Abstract
Background
Bph3, a major brown planthopper (BPH) resistance locus derived from the rice cultivar Rathu Heenati (RH), has been used as a stable donor of traits that improve highly susceptible aromatic rice varieties in Thailand. Map-based cloning was initiated using a set of isogenic lines (ILs) harboring the major Bph3 locus on chromosome 6. IL genomes were scanned with a 57 K Affymetrix Rice GeneChip to identify the gene responsible for Bph3.
Findings
Single-feature polymorphism (SFP) mapping was used to localize 84 candidate genes. An expression analysis of 15 selected candidate genes in the aromatic rice cultivar KDML105 (KD) and the ILs under normal conditions revealed two differentially expressed sequences. Following hopper feeding, only one candidate gene, Os04g27430, was differentially expressed. Os04g27430 encodes a putative sesquiterpene synthase (STPS) gene that was induced by BPH feeding in ILs. An antixenosis test in three selected ILs revealed a major role for STPS in insect preference during the first 120 hours of the rice-insect interaction. Functional SNPs in exon 5 that resulted in the deletion of seven amino acids in the susceptible rice line were identified. Moreover, three additional SNPs associated with three transcription binding sites were also identified, which might explain the differential response of Os04g27430 during the anti-feeding test.
Conclusion
Os04g27430 is the second known rice STPS induced by BPH. The gene may involve an antixenosis BPH resistance mechanism. The combination of the STPS and the Bph3 locus was more effective than Bph3 alone in the tested ILs.
Electronic supplementary material
The online version of this article (doi:10.1186/1939-8433-6-18) contains supplementary material, which is available to authorized users.
Keywords: Brown planthopper, Single-feature polymorphism (SFP), Sesquiterpene synthase (STPS), Antixenosis on feeding preference (AFP)
Findings
Microarray-based genome mapping identification of additional genes correlated with brown planthopper (BPH) resistance in rice
The stability of brown planthopper (BPH) resistance in Rathu Heenati (RH), a traditional Sri Lankan rice cultivar containing BPH 3, has made this strain one of the most popular hopper resistance donors in the Mekong subregion, where rice production is highly intensive. The Thai jasmine rice KDML105 (KD) is one of the most sensitive cultivars to BPH, and its BPH resistance has recently been improved by backcross introgression of the critical Bph3 region linked to RM589 on chromosome 6 from RH (Jairin et al. 2009). BC3F5 isogenic lines (ILs), differing primarily in the introgressed region, were developed. The Affymetrix Rice GeneChip array was used to scrutinize the critical map region on chromosome 6 to simplify the map-based cloning of BPH 3. The pool of genomic DNA from four ILs with commonly inherited BPH resistance from RH – UBN3078-101-342-4-162 (IL162), UBN3078-101-342-4-283 (IL283), UBN3078-101-342-6-302 (IL302), and UBN3078-101-432-6-308 (IL308) – was used for single-feature polymorphism (SFP) mapping comparing the genomic DNA of KD. SFP mapping was performed following the protocol developed by Kumar et al. 2007. No significant variation between samples or replicates was observed. A statistical analysis of the microarrays was performed, and SFPs were predicted by determining the hybridization differences at each perfect match (PM) probe in the array (Thongjuea et al. 2009). At a false-discovery rate of 30%, 157 PM probes were selected for further investigation (Additional file 1). Only 99 SFPs had unique locations in the rice nuclear genome; the remaining 58 SFPs were located in multiple locations, no locations, or in organellar genomes. The 99 SFPs were located on 84 annotated genes throughout the rice genome. Five SFPs were located on chromosome 6 and were all outside the critically mapped location (Figure 1A) (Jairin et al. 2007). This observation was consistent with the expression analysis of BPH-infested RH conducted by another research group that did not identify any gene in the Bph3 candidate region (Wang et al. 2012). By contrast, half of the SFPs were located on chromosome 4 (Additional file 2: Table S1), where another BPH resistance gene from RH (Bph17) as well as other BPH resistance genes (Bph12(t), Bph15, Bph20(t), Qbph2, and Qbph4) have been previously mapped (Sun et al. 2005 Yang et al 2002,2004 Rahman et al. 2009 Huang et al. 2001 Liu et al. 2009).
Figure 1.
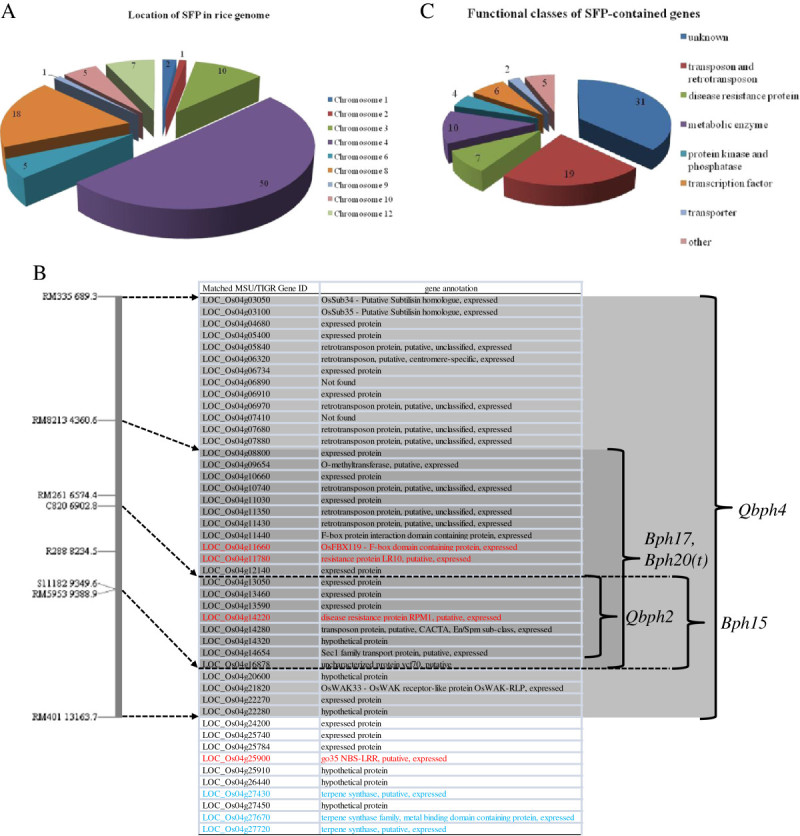
Classification of SFP and SFP-containing genes. (A) Pie chart depicting the chromosomal location of 99 predicted unique SFPs. (B) SFP-containing genes located on chromosome 4 and the location of BPH resistance genes mapped to chromosome 4. The vertical bar represents the map location on chromosome 4. RM markers and their physical location on the pseudomolecule are shown at the left. The SFP-containing genes located in the Qbph2, Qbph4, Bph15, and Bph17 regions are highlighted. The three SFP-containing TPS genes are labeled in blue. The SFP-containing resistance related genes are labeled in red. (C) Pie chart showing the functional classification of 84 SFP-containing genes. Numeric characters in the pie charts indicate the number of SFPs or genes located or classified in that chromosome/class.
Qbph4, Bph17, and Bph20(t) were mapped to the intervals RM335 – RM401 and RM8213 – RM5953 and with the linked marker RM5953, respectively. The Qbph4 region encompassed a position from 689,354 to 13,163,724 bp, and Bph17 encompassed 4,360,621 to 9,388,937 bp on pseudomolecule 4 (Os-Nipponbare-Reference-IRGSP-1.0). A total of 36 SFP-containing genes (Os04g03050 – Os04g22280) were located in the Qbph4 region (Figure 1B). Nineteen of these genes (Os04g08800 – Os04g16878) were also specifically associated with the Bph17 region. The Qbph2 and Bph15 genes were mapped in the same region by the RFLP markers C820-R288 and C820-S11182. The region located between 6,902,846 and 9,349,627 bp on pseudomolecule 4 contained eight SFP-containing genes (Os04g13050 – Os04g16878). The linked marker of Bph12(t), RM261, was adjacent to Os04g11780, the resistance protein LR10, with a physical distance of 130.5 kb. Moreover, another NBS-LRR resistance-related protein, Os04g25900, also contained an SFP.
Compared to the expression analysis of BPH-infested RH studied by Wang et al. (2012), three BPH resistance-related genes, two putative resistance proteins, Os04g11780 (LR10) and Os04g14220 (RPM1), and an F-box-containing protein, Os04g11660, were found to contain SFP (Figure 1B and Additional file 2: Table S1) in the present study. In contrast, no candidate BPH resistance gene on chromosome 3, 6, and 10 identified in the study by Wang et al. (2012) was found to contain an SFP in our study. This difference may be due to the different BPH-susceptible rice cultivars, KD and Taichung native 1 (TN1), used for the RH comparison in the two studies.
The SFP-containing genes were classified into various functional groups, as shown in Figure 1C. The largest group contained genes with unknown functions such as expressed proteins, hypothetical proteins, and uncharacterized proteins (Additional file 3: Table S2). Transposons and retrotransposons formed the second largest group. The most significant finding was the identification of 10 genes that encode metabolic enzymes in the third most abundant group, which included three genes encoding terpene synthases (TPS). These enzymes are involved in the biosynthesis of secondary metabolites known as terpenoids, a large group of volatile compounds involved in defense mechanisms against plant herbivores (Schnee et al. 2006 Yuan et al. 2008). The fourth most abundant group included seven R gene-like sequences on chromosomes 3, 4, 8, and 10. These findings suggest that several minor quantitative trait loci (QTLs) may strengthen BPH 3 in terms of stable BPH resistance in RH and ILs. The last three groups contained genes involved in protein phosphorylation processes, transcription factors, and transporters.
Expression analysis of SFP-containing genes
A total of 87 predicted SFPs were validated by comparing the hybridization intensity of each probe with the results of sequence comparisons and PCR amplifications (Additional file 4). A total of 15 genes were chosen from 24 validated SFP-containing genes in which an SNP or a small insertion/deletion between KD and RH was identified (Additional file 5: Table S3). Reverse transcription polymerase chain reaction (RT-PCR) expression analyses and the functional ontologies of 15 pre-candidate genes are shown (Figure 2A). The Os08g31970 and Os04g27430 genes clearly exhibited differential expression under normal conditions, with no expression in the susceptible KD jasmine rice. In contrast, several pre-candidate genes were differentially expressed in the susceptible parent; however, the remaining genes exhibited constitutive expression. The differential expression of Os08g31970 and Os04g27430, an NHL repeat-containing protein that plays a role in signal transduction and a TPS responsible for the biosynthesis of volatile compounds, respectively, was further verified. Os04g27670 and Os04g27720 (two SFP-containing TPS genes, Figure 1B) were also selected for a total of four genes that were evaluated in a two-day BPH feeding test using two-week-old seedlings and the Ubon Ratchathani biotype of the BPH population (UBN-BPH) (Jairin et al. 2009). Interestingly, only Os04g27430 exhibited differential expression between the control and the BPH feeding condition in IL162; however, no change in expression levels was observed for Os08g31970 in IL162 (Figure 2B). No expression was detected for the remaining two TPS genes in the rice plants, perhaps because the genes were not functional during the seedling stage or because they may be pseudogenes. We investigated the role of Os04g27430 in response to BPH attack in greater detail.
Figure 2.
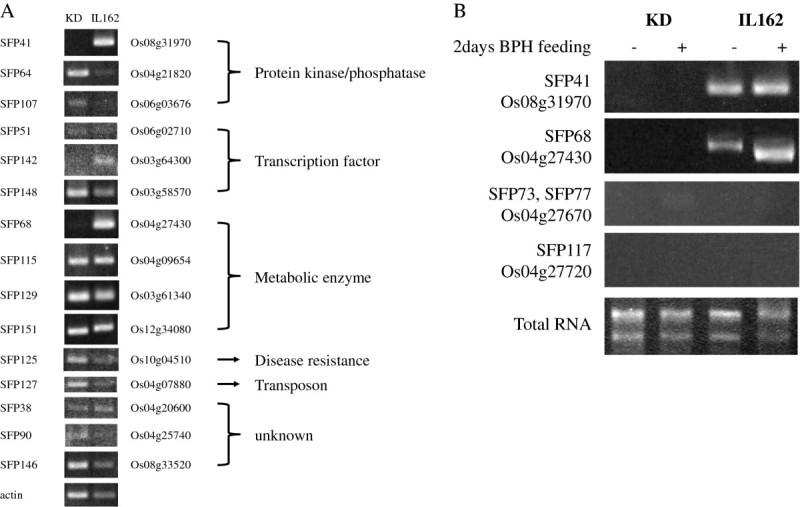
Expression analysis of SFP-containing genes by RT-PCR. (A) RT-PCR analysis of rice lines KD and IL162. SFP and LOC numbers are from (Additional file 2: Table S1). Actin was used as a positive control. The functional classes of these genes refer to the functional classes in (Additional file 3: Table S2). (B) Expression analysis of rice plants following two days of BPH infestation (+) and control plants (−). The total RNA in each lane is represented by the amount of RNA used in the reaction.
Genomic characterization of Os04g27430
The genomic region of Os04g27430 in RH, KD, and IL162 was sequenced and compared (accession nos. KC511049 – KC511051), and major polymorphisms were identified (Figure 3). Three indels were found to be located in intron 1 (645 bp) and intron 3 (679 and 32 bp, respectively). In the coding sequence, an in-frame 6-bp deletion was present in exon 2 in RH and IL162. More significantly, a 2-bp SNP was present in KD exon 5 with a strong correlation to a 21-bp deletion in its cDNA. Comparisons between the genomic DNA, cDNA, and predicted amino acid sequences (accession nos. KC511027 – KC511029) revealed a 21-bp deletion in exon 5 of the KD cDNA allele that resulted in the deletion of seven residues from the amino acid sequence in KD. The deduced amino acid sequences of the KD and RH alleles were translated, yielding protein sequences containing 500 and 505 residues, respectively. The conserved DDXXD motif that functions as a substrate binding site was present in both the KD and RH alleles (Figure 4A). The seven-amino-acid deletion was WxHQxxx, a signature motif in several plant TPS genes (Figure 4B). Based on the presence of this important deletion in KD, Os04g27430 mRNA may be subjected to post-transcriptional degradation, and the absence of this mRNA may responsible for the BPH susceptibility observed in the KD parent.
Figure 3.
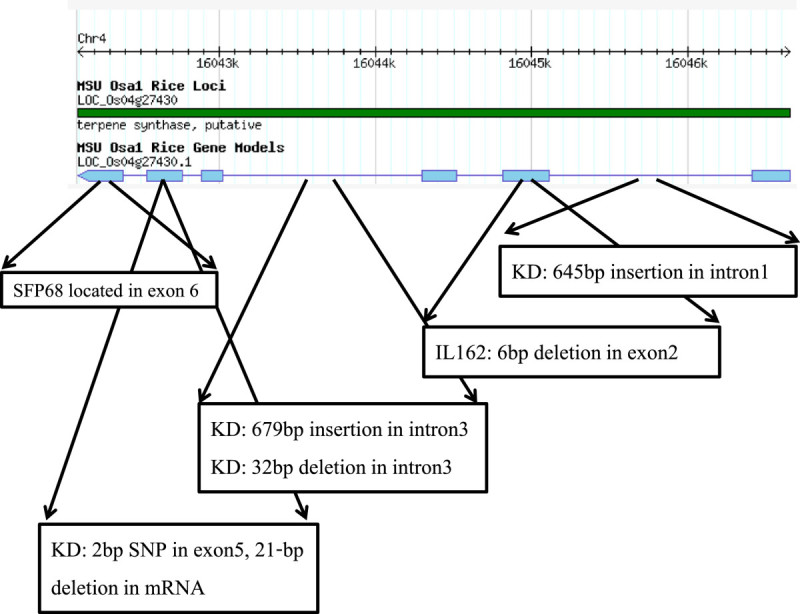
Gene construct of Os04g27430 from the Rice Genome Annotation Database (http://rice.plantbiology.msu.edu). Major polymorphisms between the KD and RH alleles (SNP or insertion/ deletion) are marked in horizontal boxes below the construct.
Figure 4.
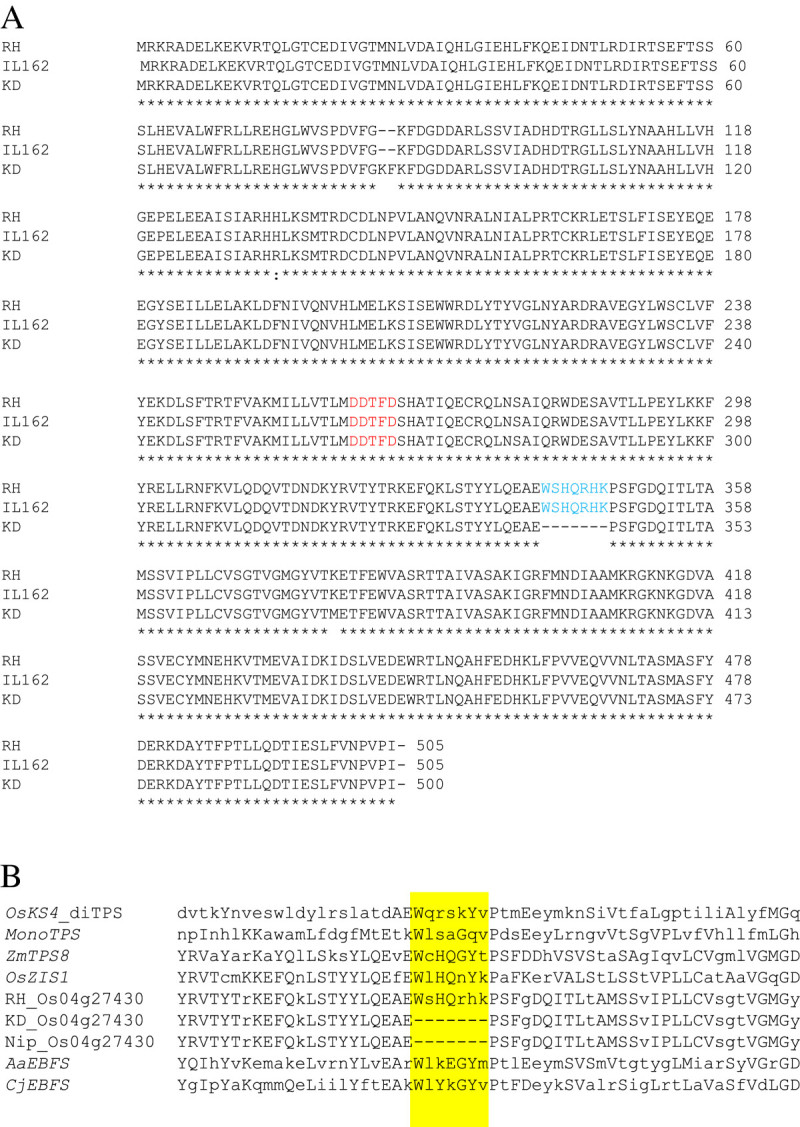
Amino acid sequence analysis. (A) Amino acid sequence alignment of the KD, RH, and IL162 alleles of Os04g27430. The conserved DDXXD domain found in the plant TPS genes is labeled in red, and the seven-amino-acid deletion in the KD allele is labeled in blue. (B) Consensus sequence detected in Os04g27430 and the TPS genes of rice and other plant species. OsKS4 and monoTPS are rice TPS s, ZmTPS8 is a terpene synthase from Zea mays (NP_001105912), OsZIS1 is a rice putative zingiberene synthase I (ACM41835), AaEBFS is the (E)-β-farnesene synthase from Artemisia annua (AAX39387), and CjEBFS is the (E)-β-farnesene synthase from Citrus junos (AAK54279).
The 5′ upstream region of Os04g27430 in RH, KD, and IL162 was sequenced (accession nos. KC511031, KC527594, KC511035), and searches for transcription factor (TF) binding sites were performed (http://www.cbrc.jp/research/db/TFSEARCH.html). Three consensus elements for the transcription factors ATHB-1 (Arabidopsis thaliana homeobox protein 1), SBF-1 (silencer-binding factor 1), and P (maize activator P) were identified in this promoter region. Interestingly, the KD allele contained one SNP in each element. These SNPs led to the non-recognition of the ATHB-1 element (score = 0) and a decreased TF search score for the SBF-1 and P elements (scores = 92.5 and 86.5) (Figure 5). These three SNPs, particularly the SNP in the ATHB-1 element, may be responsible for the low expression levels of this gene in KD because the transcription factor cannot bind at the target site to enhance gene expression. Therefore, a sequence comparison of other BPH-resistant rice varieties is needed to further explore the understanding of how this gene is controlled.
Figure 5.

Genomic sequence alignment of the 5′ region of Os04g27430. The 3 TF elements are marked in red, and the SNPs of the KD allele are marked in blue. The TF search score for each allele is indicated at the far right of the alignment.
The amino acid sequences of 10 rice TPS s (Cheng et al. 2007 Prisic et al. 2004 Sakamoto et al. 2004 Xu et al. 2004 Yuan et al. 2008) and TPS s from other plant species were included in the phylogenetic analysis of the RH allele of Os04g27430, which revealed that Os04g27430 clustered with the sesquiterpene synthase (STPS) group (Figure 6). Os04g27430 was most similar to OsZIS1, OsZIS2, and OsTPS13. OsZIS1 and 2 are putative zingiberene synthase genes whose function has not been confirmed experimentally.
Figure 6.
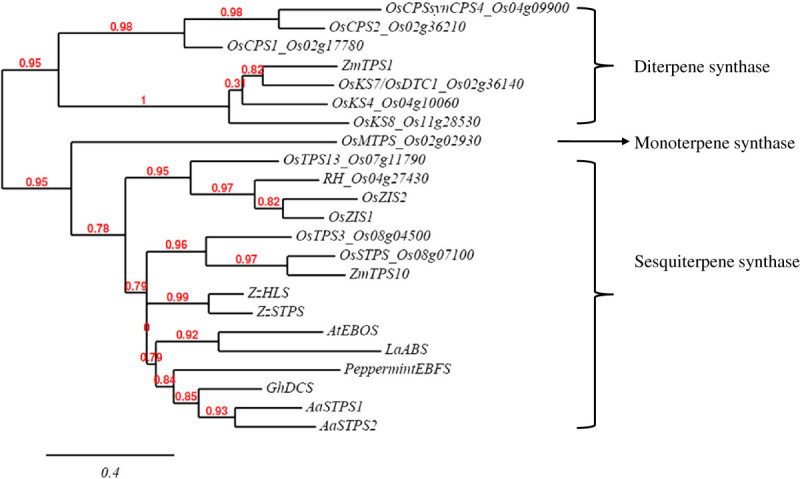
Phylogenetic analysis based on the degree of sequence similarity between Os04g27430 and other rice TPS genes. Rice gene identities are based on the gene name in the reference and the rice genome database; the prefix ‘LOC_’ is omitted. ZmTPS1 and ZmTPS10 are TPS from Zea mays (AAO18435, AAX99146), AaSTPS1 and 2 are sesquiterpene cyclases from Artemisia annua (CAC12732, AAG24640), OsZIS1 and 2 are rice putative zingiberene synthases (ACM41835, ACM41834), ZzAHS and ZzSTPS are α-humulene synthase and sesquiterpene synthase 4 from Zingiber zerumbet (BAG12020, BAG50434), PeppermintEBFS is (E)-β-farnesene synthase from peppermint tree (Mentha x piperita) (AAB95209), GhDCS is a δ-cadinene synthase from Gossypium hirsutum (AF270425), AtEBOS is (E)-β-ocimene synthase from Arabidopsis thaliana (NP_567511.3), and LaABS is α-bergamotene synthase from Lavandula angustifolia (ABB73046).
OsTPS13 is an STPS that catalyzes the formation of the sesquiterpene alcohol (E, E) farnesol (Cheng et al. 2007). The gene was identified from methyl jasmonate (MeJA)-treated rice seedlings. However, this gene was constitutively expressed in two-week-old KD and IL308 seedlings under both control and BPH feeding conditions for 1, 2, 3, 4, and 8 days (Figure 7A). In addition to BPH feeding, 24 and 72 hr of MeJA and wound stress also induced Os04g27430 expression (Figure 7B). This discovery suggests that OsTPS13 is not a BPH feeding-inducible STPS and that Os04g27430 expression is induced by both BPH feeding and other stresses, such as MeJA and wounding.
Figure 7.

Expression analysis of STPS genes. (A) The expression of three STPS s in KD and IL308 under control (3DC and 8DC) and BPH feeding (1D, 2D, 3D, 4D, and 8D) conditions; D = day(s). Ubiquitin and total RNA were used as positive controls for RT-PCR. (B) Expression of Os04g27430 in response to stress conditions by RT-PCR analysis. Actin was used as a positive control. Two-week-old RH seedlings were subjected to several stresses. For SA and MeJA, the seedlings were sprayed with 1 mM and 250 μM salicylic acid and methyl jasmonate, respectively; for the salt treatment, the seedlings were transplanted into 150 mM NaCl solution; and for wounding, the seedlings were cut into small pieces in distilled water.
Another STPS gene (Os08g07100) that is reportedly induced by BPH (Cho et al. 2005) was not polymorphic between KD and IL308 at the expression level in the present study (Figure 7A). This gene was induced by BPH feeding in both rice strains. Moreover, the gene was induced by BPH feeding and by the fall army worm (Yuan et al. 2008), suggesting that the gene plays a common role in the response to herbivore attacks on rice plants.
Os04g27430 is likely the STPS that functions as zingiberene synthase, which catalyzes the formation of a number of sesquiterpene products (Iijima et al. 2004). Sesquiterpene volatile compounds are the potential products of this gene and may play a role in BPH resistance mechanisms in RH and ILs.
Allelic variation in TPS genes leading to a volatile compound composition difference has been reported in maize (Köllner et al. 2004). In rice, several rice varieties have been shown to release different volatile blends (Lou et al. 2006); however, the gene(s) that controls these differences has not yet been identified. In our preliminary study, a total of 25 sesquiterpenes were identified by GC-MS in the mixture of volatile compounds emitted by KD and RH rice plants infested by BPH. The major sesquiterpene product that KD emitted at a significantly lower level than RH is E-β-farnesene (data not shown), the common constituent of the aphid alarm pheromone (Bowers et al. 1972 Edwards et al. 1973 Pickett and Griffiths. 1980) and the aphid repellent of wild potato (Gibson and Pickett. 1983). This variation may be due to a defect in the KD allele of Os04g27430 at both the genomic and the protein sequence levels.
Os04g27430 may play a more important role than Bph3 in the antixenosis mechanism
Three ILs were selected to elucidate the epistasis of Os04g27430 and the BPH 3 major gene within the BPH resistance mechanism. UBN03078-101-342-4-7(IL7), UBN03078-101-342-4-72 (IL72), and UBN03078-101-342-6-82 (IL82) contain a Bph3 critical region, Os04g27430, and both, respectively. The antixenosis of the BPH feeding preference (AFP) was compared between each IL with KD. Within the first 8 hr, UNB-BPHs randomly landed on each tested plant and consistently moved to KD. At 120 hr, the majority of the UBN-BPH population (> 80%) had settled on KD instead of the IL plants (Figure 8A-8C). This result as well as an AFP test between IL7 and IL72 suggest that either Bph3 or Os04g27430 is sufficient to confer protection against BPH landing within the first 120 hr of attack (Figure 8D). In a comparison between IL82, which contains both Bph3 and Os04g27430, with IL7 and IL72 (Figure 8E-8F), UBN-BPH was found to prefer IL7 over both IL72 and IL82. This result suggests that Os04g27430 is more important than Bph3 in determining BPH landing preference.
Figure 8.
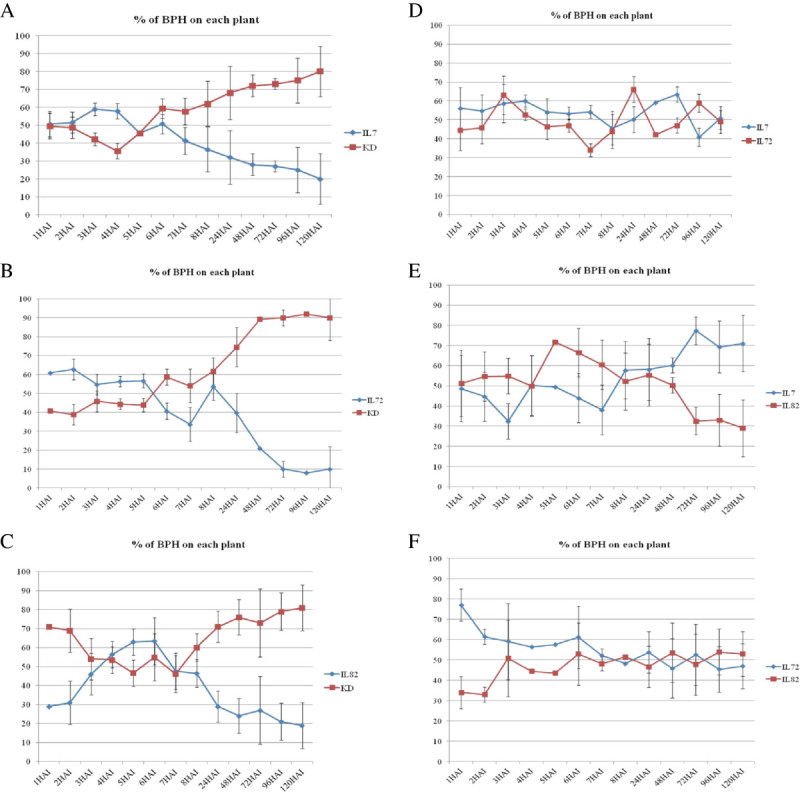
Antixenosis of BPH feeding preference (AFP) test. (A)-(C) show comparisons between KD and the ILs; (D)-(F) provide comparison between the ILs. A total of 10 second- and third-instar BPH nymphs were allowed to settle on the experimental plants, and eight replicates were performed for each comparison. The y-axis indicates the number of BPH that settled on each plant as a percentage, and the x-axis shows the progress between 1 and 120 hr after BPH infestation.
This study clearly demonstrates that the Bph3 critical region and at least 84 other genes have been transferred to ILs. This unexpected finding may be a consequence of the phenotypic selection process used in the breeding program before the implementation of marker-assisted selection. These genes may play a complementary role to BPH 3 in the BPH resistance mechanisms of IL rice plants. In this study, Os04g27430 was identified as a BPH feeding-inducible STPS that may be involved in the BPH resistance mechanism of RH. This gene may contribute in the antixenosis mechanism by interfering with BPH settling.
Electronic supplementary material
Additional file 1:Data 1A: Predicted SFP called at different threshold (delta) between KDML105 and the pool of 4 introgression lines. Data 1B: 157 predicted SFP choose in this study. (NULL 13 KB)
Additional file 2: Table S1: SFP-containing genes. (XLS 143 KB)
Additional file 3: Table S2: Functional classification of SFP-containing genes. (XLS 140 KB)
Additional file 4: SFP validation by comparison of hybridization fold change and polymorphism detected in the probe region between KD and RH. (XLS 30 KB)
Additional file 5: Table S3:Sequence polymorphisms between KD and RH in predicted SFP. (XLS 32 KB)
Below are the links to the authors’ original submitted files for images.
Acknowledgments
This work was supported by the National Center for Genetic Engineering and Biotechnology (BIOTEC) (Grant No. P0010270) and the Agriculture Research Development Agency (ARDA) (Grant No P12/2552). WK thanks the Ministry of Science and Technology, Thailand, for financially supporting his Ph.D. studies.
Abbreviations
- BPH
Brown planthopper
- IL
Isogenic line
- KDML105
KD, Khao Dowk Mali 105
- RH
Rathu Heenati
- STPS
Sesquiterpene synthase
- SFP
Single feature polymorphism
- SNP
Single nucleotide polymorphism
- TPS
Terpene synthase.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WK and WS performed the BPH infestation experiments and antixenosis tests. WK and VR performed the SFP mapping. TT provided all plant materials used in this study. WK planned and conducted all other experiments. The entire study was designed and coordinated by AV and TT. WK drafted the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Wintai Kamolsukyunyong, Email: wtkmsyy@gmail.com.
Wissarut Sukhaket, Email: aonidae@gmail.com.
Vinitchan Ruanjaichon, Email: vitchakorn.rua@gmail.com.
Theerayut Toojinda, Email: toojindatheerayut@gmail.com.
Apichart Vanavichit, Email: vanavichit@gmail.com.
References
- Bowers WS, Webb RE, Nault LR, Dutky SR. Aphid alarm pheromone: Isolation, identification, synthesis. Science. 1972;177:1121–1122. doi: 10.1126/science.177.4054.1121. [DOI] [PubMed] [Google Scholar]
- Cheng AX, Xiang CY, Li JX, Yang CQ, Hu WL, Wang LJ, Lou YG, Chen XY. The rice (E)-β-caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry. 2007;68:1632–1641. doi: 10.1016/j.phytochem.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Cho SK, Jung KW, Jeung JU, Kang KH, Shim KS, You MK, Yoo KS, Ok SH, Shin JS. Analysis of differentially expressed transcripts from planthopper-infested wild rice (Oryza minuta) Plant Cell Rep. 2005;24:59–67. doi: 10.1007/s00299-004-0905-9. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Siddall JB, Dunham LL, Uden P, Kislow CJ. Trans-β-farnesene, alarm pheromone of green peach aphid, Myzus persicae (Sulzer) Nature. 1973;241:126–127. doi: 10.1038/241126b0. [DOI] [Google Scholar]
- Gibson RW, Pickett JA. Wild potato repels aphids by release of aphid alarm pheromone. Nature. 1983;302:608–609. doi: 10.1038/302608a0. [DOI] [Google Scholar]
- Huang N, He G, Shu L, Li X, Zhang Q. Identification and mapping of two brown planthopper genes in rice. Theor Appl Genet. 2001;102:929–934. doi: 10.1007/s001220000455. [DOI] [Google Scholar]
- Iijima Y, Davidovich-Rikanati R, Fridman E, Gang DR, Bar E, Lewinsohn E, Pichersky E. The biochemical and molecular basis for the divergent patterns in the biosynthesis of terpenes and phenylpropenes in the peltate glands of three cultivars of basil. Plant Physiol. 2004;136:3724–3736. doi: 10.1104/pp.104.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jairin J, Teangdeerith S, Leelagud P, Phengrat K, Vanavichit A, Toojinda T. Physical mapping of Bph3, a brown planthopper resistance locus in rice. Mj Int J Sci Tech. 2007;1:166–177. [Google Scholar]
- Jairin J, Teangdeerith S, Leelagud P, Kothcharerk J, Sansen K, Yi M, Vanavichit A, Toojinda T. Development of rice introgression lines with brown planthopper resistance and KDML105 grain quality characteristics through marker-assisted selection. Field Crop Res. 2009;110:263–271. doi: 10.1016/j.fcr.2008.09.009. [DOI] [Google Scholar]
- Köllner T, Schnee C, Gershenzon J, Degenhardt J. The variability of sesquiterpenes emitted from two Zea mays cultivars is controlled by allelic variation of two terpene synthase genes encoding stereoselective multiple product enzymes. Plant Cell. 2004;16:1115–1131. doi: 10.1105/tpc.019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Qiu J, Joshi T, Valliyodan B, Xu D, Nguyen HT. Single feature polymorphism discovery in rice. PLoS One. 2007;2:e284. doi: 10.1371/journal.pone.0000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Su C, Jiang L, He J, Wu H, Peng C, Wan J. The distribution and identification of brown planthopper resistance genes in rice. Hereditas. 2009;146:67–73. doi: 10.1111/j.1601-5223.2009.02088.x. [DOI] [PubMed] [Google Scholar]
- Lou Y, Hua X, Turlings TCJ, Cheng J, Chen X, Ye G. Differences in induced volatile emission among rice varieties result in differential attraction and parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae in the field. J Chem Ecol. 2006;32:2375–2387. doi: 10.1007/s10886-006-9151-7. [DOI] [PubMed] [Google Scholar]
- Pickett JA, Griffiths DC. Composition of aphid alarm pheromones. J Chem Ecol. 1980;6:349–360. doi: 10.1007/BF01402913. [DOI] [Google Scholar]
- Prisic S, Xu MM, Wilderman PR, Peters RJ. Rice contains two disparate ent-copalyl diphosphate synthases with distinct metabolic functions. Plant Physiol. 2004;136:4228–4236. doi: 10.1104/pp.104.050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman ML, Jiang W, Chu SH, Qiao Y, Ham TH, Woo MO, Lee J, Khanam MS, Chin JH, Jeung JU, Brar DS, Jena KK, Koh HJ. High-resolution mapping of two rice brown planthopper resistance genes, Bph20(t) and Bph21(t), originating from Oryza minuta. Theor Appl Genet. 2009;119:1237–1246. doi: 10.1007/s00122-009-1125-z. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Agrawal GK, Takeda S, Abe K. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 2004;134:1642–1653. doi: 10.1104/pp.103.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee C, Köllner TG, Held M, Turlings TCL, Gershenzon J, Degenhardt J. The product of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA. 2006;103:1129–1134. doi: 10.1073/pnas.0508027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Su C, Wang C, Zhai H, Wan J. Mapping of a major resistance gene to the brown planthopper in the rice cultivar Rathu Heenati. Breeding Sci. 2005;55:391–396. doi: 10.1270/jsbbs.55.391. [DOI] [Google Scholar]
- Thongjuea S, Ruanjaichon V, Bruskiewich R, Vanavichit A. RiceGeneThresher: a web-based application for mining genes underlying QTL in rice genome. Nucl Acids Res. 2009;37:D996–D1000. doi: 10.1093/nar/gkn638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li H, Si Y, Zhang H, Guo H, Miao X. Microarray analysis of broad-spectrum resistance derived from an indica cultivars Rathu heenati. Planta. 2012;235:829–840. doi: 10.1007/s00425-011-1546-1. [DOI] [PubMed] [Google Scholar]
- Xu M, Hillwig ML, Prisic S, Coates RM, Peters RJ. Functional identification of rice syn-copalyl diphosphate synthase and its role in initiating biosynthesis of diterpenoid phytoalexin/allelopathic natural products. Plant J. 2004;39:309–318. doi: 10.1111/j.1365-313X.2004.02137.x. [DOI] [PubMed] [Google Scholar]
- Yang HY, Ren X, Weng QM, Zhu LL, He GC. Molecular mapping and genetic analysis of a rice brown planthopper (Nilarparvata lugen Stål) resistance gene. Hereditas. 2002;136:39–43. doi: 10.1034/j.1601-5223.2002.1360106.x. [DOI] [PubMed] [Google Scholar]
- Yang H, You A, Yang Z, Zhang F, He R, Zu L, He G. High resolution genetic mapping at the Bph15 locus for brown planthopper resistance in rice (Oryza sativa L.) Theor Appl Genet. 2004;110:182–191. doi: 10.1007/s00122-004-1844-0. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Killner TG, Wiggins G, Grant J, Degenhardt J, Chen F. Molecular and genomic basis of volatile-mediated indirect defense against insect in rice. Plant J. 2008;55:491–503. doi: 10.1111/j.1365-313X.2008.03524.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1:Data 1A: Predicted SFP called at different threshold (delta) between KDML105 and the pool of 4 introgression lines. Data 1B: 157 predicted SFP choose in this study. (NULL 13 KB)
Additional file 2: Table S1: SFP-containing genes. (XLS 143 KB)
Additional file 3: Table S2: Functional classification of SFP-containing genes. (XLS 140 KB)
Additional file 4: SFP validation by comparison of hybridization fold change and polymorphism detected in the probe region between KD and RH. (XLS 30 KB)
Additional file 5: Table S3:Sequence polymorphisms between KD and RH in predicted SFP. (XLS 32 KB)


