Abstract
Embryonic synchronous and asynchronous cells were successfully transplanted into preimplanted mouse blastocysts. At 15–17 days of fetal development, 19% (32/167), 15% (20/130) and 3% (4/117) of the fetuses expressed chimaerism of the ocular pigmentation when transplanted cells were 0, 48, and 96 hours asynchronous respectively. At birth and during postnatal development, 15% (23/153) and 13% (14/111) of the young expressed chimaerism of ocular pigmentation, coat color, and/or functional germ lines when transplanted cells were 0 and 48 hours asynchronous respectively. Five animals from synchronous and two animals from asynchronous cell transplants were chimaeric in functional germ lines but resembled recipient blastocysts in phenotype color. One overtly chimaeric male had progeny with color phenotype of the albino donor cells only. This report provides evidence that transplanted asynchronous as well as synchronous embryonic cells do participate in the final structure of the embryo. In addition, the results of these studies suggest that the micrurgy and transplantation of the cells does not affect substantially subsequent development of the recipient blastocysts.
Micromanipulation and micrurgical techniques have been applied by various biologists to provide a means for the study of cell differentiation and regulation of gene action in mammalian embryos. Fusing whole zygotes or cleaving embryos is generally believed to have been attempted first in the latter part of the last century (reviewed by Morgan, ’27). Attempts to combine rat zygotes were made by Nicholas and Hall (’42), and one fetus developed that might have originated from two zygotes. Tarkowski (’61) and Mintz (’62) were able to obtain living chimaeric animals by fusing synchronously cleaving mouse embryos. Transfer of somatic nuclei to the unfertilized mammalian ovum and 1- and 2-cell mouse embryos has also been used in an attempt to study differentiation and regulation of gene action in the early embryo (Graham, ’69; Baranska and Koprowski, ’70). However, this latter technique has proved difficult.
Recently, Gardner (’68) successfully transplanted single synchronous embryonic cells into preimplanted mouse blastocysts, and some of these blastocysts developed into chimaeric young. Using autoradiographic techniques, we were able to show that older asynchronous cells as well as synchronous cells were mitotically active and divided after being transplanted into the mouse blastocyst (Moustafa and Brinster, ’71, ’72). This demonstration that asynchronous cells do multiply encouraged us to examine whether they also participated in embryogenesis as they appeared to do in the autoradiographic studies. This report contains the results of experiments in which micromanipulated blastocysts were transferred into foster mothers and provides evidence that the older asynchronous cells do contribute to the structure of the embryo.
MATERIALS AND METHODS
Transplantation of donor cells into the blastocysts
Donor cells obtained from mouse embryos at 4, 5.5, 8 and 12 days after ovulation were transplanted into recipient mouse blastocysts. Recipient blastocysts for the five and one-half-day donor cell transplants were collected three and one-half days after ovulation. Those for other developmental stages of donor cell transplants were collected four days after ovulation. The donor cells were obtained from either albino [Ha(ICR) × Ha(ICR)], true black (C57B1/6J × C57B1/6J) or hybrid black [C57B1/6J × Ha(ICR)] embryos, and the recipient blastocysts were albino or true black embryos.
With the exception of the five and one-half-day donor cells, all donor cell stages and recipient blastocysts were treated and prepared for cell transplantation as previously described (Moustafa and Brinster, ’72). Culture medium used was Brinster’s Medium for Ovum Culture (BMOC-3) (Brinster, ’71) supplemented with 10% fetal calf serum (modified BMOC-3). The five and one-half-day donor cells were obtained by collecting late four and one-half-day blastocysts and culturing them for 20 to 24 hours in a microdrop of culture medium under oil in a humid chamber at 37.5° C under 5% CO2 in air. At the end of the incubation period, the five and one-half-day blastocysts were transferred to a small drop of 0.25% trypsin in calcium-magnesium-free, phosphate-buffered saline (CMF-PBS) on a thin layer of agar gel under oil. After 15 minutes in trypsin the blastocysts were washed four times with CMF-PBS and maintained in the last wash at 37° C until transplantation.
Micrurgical tools and the well were prepared as previously described (Moustafa and Brinster, ’72). With the aid of a Leitz micromanipulator, one to five donor cells (from the inner cell mass of the 4- or 5.5-day donor blastocysts, or randomly picked from the suspended 8- or 12-day embryonic cells) were transplanted into the blastocoele adjacent to or within the inner cell mass of the recipient blastocysts. These blastocysts were removed from the well and placed in a drop of modified BMOC-3 under oil and maintained at 37.5° C under 5% CO2 in air until transfer to a foster mother. Control blastocysts, selected randomly during each experiment, were subjected to all phases of the micrurgical procedures except actual donor cell transplantation.
Transfer of recipient blastocysts into foster mothers
Five to six manipulated recipient blastocysts were transferred into the right uterine horn of pseudopregnant albino females mated three to three and one-half days earlier to vasectomized albino males. The uteri of the foster mothers were examined at 15–17 days of fetal development, or the females were allowed to give natural birth. Occasionally, because of a shortage of foster mothers and/or manipulated recipient blastocysts, four or five manipulated recipient blastocysts were transferred into the right uterine horn, and four or five control blastocysts were simultaneously transferred into the left uterine horn. There is no evidence of transuterine migration in the mouse (Boyd and Hamilton, ’52). The young were removed from each uterine horn by Cesarean section and placed with different lactating mothers that had given birth within the previous 24 hours.
Experimental design
The experiments were divided into two series. The first series dealt specifically with synchronous donor cell transplants and the second with asynchronous cell transplants. Within each of the two series, prenatal and postnatal observations were made. In the prenatal observations (15–17 days of fetal development), emphasis was on determining the percentages of implantation, fetal viability, and ocular pigmentation (chimaerism). In the postnatal observations (performed immediately after birth and continued during subsequent growth), emphasis was on determining the percentages of viable fetuses at delivery, survival of the offspring, sex ratio, and chimaerism as expressed in the eyes, skin and subsequent hair pigmentation. The fertility of surviving offspring was determined by breeding to recessive albino mice. F1 progeny were examined for black and albino color distribution which would reflect chimaerism of the germ cell line in the experimental animals.
RESULTS
Synchronous transplants
In the first series of experiments, cells from the inner cell mass of four-day black donor blastocysts, were transplanted within or near the inner cell mass of synchronous albino recipient blastocysts. The results of observations made at 15–17 days of prenatal development are shown in table 1. Seventy per cent (35/50) of the foster mothers receiving blastocysts with synchronous cell transplants became pregnant. This percentage was similar to the 75% pregnancy rate for foster mothers of the control blastocysts (30/40). Seventy-nine per cent (143/180) of the blastocysts which received synchronous donor cells developed into live fetuses compared to 82% (123/150) of the sham-manipulated blastocysts. These pregnancy rates in both control and synchronous transplant experiments are as high as or higher than those cited in many other reports of blastocyst transfers in the literature (Testart, ’69; McLaren, ’70), suggesting that neither the micrurgy of the recipient blastocysts nor the transplanted donor cells in the blastocysts harms most embryos.
TABLE 1.
Fate of transferred blastocysts at 15–17 days of fetal development
| Experiment | Number of pregnant to total females receiving RB |
Number of blastocysts transferred1 |
Number of fetuses which developed from transferred blastocysts |
Number of fetuses with ocular chimaerism |
||
|---|---|---|---|---|---|---|
| Dead | Alive | Dead | Alive | |||
| Control | 30/40 | 150 | 9 | 123 | — | — |
| Synchronous | ||||||
| 4 days DC | 35/50 | 180 | 24 | 143 | 12 | 20 |
| 4 days RB | ||||||
| Asynchronous | ||||||
| 5.5 days DC | 30/40 | 150 | 20 | 110 | 8 | 12 |
| 3.5 days RB | ||||||
| 8 days DC | 26/40 | 130 | 24 | 93 | 3 | 1 |
| 4 days RB | ||||||
| 12 days DC | 25/38 | 125 | 16 | 88 | 0 | 0 |
| 4 days RB | ||||||
DC designates donor cells, which were all black in these experiments.
RB designates recipient blastocysts, which were all albino in these experiments.
These values do not include blastocysts transferred into females which did not become pregnant.
Of the 143 live fetuses, 20 exhibited varying degrees of ocular chimaerism (fig. 1). In several cases the density of pigmentation was not the same in the two eyes. Ocular pigmentation was also discernible in 12 dead fetuses (table 1). These 32 cases represent 18% (32/180) of the total number of transferred recipient blastocysts. The remaining fetuses lacked ocular pigmentation.
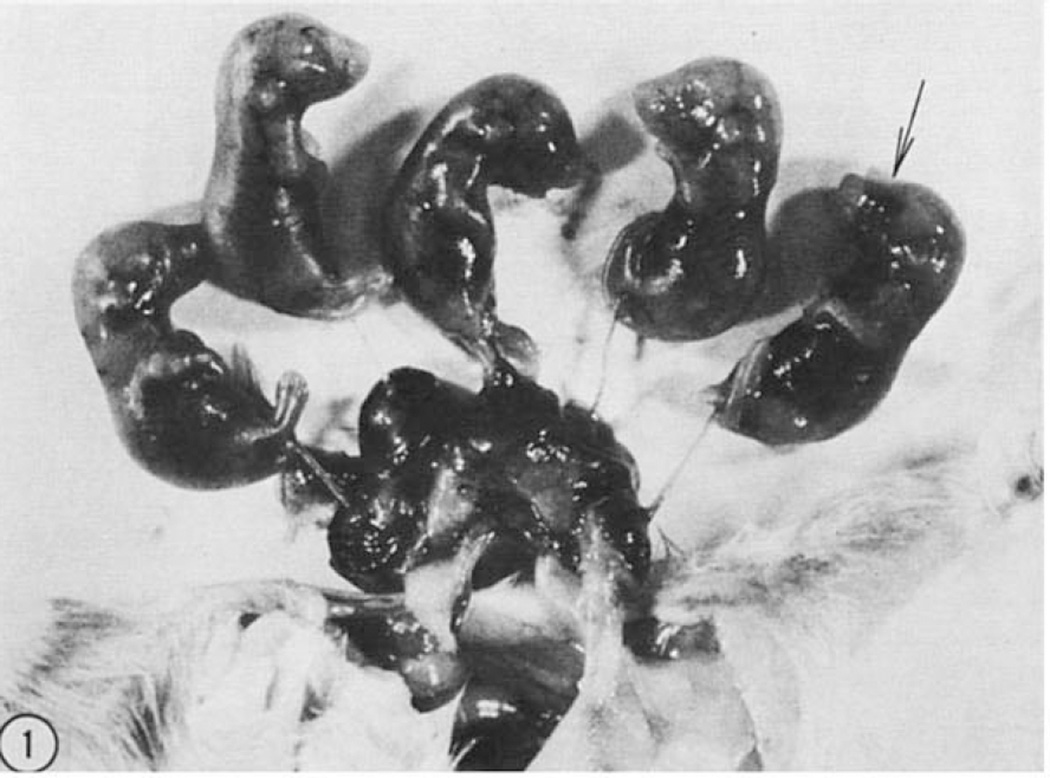
Figure 1. Results of synchronous transplants of four day embryonic cells.
Five fetuses 17 days old that developed from eight albino blastocysts which received synchronous black donor cells. One fetus (arrow) exhibited ocular chimaerism.
Some foster mothers receiving blastocysts containing synchronous donor cells were allowed to give birth and postnatal observations were made on these young. These results are shown in table 2. Eight live, overtly chimaeric neonates were obtained from transplanting black donor cells into albino recipient blastocysts. Of these eight, two animals expressed ocular chimaerism only, one in the coat color only, and the remaining five in both the eye and coat. The density of ocular chimaerism varied. Coat color variegations ranged from distinct large spots, as shown in figure 2, to a mixture of black and white hairs over most of the body as seen in figure 3. Ocular chimaerism was observed in six stillborn. Because of cannibalism, it was not possible to obtain complete records.
TABLE 2.
Fate of transferred blastocysts at term and during postnatal development
| Experiment | Number of RB transferred1 |
Number of young born |
Number of chimaeric animals | |||
|---|---|---|---|---|---|---|
| Overtly chimaeric | Non-overtly chimaeric2 |
|||||
| Dead | Alive | Dead | Alive | |||
| Control | 140 | 10 | 85 | — | — | — |
| Synchronous | ||||||
| 4 day black DC | 100 | 12 | 69 | 6 | 8 | 2 |
| 4 day albino RB | ||||||
| 4 day albino DC | 92 | 15 | 57 | 0 | 4 | 3 |
| 4 day black RB | ||||||
| Asynchronous | ||||||
| 5.5 day black DC | 72 | 18 | 40 | 3 | 6 | 1 |
| 3.5 day albino RB | ||||||
| 5.5 day albino DC | 63 | 16 | 37 | 0 | 3 | 1 |
| 3.5 day black RB | ||||||
| 8 day black DC | 60 | 21 | 28 | 0 | 0 | 0 |
| 4 day albino RB | ||||||
| 8 day albino DC | 72 | 23 | 32 | 0 | 0 | 0 |
| 4 day black RB | ||||||
DC designates donor cells.
RB designates recipient blastocysts.
These values do not include blastocysts transferred into females which did not become pregnant.
These animals resembled recipient blastocysts in phenotype color but were chimaeric in functional germ lines.
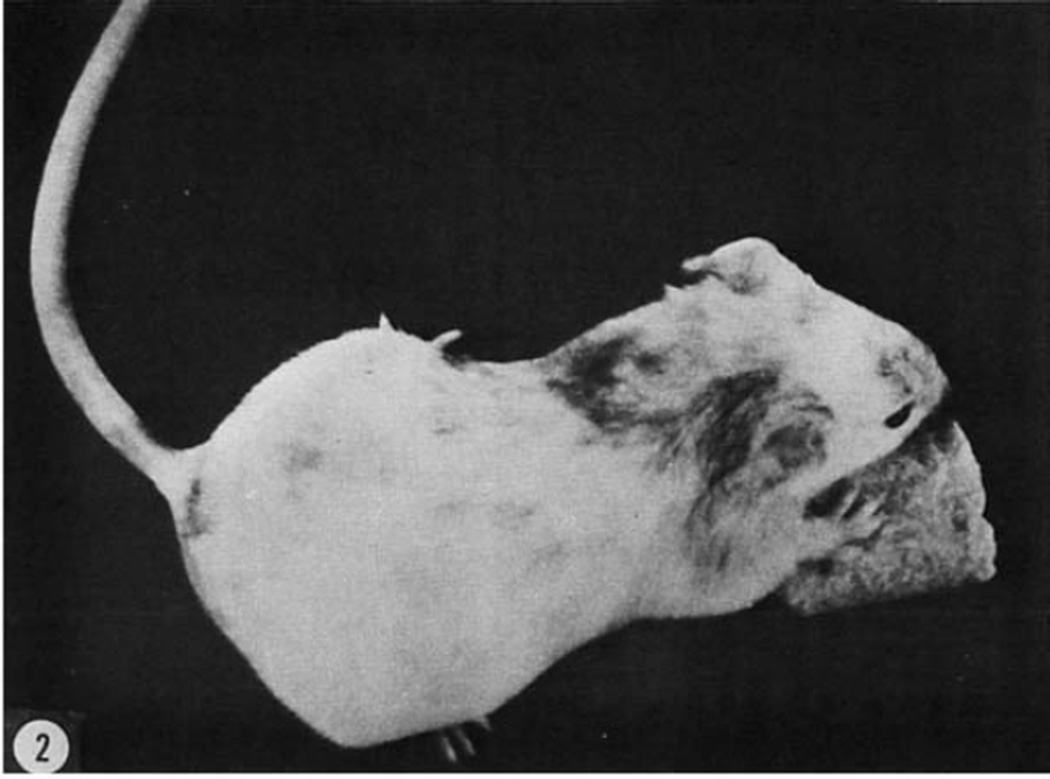
Figure 2. Results of synchronous transplants of four day embryonic cells.
A female at six weeks of postnatal life that developed from an albino blastocyst which received true black donor cells. Note the distinct mottling. The left eye was maroon; the right one was black. The progeny were all albino.
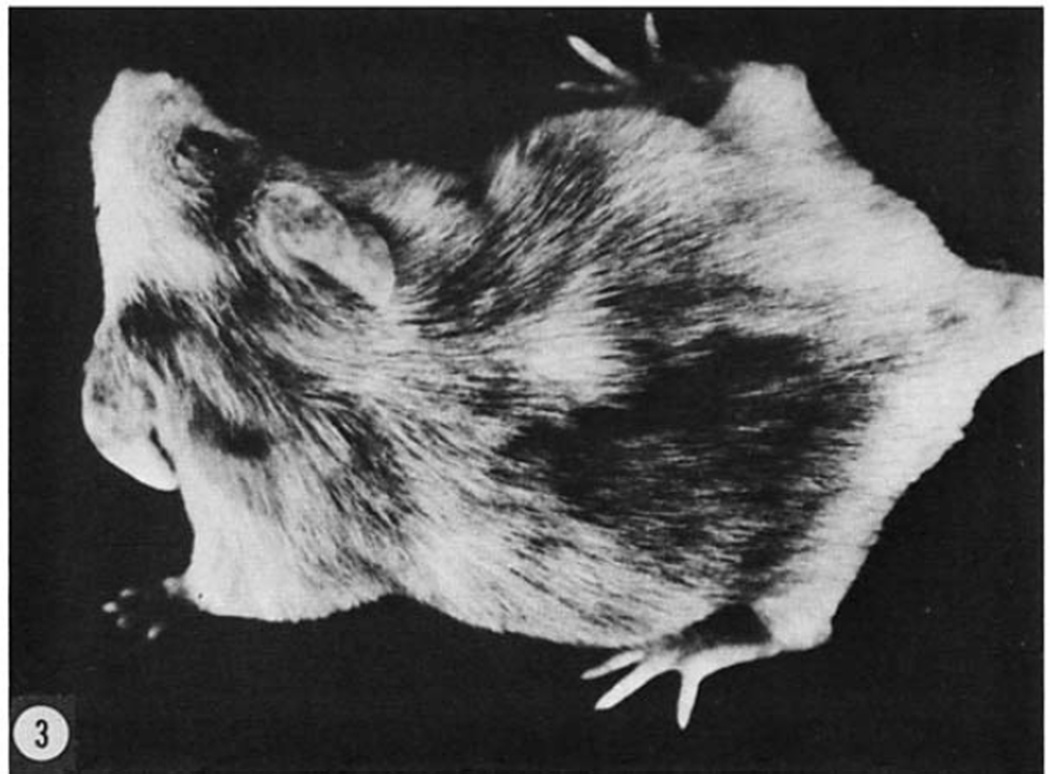
Figure 3. Results of synchronous transplants of four day embryonic cells.
A male at seven weeks of postnatal life that developed from an albino blastocyst which received hybrid black donor cells. Eye pigmentation was dark maroon. This male had both black progeny and albino progeny.
In reciprocal experiments, in which albino donor cells were transplanted into black recipient blastocysts, four overtly chimaeric neonates were obtained. Their coat color variegations were similar to the above group. All four animals had very dark ocular pigmentation; therefore, histological analysis will be needed to determine the possible existence of ocular chimaerism.
The overall sex distribution among the 126 young resulting from blastocysts containing synchronous donor cells was 65 males, 59 females and two intersex. Among the 12 living, overtly chimaeric animals, five were males, five were females, and two were intersex. All chimaeric animals reached maturity (7 to 8 weeks) except one intersex, which died at the age of five weeks.
Asynchronous transplants
In the second series of experiments, asynchronous black donor cells were collected from 5.5-, 8-, and 12-day embryos and transplanted into three and one-half or four day recipient blastocysts (depending upon the experiment). The results at 15–17 days of prenatal development are summarized in table 1. Of the total manipulated recipient blastocysts transferred, the percentages of implanted and subsequently developed fetuses for the 5.5-, 8-, and 12-day asynchronous transplants were 87% (130/150), 90% (117/130), and 83% (104/125) respectively, compared to 93% (167/180) and 88% (132/150) for the synchronous and control groups respectively.
Among the fetuses examined at 15–17 days from the five and one-half day asynchronous transplants, 12 live and 8 dead fetuses exhibited ocular chimaerism. In the eight day asynchronous transplants, one live and three dead fetuses exhibited obvious ocular pigmentation. In the 12-day asynchronous transplants, none of the developed fetuses exhibited any macroscopic ocular chimaerism.
The second group of foster mothers was allowed to deliver, and postnatal observations were made on the young. These results are summarized in table 2. Of the births obtained from the five and one-half day asynchronous transplants, six neonates were overtly chimaeric. Of these, one animal expressed ocular chimaerism only and one in the coat color only. The remaining four animals exhibited distinct spotting in the coat color. Ocular chimaerism varied in density. Ocular chimaerism was obvious in three animals stillborn. In the reciprocal. experiments (albino donor cells transplanted into true black recipient blastocysts), three live, overtly chimaeric males were born.
The young of the eight day asynchronous transplants displayed no overt chimaerism. One factor which may account for the absence of chimaerism in this group is the high rate of cannibalism at birth. The small number of overtly chimaeric young, that might be expected from the observations at 15–17 days, could have been cannibalized, particularly if these young were weaker than their litter mates. None of the blastocysts containing 12-day donor cells produced any overtly chimaeric young.
The overall sex distribution among the 77 living young resulting from five and one-half day asynchronous transplant experiments was 41 males, 35 females and one intersex. However, the sex distribution among the nine live, overtly chimaeric animals was six males, two females, and one intersex. Overall sex ratio in the animals which received eight day asynchronous transplants was 38 males to 22 females.
Fertility and chimaerism of the germ cell line
All 21 overtly chimaeric animals (12 from synchronous and nine from asynchronous cell transplants) were caged with albino mates. Three animals never produced offspring after three months and three changes of mates. Two of these grossly appeared to be true hermaphrodites; the third has not been sacrificed and could also be histologically hermaphroditic. All other animals have produced at least four litters ranging in size from 5 to 19. Of the ten fertile chimaeras from synchronous cell transplants, six produced progeny with color phenotype of both recipient blastocyst and donor cells, three produced progeny with color phenotype of the recipient blastocyst only, and one produced progeny with color phenotype of the donor cells only. Of the eight fertile chimaeras from asynchronous transplants (all developed from 3.5-day blastocysts that had received 5.5-day donor cells), five produced progeny with color phenotype of both recipient blastocyst and donor cells, three produced progeny with color phenotype of the recipient blastocyst only, and none produced young with the color phenotype of the donor cells only.
Two hundred and ten non-overtly chimaeric animals produced from blastocysts that received donor cells reached maturity. These all resembled the recipient blastocysts in color and were caged with albino mates. Five of these pairs have not produced offspring after three months and three changes of mates. None shows gross morphological signs of hermaphroditism, but they have not yet been sacrificed for histological observations. All other animals produced at least four litters. Of the 110 non-overtly chimaeric fertile animals that developed from synchronous cell transplants and reached maturity, five produced progeny with color phenotype of both recipient blastocyst and donor cells. The rest produced progeny with color phenotype of the blastocyst only. None produced young with color phenotype of the donor cells only. Of the 58 non-overtly chimaeric fertile animals that developed from asynchronous transplants of five and one-half day donor cells into three and one-half day blastocysts, two produced progeny with color phenotype of both recipient blastocyst and donor cells, and the rest produced progeny with color phenotype of the blastocysts only. Of the 37 non-overtly chimaeric fertile animals that developed from asynchronous transplants of 8- and 12-day donor cells into recipient blastocysts and reached maturity, none produced young with color different from that of the blastocyst phenotype. Fertility and survival in these last pairs seem to be low, and litter size was below average.
DISCUSSION
The percentage of blastocysts which implanted and developed in these experiments, compares favorably with Bowman and McLaren (’70) who found that 50% of their transferred control blastocysts developed into viable fetuses. Mintz (’67) reported that 45% of her fused blastocysts survived at least until birth, while Tarkowski (’61), and Mystkowska and Tarkowski (’68) reported respectively 25% and 7% successful development from fused embryos. Gardner (’68) reported 50% recovery at mid-term and 22% viable births from blastocysts that had been injected with synchronous cells. However, none of these reports give any control data on untreated blastocysts, and the percentages are below those found in the experiments reported here. Our findings would suggest that the micrurgy of the blastocysts did not decrease significantly the embryo’s chance for survival. In fact, even the injection of the donor cells into the blastocyst did not appreciably affect subsequent development of the blastocyst. These findings suggest that the early embryo is quite adaptable to such physiological stress. Thus, the technique of blastocyst micrurgy and cell transplant appears to be a feasible experimental technique.
The results of experiments in which synchronous cells were transplanted into blastocysts confirm and extend the findings of Gardner (’68) and our previous autoradiographic studies (Moustafa and Brinster, ’72). In Gardner’s experiments the donor cells were shown to participate in skin, hair, and retinal pigmentation. This was confirmed in our experiments, and we further showed that the donor cells participate in the formation of the embryo’s germ cell line.
The sex ratio of 1:l in the offspring from the synchronous cell transplants and from the two day asynchronous cell transplants agrees with the results of whole eight cell embryo fusion by Mintz (’68). However, the sex ratio of 38/22 (male/female) in the animals which received four day asynchronous cell transplants was significantly different from 1:l and the litter size was significantly smaller than in other groups. The reduction in litter size appeared to be at the expense of the females. In this group of offspring the sex ratio was thus similar to the results obtained from whole embryo fusion by Tarkowski (’64), Mystkowska and Tarkowski (’68), and McLaren and Bowman (’69). These authors explain the excess of males on the basis of Y-chromosome domination, in which case the XX/XY chimaeric embryos become males. Mullen and Whitten (’71) have recently suggested that the sex ratio varies depending on the strains of mice used in studies of whole embryo fusion. This may explain the different ratios found in whole embryo fusion experiments by different workers. However, our studies suggest an effect of the donor cells on sex ratio that is dependent on the age of the donor cell. Perhaps older XY donor cells dominate the XX cells of potentially female blastocysts, but older XX donor cells do not dominate XY cells of potentially male blastocysts. If there are small differences in the time of maturation of the primordial germ cells among different strains, this could explain the findings of Mullen and Whitten (’71). Very few cells must be involved in this early determination of sex since we transplanted only one to five cells into each blastocyst. From her work with whole embryo fusion Mintz (’68) also feels that only a few embryonic cells are involved in sex determination.
The appearance of the intersexes also indicates that donor cells opposite in sex from that of the blastocyst cooperated in the organogenesis of some embryos. It appears that the donor cells may participate in the formation of a variety of tissues, and studies are now underway with chromosome markers to determine the extent to which the donor cells participate in the formation of different tissues, It also seems possible that both the origin and age of donor cells could determine the potentiality of the tissues to participate in organogenesis.
Our previous autoradiographic findings suggested that asynchronous cells transplanted into blastocysts will divide and participate in embryogenesis. The results reported here definitely show that the 48-hour asynchronous cells participate in embryo formation; the 96-hour asynchronous cells participate to a lesser extent. The 192-hour asynchronous cells have not yet been shown to contribute to the structure of the embryo. As the asynchrony increases, it appears as though the ability of the donor cell to influence embryo development and structure decreases. This seems a logical occurrence since many cells from the older embryos may not be able to dedifferentiate. It also seems logical or at least quite probable that different types of cells may vary in their ability to dedifferentiate. Thus, cells from different areas of the late embryo or adult may differ in their ability to influence blastocyst development and clone the embryo. The studies with the asynchronous cells suggest it is possible to begin testing the potential of different early embryonic cell lines for their ability to dedifferentiate and clone the blastocyst.
Acknowledgments
This investigation was supported by PHS Training grant 00239 and PHS Research grant 03071 from the National Institute of Child Health and Human Development.
LITERATURE CITED
- Baranska W, Koprowski H. Fusion of unfertilized eggs with somatic cells. J. Exp. Zool. 1970;174:1–14. doi: 10.1002/jez.1401740102. [DOI] [PubMed] [Google Scholar]
- Bowman P, McLaren A. Viability and growth of mouse embryos after in vitro culture and fusion. J. Embryol. exp. Morph. 1970;23:693–704. [PubMed] [Google Scholar]
- Boyd JD, Hamilton WJ. Cleavage, early development and implantation of the egg. In: Parkes AS, editor. Marshall’s Physiology of Reproduction. II. Little, Brown and Co.; 1952. pp. 44–49. [Google Scholar]
- Brinster RL. In vitro culture of the embryo. In: Sherman AI, editor. Pathways to Conception. Springfield: Charles C Thomas; 1971. pp. 245–277. [Google Scholar]
- Gardner RL. Mouse chimaeras obtained by the injection of cells into the blastocyst. Nature. 1968;220:596–597. doi: 10.1038/220596a0. [DOI] [PubMed] [Google Scholar]
- Graham CF. The fusion of cells with one- and two-cell mouse embryos. In: Defendi V, editor. The Wistar Institute Symposium Monograph No. 9, Heterospecific Genome Interaction. The Wistar Institute Press; 1969. pp. 19–35. [PubMed] [Google Scholar]
- McLaren A. The fate of very small litters produced by egg transfer in mice. J. Endocrinol. 1970;47:87–94. doi: 10.1677/joe.0.0470087. [DOI] [PubMed] [Google Scholar]
- McLaren A, Bowman P. Mouse chimaeras derived from fusion of embryos differing by nine genetic factors. Nature. 1969;224:238–240. doi: 10.1038/224238a0. [DOI] [PubMed] [Google Scholar]
- Mintz B. Formation of genotypically mosaic mouse embryos. Amer. Zool. 1962;2:432. [Google Scholar]
- Mintz B. Gene control of mammalian pigmentary differentiation. I. Clonal origin of melanocytes. Proc. Natl. Acad. Sci., U.S.A. 1967;58:344–351. doi: 10.1073/pnas.58.1.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B. Hermaphroditism, sex chromosomal mosaicism and germ cell selection in allophenic mice. Eighth Biennial Symposium on Animal Reproduction. J. Anim. Sci. 1968;27(Suppl.1):51–60. [PubMed] [Google Scholar]
- Morgan TH. Experimental Embryology. Columbia University Press; 1927. p. 766. [Google Scholar]
- Moustafa LA, Brinster RL. Isoplastic cell transplant into mouse blastocysts. The Fortieth Annual Meeting of the Genetics Society of America, Rochester, N. Y., August, 1971. Genetics. 1971a;68(Suppl):45. [Google Scholar]
- Moustafa LA, Brinster RL. The fate of transplanted cells in mouse blastocysts in vitro. J. Exp. Zool. 1972;181:181–192. doi: 10.1002/jez.1401810205. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Whitten WK. Sex ratios of chimaeric mice. Society for the Study of Reproduction, Fourth Annual Meeting and Symposium on Immunoreproduction; June, 1971; Boston, Mass. 1971. [Google Scholar]
- Mystkowska ET, Tarkowski AK. Observations on CBA-p/CBA-T6T6 mouse chimaeras. J. Embryol. exp. Morph. 1968;20:33–52. [PubMed] [Google Scholar]
- Nicholas JS, Hall BV. Experiments on developing rats. II. The development of isolated blastomeres and fused eggs. J. Exp. Zool. 1942;90:441–459. [Google Scholar]
- Tarkowski AK. Mouse chimaeras developed from fused eggs. Nature. 1961;190:857–860. doi: 10.1038/190857a0. [DOI] [PubMed] [Google Scholar]
- Tarkowski AK. True hermaphroditism in chimaeric mice. J. Embryol. exp. Morph. 1964;12:735–757. [PubMed] [Google Scholar]
- Testart J. Comparison de differentes techniques de transplantation des blastocystes chez la lapine. Ann. Biol. anim. Bioch. Biophys. 1969;9:351–360. [Google Scholar]