Abstract
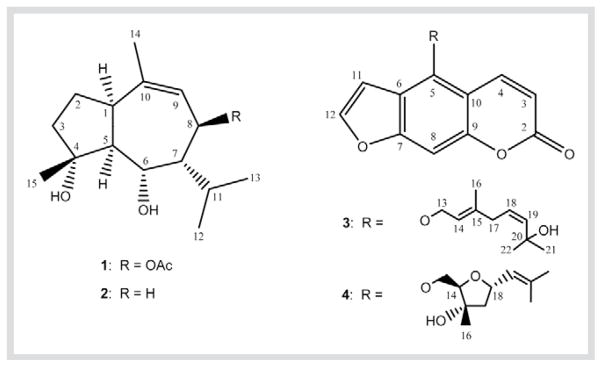
In addition to twenty-nine known compounds, two new guaiane sesquiterpenes and two new furanocoumarins were isolated from the chloroform extract of the rhizomes of Notopterygium incisum. The new structures were elucidated by means of spectroscopic methods including 2 D NMR techniques and mass spectrometry to be 8β-acetoxy-4α,6α-dihydroxy-1α,5α(H)-guai-9-ene (incisumdiol A, 1), 4α,6α-dihydroxy-1α,5α(H)-guai-9-ene (incisumdiol B, 2), 5-[(2E,5Z)-7-hydroxy- 3,7-dimethyl-2,5-octadienoxy]psoralene (3) and 5-[(2,5)-epoxy-3-hydroxy-3,7-dimethyl-6-octenoxy]psoralene (4).
Keywords: Notopterygium incisum Ting ex H. T. Chang, Apiaceae, structure elucidation, guaiane sesquiterpenes, incisumdiols, furanocoumarins
Introduction
The rhizomes of the plant of Notopterygium incisum Ting ex H. T. Chang (Apiaceae), known as “Qianghuo” in Chinese, have been used for centuries as a common traditional Chinese medicine (TCM) to relieve arthritic pain and to alleviate headache from sinus infections [1]. The rhizomes were previously reported to afford furanocoumarins [2], [3], [4]; however, no sesquiterpenoid has been isolated, although oxygenated sesquiterpenes have been recently detected by GC-MS from the essential oil in the rhizomes [5]. In this paper, we report the isolation and structure elucidation of two new guaiane sesquiterpenes (1, 2) and two new furanocoumarins (3, 4) from the chloroform extract of the rhizomes of N. incisum.
Materials and Methods
General experimental procedures
Melting points were measured on aWRS-1A Digital Melting-Point Apparatus (Shanghai YICE Apparatus & Equipments CO., LTD). Optical rotations were determined by using a Perkin-Elmer 341 polarimeter. IR spectra were measured on a Nicolet NEXUS-670 FT-IR spectrophotometer. UV spectra were recorded on a Shimadzu UV-2550 spectrophotometer. 1 D and 2 D NMR spectra were recorded on a Bruker Avance DRX-500 spectrometer using TMS as the internal reference. EIMS analyses were performed on a Thermo Trace GC Ultra-DSQ instrument. LR-ESI-MS were carried out on a Bruker Esquire 3000plus instrument. HR-ESI-MS were measured on a Bruker Daltonics micrOTOF mass spectrometer. All the solvents were of analytical grade and were purchased from Shanghai Chemical Reagents Company Ltd. Column chromatography was performed using silica gel (Qingdao Ji-Yi-Da Silysia Chemical Ltd.; 200–300 mesh) or Sephadex LH-20 (Pharmacia Biotech Ltd.).
Plant material
The dry rhizomes of N. incisum were purchased from Shanghai Kang-Qiao traditional Chinese medicinal (TCM) materials Co. Ltd, and were originally collected from Sichuan province, China. The plant was identified by Prof. Jian-Wei Chen (College of Pharmacy, Nanjing University of TCM). A voucher specimen (No. 060305) was deposited at the Herbarium of the Shanghai Key Laboratory of Brain Functional Genomics, East China Normal University.
Extraction and isolation
Dry crude material (10 kg) was extracted with chloroform (10×2000 mL, total amount 20 L) at room temperature. After filtration, the extracts were combined and evaporated under vacuum. The residue (ca. 1000 g) was chromatographed on silica gel (7 kg, column: 150×14 cm) with an n-hexane-acetone gradient (15:1, 8:1, 4:1, 1:1, 0:1, each 6 L) to yield twelve fractions (Fr.1–Fr.12). Fr.2 (between 0.8 and 2.0 L, 1.8 g) was chromatographed on silica gel (200 g, column: 70×4 cm) with a petroleum ether-chloroform gradient (8 :1, 6:1, 4:1, each 600 mL) to yield compounds 9 (between 120 and 800 mL, 60 mg) and 31 (between 1200 and 1800 mL, 19 mg); Fr.3 (between 2.0 and 3.6 L, 1.5 g) was chromatographed on silica gel (180 g, column: 70×4 cm) with a petroleum ether-chloroform gradient (8 :1, 6:1, 4:1, each 500 mL) to yield compounds 24 (between 600 and 800 mL, 4 mg) and 25 (between 1000 and 1300 mL, 8 mg); Fr.4 (between 3.6 and 5.0 L, ca. 8 g)was chromatographed on silica gel (1000 g, column: 70×5 cm) with a petroleum ether-ethyl acetate gradient (10:1, 8:1, 6:1, 5:1, each 800 mL) to yield compounds 11 (between 500 and 1200 mL, 38 mg), 12 (between 1200 and 1800 mL, 30 mg), 14 (between 1800 and 2100 mL, 900 mg) and 16 (between 2100 and 2800 mL, 35 mg). Fr.5 (between 5.0 and 8.0 L, ca. 10 g) was chromatographed on silica gel (1000 g, column: 70×5 cm) with a petroleum ether-ethyl acetate gradient (8 :1, 6:1, 5:1, 3:1, each 700 mL) to yield compounds 19 (between 1200 and 1300 mL, 15 mg), 20 (between 1800 and 2100 mL, 30 mg), 27 (between 800 and 1000 mL, 90 mg), 28 (between 1300 and 1600 mL, 15 mg), 30 (between 1600 and 1800 mL, 35 mg) and 33 (between 600 and 1100 mL, 30 mg).
Fr.6 (between 8.0 and 14 L, ca. 11 g) was chromatographed on silica gel (1000 g, column: 70×6 cm) using a gradient of chloroform-ethyl acetate (5 :1, 4:1, 2:1, 0:1, each 800 mL) to yield six subfractions (Fr.6–1 to Fr.6–6). Compounds 4 (15 mg) and 13 (38 mg) were obtained from Fr.6–1 (between 0 and 1100 mL, 1.2 g) by gel permeation chromatography on Sephadex LH-20 (column: 110×4 cm) in acetone. Compounds 7 (25 mg) and 32 (15 mg) were obtained from Fr.6–2 (between 1100 and 1800 mL, 1.4 g) on Sephadex LH-20 (column: 120×4 cm) in methanol. Compounds 3 (20 mg), 5 (8 mg) and 6 (11 mg) were isolated from Fr.6–3 (between 1800 and 2100 mL, 1.0 g) by preparative TLC (chloroform-ethyl acetate 5:1, 3: Rf = 0.2, 5:Rf = 0.4, 6: Rf = 0.8) and further purified on Sephadex LH-20 (column: 120×4 cm) in methanol. Compounds 1 (between 200 and 650 mL, 32 mg), 2 (between 0 and 200 mL, 30 mg), 17 (between 1000 and 1200 mL, 32 mg) and 18 (between 1200 and 1800 mL, 30 mg) were isolated from Fr.6–5 (between 2700 and 3100 mL, 1.8 g) by repetitive silica gel column (50×4 cm) chromatography with n-hexane-ethyl acetate (10:1, 2000 mL), and were further purified on Sephadex LH-20 (column: 100×3 cm) in methanol. Fr.7 (between 14 and 16.5 L, ca. 8 g) was chromatographed on silica gel (column: 70×4 cm) with a gradient of n-hexane-ethyl acetate (10:1, 8:1, 5:1, each 1000 mL) to yield compounds 22 (between 1500 and 1800 mL, 15 mg) and 23 (between 1200 and 1500 mL, 40 mg). Compound 15 (1 g)was obtained from Fr.8 (between 16.5 and 18 L, ca 12 g) by crystallization from ethyl acetate. Compounds 8 (between 800 and 1000 mL,15 mg), 10 (between 500 and 800 mL, 40 mg), 21 (between 1200 and 1350 mL, 40 mg) and 26 (between 1500 and 1750 mL, 110 mg) were isolated from Fr.9 (between 18 and 21.5 L, ca. 8 g) by silica gel column (50×4 cm) chromatography with a gradient of n-hexane-acetone (4 :1, 3:1, 2:1, each 800 mL). Compound 29 (300 mg) was obtained from Fr.10 (between 21.5 and 22 L, 2:1, ca. 3 g) by crystallization from ethyl acetate.
Incisumdiol A (1)
Yellow gum; : −30.6 (c 2.45, CHCl3); IR (CHCl3) νmax = 3451, 2954, 2928, 2867, 1734, 1670, 1459, 1383, 1374, 1248, 1183, 1150, 1025 cm−1; 1H-NMR and 13C-NMR data, see Table 1; ESI-MS: m/z=319 [M+Na]+, 615 [2 M+Na]+; HR-ESI-MS: m/z=319.1881 [M+Na]+ (calcd. for C17H28O4Na: 319.1885).
Table 1.
1H- (500 MHz) and 13C-NMR (125 MHz) data of 1 and 2a
| No. | 1 | 1 | 2 | |||
|---|---|---|---|---|---|---|
| dH (mult, J in Hz) b | dCb | dH (mult, J in Hz) c | dCd | dH (mult, J in Hz)c | dCd | |
| 1 | 2.47 (1H, brd, 10.5) | 42.4e | 2.58 (1H, brs) | 42.0f | 2.23 (1H, brs) | 39.1 f |
| 2 | 2.22 (2H, m) | 23.2e | 2.24 (2H, m) | 24.3f | 2.36 (1H, m), 2.04 (1H, m) | 23.1 f |
| 3 | 1.18 (1H, m), 1.97 (1H, m) | 37.6e | 1.20 (1H, m), 2.09 (1H, overlapped) | 37.8f | 1.26 (1H, m), 2.10 (1H, m) | 37.3 f |
| 4 | 58.8 | 59.7 | 60.5 | |||
| 5 | 2.57 (1H, brd, 7.4) | 68.3 | 2.76 (1H, brs) | 68.4 | 2.63 (1H, brs) | 68.7 |
| 6 | 3.40 (1H, brd, 7.4) | 69.8 | 3.47 (1H, brd, 8.2) | 71.0 | 3.63 (1H, brd, 8.0) | 71.8 |
| 7 | 1.30 (1H, brdd, 11.5, 6.3) | 48.8e | 1.32 (1H, m) | 50.1f | 0.97 (1H, m) | 47.0 f |
| 8 | 5.16 (1H, brd, 11.5) | 71.1e | 5.07 (1H, brs) | 73.1f | 1.79 (1H, m), 1.43 (1H, m) | 25.8f |
| 9 | 5.30 (1H, brs) | 128.2e | 5.27 (1H, brs) | 128.1f | 5.27 (1H, brs) | 122.8f |
| 10 | 131.1e | 129.8f | 137.3f | |||
| 11 | 2.01 (1H, m) | 25.3 | 2.09 (1H, overlapped) | 26.1 | 1.72 (1H, m) | 31.7 |
| 12 | 1.07 (3H, d, 6.5) | 23.0 | 1.07 (3H, d, 6.4) | 23.4 | 1.01 (3H, d, 6.5) | 20.9 |
| 13 | 0.91 (3H, d, 6.5) | 20.8 | 0.93 (3H, d, 6.4) | 21.3 | 0.94 (3H, d, 6.5) | 21.4 |
| 14 | 1.71 (3H, brs) | 19.3e | 1.75 (3H, brs) | 20.4f | 1.67 (3H, brs) | 18.3f |
| 15 | 1.09 (3H, s) | 16.0 | 1.13 (3H, s) | 16.1 | 1.21 (3H, s) | 16.9 |
| OAc | 1.99 (3H, s) | 170.3, 20.7 | 2.09 (3H, s) | 172.3, 21.1 | ||
Assignments were based on 1 D and 2 D NMR experiments (COSY, HSQC and HMBC).
In DMSO-d6 at 343K.
In CDCl3 at 298K.
In CDCl3 at 310K.
Signals with medium intensity.
Signals with weaker intensity and broad.
Incisumdiol B (2)
Yellow gum; : + 62.1 (c 3.25, CHCl3); IR (CHCl3): νmax = 3434, 2927, 2868, 1637, 1460, 1385, 1374, 1137 cm−1; 1H-NMR and 13C-NMR data, see Table 1; ESI-MS: m/z= 261 [M + Na]+, 499 [2 M + Na]+.
5-[(2E,5Z)-7-Hydroxy-3,7-dimethyl-2,5-octadienoxy]psoralene (3)
Yellow powder; m.p. 80–81°C; : = 0 (c 0.43, CHCl3); UV (MeOH): λmax = 221 (2.94), 248 (2.99), 258 (2.92), 268 (2.90), 311 (2.78) nm; IR (KBr): νmax = 3476, 3125, 2974, 2925, 2853, 1721, 1626, 1608, 1579, 1544, 1455, 1400, 1203, 1166, 1093, 1072 cm−1; 1H-NMR and 13C-NMR data, see Table 2; ESI-MS: m/z = 377 [M + Na]+, 731 [2 M+ Na]+; HR-ESI-MS: m/z = 377.1359 [M + Na]+ (calcd. for C21H22O5Na: 377.1364).
Table 2.
1H- (500 MHz) and 13C-NMR (125 MHz) data of 3 and 4 (in CDCl3, 298 K)a
| No. | 3 | 4 | ||
|---|---|---|---|---|
| 1H (J values in Hz) | 13Cb | 1H (J values in Hz) | 13C | |
| 2 | 161.2 (161.1) | 161.2 | ||
| 3 | 6.27 (1H, d, 9.5) | 112.6 (112.4) | 6.26 (1H, d, 9.6) | 112.7 |
| 4 | 8.14 (1H, d, 9.5) | 139.5 (139.5) | 8.21 (1H, d, 9.6) | 139.5 |
| 5 | 148.9 (148.8) | 148.6 | ||
| 6 | 114.1 (114.3) | 114.1 | ||
| 7 | 158.1 (158.0) | 158.0 | ||
| 8 | 7.15 (1H, s) | 94.2 (94.0) | 7.16 (1H, s) | 94.6 |
| 9 | 152.6 (152.5) | 152.5 | ||
| 10 | 107.5 (107.4) | 107.3 | ||
| 11 | 6.95 (1H, d, 2.2) | 105.0 (105.0) | 6.99 (1H, d, 2.0) | 104.8 |
| 12 | 7.60 (1H, d, 2.2) | 144.9 (144.9) | 7.59 (1H, d, 2.0) | 145.1 |
| 13 | 4.96 (2H, d, 6.7) | 69.7 (69.6) | 4.44 (1 h, dd, 9.9, 6.3) | 72.8 |
| 4.47 (1H, dd, 9.9, 4.7) | ||||
| 14 | 5.59 (1H, brd, 6.7) | 119.1 (119.6) | 4.23 (1H, dd, 6.3, 4.7) | 83.4 |
| 15 | 142.6 (141.7) | 78.6 | ||
| 16 | 1.73 (3H, brs) | 17.1 (16.7) | 1.45 (3H, s) | 22.2 |
| 17 | 3.15 (2H, brd, 7.7) | 37.4 (42.0) | 1.98 (1H, dd, 12.7, 7.8) | 47.9 |
| 2.25 (1H, dd, 12.7, 7.2) | ||||
| 18 | 5.34 (1H, dt, 11.7, 7.7) | 126.9 (123.5) | 4.85 (1H, ddd, 8.5, 7.8, 7.2) | 74.3 |
| 19 | 5.61 (1H, d, brd, 11.7) | 138.7 (140.6) | 5.39 (1H, brd, 8.5) | 126.1 |
| 20 | 71.8 (70.5) | 136.4 | ||
| 21 | 1.36 (3H, s) | 31.2 (29.8) | 1.75 (3H, brs) | 25.8 |
| 22 | 1.36 (3H, s) | 31.2 (29.8) | 1.68 (3H, brs) | 18.1 |
Assignments were based on 1 D and 2 D NMR experiments (COSY, HSQC and HMBC).
The 13C-NMR chemical shifts of compound 13 in the parentheses are consistent with the reported data for notoptol [4].
5-[(2,5)-Epoxy-3-hydroxy-3,7-dimethyl-6-octenoxy]psoralene (4)
Yellow gum; : +1.5 (c 0.62, CHCl3); UV (MeOH): λmax = 220 (3.11), 249 (3.08), 258 (2.97), 267 (3.04), 308 (3.12) nm; IR (KBr): νmax = 3429, 3119, 2973, 2924, 2853, 1721, 1624, 1578, 1546, 1400, 1205, 1155, 1069 cm−1; 1H-NMR and 13C-NMR data, see Table 2; ESI-MS: m/z = 371 [M+ H]+, 741 [2 M+ H]+, 763 [2 M+ Na]+, 785 [2 M+ HCOO−]−.
Supporting Information
Structures of the isolates (1–33) (Fig. 1S), 1H- and 13C-NMR spectra of compounds 1 (Fig. 2S–Fig. 5S), 2 (Fig. 6S–Fig. 8S), 3 (Fig. 9S and Fig. 10S), and 4 (Fig. 11S and Fig. 12S) are available as Supporting Information.
Results and Discussion
In a reinvestigation of the chemical components of N. incisum, the chloroform extract from the plant’s rhizomes was subjected to column chromatography (CC) over silica gel and Sephadex LH-20 to furnish two new cis-fused Δ9-guaienes (1, 2) and two new furanocoumarins (3, 4) (Fig. 1), along with eight known guaiane sesquiterpenes (5–12) and fourteen known coumarins (13–26) as well as seven miscellaneous constituents (27–33). Comparing their MS and NMR data as well as the optical activity with the literature, the known compounds were identified as 4β,6β-dihydroxy-1α,5β(H)-guai-9-ene (5) [6], teucladiol (6) [7], chrysothol (7) [8], teuclatriol (8) [7], hanamyol (9) [9], 1,5,11-trihydroxyguaiane (10) [10], grilactone (11) [11], 3,7(11),10(14)-guaiatrien-1β,5β(H)-12,6β-olide (12) [12], notoptol (13) [4], isoimperatorin (14) [3], [13], notopterol (15) [2], [4], bergamottin (16) [14], anhydronotoptol (17) [4], bergapten (18) [3], [13], 6-methoxy-7-geranyloxycoumarin (19) [15], ostruthin (20) [16], scopoletin (21) [17], isofraxidin (22) [18], 7-isopentenyloxy-6-methoxycoumarin (23) [19], 5-geranyloxy-7-methoxycoumarin (24) [20], 5-isopentenyloxy-7-methoxycoumarin (25) [21], marmesin (26) [15], [22], phenethyl ferulate (27) [23], [24], pterostilbene (28) [25], pregnenolone (29) [26], falcarindiol (30) [2], falcarinol (31) [27], (2E,9Z)-heptadecadiene-4,6-diyn-1-ol (32) [28] and 8-acetoxy-(1,9Z)-heptadecadiene-4,6-diyn-3-ol (33) [29]. Among them, compounds 5–12, 20–25 and 31–33 were reported from N. incisum for the first time.
Fig. 1.
Structures of guaiane sesquiterpenes (1, 2) and furanocoumarins (3, 4).
The molecular weight of compound 1 and its chemical formula of C17H28O4 were deduced from the positive mode high-resolution electrospray ionization mass spectrum (HR-ESI-MS), which resulted in an [M + Na]+ ion peak at m/z= 319.1881 (C17H28O4Na requires 319.1885). The IR spectrum of 1 showed absorptions (νmax) at 3451 (hydroxy groups), 1734 and 1025 (ester carbonyl), 1670 and 1459 (double bond), 1383 and 1374 (isopropyl group) cm−1. In the high field region of the 1H-NMR spectrum (CDCl3, 298 K), five methyl signals were observed: two secondary methyls for an isopropyl group (δ = 1.07 and 0.93), one tertiary methyl (δ = 1.13), one olefinic methyl (δ = 1.75), and one acetyl methyl (δ = 2.09). Based on these spectroscopic data and on the degrees of unsaturation, the backbone structure of 1was elucidated to be a guaiane-type skeleton that was also found for the known compounds 5–12 [6], [7], [8], [9], [10], [11], [12].
In the 1H-NMR spectrum (CDCl3, 298 K), the signals for H-1, H-5, and H-8 appeared very broad. Similarly, in the 13C-NMR spectrum recorded in CDCl3 at 310 K, extremely weak and broad signals were observed for C-1, C-2, C-3, C-7, C-8, C9, C10, and C-14. These broadened signals suggested that 1 exists as a mixture of conformations that interconvert at a moderate rate on the NMR time scale. To test this hypothesis, we re-acquired the NMR spectra at a higher temperature (343 K, DMSO-d6). Indeed, at higher temperatures, the signals for H-1, H-5 and H-8 coalesce to sharp, averaged multiplets at δ = 2.47, 2.57, and 5.16, respectively (Table 1). In the 13C-NMR spectrum acquired under these conditions, the intensity of the eight carbon signals of C-1, C-2, C-3, C-7, C-8, C9, C10, and C-14 was significantly increased, although they were still less intense than the other carbon signals (Table 1).
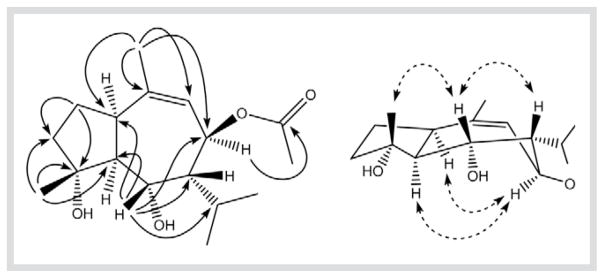
The 13C-NMR and DEPT spectra recorded in DMSO-d6 at 343 K confirmed that compound 1 contained seventeen carbons classified as three quaternary carbons at δ = 170.3, 131.1 and 58.8, seven methines at δ = 128.2, 71.1, 69.8, 68.3, 48.8, 42.4 and 25.3, two methylenes at δ = 37.6 and 23.2, and five methyls at δ = 23.0, 20.8, 20.7,19.3 and 16.0 (Table 1). In the COSY spectrum (DMSO-d6), a clear proton spin systemwas observed between H-5 at δ = 2.57 and H-6 at δ = 3.40, between H-6 and H-7 at δ = 1.30, as well as between H-7 and H-8 at δ = 5.16. The correlation between H-8 and its vicinal olefinic proton H-9 is ambiguous due to the dihedral angle (ca. 90 °) between H-8 and H-9. The chemical shifts of the corresponding methine carbons were determined through the HSQC NMR experiment. The unusual lowfield chemical shift (68.3 ppm) of C-5, a non-oxygenated methine, was also found in chrysothol (7) and its derivatives [8]. In the HMBC spectrum (Fig. 2), the correlations (nJ, n = 2 or 3) between H-15 and C-3, C-4 and C-5, as well as between H-6 and C-1, C-5, C-7, C-8 and C-11 indicated that the two hydroxy groups were linked to C-4 and C-6, respectively. The correlation between H-8 at δ = 5.16 and the carbonyl carbon at δ = 170.3 showed that the acetoxy group is located at C-8. The double bond at C-9 was also confirmed by the HMBC correlations (nJ, n = 2 to 4) between H-14 and C-1, C-8, C-9 and C-10 (Fig. 2).
Fig. 2.
Key HMBC and NOE correlations of 1.
The relative configuration of 1 was established by the coupling constants (Table 1) in the 1H-NMR and the correlations in the NOESY spectrum (DMSO-d6, 343 K). When H-5 was assumed to be in the α-orientation, H-1 should be also α, and both H-6 and H-7 should be β due to the coupling constants between H-5 and H-1 (J1,5 = ca. 0 Hz), between H-5 and H-6 (J5,6 = 7.4 Hz), and between H-6 and H-7 (J6,7 = ca. 0 Hz). Moreover, the large coupling constant (11.5 Hz) between H-7 and H-8 requires that these two protons be trans-diaxial. Clear NOE correlations (Fig. 2) were observed between H-1 and H-8, between H-5 and H-8, between H-6 and H-7, and between H-6 and H-15. Thus, the structure of 1 was established to be 8β-acetoxy-4α,6α-dihydroxy-1α,5α(H)-guai-9-ene.
The molecular formula C15H26O2 of compound 2 was determined by the ESI-mass spectrum with an [M + Na]+ peak at m/z= 261, together with its 13C- and 1H-NMR data (Table 1). Its 1H-NMR spectrum recorded in CDCl3 showed general features similar to those of 1, indicating that 2 also has a cis-fused Δ9-guaiene skeleton. Compound 2 also showed broadened signals at lower acquisition temperatures as described for 1. The most obvious difference between these two compounds is that the proton signal at δ = 5.07 (H-8) and the acetyl methyl at δ = 2.09 in 1 are absent in 2. Thiswas confirmed by the 13C-NMR (CDCl3, 310 K) spectrum of 2 (Table 1), which only showed fifteen carbon signals. All the above data indicated that 2 is an 8-deacetoxy derivative of 1. This is further supported by its IR spectrum, which did not show any absorption for the ester carbonyl. Therefore, compound 2 was deduced to be 4α,6α-dihydroxy-1α,5α(H)-guai-9-ene. Detailed 2 D NMR (COSY, HMQC, HMBC and NOESY) spectra of 2 were in full agreement with the proposed structure.
The molecular formula C21H22O5 of compound 3 was deduced from the positive mode HR-ESI-MS, with an [M + Na]+ ion peak at m/z = 377.1359 (C21H22O5Na requires 377.1364). The IR spectrum of 3 showed absorptions (νmax) at 3476 (hydroxy group), 1721 (ester carbonyl), and 1626, 1608, 1544 (aromatic ring) cm−1. Its UV spectrum exhibited absorption maxima at 221, 248, 258, 268 and 311 nm, which is very similar to those of the furanocoumarins notoptol (13) [4] and notopterol (15) [2], [4]. In the low-field region of the 1H-NMR spectrum (500 MHz, CDCl3), the proton signals of the coumarin part were observed at δ = 6.27, 7.15 and 8.14, and the furan part at δ = 6.95 and 7.60. Both compounds 3 and 13 have a similar monoterpenyloxy side chain at the C-5 position. The smaller coupling constant in 3 (JH-18,H-19 = 11.7 Hz) compared with that in 13 (16.0 Hz) [4] indicated that 3 is an isomer of 13 with a cis-double bond at C-18 rather than a trans-double bond as in 13. Furthermore, in its 13C-NMR spectrum, the chemical shifts of C-13 to C-16 of 3 are close to those of 13, but these of C-17 through C-22 are quite different (Table 2). Therefore, both compounds 3 and 13 contain the same geometric configuration of the double bond at C-14, but possess a different geometric configuration of the double bond at C-18. Thus, compound 3 was deduced to be 5-[(2E,5Z)-7-hydroxy-3,7-dimethyl- 2,5-octadienoxy]psoralene.
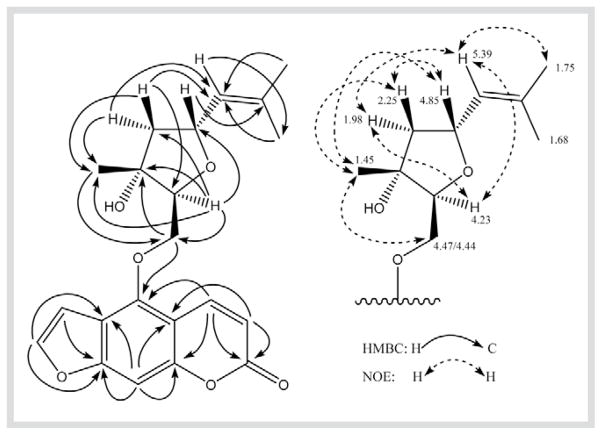
The molecular formula C21H22O6 of compound 4 was assigned by ESI-MS with a clear [M + H]+ peak at m/z = 371, together with its 13C- and 1H-NMR data (Table 2). The IR and UV spectra of 4 were very similar to those of 3, indicating the presence of hydroxy and furanocoumarin moieties in 4. The 1H- and 13C-NMR data (Table 2) showed that both compounds 4 and 3 have the same mono-substituted furanocoumarin skeleton with a different monoterpenyloxy side chain at C-5. The structure of the side chain in 4 was determined by 1 D and 2 D NMR experiments. In the COSY spectrum, two proton spin systems were observed for the side chain: H2-13 and H-14 comprise one spin system, while H2-17, H-18, H-19, H-21, and H-22 comprise the other. The proton signal of the tertiary Me-16 in 4 exhibited a sharp singlet at δ = 1.45, rather than the broad singlet at δ = 1.73 in 3 (Table 2). The 13C-NMR data indicated that C-15 is an oxygenated quaternary carbon (δ = 78.6). Based on these data and the molecular formula, there should be a ring system in the side chain; we therefore deduced that an ether bond is formed between C-14 (δC = 83.4) and C-18 (δC = 74.3). This tetrahydrofuran ring structure was confirmed by an HMBC correlation across the oxygen (Fig. 3). Clear HMBC correlations were observed between H-14 and C-13, C-15, C-16, C-17 and C-18, respectively. Therefore, compound 4 was elucidated to be 5-[(2,5)-epoxy-3-hydroxy-3,7-dimethyl-6-octenoxy]psoralene. The relative configuration of the chiral centers at C-14, C-15 and C-18 in the tetrahydrofuran ring were determined by the NOESY spectrum, which showed clear correlations between H-14α and H-17α, between H-16β and H-17β, between H-16β and H-13/H-13′, between H-16β and H-18β, and between H-17β and H-18β. Moreover, the olefinic proton of H-19 at δ = 5.39 was also detected to show NOE correlation with H-14α, H-17α, and H-21, respectively (Fig. 3).
Fig. 3.
Key HMBC and NOE correlations of 4.
Supplementary Material
Acknowledgments
The authors are grateful to Prof. Jian-Ming Yue (Shanghai Institute of Materia Medica, Chinese Academy of Sciences), Dr. Yong-Ming Xie [Bruker Daltonics (Beijing) Inc.] and Dr. Zheng Zhao (Shanghai Key Laboratory of Brain Functional Genomics) for their assistance with the measurement of mass spectra. The authors wish to thank Prof. Jian-Wei Chen (Nanjing University of Traditional Chinese Medicine) for the plant identification. This work was supported, in part, by NSFC grants (No. 90713040, 30640 068), MOST grant (No. 2003CB716601), MOE grant (No. NCET-06-0422), and STCSM grants (No. 06DZ19002, 06PJ14033, 07DZ22006).
Footnotes
Supporting information available online at http://www.thieme-connect.de/ejournals/toc/plantamedica
References
- 1.China Pharmacopoeia Committee. Pharmacopoeia of the P.R.C., the first division of 2000 Edition (English) Beijing: Chemical and Technologic Press; 2000. p. 232. [Google Scholar]
- 2.Okuyama E, Nishimura S, Ohmori S, Ozaki Y, Satake M, Yamazaki M. Analgesic component of Notopterygium incisum Ting. Chem Pharm Bull. 1993;41:926–9. doi: 10.1248/cpb.41.926. [DOI] [PubMed] [Google Scholar]
- 3.Gu ZM, Zhang DX, Yang XW, Hattori M, Namba T. Isolation of two new coumarin glycosides from Notopterygium forbesii and evaluation of a Chinese crude drug, qiang-huo, the underground parts of N. incisum and N. forbesii, by high-performance liquid chromatography. Chem Pharm Bull. 1990;38:2498–502. doi: 10.1248/cpb.38.2498. [DOI] [PubMed] [Google Scholar]
- 4.Kozawa M, Fukumoto M, Matsuyama Y, Baba K. Chemical studies on the constituents of the Chinese crude drug “Quiang Huo”. Chem Pharm Bull. 1983;31:2712–7. [Google Scholar]
- 5.Qiu YQ, Lu X, Pang T, Zhu SK, Kong HW, Xu GW. Study of traditional Chinese medicine volatile oils from different geographical origins by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GC × GC-TOFMS) in combination with multivariate analysis. J Pharm Biomed Anal. 2007;43:1721–7. doi: 10.1016/j.jpba.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Mahmoud AA. 7-epi-Eudesmanes, eudesmanoic acids, eudesmanolides and other sesquiterpenes from Pluchea dioscoridis. Phytochemistry. 1997;45:1633–8. [Google Scholar]
- 7.Bruno M, De la Torre MC, Rodriguez B, Omar AA. Guaiane sesquiterpenes from Teucrium leucocladum. Phytochemistry. 1993;34:245–7. [Google Scholar]
- 8.Ahmed AA, Hegazy MEF, Hassan NM, Wojcinska M, Karchesy J, Pare PW, et al. Constituents of Chrysothamnus viscidiflorus. Phytochemistry. 2006;67:1547–53. doi: 10.1016/j.phytochem.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Itokawa H, Morita H, Watanabe K, Takase A, Iitaka Y. Two new sesquiterpenoids (alpinolide and hanamyol) from Alpinia japonica (Thunb.) Miq. Chem Lett. 1984:1687–90. [Google Scholar]
- 10.Anh NH, Ripperger H, Sung TV, Adam G. Neolignans and a sesquiterpene from Caryodaphnopsis tonkinensis. Phytochemistry. 1996;42:1167–9. [Google Scholar]
- 11.Malone JF, Parves M, Karim A, McKervey MA, Ahmad I, Bhatty MK. Isolation and crystal structure of grilactone, a new guaianolide from Ferula oopoda. J Chem Soc [Perkin II] 1980:1683–5. [Google Scholar]
- 12.Casinovi CG, Tomassini L, Nicoletti M. A new guaianolide from Ferula arrigonii Bocchieri. Gazz Chim Ital. 1989;119:563–4. [Google Scholar]
- 13.Masuda T, Takasugi M, Anetai M. Psoralen and other linear furanocoumarins as phytoalexins in Glehnia littoralis. Phytochemistry. 1998;47:13–6. [Google Scholar]
- 14.Adams M, Ettl S, Kunert O, Wube AA, Haslinger E, Bucar F, et al. Antimycobacterial activity of geranylated furocoumarins from Tetradium daniellii. Planta Med. 2006;72:1132–5. doi: 10.1055/s-2006-947239. [DOI] [PubMed] [Google Scholar]
- 15.Talapatra SK, Chaudhuri MK, Talapatra B. Coumarins on the root bark of Feronia elephantum. Phytochemistry. 1973;12:236–7. [Google Scholar]
- 16.Liu RM, Sun QH, Shi YR, Kong LY. Isolation and purification of coumarin compounds from the root of Peucedanum decursivum (Miq) Maxim by high-speed counter-current chromatography. J Chromatogr A. 2005;1076:127–32. doi: 10.1016/j.chroma.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 17.Cassady JM, Ojima N, Chang CJ, McLaughlin JL. An investigation of the antitumor activity of Micromelum integerrimum (Rutaceae) J Nat Prod. 1979;42:274–8. doi: 10.1021/np50003a005. [DOI] [PubMed] [Google Scholar]
- 18.Panichayupakaranant P, Noguchi H, De-Eknamkul W, Sankawa U. Naphthoquinones and coumarins from Impatiens balsamina root cultures. Phytochemistry. 1995;40:1141–3. [Google Scholar]
- 19.Cardona L, Garcia B, Pedro JR, Pérez J. 6-Prenyloxy-7-methoxycoumarin, a coumarin-hemiterpene ether from Carduus tenuiflorus. Phytochemistry. 1992;31:3989–91. [Google Scholar]
- 20.Miyake Y, Murakami A, Sugiyama Y, Isobe M, Koshimizu K, Ohigashi H. Identification of coumarins from lemon fruit (Citrus limon) as inhibitors of in vitro tumor promotion and superoxide and nitric oxide generation. J Agric Food Chem. 1999;47:3151–7. doi: 10.1021/jf980999y. [DOI] [PubMed] [Google Scholar]
- 21.Murray RDH, Ballantyne MM, Hogg TC, McCabe PH. Claisen rearrangements – VI. Synthesis of the coumarins, sesibiricin and toddaculin. Tetrahedron. 1975;31:2960–5. [Google Scholar]
- 22.Nemoto T, Ohshima T, Shibasaki M. Enantioselective total syntheses of (+)-decursin and related natural compounds using catalytic asymmetric epoxidation of an enone. Tetrahedron. 2003;59:6889–97. [Google Scholar]
- 23.Zschocke S, Lehner M, Bauer R. 5-Lipoxygenase and cyclooxygenase inhibitory active constituents from Qianghuo (Notopterygium incisum) Planta Med. 1997;63:203–6. doi: 10.1055/s-2006-957653. [DOI] [PubMed] [Google Scholar]
- 24.Zdero C, Jakupovic J, Bohlmann F. Diterpenes and other constituents from Pteronia species. Phytochemistry. 1990;29:1231–45. [Google Scholar]
- 25.Fuendjiep V, Wandji J, Tillequin F, Mulholland DA, Budzikiewicz H, Fomum ZT, et al. Chalconoid and stilbenoid glycosides from Guibourtia tessmanii. Phytochemistry. 2002;60:803–6. doi: 10.1016/s0031-9422(02)00108-5. [DOI] [PubMed] [Google Scholar]
- 26.Szendi Z, Forgó P, Sweet F. Complete 1H and 13C NMR spectra of pregnenolone. Steroids. 1995;60:442–6. doi: 10.1016/0039-128x(94)00047-g. [DOI] [PubMed] [Google Scholar]
- 27.Mayer SF, Steinreiber A, Orru RVA, Faber K. Chemoenzymatic asymmetric total syntheses of antitumor agents (3R,9R,10R)- and (3S,9R,10R)-panaxytriol and (R)- and (S)-falcarinol from Panax ginseng using an enantioconvergent enzyme-triggered cascade reaction. J Org Chem. 2002;67:9115–21. doi: 10.1021/jo020073w. [DOI] [PubMed] [Google Scholar]
- 28.Bohlmann F, Rode KM. Polyacetylenverbindungen, 147. Die Polyine aus Oenanthe crocata L. Chem Ber. 1968;101:1163–75. [Google Scholar]
- 29.Bohlmann F, Zdero C. Polyacetylenverbindungen, 230. Ein neues Polyin aus Centella-Arten. Chem Ber. 1975;108:511–4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.