactan Hybrid is an Efficient Preventing Agent for In Vitro Dengue Virus Infection. Planta Med. 2007;73:1464–1468. doi: 10.1055/s-2007-990241. [DOI] [PubMed] [Google Scholar]
- 347.Talarico LB, Noseda MD, Ducatti DRB, Duarte MER, Damonte EB. Differential Inhibition of Dengue Virus Infection in Mammalian and Mosquito Cells by Iota-Carrageenan. J Gen Virol. 2011;92:1332–1342. doi: 10.1099/vir.0.028522-0. [DOI] [PubMed] [Google Scholar]
- 348.Arena A, Maugeri TL, Pavone B, Iannello D, Gugliandolo C, Bisignano G. Antiviral and Immunoregulatory Effect of a Novel Exopolysaccharide from a Marine Thermotolerant Bacillus licheniformis. Int Immunopharmacol. 2006;6:8–13. doi: 10.1016/j.intimp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 349.Arena A, Gugliandolo C, Stassi G, Pavone B, Iannello D, Bisignano G, Maugeri TL. An Exopolysaccharide Produced by Geobacillus thermodenitrificans Strain B3-72: Antiviral Activity on Immunocompetent Cells. Immunol Lett. 2009;123:132–137. doi: 10.1016/j.imlet.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 350.Baba M, Snoeck R, Pauwels R, De Clercq E. Sulfated Polysaccharides are Potent and Selective Inhibitors of Various Enveloped Viruses, Including Herpes Simplex Virus, Cytomegalovirus, Vesicular Stomatitis Virus, and Human Immunodeficiency Virus. Antimicrob Agents Chemother. 1988;32:1742–1745. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Béress A, Wassermann O, Bruhn T, Béress L, Kraiselburd EN, Gonzalez LV, de Motta GE, Chavez PI. A New Procedure for the Isolation of Anti-HIV Compounds (Polysaccharides and Polyphenols) from the Marine Alga Fucus vesiculosus. J Nat Prod. 1993;56:478–488. doi: 10.1021/np50094a005. [DOI] [PubMed] [Google Scholar]
- 352.Rowley DC, Kelly S, Kauffman CA, Jensen PR, Fenical W, Halovirs A-E. New Antiviral Agents from a Marine-derived Fungus of the Genus Scytalidium. Bioorg Med Chem. 2003;11:4263–4274. doi: 10.1016/s0968-0896(03)00395-x. [DOI] [PubMed] [Google Scholar]
- 353.Kobayashi J, Harbour GC, Gilmore J, Rinehart KL., Jr Eudistomins A, D, G, H, I, J, M, N, O, P, Q, Bromo, Hydroxy, Pyrrolyl Iminoazepino β-carbolines from the Antiviral Caribbean Tunicate Eudistoma olivaceum. J Am Chem Soc. 1984;106:1526–1528. [Google Scholar]
- 354.Hudson JB, Saboune H, Abramowski Z, Towers GHN, Rinehart KL., Jr The Photoactive Antimicrobial Properties of Eudistomins from the Caribbean Tunicate Eudistoma olivaceum. Photochem Photobiol. 1988;47:377–381. doi: 10.1111/j.1751-1097.1988.tb02740.x. [DOI] [PubMed] [Google Scholar]
- 355.Sakai R, Rinehart KL, Kishore V, Kundu B, Faircloth G, Gloer JB, Carney JR, Namikoshi M, Sun F, Hughes RG, Jr, Grávalos DG, de Quesada TG, Wilson GR, Heid RM. Structure-Activity Relationships of the Didemnins. J Med Chem. 1996;39:2819–2834. doi: 10.1021/jm960048g. [DOI] [PubMed] [Google Scholar]
- 356.Rinehart KL, Kishore V, Bible KC, Sakai R, Sullins DW, Li KM. Didemnins and Tunichlorin: Novel Natural Products from the Marine Tunicate Trididemnum solidum. J Nat Prod. 1988;51:1–21. doi: 10.1021/np50055a001. [DOI] [PubMed] [Google Scholar]
- 357.Copeland R, Balasubramaniam A, Tiwari V, Zhang F, Bridges A, Linhardt RJ, Shukla D, Liu J. Using a 3-O-Sulfated Heparin Octasaccharide to Inhibit the Entry of Herpes Simplex Virus Type 1. Biochemistry. 2008;47:5774–5783. doi: 10.1021/bi800205t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Ramos-Kuri M, Barron RBL, Aguilar-Setien A. Inhibition of Three Alphaherpesviruses (Herpes Simplex 1 and 2 and Pseudorabies Virus) by Heparin, Heparan and Other Sulfated Polyelectrolytes. Arch Med Res. 1996;27:43–48. [PubMed] [Google Scholar]
- 359.Neyts J, Snoeck R, Schols D, Balzarini J, Esko JD, Van Schepdael A, De Clercq E. Sulfated Polymers Inhibit the Interaction of Human Cytomegalovirus with Cell Surface Heparan Sulfate. Virology. 1992;189:48–58. doi: 10.1016/0042-6822(92)90680-n. [DOI] [PubMed] [Google Scholar]
- 360.Okazaki K, Matsuzaki T, Sugahara Y, Okada J, Hasebe M, Iwamura Y, Ohnishi M, Kanno T, Shimizu M, Honda E, Kong Y. BHV-1 Adsorption is Mediated by the Interaction of Glycoprotein gIII with Heparinlike Moiety on the Cell Surface. Virology. 1991;181:666–670. doi: 10.1016/0042-6822(91)90900-v. [DOI] [PubMed] [Google Scholar]
- 361.Kari B, Gehrz R. A Human Cytomegalovirus Glycoprotein Complex Designated gC-II is a Major Heparin-Binding Component of the Envelope. J Virol. 1992;66:1761–1764. doi: 10.1128/jvi.66.3.1761-1764.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Nan Y, Sai L, Jian-Jian H. The Depressive Effect of Glycosaminoglycan from Scallop on Type-1 Herpes Simplex Virus. Acta Acad Med Qingdao Univ. 2008;2:111–114. [Google Scholar]
- 363.Carlucci MJ, Scolaro LA, Damonte EB. Herpes Simplex Virus Type 1 Variants Arising after Selection with an Antiviral Carrageenan: Lack of Correlation Between Drug Susceptibility and Syn Phenotype. J Med Virol. 2002;68:92–98. doi: 10.1002/jmv.10174. [DOI] [PubMed] [Google Scholar]
- 364.Carlucci MJ, Ciancia M, Matulewicz MC, Cerezo AS, Damonte EB. Antiherpetic Activity and Mode of Action of Natural Carrageenans of Diverse Structural Types. Antiviral Res. 1999;43:93–102. doi: 10.1016/s0166-3542(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 365.Carlucci MJ, Pujol CA, Ciancia M, Noseda MD, Matulewicz MC, Damonte EB, Cerezo AS. Antiherpetic and Anticoagulant Properties of Carrageenans from the Red Seaweed Gigartina skottsbergii and their Cyclized Derivatives: Correlation Between Structure and Biological Activity. Int J Biol Macromol. 1997;20:97–105. doi: 10.1016/s0141-8130(96)01145-2. [DOI] [PubMed] [Google Scholar]
- 366.Harden EA, Falshaw R, Carnachan SM, Kern ER, Prichard MN. Virucidal Activity of Polysaccharide Extracts from Four Algal Species against Herpes Simplex Virus. Antiviral Res. 2009;83:282–289. doi: 10.1016/j.antiviral.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Damonte EB, Matulewicz MC, Cerezo AS. Sulfated Seaweed Polysaccharides as Antiviral Agents. Curr Med Chem. 2004;11:2399–2419. doi: 10.2174/0929867043364504. [DOI] [PubMed] [Google Scholar]
- 368.Kanekiyo K, Hayashi K, Takenaka H, Lee J-B, Hayashi T. Anti-herpes Simplex Virus Target of an Acidic Polysaccharide, Nostoflan, from the Edible Blue-Green Alga. Biol Pharm Bull. 2007;30:1573–1575. doi: 10.1248/bpb.30.1573. [DOI] [PubMed] [Google Scholar]
- 369.Canadas’s Source for HIV and Hepatitis C Information [CATIE] [accessed April 03, 2013];Treatment Update. 194 http://www.catie.ca/sites/default/files/tu194.pdf. [Google Scholar]
- 370.Medicines Patent Pool. [accessed April 01, 2013];Patent Status of ARVs. http://www.medicinespatentpool.org/patent-data/patent-status-of-arvs/
- 371.Van Rompay KK, Kearney BP, Sexton JJ, Colón R, Lawson JR, Blackwood EJ, Lee WA, Bischofberger N, Marthas ML. Evaluation of Oral Tenofovir Disoproxil Fumarate and Topical Tenofovir GS-7340 to Protect Infant Macaques against Repeated Oral Challenges with Virulent Simian Immunodeficiency Virus. JAIDS, J Acquired Immune Defic Syndr. 2006;43:6–14. doi: 10.1097/01.qai.0000224972.60339.7c. [DOI] [PubMed] [Google Scholar]
- 372.Eron JJ., Jr Antiretroviral Therapy: New Drugs Formulations, Ideas and Strategies. Top HIV Med. 2009;17:146–150. [PubMed] [Google Scholar]
- 373.Gulick RM. New Antiretroviral Drugs. Clin Microbiol Infect. 2003;9:186–193. doi: 10.1046/j.1469-0691.2003.00570.x. [DOI] [PubMed] [Google Scholar]
- 374.Corbett JW, Ko SS, Rodgers JD, Jeffrey S, Bacheler LT, Klabe RM, Diamond S, Lai CM, Rabel SR, Saye JA, Adams SP, Trainor GL, Anderson PS, Erickson-Viitanen SK. Expanded-Spectrum Nonnucleoside Reverse Transcriptase Inhibitors Inhibit Clinically Relevant Mutant Variants of Human Immunodeficiency Virus Type 1. Antimicrob Agents Chemother. 1999;43:2893–2897. doi: 10.1128/aac.43.12.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Corbett JW, Ko SS, Rodgers JD, Gearhart LA, Magnus NA, Bacheler LT, Diamond S, Jeffrey S, Klabe RM, Cordova BC, Garber S, Logue K, Trainor GL, Anderson PS, Erickson-Viitanen SK. Inhibition of Clinically Relevant Mutant Variants of HIV-1 by Quinazolinone Non-nucleoside Reverse Transcriptase Inhibitors. J Med Chem. 2000;43:2019–2030. doi: 10.1021/jm990580e. [DOI] [PubMed] [Google Scholar]
- 376.Ruiz N, Nusrat R, Lauenroth-Mai E, Berger D, Walworth C, Bacheler L, Ploughman L, Tsang P, Labriola D, Echols R. Study DPC 083-203, a Phase II Comparison of 100 and 200 mg once-daily DPC 083 and 2 NRTIs in Patients Failing a NNRTI-Containing Regimen. Presented at Program and abstracts of the 9:h Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2002. [Google Scholar]
- 377.Allaway GP, Davis-Bruno KL, Beaudry GA, Garcia EB, Wong EL, Ryder AM, Hasel KW, Gauduin MC, Koup RA, McDougal JS, Maddon PJ. Expression and Characterization of CD4-IgG2, a Novel Heterotetramer that Neutralizes Primary HIV Type 1 Isolates. AIDS Res Hum Retroviruses. 1995;11:533–539. doi: 10.1089/aid.1995.11.533. [DOI] [PubMed] [Google Scholar]
- 378.Strizki JM, Xu S, Wagner NE, Wojcik L, Liu J, Hou Y, Endres M, Palani A, Shapiro S, Clader JW, Greenlee WJ, Tagat JR, McCombie S, Cox K, Fawzi AB, Chou CC, Pugliese-Sivo C, Davies L, Moreno ME, Ho DD, Trkola A, Stoddart CA, Moore JP, Reyes GR, Baroudy BM. SCH-C (SCH 351125), an Orally Bioavailable, Small Molecule Antagonist of the Chemokine Receptor CCR5, is a Potent Inhibitor of HIV-1 Infection In Vitro and In Vivo. Proc Natl Acad Sci U S A. 2001;98:12718–12723. doi: 10.1073/pnas.221375398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Eron JJ, Gulick RM, Bartlett JA, Merigan T, Arduino R, Kilby JM, Yangco B, Diers A, Drobnes C, DeMasi R, Greenberg M, Melby T, Raskino C, Rusnak P, Zhang Y, Spence R, Miralles GD. Short-term Safety and Antiretroviral Activity of T-1249, a Second-generation Fusion Inhibitor of HIV. J Infect Dis. 2004;189:1075–1083. doi: 10.1086/381707. [DOI] [PubMed] [Google Scholar]
- 380.Do Canto AMTM, Carvalho AJP, Ramalho JPP, Loura LMS. T-20 and T-1249 HIV Fusion Inhibitors’ Structure and Conformation in Solution: A Molecular Dynamics Study. J Pept Sci. 2008;14:442–447. doi: 10.1002/psc.982. [DOI] [PubMed] [Google Scholar]
- 381.Rosemond MJC, St John-Williams L, Yamaguchi T, Fujishita T, Walsh JS. Enzymology of a Carbonyl Reduction Clearance Pathway for the HIV Integrase Inhibitor, S-1360: Role of Human Liver Cytosolic Aldo-Keto Reductases. Chem-Biol Interact. 2004;147:129–139. doi: 10.1016/j.cbi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 382.Saag MS. New and Investigational Antiretroviral Drugs for HIV Infection: Mechanisms of Action and Early Research Findings. Top Antivir Med. 2012;20:162–167. [PMC free article] [PubMed] [Google Scholar]
- 383.Merck. [accessed April 05, 2013];Merck Pipeline. http://www.merck.com/research/pipeline/home.html.
- 384.Garrido C, Soriano V, de Mendoza C. New therapeutic strategies for raltegravir. J Antimicrob Chemother. 2010;65:218–223. doi: 10.1093/jac/dkp447. [DOI] [PubMed] [Google Scholar]
- 385.GlaxoSmithKline. [accessed April 05, 2013];Our Product Pipeline. http://www.gsk.com/en-gb/research/what-we-are-working-on/product-pipeline/
- 386.Pockros P, Jensen D, Tsai N, Taylor RM, Ramji A, Cooper C, Dickson R, Tice A, Stande S, Ipe D, Thommes JA, Vierling JM. First SVR Data with the Nucleoside Analogue Polymerase Inhibitor Mericitabine (RG7128) Combined with Peginterferon/ribavirin in Treatment-naive HCV G1/4 Patients: Interim Analysis from the JUMP-C Trial. J Hepatol. 2011;54:S538. [Google Scholar]
- 387.Roche Research & Development. [accessed April 04, 2013];Pharma Pipeline. http://www.roche.com/research_and_development/who_we_are_how_we_work/pipeline.htm.
- 388.Gane EJ, Roberts SK, Stedman CAM, Angus PW, Ritchie B, Elston R, Ipe D, Morcos PN, Baher L, Najera I, Chu T, Lopatin U, Berrey MM, Bradford W, Laughlin M, Shulman NS, Smith PF. Oral Combination Therapy with a Nucleoside Polymerase Inhibitor (RG7128) and Danoprevir for Chronic Hepatitis C Genotype 1 Infection (INFORM-1): A Randomised, Double-blind, Placebo-controlled, Dose-escalation Trial. Lancet. 2010;376:1467–1475. doi: 10.1016/S0140-6736(10)61384-0. [DOI] [PubMed] [Google Scholar]
- 389.Cartwright H. Roche Expands its HCV Pipeline with Anadys Pharmaceuticals Acquisition. PharmaDeals Rev. 2011;2011:109. [Google Scholar]
- 390.Zeuzem S, Asselah T, Angus P, Zarski JP, Larrey D, Müllhaupt B, Gane E, Schuchmann M, Lohse A, Pol S, Bronowicki JP, Roberts S, Arasteh K, Zoulim F, Heim M, Stern JO, Kukolj G, Nehmiz G, Haefner C, Boecher WO. Efficacy of the Protease Inhibitor BI 201335, Polymerase Inhibitor BI 207127, and Ribavirin in Patients with Chronic HCV Infection. Gastroenterology. 2011;141:2047–2055. doi: 10.1053/j.gastro.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 391.LaPlante SR, Bös M, Brochu C, Chabot C, Coulombe R, Gillard JR, Jakalian A, Poirier M, Rancourt J, Stammers T, Thavonekham B, Beaulieu PL, Kukolj G, Tsantrizos YS. Conformation-Based Restrictions and Scaffold Replacements in the Design of Hepatitis C Virus Polymerase Inhibitors: Discovery of Deleobuvir (BI 207127) J Med Chem. 2014;57:1845–1854. doi: 10.1021/jm4011862. [DOI] [PubMed] [Google Scholar]
- 392.Boehringer Ingelheim. [accessed April 05, 2013];R&D Pipeline. http://www.boehringer-ingelheim.com/research_development/drug_discovery/pipeline.html.
- 393.Summa V, Ludmerer SW, McCauley JA, Fandozzi C, Burlein C, Claudio G, Coleman PJ, DiMuzio JM, Ferrara M, Di Filippo M, Gates AT, Graham DJ, Harper S, Hazuda DJ, McHale C, Monteagudo E, Pucci V, Rowley M, Rudd MT, Soriano A, Stahlhut MW, Vacca JP, Olsen DB, Liverton NJ, Carroll SS. MK-5172, a Selective Inhibitor of Hepatitis C Virus NS3/4a Protease with Broad Activity across Genotypes and Resistant Variants. Antimicrob Agents Chemother. 2012;56:4161–4167. doi: 10.1128/AAC.00324-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Coburn CA, Meinke PT, Chang W, Fandozzi CM, Graham DJ, Hu B, Huang Q, Kargman S, Kozlowski J, Liu R, McCauley JA, Nomeir AA, Soll RM, Vacca JP, Wang D, Wu H, Zhong B, Olsen DB, Ludmerer SW. Discovery of MK-8742: An HCV NS5A Inhibitor with Broad Genotype Activity. ChemMedChem. 2013;8:1930–1940. doi: 10.1002/cmdc.201300343. [DOI] [PubMed] [Google Scholar]
- 395.Liverton NJ, Carroll SS, DiMuzio J, Fandozzi C, Graham DJ, Hazuda D, Holloway MK, Ludmerer SW, McCauley JA, McIntyre CJ, Olsen DB, Rudd MT, Stahlhut M, Vacca JP. MK-7009, a Potent and Selective Inhibitor of Hepatitis C Virus NS3/4A Protease. Antimicrob Agents Chemother. 2010;54:305–311. doi: 10.1128/AAC.00677-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.McCauley JA, McIntyre CJ, Rudd MT, Nguyen KT, Romano JJ, Butcher JW, Gilbert KF, Bush KJ, Holloway MK, Swestock J, Wan BL, Carroll SS, DiMuzio JM, Graham DJ, Ludmerer SW, Mao SS, Stahlhut MW, Fandozzi CM, Trainor N, Olsen DB, Vacca JP, Liverton NJ. Discovery of Vaniprevir (MK-7009), a Macrocyclic Hepatitis C Virus NS3/4a Protease Inhibitor. J Med Chem. 2010;53:2443–2463. doi: 10.1021/jm9015526. [DOI] [PubMed] [Google Scholar]
- 397.Bechtel J, Crosby R, Wang A, Woldu E, Van Horn S, Horton J, Creech K, Caballo LH, Voitenleitner C, Vamathevan J, Duan M, Spaltenstein A, Kazmierski W, Roberts C, Hamatake R. In Vitro Profiling of GSK2336805, A Potent and Selective Inhibitor of HCV NS5A. J Hepatol. 2011;54:S307–S308. [Google Scholar]
- 398.Merck. [accessed August 11, 2014];Merck Pipeline. http://www.merck.com/research/pipeline/home.html.
- 399.Margot N, Liu Y, Babusis D, Miller MD, Callebaut C. Antiviral Activity of Tenofovir Alafenamide (TAF) against Major NRTI-resistant Viruses: Improvement over TDF/TFV is Driven by Higher TFV-DP Loading in Target Cells. Presented at the International Workshop on HIV and Hepatitis Virus Drug Resistance and Curative Strategies; Toronto, Canada. 2013. [Google Scholar]
- 400.Gilead. [accessed August 11, 2014];Pipeline. http://gilead.com/research/pipeline.
- 401.Gaggar A, Coeshott C, Apelian D, Rodell T, Armstrong BR, Shen G, Subramanian GM, McHutchison JG. Safety, Tolerability and Immunogenicity of GS-4774, a Hepatitis B Virus-specific Therapeutic Vaccine, in Healthy Subjects: A Randomized Study. Vaccine. 2014;32:4925–4931. doi: 10.1016/j.vaccine.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 402.Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G, Halcomb RL, Tumas DB. GS-9620, an Oral Agonist of Toll-like Receptor-7, Induces Prolonged Suppression of Hepatitis B Virus in Chronically Infected Chimpanzees. Gastroenterology. 2013;144:1508–1517. doi: 10.1053/j.gastro.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Rossignol JF, El-Gohary YM. Nitazoxanide in the Treatment of Viral Gastroenteritis: A Randomized Double-blind Placebo-controlled Clinical Trial. Aliment Pharmacol Ther. 2006;24:1423–1430. doi: 10.1111/j.1365-2036.2006.03128.x. [DOI] [PubMed] [Google Scholar]
- 404.Rossignol JF. Nitazoxanide: A First-in-class Broad-spectrum Antiviral Agent. Antiviral Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Petit J, Meurice N, Kaiser C, Maggiora G. Softening the Rule of Five - Where to Draw the Line? Bioorg Med Chem. 2012;20:5343–5351. doi: 10.1016/j.bmc.2011.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.U.S. Food and Drug Administration. [accessed December 02, 2012];Draft Guidance on Didanosine. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm085597.pdf.
- 407.U.S. Food and Drug Administration. [accessed December 01, 2012];Draft Guidance on Zalcitabine. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm091313.pdf.
- 408.Bhadury P, Mohammad BT, Wright PC. The Current Status of Natural Products from Marine Fungi and their Potential as Anti-infective Agents. J Ind Microbiol Biotechnol. 2006;33:325–337. doi: 10.1007/s10295-005-0070-3. [DOI] [PubMed] [Google Scholar]
- 409.Yasuhara-Bell J, Lu Y. Marine Compounds and their Antiviral Activities. Antiviral Res. 2010;86:231–240. doi: 10.1016/j.antiviral.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Yin PD, Das D, Mitsuya H. Overcoming HIV Drug Resistance through Rational Drug Design Based on Molecular, Biochemical, and Structural Profiles of HIV Resistance. Cell Mol Life Sci. 2006;63:1706–1724. doi: 10.1007/s00018-006-6009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Greenblatt DJ. Antiretroviral Boosting by Cobicistat, a Structural Analog of Ritonavir. Clin Pharmacol Drug Dev. 2014;3:335–337. doi: 10.1002/cpdd.159. [DOI] [PubMed] [Google Scholar]
- 412.Matthews T, Salgo M, Greenberg M, Chung J, DeMasi R, Bolognesi D. Enfuvirtide: The First Therapy to Inhibit the Entry of HIV-1 into Host CD4 Lymphocytes. Nat Rev Drug Discovery. 2004;3:215–225. doi: 10.1038/nrd1331. [DOI] [PubMed] [Google Scholar]
- 413.PDB ID: 1HRH: Davies JF, 2nd, Hostomska Z, Hostomsky Z, Jordan SR, Matthews DA. Crystal Structure of the Ribonuclease H Domain of HIV-1 Reverse Transcriptase. Science. 1991;252:88–95. doi: 10.1126/science.1707186.
- 414.PDB ID: 1EX4: Chen JCH, Krucinski J, Miercke LJW, Finer-Moore JS, Tang AH, Leavitt AD, Stroud RM. Crystal Structure of the HIV-1 Integrase Catalytic Core and C-terminal Domains: A Model for Viral DNA Binding. Proc Natl Acad Sci U S A. 2000;97:8233–8238. doi: 10.1073/pnas.150220297.
- 415.PDB ID: 2R92: Lehmann E, Brueckner F, Cramer P. Molecular Basis of RNA-dependent RNA Polymerase II Activity. Nature. 2007;450:445–449. doi: 10.1038/nature06290.
- 416.PDB ID: 3HVP: Wlodawer A, Miller M, Jaskolski M, Sathyanarayana BK, Baldwin E, Weber IT, Selk LM, Clawson L, Schneider J, Kent SB. Conserved Folding in Retroviral Proteases: Crystal Structure of a Synthetic HIV-1 Protease. Science. 1989;245:616–621. doi: 10.1126/science.2548279.
- 417.National Institute of Allergy and Infectious Diseases. [accessed November 02, 2014];Scanning electron micrograph of HIV particles infecting a human T cell. http://www.nih.gov/science/hiv/full_images/infected1_image.htm.
- 418.Link JO, Taylor JG, Xu L, Mitchell M, Guo H, Liu H, Kato D, Kirschberg T, Sun J, Squires N, Parrish J, Keller T, Yang ZY, Yang C, Matles M, Wang Y, Wang K, Cheng G, Tian Y, Mogalian E, Mondou E, Cornpropst M, Perry J, Desai MC. Discovery of Ledipasvir (GS-5885): A Potent, Once-Daily Oral NS5A Inhibitor for the Treatment of Hepatitis C Virus Infection. J Med Chem. 2014;57:2033–2046. doi: 10.1021/jm401499g. [DOI] [PubMed] [Google Scholar]
- 419.Vaidya A, Perry CM. Simeprevir: First Global Approval. Drugs. 2013;73:2093–2106. doi: 10.1007/s40265-013-0153-9. [DOI] [PubMed] [Google Scholar]
- 420.Henderson JA, Bilimoria D, Bubenik M, Cadilhac C, Cottrell KM, Denis F, Dietrich E, Ewing N, Falardeau G, Giroux S, L’Heureux C, Liu B, Mani N, Morris M, Nicolas O, Pereira OZ, Poisson C, Reddy JT, Selliah S, Shawgo RS, Vaillancourt L, Wang J, Xu J, Chauret N, Berlioz-Seux F, Chan LC, Das SK, Grillot AL, Bennani YL, Maxwell JP. Synthesis and Evaluation of NS5A Inhibitors Containing Diverse Heteroaromatic Cores. Bioorg Med Chem Lett. 2014;25:948–951. doi: 10.1016/j.bmcl.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 421.Pilot-Matias T, Tripathi R, Cohen D, Gaultier I, Dekhtyar T, Lu L, Reisch T, Irvin M, Hopkins T, Pithawalla R, Middleton T, Ng T, McDaniel K, Or YS, Menon R, Kempf D, Molla A, Collins C. In Vitro and In Vivo Antiviral Activity and Resistance Profile of the Hepatitis C Virus NS3/4A Protease Inhibitor ABT-450. Antimicrob Agents Chemother. 2015;59:988. doi: 10.1128/AAC.04227-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Kati W, Koev G, Irvin M, Beyer J, Liu Y, Krishnan P, Reisch T, Mondal R, Wagner R, Molla A, Maring C, Collins C. In Vitro Activity and Resistance Profile of Dasabuvir, a Non-nucleoside HCV Polymerase Inhibitor. Antimicrob Agents Chemother. 2015;59:1505. doi: 10.1128/AAC.04619-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Ghosh AK, Dawson ZL, Mitsuya H. Darunavir, a Conceptually New HIV-1 Protease Inhibitor for the Treatment of Drug-resistant HIV. Bioorg Med Chem. 2007;15:7576–7580. doi: 10.1016/j.bmc.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Black DM, Davis R, Doan BD, Lovelace TC, Millar A, Toczko JF, Xie S. Highly Diastereo- and Enantioselective Catalytic Synthesis of the Bis-tetrahydrofuran Alcohol of Brecanavir and Darunavir. Tetrahedron: Asymmetry. 2008;19:2015–2019. [Google Scholar]
- 425.Nowicka-Sans B, Gong YF, McAuliffe B, Dicker I, Ho HT, Zhou N, Eggers B, Lin PF, Ray N, Wind-Rotolo M, Zhu L, Majumdar A, Stock D, Lataillade M, Hanna GJ, Matiskella JD, Ueda Y, Wang T, Kadow JF, Meanwell NA, Krystal M. In Vitro Antiviral Characteristics of HIV-1 Attachment Inhibitor BMS-626529, the Active Component of the Prodrug BMS-663068. Antimicrob Agents Chemother. 2012;56:3498–3507. doi: 10.1128/AAC.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Hightower KE, Wang R, DeAnda F, Johns BA, Weaver K, Shen Y, Tomberlin GH, Carter HL, III, Broderick T, Sigethy S, Seki T, Kobayashi M, Underwood MR. Dolutegravir (S/GSK1349572) Exhibits Significantly Slower Dissociation than Ralte-gravir and Elvitegravir from Wild-Type and Integrase Inhibitor-Resistant HIV-1 Integrase-DNA Complexes. Antimicrob Agents Chemother. 2011;55:4552–4559. doi: 10.1128/AAC.00157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.PDB ID: 3O2D: Freeman MM, Seaman MS, Rits-Volloch S, Hong X, Kao CY, Ho DD, Chen B. Crystal Structure of HIV-1 Primary Receptor CD4 in Complex with a Potent Antiviral Antibody. Structure. 2010;18:1632–1641. doi: 10.1016/j.str.2010.09.017.
- 428.PDB ID: 4MBS: Tan Q, Zhu Y, Li J, Chen Z, Han GW, Kufareva I, Li T, Ma L, Fenalti G, Li J, Zhang W, Xie X, Yang H, Jiang H, Cherezov V, Liu H, Stevens RC, Zhao Q, Wu B. Structure of the CCR5 Chemokine Receptor-HIV Entry Inhibitor Maraviroc Complex. Science. 2013;341:1387–1390. doi: 10.1126/science.1241475.
- 429.PDB ID: 3V6Z: DiMattia MA, Watts NR, Stahl SJ, Grimes JM, Steven AC, Stuart DI, Wingfield PT. Antigenic Switching of Hepatitis B Virus by Alternative Dimerization of the Capsid Protein. Structure. 2013;21:133–142. doi: 10.1016/j.str.2012.10.017.
- 430.PDB ID: 3J2V: Yu X, Jin L, Jih J, Shih C, Zhou ZH. 3.5Å cryoEM Structure of Hepatitis B Virus Core Assembled from Full-Length Core Protein. PLoS One. 2013;8:e69729. doi: 10.1371/journal.pone.0069729.
- 431.PDB ID: 2WJ8: Tawar RG, Duquerroy S, Vonrhein C, Varela PF, Damier-Piolle L, Castagné N, MacLellan K, Bedouelle H, Bricogne G, Bhella D, Eléouët JF, Rey FA. Crystal Structure of a Nucleocapsid-like Nucleoprotein-RNA Complex of Respiratory Syncytial Virus. Science. 2009;326:1279–1283. doi: 10.1126/science.1177634.
- 432.PDB ID: 2YKD: McPhee HK, Carlisle JL, Beeby A, Money VA, Watson SMD, Yeo RP, Sanderson JM. Influence of Lipids on the Interfacial Disposition of Respiratory Syncytial Virus Matrix Protein. Langmuir. 2011;27:304–311. doi: 10.1021/la104041n.
- 433.PDB ID: 4CCF: Peat TS. Structure of Respiratory Syncytial Virus F Protein Head Domain. doi: 10.2210/pdb4ccf/pdb.
- 434.PDB ID: 3VTT: Elahi M, Islam MM, Noguchi K, Yohda M, Kuroda Y. High Resolution Crystal Structure of Dengue-3 Envelope Protein Domain III Suggests Possible Molecular Mechanisms for Serospecific Antibody Recognition. Proteins: Struct, Funct, Genet. 2013;81:1090–1095. doi: 10.1002/prot.24237.
- 435.PDB ID: 1WYY: Koellhoffer JF, Dai Z, Malashkevich VN, Stenglein MD, Liu Y, Toro R, Harrison JS, Chandran K, DeRisi JL, Almo SC, Lai JR. Structural Characterization of the Glycoprotein GP2 Core Domain from the CAS Virus, a Novel Arenavirus-like Species. J Mol Biol. 2014;426:1452–1468. doi: 10.1016/j.jmb.2013.12.009.
- 436.PDB ID: 3I6L: Liu J, Wu P, Gao F, Qi J, Kawana-Tachikawa A, Xie J, Vavricka CJ, Iwamoto A, Li T, Gao GF. Novel Immunodominant Peptide Presentation Strategy: A Featured HLA-A*2402-Restricted Cytotoxic T-Lymphocyte Epitope Stabilized by Intrachain Hydrogen Bonds from Severe Acute Respiratory Syndrome Coronavirus Nucleocapsid Protein. J Virol. 2010;84:11849–11857. doi: 10.1128/JVI.01464-10.
- 437.PDB ID: 1XJR: Robertson MP, Igel H, Baertsch R, Haussler D, Ares M, Jr, Scott WG. The Structure of a Rigorously Conserved RNA Element within the SARS Virus Genome. PLoS Biol. 2004;3:e5. doi: 10.1371/journal.pbio.0030005.
- 438.Zhang XN, Song ZG, Jiang T, Shi BS, Hu YW, Yuan ZH. Rupintrivir is a Promising Candidate for Treating Severe Cases of Enterovirus-71 Infection. World J Gastroenterol. 2010;16:201–209. doi: 10.3748/wjg.v16.i2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Nakajima M, DeChavigny A, Johnson CE, Hamada J, Stein CA, Nicolson GL. Suramin: A Potent Inhibitor of Melanoma Heparanase and Invasion. J Biol Chem. 1991;266:9661–9666. [PubMed] [Google Scholar]
- 440.PDB ID: 3FMG: Aoki ST, Settembre EC, Trask SD, Greenberg HB, Harrison SC, Dormitzer PR. Structure of Rotavirus Outer-layer Protein VP7 Bound with a Neutralizing Fab. Science. 2009;324:1444–1447. doi: 10.1126/science.1170481.
- 441.PDB ID: 2R7R: Lu X, McDonald SM, Tortorici MA, Tao YJ, Vasquez-Del Carpio R, Nibert ML, Patton JT, Harrison SC. Mechanism for Coordinated RNA Packaging and Genome Replication by Rotavirus Polymerase VP1. Structure. 2008;16:1678–1688. doi: 10.1016/j.str.2008.09.006.
- 442.PDB ID: 1QHD: Mathieu M, Petitpas I, Navaza J, Lepault J, Kohli E, Pothier P, Prasad BVV, Cohen J, Rey FA. Atomic Structure of the Major Capsid Protein of Rotavirus: Implications for the Architecture of the Virion. EMBO J. 2001;20:1485–1497. doi: 10.1093/emboj/20.7.1485.
- 443.PDB ID: 4IDD: Bornholdt ZA, Noda T, Abelson DM, Halfmann P, Wood MR, Kawaoka Y, Saphire EO. Structural Rearrangement of Ebola Virus VP40 Begets Multiple Functions in the Virus Life Cycle. Cell. 2013;154:763–774. doi: 10.1016/j.cell.2013.07.015.
- 444.PDB ID: 1H2C: Gomis-Rüth FX, Dessen A, Timmins J, Bracher A, Kolesnikowa L, Becker S, Klenk HD, Weissenhorn W. The Matrix Protein VP40 from Ebola Virus Octamerizes into Pore-like Structures with Specific RNA Binding Properties. Structure. 2003;11:423–433. doi: 10.1016/S0969-2126(03)00050-9.
- 445.National Institute of Allergy and Infectious Diseases. [accessed November 02, 2014];Ebola Outbreak Highlights Global Disparities in Healthcare Resources. http://www.niaid.nih.gov/news/newsreleases/2014/Pages/EbolaDisparities.aspx.
- 446.PDB ID: 1VCL: Uchida T, Yamasaki T, Eto S, Sugawara H, Kurisu G, Nakagawa A, Kusunoki M, Hatakeyama T. Crystal Structure of the Hemolytic Lectin CEL-III Isolated from the Marine Invertebrate Cucumaria echinata: Implications of Domain Structure for its Membrane Pore-formation Mechanism. J Biol Chem. 2004;279:37133–37141. doi: 10.1074/jbc.M404065200.
- 447.Delattre C, Fenoradosoa TA, Michaud P. Galactans: An Overview of their Most Important Sourcing and Applications as Natural Polysaccharides. Braz Arch Biol Technol. 2011;54:1075–1092. [Google Scholar]
- 448.Plaza A, Bifulco G, Keffer JL, Lloyd JR, Baker HL, Bewley CA. Celebesides A–C and Theopapuamides B–D, Depsipep-tides from an Indonesian Sponge that Inhibit HIV-1 Entry. J Org Chem. 2009;74:504–512. doi: 10.1021/jo802232u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Eisenberg EJ, He GX, Lee WA. Metabolism of GS-7340, A Novel Phenyl Monophosphoramidate Intracellular Prodrug of PMPA, in Blood. Nucleosides, Nucleotides Nucleic Acids. 2001;20:1091–1098. doi: 10.1081/NCN-100002496. [DOI] [PubMed] [Google Scholar]
- 450.Côté B, Burch JD, Asante-Appiah E, Bayly C, Bédard L, Blouin M, Campeau LC, Cauchon E, Chan M, Chefson A, Coulombe N, Cromlish W, Debnath S, Deschênes D, Dupont-Gaudet K, Falgueyret JP, Forget R, Gagné S, Gauvreau D, Girardin M, Guiral S, Langlois E, Li CS, Nguyen N, Papp R, Plamondon S, Roy A, Roy S, Seliniotakis R, St-Onge M, et al. Discovery of MK-1439, an Orally Bioavailable Non-nucleoside Reverse Transcriptase Inhibitor Potent against a Wide Range of Resistant Mutant HIV Viruses. Bioorg Med Chem Lett. 2014;24:917–922. doi: 10.1016/j.bmcl.2013.12.070. [DOI] [PubMed] [Google Scholar]
- 451.Yoshinaga T, Kobayashi M, Seki T, Miki S, Wakasa-Morimoto C, Suyama-Kagitani A, Kawauchi-Miki S, Taishi T, Kawasuji T, Johns BA, Underwood MR, Garvey EP, Sato A, Fujiwara T. Antiviral Characteristics of GSK1265744, an HIV Integrase Inhibitor Dosed Orally or by Long-acting Injection. Antimicrob Agents Chemother. 2015;59:397. doi: 10.1128/AAC.03909-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Pawlotsky JM, Najera I, Jacobson I. Resistance to Mericitabine, a Nucleoside Analogue Inhibitor of HCV RNA-dependent RNA Polymerase. Antiviral Ther. 2012;17:411–423. doi: 10.3851/IMP2088. [DOI] [PubMed] [Google Scholar]
- 453.Jiang Y, Andrews SW, Condroski KR, Buckman B, Serebryany V, Wenglowsky S, Kennedy AL, Madduru MR, Wang B, Lyon M, Doherty GA, Woodard BT, Lemieux C, Do MG, Zhang H, Ballard J, Vigers G, Brandhuber BJ, Stengel P, Josey JA, Beigelman L, Blatt L, Seiwert SD. Discovery of Danoprevir (ITMN-191/R7227), a Highly Selective and Potent Inhibitor of Hepatitis C Virus (HCV) NS3/4A Protease. J Med Chem. 2014;57:1753–1769. doi: 10.1021/jm400164c. [DOI] [PubMed] [Google Scholar]
- 454.Aghemo A, De Francesco R. New Horizons in Hepatitis C Antiviral Therapy with Direct-Acting Antivirals. Hepatology. 2013;58:428–438. doi: 10.1002/hep.26371. [DOI] [PubMed] [Google Scholar]
- 455.White PW, Llinàs-Brunet M, Amad M, Bethell RC, Bolger G, Cordingley MG, Duan J, Garneau M, Lagacé L, Thibeault D, Kukolj G. Preclinical Characterization of BI 201335, a C-Terminal Carboxylic Acid Inhibitor of the Hepatitis C Virus NS3-NS4A Protease. Antimicrob Agents Chemother. 2010;54:4611–4618. doi: 10.1128/AAC.00787-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Mangion IK, Chen CY, Li H, Maligres P, Chen Y, Christensen M, Cohen R, Jeon I, Klapars A, Krska S, Nguyen H, Reamer RA, Sherry BD, Zavialov I. Enantioselective Synthesis of an HCV NS5a Antagonist. Org Lett. 2014;16:2310–2313. doi: 10.1021/ol500971c. [DOI] [PubMed] [Google Scholar]
- 457.Kazmierski WM, Maynard A, Duan M, Baskaran S, Botyanszki J, Crosby R, Dickerson S, Tallant M, Grimes R, Hamatake R, Leivers M, Roberts CD, Walker J. Novel Spiroketal Pyrrolidine GSK2336805 Potently Inhibits Key Hepatitis C Virus Genotype 1b Mutants: From Lead to Clinical Compound. J Med Chem. 2014;57:2058–2073. doi: 10.1021/jm4013104. [DOI] [PubMed] [Google Scholar]
- 458.Fosdick A, Zheng J, Pflanz S, Frey CR, Hesselgesser J, Halcomb RL, Wolfgang G, Tumas DB. Pharmacokinetic and Pharmacodynamic Properties of GS-9620, a Novel Toll-like Receptor 7 Agonist, Demonstrate Interferon-Stimulated Gene Induction without Detectable Serum Interferon at Low Oral Doses. J Pharmacol Exp Ther. 2014;348:96–105. doi: 10.1124/jpet.113.207878. [DOI] [PubMed] [Google Scholar]
- 459.Fox LM, Saravolatz LD. Nitazoxanide: A New Thiazolide Antiparasitic Agent. Clin Infect Dis. 2005;40:1173–1180. doi: 10.1086/428839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.