Abstract
Background
Fusarium head blight (FHB) caused by Fusarium graminearum not only causes severe losses in yield, but also reduces quality of wheat grain by accumulating mycotoxins. Breeding for host plant resistance is considered as the best strategy to manage FHB. Resistance in wheat to FHB is quantitative in nature, involving cumulative effects of many genes governing resistance. The poor understanding of genetics and lack of precise phenotyping has hindered the development of FHB resistant cultivars. Though more than 100 QTLs imparting FHB resistance have been reported, none discovered the specific genes localized within the QTL region, nor the underlying mechanisms of resistance.
Findings
In our study recombinant inbred lines (RILs) carrying resistant (R-RIL) and susceptible (S-RIL) alleles of QTL-Fhb2 were subjected to metabolome and transcriptome profiling to discover the candidate genes. Metabolome profiling detected a higher abundance of metabolites belonging to phenylpropanoid, lignin, glycerophospholipid, flavonoid, fatty acid, and terpenoid biosynthetic pathways in R-RIL than in S-RIL. Transcriptome analysis revealed up-regulation of several receptor kinases, transcription factors, signaling, mycotoxin detoxification and resistance related genes. The dissection of QTL-Fhb2 using flanking marker sequences, integrating metabolomic and transcriptomic datasets, identified 4-Coumarate: CoA ligase (4CL), callose synthase (CS), basic Helix Loop Helix (bHLH041) transcription factor, glutathione S-transferase (GST), ABC transporter-4 (ABC4) and cinnamyl alcohol dehydrogenase (CAD) as putative resistance genes localized within the QTL-Fhb2 region.
Conclusion
Some of the identified genes within the QTL region are associated with structural resistance through cell wall reinforcement, reducing the spread of pathogen through rachis within a spike and few other genes that detoxify DON, the virulence factor, thus eventually reducing disease severity. In conclusion, we report that the wheat resistance QTL-Fhb2 is associated with high rachis resistance through additive resistance effects of genes, based on cell wall enforcement and detoxification of DON. Following further functional characterization and validation, these resistance genes can be used to replace the genes in susceptible commercial cultivars, if nonfunctional, based on genome editing to improve FHB resistance.
Introduction
Crop plants in natural environment significantly suffer from several devastating diseases, leading to severe economic losses. Plants resist pathogens through both constitutive or pre-existing and induced or de-novo synthesized metabolites and proteins, following pathogen invasion [1]. These resistance metabolites and proteins may be either structural or biochemical. The constitutive structural barriers include thick cuticles [2] and cell walls [3], and the resistance biochemicals include preformed antimicrobial or toxic secondary metabolites and proteins called phytoanticipins [1,4]. Phytoanticipins are constitutively synthesized secondary plant metabolites that provide defense at the outer layers in plants. These may be stored in nontoxic forms, but are released in active forms upon pathogen attack, with simple hydrolysis, as antimicrobial compounds [5]. The induced biochemicals, also known as phytoalexins, include hundreds of resistance metabolites, monomers and polymers, produced following pathogen invasion [6,7]. The induced biochemicals can also be proteins, also known as pathogenesis related (PR) proteins [3,8]. The induced structural barriers include formation and deposition of cell wall enforcing compounds such as hydroxycinnamic acid amides (HCAAs) which contain the pathogen to initial infection site [6].
Resistance in plants against pathogen attack has been considered to be qualitative or hypersensitive response, and quantitative or reduced susceptibility [9, 10]. But the distinction between them are not always clear, rather they are shades of gray [11]. Recently a novel unifying concept of resistance has been proposed and the resistance has been defined as the reduced susceptibility. The resistance is controlled by hierarchies of R genes, with regulatory and resistance related (RR) metabolite and protein biosynthetic roles [12]. The resistance is mainly due to resistance related (RR) metabolites and RR proteins, due to their antimicrobial or cell wall reinforcement properties. These RR metabolites and RR proteins can be constitutive (RRC) or induced (RRI) [13]. The pathogens, following inoculation, produce elicitors that are recognized by the membrane localized receptors called elicitor recognition receptors (ELRRs), encoded by RELRR genes, which then trigger downstream genes to induce elicitor triggered immunity (ELTI), the first line of defense [9–12]. Specialized pathogens enter into the cell, produce effectors by avirulence (AVR) genes, which in turn are recognized by the host effector recognition receptors (ERRs), encoded by RERR genes, to induce effector triggered immunity (ETI), the second line of defense [9–12]. Both ELTI and ETI result in hypersensitive response, thus considered to be qualitative resistance [12]. The ETI is considered to be due to RERR genes, and because of simple inheritance it has been extensively used in developing resistant plants. However, this resistance often breaks down because these genes are only receptor genes, and thus, their association with downstream resistance genes must be confirmed in a given cultivar [12,14]. The resistance is mainly due to RR metabolites and RR proteins, which are either constitutively produced (RRC) or induced (RRI), following pathogen invasion [13].
Wheat [Triticum aestivum L. (2n = 6x = 42)] is the second most important cereal crop with multi-utilitarian value, feeding 40% of the world’s population. Fusarium head blight (FHB) caused by Fusarium graminearum Schwabe [telomorph: Gibberella zeae Schw. (Petch)] is one of the most devastating and alarming diseases of wheat ruining harvests, in many wheat producing regions of the world, including Canada [15,16]. The accumulation of mycotoxins such as deoxynivalenol (DON) and nivalenol (NIV) is of major concern due to their detrimental effects on humans and animals [17]. The development of FHB resistant cultivars is considered to be the best way to manage this disease and the accumulation of mycotoxins in grains, as it is the most efficient, economic, and ecofriendly approach to manage FHB [15].
FHB resistance is quantitative in nature, involving several genes, each with small or large effects, and the phenotype is the result of their additive effects. Three different types of FHB resistance in wheat have been recognized and used in breeding: (i) resistance to initial infection or spikelet resistance (type-I), (ii) resistance to spread within the spike or rachis resistance (type-II), and (iii) resistance to mycotoxin accumulation in grains (type-III) [18]. The development of resistant cultivars is very challenging because of limited understanding of genetics of resistance and lack of cost-effective means to phenotype [19]. The screening is generally done based on spray inoculation which leads to high experimental errors, leading to inconsistent ranking of genotypes over years. Inoculation under controlled conditions can significantly reduce the experimental error and can enable quantification of both spikelet and rachis resistance [20].
More than 100 quantitative trait loci (QTLs) for FHB resistance have been identified in wheat [21]. The FHB resistant QTLs, with major and/or minor effects, have been mapped on all the wheat chromosomes, except on 7D [21]. Major QTLs on chromosome 3B (QTL-Fhb1) [22], 6B (QTL-Fhb2) [23] and 2D (QTL-2DL) [16], exhibit rachis resistance, and on chromosomes 5A (QTL-Fhb5) [22] and 4B (QTL-Fhb4) [16] confer spikelet resistance. The transfer of resistant QTLs based on marker assisted breeding is not very practical because these QTLs, in general, contain undesirable genes that are also transferred due to linkage drag.
The QTL-Fhb2 localized on the short arm of chromosome 6B is the second major QTL conferring rachis resistance [23]. The QTL-Fhb2 has been mapped as a Mendelian factor, spanning a region of 4.2 cM flanked by two simple sequence repeat (SSR) markers, GWM-133 and GWM-644, using recombinant inbred (RIL) population derived from a cross between BW278 (resistant parent) and AC foremost (susceptible parent), and the QTL explained 24.1% of resistance to FHB [23]. Resistant parent BW278 is a descendant of Sumai-3, a Chinese bread wheat cultivar which possesses high level of rachis resistance [22]. Similarly, the QTL-Fhb2 was mapped on 6BS using double haploid population, which conferred 21% resistance to FHB [24]. Both the studies reported high levels of rachis resistance, but no study has reported the specific genes localized in the QTL-Fhb2 region conferring resistance, nor the mechanisms of resistance. As the QTL regions contain several genes, the dissection and functional characterization of each gene localized in the QTL region on genomic scale is a very challenging task, especially in wheat which possesses a highly complex genome and lacks a complete genome sequence [25]. Therefore, genes in the QTL regions and the resistance mechanisms governed by them are not studied in detail. Even though significant attempts have been made to identify candidate genes localized within the QTL-Fhb1 [6,26], QTL-Fhb5 [27], so far no FHB resistance gene has been identified and validated.
Technological advancements in genome sequencing and integration of omics platforms have offered novel insights to explore the regulation of metabolic pathways and their biosynthetic genes underlying disease resistance mechanisms [6,28–30]. Metabolomics is a potential post-genomics tool to elucidate the host biochemical responses under biotic stress, to identify the candidate R genes, and to validate gene functions [6,31–33]. Non-targeted metabolomics has been applied to reveal the host biochemical mechanisms of quantitative resistance in crop plants such as wheat [6,34], and barley against F. graminearum [35–38], and potato against Phytophthora infestans [28–30,33]. Non-targeted metabolic profiling of wheat near isogenic lines (NILs) with FHB resistant QTL-Fhb1 revealed deposition of HCCAs in the cell wall that reduced further spread of pathogen within rachis, thus imparting resistance [6]. Higher abundance of several resistance metabolites belonging to phenylpropanoids, flavonoids, fatty acids, and terpenoids that inhibited the growth of F. graminearum and trichothecene biosynthesis was identified in barley against F. graminearum [35,38]. Metabolic profiling of resistant and susceptible potato cultivars against late blight identified phenylpropanoids and their biosynthetic genes regulated by StWRKY1 [28].
The transcriptome is highly active and instantly changes with response to cellular perturbations. The study of wheat transcriptome under Fusarium stress revealed the expressed genes following FHB infection [39,40]. QTL-Fhb1 specific RNA-seq of Wangshuibai and its mutant NAUH117 (lacking a chromosome region including QTL-Fhb1 segment), revealed association of PR5, PR14, ABC transporter and jasmonic acid pathway genes in FHB resistance in wheat [39]. Transcriptome analysis using RNA-seq in maize upon Fusarium verticillioides inoculation revealed the involvement of shikimate, flavonoid, lignin and terpenoid biosynthetic pathways in imparting FHB resistance [40]. A lipid transfer protein (LTP) was found to be constitutively more abundant in NIL carrying QTL-Fhb5, based on microarray [27]. QTL-specific microarray analysis of Sumai-3 and two susceptible NILs showed up-regulation of 25 genes and the genes encoding PR proteins, like β-1-3 glucanase (PR-2), thaumatin like proteins (PR-5) and wheatwins (PR-4) were significantly over-expressed in genotypes containing Sumai-3 3BS region [41]. Microarray analysis of near isogenic lines carrying QTL-3BS, showed the up-regulation of genes involved in cell wall biogenesis upon fusarium infection [42]. A gene UDP-glycosyltransferase was reported to be highly over-expressed in NILs harboring two QTL-Fhb1 and QTL-Fhb5, based on microarray analysis, which has a major role in the detoxification of deoxynivalenol [42].
Considering this background, RILs carrying resistant and susceptible alleles of genes in QTL-Fhb2 were subjected to metabolome and transcriptome profiling upon F. graminearum inoculation. Our study is the first report that revealed six putative resistance genes localized within the QTL-Fhb2 region and also the plausible association of cell wall enforcing metabolites, explaining the underlying mechanisms of resistance, based on the integration of metabolomics and transcriptomics. The application of these genes, following validation, is discussed.
Results
Disease severity of RILs
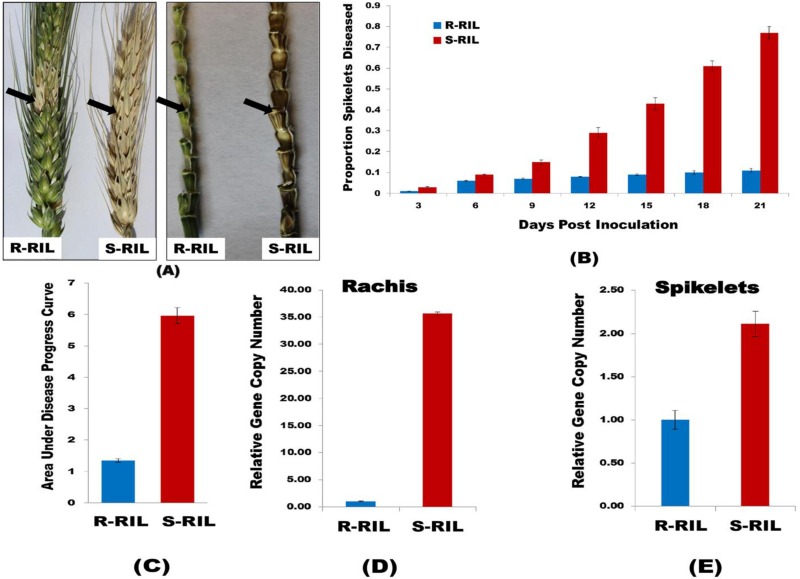
Spikelets showing necrotic lesions and bleaching symptoms were considered as diseased. The diseased symptoms were visible at 3 dpi as small, tiny necrotic spots on the inoculated pair of spikelets. The numbers of spikelets diseased in R-RIL were very few; the fungus was able to colonize only spikelets adjacent to the inoculated pair of spikelet, not further, even at 21 dpi. On other hand, in S-RIL the fungus was able to spread through rachis making the whole spike diseased at 21 dpi (Fig 1A). The rachis was lush green in R-RIL without any disease symptoms in rest of the spikelets, unlike in S-RIL where both rachis and spikelets were diseased showing blackish brown discoloration with necrotic lesions. This clearly demonstrated that the QTL imparts high rachis resistance as the fungus was unable to spread through rachis in R-RIL. The PSD in S-RIL was highly significant than in R-RIL (Fig 1B). The AUDPC calculated from PSD was significantly higher (P < 0.001) in S-RIL (AUDPC = 5.95) than in R-RIL (AUDPC = 1.34) (Fig 1C).
Fig 1. Phenotyping of RILs.
(A). Spike and rachis of R-RIL and S-RIL, 21 dpi with F. graminearum spore suspension. A single alternate pair of spikelets in a spike was inoculated; black arrows indicate the site of inoculation. The spike and rachis in R-RIL shows only necrotic spots or diseased symptoms limited to the inoculated spikelet, while in S-RIL both spikelet and rachis are entirely diseased. (B). Proportion of Spikelets Diseased (PSD). A single pair of spikelets of a spike was inoculated in both the RILs and the proportion of spikelets diseased was recorded at 3 days intervals until 21 days, from which the PSD was calculated. The bar graph shows high PSD in S-RIL as compared to R-RIL. (C). The bar graph shows area under disease progress curve (AUDPC) calculated from PSD, significantly higher in S-RIL. (D) and (E). Fungal Biomass quantification in RILs. Three alternate pair of spikelets were inoculated with F. graminearum spore suspension and samples were collected at 7 dpi. The total genomic DNA was extracted and the relative gene copy number of Tri6 was estimated using 2−ΔΔCT method. (D). Shows the relative gene copy number of Tri6 in rachis tissues; (E). Shows the relative gene copy number of Tri6 in spikelet tissues. In both graphs, the gene copy number of Tri6 is significantly higher in S-RIL as compared to R-RIL.
Quantity of fungal biomass in RILs
The quantity of fungal biomass, quantified as relative copy number of Tri6 gene, was highly significant in rachis of S-RIL as compared to R-RIL (Fig 1D). The reduced amount of fungal biomass in rachis of R-RIL, due to reduced ability of the pathogen to colonize the rachis, clearly demonstrates the role of QTL-Fhb2 in the R-RIL. Whereas, in the S-RIL the Tri6 gene copy number was high, as the fungus was able to move through the rachis to the adjacent spikelets, meaning absence of rachis resistance. The relative gene copy number of Tri6 was also calculated for the spikelets collected from both R-RIL and S-RIL, and it was found that the Tri6 gene copy number was also significantly higher in S-RIL than in R-RIL, but the fold change was comparatively lower in spikelets than in rachis (Fig 1E). These results clearly demonstrate that the resistant alleles of genes in QTL-Fhb2 impart high rachis resistance.
Resistance related induced (RRI) metabolites associated with QTL-Fhb2
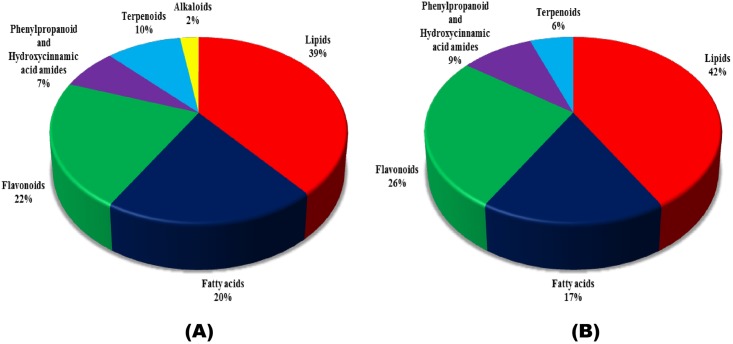
A total of 546 RRI metabolites were differentially accumulated, of which 41 had relatively high fold change in R-RIL (S1 Table). The RRI metabolites accumulated in higher abundance mainly belonged to phenylpropanoid, flavonoid, lignin, lipid, fatty acid, and terpenoid class of compounds (Table 1; S2 Table; Fig 2A). Metabolites belonging to lipids, in particular, the glycerophospholipids such as, phosphatidic acid (FC = 17.72 & 9.86), phosphatidylcholine (FC = 6.56), phosphatidylinositol (FC = 5.77) were significantly higher in abundance (Table 1). Metabolites belonging to phenylpropanoid biosynthetic pathway, in particular hydroxycinnamic acids (HCAs) such as N-caffeoylputrescine (FC = 5.03) and feruloyl-2-hydroxyputrescine (FC = 3.33) were found to be higher in abundance. Metabolites belonging to flavonoid biosynthetic pathway such as quercetin 3-O-[beta-D-xylosyl-(1->2)-beta-D-glucoside] (FC = 2.59), isovitexin 2''-O-(6‴-feruloyl) glucoside (FC = 2.20), quercitrin (FC = 1.82), 5,7-dimethoxyflavone (FC = 1.64) were few among many that were found in higher abundance. Seven metabolites belonging to fatty acids class of compounds such as 9Z)-(7S, 8S)-Dihydroxyoctadecenoic acid (FC = 6.44), 2,3-Bis (Trimethylsilyl) Oxy-Butanedioic acid Bis (Trimethylsilyl) Ester (FC = 4.10), and cucurbic acid (FC = 2.69). Delcosine (FC = 1.59) an alkaloid was also high in abundance. The compounds identified are known to be involved either in cell wall reinforcement or act as antifungal, antibacterial and antimicrobial compounds, depicting role in FHB resistance.
Table 1. High fold change resistance related induced (RRI) and resistance related constitutive (RRC) metabolites identified upon F. graminearum and mock inoculation of RILs carrying resistant and susceptible alleles of QTL-Fhb2.
| Observed Mass (Da) | Exact Mass (Da) | Compound Name | FC | |
|---|---|---|---|---|
| RRI | RRC | |||
| Flavonoids | ||||
| 596.14 | 596.14 | Quercetin 3-O-[beta-D-xylosyl-(1->2)-beta-D-glucoside] | 2.59** | 1.47* |
| 770.20 | 770.21 | Isovitexin 2''-O-(6‴-feruloyl)glucoside | 2.20** | |
| 448.10 | 448.10 | Quercitrin | 1.82** | |
| 282.09 | 282.09 | 5,7-Dimethoxyflavone | 1.64** | |
| 282.09 | 282.09 | 7,4'-Di-O-methyldaidzein | 1.64** | |
| 338.12 | 338.12 | Psoralenol | 1.58** | |
| 596.15 | 596.15 | Okanin 4'-(6''-p-coumarylglucoside) | 2.17* | |
| 597.14 | 597.15 | Delphinidin 3-O-beta-D-sambubioside | 1.87* | |
| 614.16 | 614.16 | Safflomin C | 1.80* | |
| 596.15 | 596.15 | Okanin 4'-(6''-p-coumarylglucoside) | 1.79* | |
| 652.16 | 652.16 | Luteolin 7-(6‴-acetylallosyl-(1->2)-glucoside) | 1.77* | |
| 524.15 | 524.15 | Barbatoflavan | 1.69** | |
| Hydroxycinnamic acid amides and Phenylpropanoids | ||||
| 250.13 | 250.13 | N-Caffeoylputrescine | 5.03** | |
| 280.14 | 280.14 | Feruloyl-2-hydroxyputrescine | 3.31** | |
| 165.08 | 165.08 | L-Phenylalanine | 3.14** | |
| 570.20 | 570.19 | Decuroside III | 3.74** | |
| 330.09 | 330.10 | 1-O-Vanilloyl-beta-D-glucose | 2.00* | |
| 578.20 | 578.20 | Podorhizol beta-D-glucoside | 1.94* | |
| 386.12 | 386.12 | 1-O-Sinapoyl-beta-D-glucose | 1.78* | |
| 194.06 | 194.06 | Ferulic acid | 1.32** | |
| Terpenoids | ||||
| 566.33 | 566.32 | 25-Cinnamoyl-vulgaroside | 2.58** | |
| 294.19 | 294.18 | Phytuberin | 1.81** | |
| 360.16 | 360.16 | Triptolide | 1.49** | |
| 520.20 | 520.19 | Brusatol | 2.23* | |
| 448.23 | 448.23 | Atractyloside A | 2.43** | |
| 758.44 | 758.44 | Astaxanthin glucoside | 1.39* | |
| Fatty acids | ||||
| 314.25 | 314.25 | (9Z)-(7S,8S)-Dihydroxyoctadecenoic acid | 6.44* | |
| 438.17 | 438.17 | 2,3-Bis(Trimethylsilyl)Oxy-Butanedioic acid Bis (Trimethylsilyl) Ester | 4.10* | |
| 212.14 | 212.14 | Cucurbic acid | 2.69* | |
| 338.32 | 338.32 | 2,4-dimethyl-2-eicosenoic acid | 1.62* | |
| 403.31 | 403.31 | N-palmitoyl phenylalanine | 2.89** | |
| 375.28 | 375.28 | N-arachidonoyl alanine | 2.24** | |
| 340.33 | 340.33 | Docosanoic acid | 1.69* | |
| 386.19 | 386.19 | 12-hydroxyjasmonic acid 12-O-beta-D-glucoside | 1.64** | |
| 554.30 | 554.29 | 2-O-(beta-D-galactopyranosyl-(1->6)-beta-D-galactopyranosyl) 2S-hydroxytridecanoic acid | 1.61* | |
| 248.18 | 248.18 | 4Z,7Z,10Z,13Z-hexadecatetraenoic acid | 1.44** | |
| Lipids | ||||
| 706.46 | 706.46 | PA(15:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | 17.73* | |
| 682.46 | 682.46 | PA(13:0/22:4(7Z,10Z,13Z,16Z)) | 9.86* | |
| 739.52 | 739.52 | PC(13:0/20:4(5Z,8Z,11Z,14Z)) | 6.56* | |
| 834.53 | 834.53 | PI(15:1(9Z)/19:1(9Z)) | 5.77* | |
| 739.52 | 739.52 | PE(16:0/20:4(5Z,8Z,11Z,14Z)) | 5.27* | |
| 706.46 | 706.46 | PA(15:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | 5.81* | |
| 686.49 | 686.49 | DG(20:5(5Z,8Z,11Z,14Z,17Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0)[iso] | 5.44** | |
| 854.50 | 854.49 | PI(16:1(9Z)/20:5(5Z,8Z,11Z,14Z,17Z)) | 3.30** | |
| Alkaloids | ||||
| 452.26 | 453.27 | Delcosine | 1.59** | |
Fold change (FC) was calculated based on relative intensity of metabolites: RRC = RM/SM and RRI = (RP/RM)/(SP/SM), where RM = Resistant Mock; SM = Susceptible Mock; RP = Resistant Pathogen; RM = Resistant Mock.
Note:
** significant at P < 0.01;
*significant at P < 0.05; the significance of RRI was based on RP>RM and SP>SM.
Fig 2. Classification of metabolites detected at 72 hours post Fusarium graminearum and water inoculations.
Resistant Related Induced (RRI) and Resistant Related Constitutive (RRC) metabolites identified in the study were classified according to their chemical groups. (A) Pie chart shows RRI and (B) shows RRC metabolites classified into various chemical groups.
Resistance related constitutive (RRC) metabolites associated with QTL-Fhb2
A total of 550 RRC metabolites were differentially accumulated, of which 57 were putatively identified (S1 Table). These metabolites belonged to different chemical groups (Table 1; S2 Table; Fig 2B): Flavonoids (13): okanin 4'-(6''-p-coumarylglucoside) (FC = 2.17), delphinidin 3-O-beta-D-sambubioside (FC = 1.87), safflomin C (FC = 1.80); Phenylpropanoids (5): decuroside III (FC = 3.74), 1-O-vanilloyl-beta-D-glucose (FC = 2.0), podorhizol beta-D-glucoside (FC = 1.94), 1-O-sinapoyl-beta-D-glucose (FC = 1.78) and ferulic acid (FC = 1.32); Fatty acids (9): N-palmitoyl phenylalanine (FC = 2.89), N-arachidonoyl alanine (FC = 2.24), and docosanoic acid (FC = 1.69); Terpenoids (3): brusatol (FC = 2.23), atractyloside-A (FC = 2.43), astaxanthin glucoside (FC = 1.39); Glycerophospholipids (22): phosphatidic acid (FC = 5.81), phosphatidylinositol (FC = 3.30), and phosphatidylcholine (FC = 3.26). These high fold change constitutive (RRC) and induced (RRI) metabolites, mainly belonged to phenylpropanoid (hydroxycinnamic acids), flavonoid, glycerophospholipid, and fatty acid classes of compounds, are considered to be responsible for FHB resistance.
Comparative transcriptome of RILs based on RNA-seq
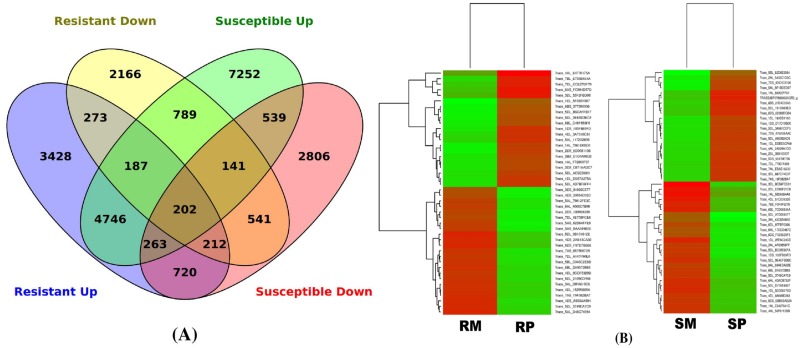
Whole transcriptome analysis (RNA-seq) was done with two genotypes, viz, R-RIL and S-RIL, across four treatments (RP, RM, SP, SM) with three biological replicates for each, collected at 48 hpi. The assembly of sequences, from the 12 sequenced samples, were compared and annotated. Transcriptome analysis using RNA-seq generated 155012 and 165036 transcripts in R-RIL and S- RIL, respectively. The number of transcripts up-regulated and down-regulated in R-RIL and S-RIL are depicted in Fig 3A. The gene ontology analysis classified the transcripts up-regulated in R-RIL and S-RIL based on their involvement in biological processes, molecular functions and cellular component in which they are localized (S1 Fig). The majority of the transcripts depicted their involvement in biological processes such as translation, transcription, response to oxidative stress, lipid metabolism, photosynthesis, DNA repair, protein folding, carbohydrate metabolic processes, trans-membrane transport, suggesting the involvement of various pathways and/or interactions in disease resistance. Differential gene expression (DGEs) analysis classified transcripts as differentially expressed with Log2FC values, or transcripts expressed only in either of the genotypes or expressed only upon pathogen inoculation with FPKM values (Table 2; S2 Table). The differentially expressed transcripts (highly up-regulated and highly down-regulated) in R-RIL and S-RIL are shown in the form of heat maps with the respective gene IDs (Fig 3B). Pathway analysis of transcripts showed that majority of transcripts belonged to phenylpropanoid, flavonoid, fatty acid, oxylipin/jasmonic acid, and phospholipid pathways.
Fig 3. Differentially expressed transcripts in RILs.
(A). Venn diagram showing number of transcripts detected as up and down-regulated (P < 0.01) in R-RIL and S-RIL, 48 hours post Fusarium graminearum inoculation. (B). Heat Maps showing differentially expressed transcripts in R-RIL and S-RIL upon Fusarium graminearum and mock inoculations. They show transcripts differentially expressed transcripts between resistant mock (RM) and resistant pathogen (RP) and between susceptible mock (SM) and susceptible pathogen (SP), respectively.
Table 2. Differentially expressed transcripts in R-RIL and S-RIL upon Fusarium graminearum and mock inoculation at 48 hpi.
The differential expressions are in log2FC values for the resistant genotype and in FPKM values following pathogen inoculation. The up-regulated transcripts were classified according to their biosynthetic pathways. The transcripts localized within QTL-Fhb2 are marked with an asterisk (*).
| Gene ID | Annotations | Expression Profile | |||
|---|---|---|---|---|---|
| Log2FC values (RP/RM)/ (SP/SM) | R-RIL (RP/RM) Log2FC values | RP with FPKM values | FPKM fold change (RP/SP) | ||
| Phenylpropanoid and monolignol biosynthesis | |||||
| Traes_5AL_E23B0E6C4 | agmatine coumaroyltransferase-2 | 11.08 | |||
| Traes_5DL_7F0CD0F79 | caffeic acid 3-o-methyltransferase | 10.16 | |||
| Traes_3AL_BF387E832 | laccase-19 | 3.76 | |||
| Traes_2DL_8BA1CE63D | phenylalanine ammonia-lyase | 4.15 | |||
| Traes_6BS_CC8E63D7F | 4-coumarate: CoA ligase* | 1.23 | |||
| Traes_7DS_D313DB9ED | laccase | 2.26 | |||
| Traes_6BS_6477278C5 | cinnamyl alcohol dehydrogenase* | 1.05 | |||
| Flavonoid biosynthesis | |||||
| Traes_6AS_4D2E3A85C | chalcone synthase 8 | 4.14 | |||
| Traes_7DS_3EE1E7974 | cinnamoyl reductase | 1.47 | |||
| Traes_7DL_33BB5BE33 | trans-cinnamate 4-monooxygenase | 1.30 | |||
| Traes_6AL_C82FB1662 | dihydroflavonol 4-reductase | 3.52 | |||
| Phosphoglycerolipid biosynthesis | |||||
| Traes_1AL_7C3E4A07E | diacylglycerol kinase | 1.05 | |||
| Transcription factors | |||||
| Traes_6BS_00E54518B | transcription factor bHLH041* | 0.42 | |||
| Traes_7DL_5968FA56C | WRKY transcription factor | 1.30 | |||
| Traes_1AS_36AF74187 | R2R3 MYB transcription factor | 1.19 | |||
| Traes_6AL_BB730675A | MYB-related protein MYB4 | 1.22 | |||
| Receptor Kinases | |||||
| Traes_4AL_7CC0FB23A | lectin receptor kinase | 4.08 | |||
| Traes_5AL_6640183AF | serine threonine-protein kinase | 2.93 | |||
| Traes_6DL_BCE522BB4 | proline-rich receptor-like protein kinase perk1 | 2.60 | |||
| Traes_5DL_61ECD451B | wall-associated receptor kinase 3 | 2.45 | |||
| Pathogenesis related proteins | |||||
| Traes_3AL_C932A3F30 | peroxidase 2 | 45.08 | |||
| Traes_7DS_F962AB6D6 | pathogenesis-related protein 1 | 4.14 | |||
| Traes_7BL_408E23082 | chitinase 1 | 1.41 | |||
| Traes_5AS_FAD05211F | pathogenesis-related protein sth-21 | 1.34 | |||
| Detoxification related transcripts | |||||
| Traes_6BS_4723C124F | abc transporter b family member 4* | 1.03 | |||
| Traes_2DS_DD9B280C5 | udp-glycosyltransferase 85a2 | 2.65 | |||
| Traes_5AS_067CB4CF9 | udp-glycosyltransferase 74e1 | 4.51 | |||
| Traes_7BL_D3A25B6C7 | pleiotropic drug resistance protein 4 (PDR) | 3.61 | |||
| Traes_6BS_4EED05084 | glutathione s-transferase* | 1.95 | |||
| Callose Synthesis | |||||
| Traes_6BS_1668BA98C | callose synthase 7-like* | 1.54 | |||
RP = Resistant pathogen, RM = Resistant Mock, SP = Susceptible pathogen, SM = Susceptible Mock. FPKM = Fragment per kilobase of transcript per million mapped reads.
Genotype-specific transcriptional changes in response to F. graminearum
The genotype-specific transcriptional modulations are clear indications from the gene ontology classification (S1 Fig), showing the differences in the functional categories of the transcripts in each RIL. The R-RIL dataset, in particular, upon pathogen treatment are considered best to identify the transcripts that would possibly be involved in imparting FHB resistance. Nevertheless, in S-RIL pathogen inoculated dataset, the transcripts down-regulated were not overlooked. The transcripts up-regulated in R-RIL were further classified according to their involvement in various biosynthetic pathways or regulators of gene expression (transcription factors, protein kinases, and secondary messengers) or transcripts belonging to RR-proteins involved in DON detoxification (Table 2). The transcription factor bHLH041 (FPKM = 0.42) was detected only in RP, suggesting its role in FHB defense. Apart from bHLH, transcription regulatory genes like WRKY, R2R3 MYB and MYB-4 were up-regulated in R-RIL (Table 2). The transcripts belonging to phenylpropanoid pathway genes such as agmatine coumaroyltransferase-2 (ACT, FPKM = 11.08), caffeic acid 3-o-methyltransferase (CoMT, FPKM = 10.16), laccase (FPKM = 3.19) were detected only in RP, while phenylalanine ammonia-lyase (PAL, FC = 4.15) and 4-coumarate: CoA ligase (4CL, FC = 1.23) were detected only in R-RIL. Cinnamyl alcohol dehydrogenase (CAD) was detected in both the RP and SP, with higher expression in RP. Chalcone synthase 8 (CS8), cinnamoyl reductase (CR), and dihydroflavonol 4-reductase (DHFR) genes of flavonoid biosynthetic pathway were up-regulated in RP. Receptors kinases like lectin receptor kinase (LRK, FC = 4.08), proline-rich receptor-like protein kinase perk (FC = 2.60), wall-associated receptor kinase 3 (WAK3, FC = 2.45) were also up-regulated in RP. Transcripts belonging to PR protein, peroxidase 2 (PR2, FPKM = 45.08) was detected only in RP. Several other PR proteins such as PR1, PR2, and chitinases were also up-regulated in RP. Transcripts involved in the detoxification were highly up-regulated in RP such as, UDP-glycosyltransferases, multidrug resistance proteins, pleiotropic drug resistance proteins, ABC transporters and glutathione S- transferases (Table 2; S2 Table). All the transcripts up-regulated in resistant genotype were reported to be involved in FHB resistance, thus implicating the involvement of a hierarchy of genes and/or interactions in imparting FHB resistance in wheat.
Genetic controls underlying QTL-Fhb2
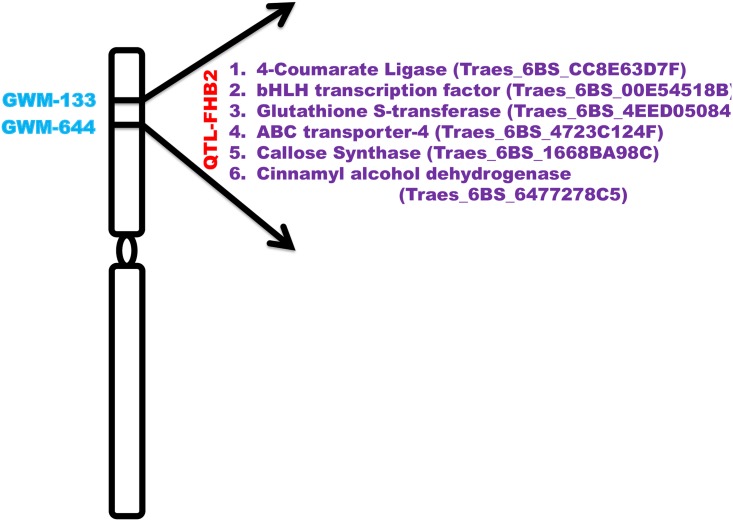
The markers flanking the QTL were sequenced and the region (sequence) within the two flanking markers was considered as QTL-Fhb2 region using wheat survey sequence available (http://wheat-urgi.versailles.inra.fr/). The transcripts aligning to the 6BS reference genome were pulled out separately and furthermore, the transcripts aligning to the sequence within the flanking marker co-ordinates were considered as genes localized within QTL-Fhb2 region. Based on high FC metabolites and transcripts in R-RIL we were able to localize six putative candidate genes within the QTL-Fhb2 region that were associated with biotic stress resistance functions (Fig 4). The putative candidate genes localized within the region were: 4CL, bHLH041 TF, GST, ABC-4, CS, and CAD. The expression values for these genes localized within the QTL-Fhb2 region are presented in Table 2 (transcripts marked with asterisk (*)). The list of all the genes localized within the QTL-Fhb2 region is provided in S3 Table.
Fig 4. Wheat chromosome 6B, depicting the physical location of QTL-Fhb2 (marked red), on short arm of the chromosome 6B, flanked by two SSR markers GWM-133 and GWM-644 (marked blue).
The genes localized within the QTL locus were identified using metabolo-transcriptomics approach, which are shown within two arrow marks, marked purple in text. The corresponding gene Ids are given in parenthesis.
Confirmation of gene expression based on qRT-PCR
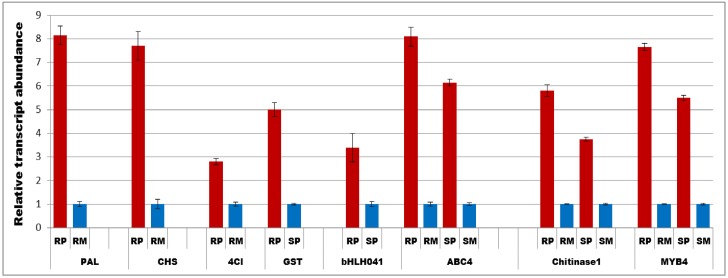
To validate the RNA-seq data a qRT-PCR analysis was carried out for a few selected genes, such as, 4CL, bHLH041, ABC-4, GST, chitinase1, CHS, PAL and MYB4. The expression values obtained from qRT-PCR analysis for the selected genes were similar to RNA-seq confirming the reproducibility of transcriptome data (Fig 5).
Fig 5. Quantitative Real time PCR (qRT-PCR) showing transcript abundances of selected genes at 48 h post F. graminearum and water inoculation.
The relative transcript abundance were calculated compared to mock treatments and for transcripts which were only expressed in pathogen treated treatments, susceptible pathogen was used to compare the abundance in resistant pathogen. RP = Resistant pathogen, RM = Resistant mock, SP = Susceptible pathogen and SM = Susceptible mock. PAL-Phenylammonia lyase, CHS = chalcone synthase, 4Cl = 4 coumarate CoA-ligase, GST = Glutathione S-transferase, bHLH041 = basic helix loop helix transcription factor, ABC4 = ABC transporter4.
Discussion
Resistance in wheat against FHB is quantitative and more than 100 QTLs with major or minor effects have been identified and mapped on all wheat chromosomes, expect on 7D. However, the genetic controls underlying these resistant QTLs are yet to be revealed. These QTL regions, even when they are fine mapped, contain several genes, including those conferring resistance. Identification of candidate genes and their resistance functions are crucial to map the hierarchical network of genes involved in the biosynthesis of a given or set of RR metabolite(s), which directly suppresses the pathogen progress. The R genes biosynthesizing these RR metabolites are regulated by other R genes; thus the functional hierarchy must be confirmed before an individual gene can be transferred to a susceptible genotype [12]. Also these specific candidate genes can be transferred without linkage drag effects based on genome editing tools. In view of this, an attempt was made to dissect one of the major FHB resistant QTL-Fhb2 to identify the underlying genetic controls. Integrating two systems biology disciplines, metabolomics and transcriptomics, we were able to identify putative genes localized within the QTL-Fhb2 region and their plausible mechanisms of resistance.
Metabolic profiling identified potential FHB resistance biomarkers
Non-targeted metabolic profiling of genotypes with contrasting levels of FHB resistance identified several RR metabolites, highly accumulated, in different metabolic pathways such as glycerophospholipid, phenylpropanoid (HCAAs), flavonoid, fatty acid and terpenoid. Glycerophospholipids like phosphatidic acid (PA) (FC = 17.72), phosphatidylcholine (FC = 6.56), phosphatidylinositol (FC = 5.77) are known to be deposited to enforce cell walls. Furthermore, these are also known to perceive and transmit signals activating downstream genes eventually regulating R genes to biosynthesize RR metabolites and RR proteins [43]. These compounds are either converted into bioactive lipids (components of lipid bilayer of cell membrane) or stay as soluble molecules (messengers), further binding to the downstream mitogen activated protein kinases (MAPK), further affecting the enzymatic activities, vesicle trafficking and ion fluxes [43]. PA acid is an important secondary messenger in plant stress signaling [44]. These stresses involve pathogen attack, salinity, cold, drought, heat and wounding. In regard to pathogen response, PA acid has been shown to accumulate in response to various elicitors such as xylanase, flagellin, and chitosan [45]. Interestingly, a gene diacylglycerol kinase (DGK) that catalyzes the conversion of structural lipids (PC, PE, PS) into PA was upregulated in our study. This clearly illustrates that the early membrane modifications and their involvement in further activating defense responses are very crucial.
Rachis resistance in wheat is mainly due to the deposition of HCCAs [6]. In our study, we detected a higher abundance of two HCCAs in particular, N-Caffeoylputrescine (FC = 5.03) and Feruloyl-2-hydroxyputrescine (FC = 3.31) upon F. graminearum inoculation. Suberin, a complex, intractable biopolymer, with polyaromatic domain of HCCAs, is deposited apoplastically between the primary cell wall and plasma membrane to enforce cell walls [46]. Once, the cell walls are thickened the pathogen won’t be able to spread within the spike, through the rachis, thus imparting high levels of rachis resistance. Resistance in NIL carrying resistant alleles of QTL-Fhb1 was reported to be due to the deposition coumaroylputrescine, feruloylputrescine, coumaroylagmatine, cinnamoylserotonin, feruloylagmatine, p-coumaroylserotonin [6]. HCCAs such as feruloylputrescine, p-coumaroyltyramine, N-feruloyltyramine, 4-coumaroyl-3-hydroxyagmatine, feruloylagmatine, 4- coumaroylagmatine, terrestriamide, and feruloylserotonin were reported to impart late blight resistance in potato [30,33]. HCCAs imparted resistance in tomato against Pseudomonas syringae [47], in maize against F. graminearum [48], in onion against Botrytis allii [49]. These HCCAs are not only involved in cell wall thickening, but also they possess antioxidant, antiviral, antibacterial and antifungal activities [47]. Silencing of St-WRKY1 reduced not only HCAAs, but also increased susceptibility of potato to late blight, thus confirming the role of HCAAs in resistance. These HCCAs can be used as markers to screen FHB resistance genotypes, because RR metabolites being the end products of R gene function, guarantee resistance [14].
Apart from HCCAs, we also have identified several flavonoids with high abundance, as RRI and RRC metabolites, such as, quercetin 3-O-[beta-D-xylosyl-(1->2)-beta-D-glucoside], isovitexin 2''-O-(6‴-feruloyl) glucoside, quercitrin, 5,7-dimethoxyflavone, 7,4'-di-O-methyldaidzein, psoralenol, fisetinidol-4beta-ol 3,4,7,3',4'-pentamethyl ether, glyceollin I, and 3,7-di-O-methylquercetin. The growth of Fusarium and macroconidia formation is completely inhibited by dihydroxyquercetin [50], and of the fungus Neurospora crassa by quercetin 3-methyl ether and its conjugated glucosides [51]. The deposition of flavonoid conjugates (of glucoside and methoxy) was higher in rachis tissues of NIL carrying resistance alleles of genes in QTL-Fhb1 [6]. The involvement of both preformed and induced flavonoids in plant defense against pathogens, herbivores, and environmental stress is well documented [52]. In resistant barley several flavonoids were accumulated in high abundance upon F. graminearum inoculation [35,38].
We detected high fold accumulation of preformed and induced free fatty acids such as dihydroxyoctadecenoic acid, 2,3-bis(trimethylsilyl)oxy-butanedioic acid bis (trimethylsilyl) ester, cucurbic acid, 2,4-dimethyl-2-eicosenoic acid, N-palmitoyl phenylalanine, and N-arachidonoyl alanine. Fatty acids are not only part of structural constituents, but also act as secondary messengers and regulators of signal transducing molecules or transcription factors [53]. Arachidonic acid acts as an elicitor in plant defense response to phytopathogens [54]. Several free fatty acids were accumulated in barley upon F. graminearum invasion [38]. The antifungal capabilities of octadecenoic, tetradecanoic, docosanoic, butenoic acid have been reported [55]. Hence, these fatty acids may be crucial components of FHB resistance in wheat, not only acting as physical barriers, but also as antimicrobials.
Transcriptome changes provided key insights to genetic reprogramming upon pathogen invasion
Transcripts belonging to phenylpropanoid and flavonoid pathways, including receptor kinases, transcription factors, detoxification, and signaling genes were highly regulated, following pathogen inoculation (Table 2). The elicitors produced by pathogen are perceived by plant membrane receptors. In our study, we found higher transcript abundances of lectin receptor kinase (LRK), serine threonine-protein kinase (STPK), proline-rich receptor-like protein kinase (PERK1), and wall-associated receptor kinase 3 (WAK3). The role of LRKs [56], STPKs [57], and WAKs [58] in plant defense is well documented. The overexpression of WAK1 in Arabidopsis thaliana conferred higher resistance to Botrytis cinerea [58]. The resistance to fungal pathogen Magnaporthe grisea was due to a G-type lectin receptor kinase (Pi-d2) in rice [59]. These receptor kinases transduce signals downstream, activating several groups of TFs. The TFs belonging to bHLH, WRKY and MYB groups were up-regulated, depicting their involvement in regulating downstream R genes that biosynthesize RR metabolites and proteins. In our study, the phenylpropanoid pathway genes, such as, agmatine coumaroyltransferase (ACT), caffeic acid 3-o-methyltransferase (CoMT), laccase, phenylalanine ammonia-lyase (PAL), 4-coumarate CoA ligase (4CL), and cinnamyl alcohol dehydrogenase (CAD) were highly expressed in R-RIL. PAL, a hub gene, that biosynthesizes precursor for phenylpropanoid biosynthetic pathway, was highly up-regulated in Sumai-3 upon F. graminearum invasion [41]. ACT which is localized within wheat FHB resistant QTL-2DL imparts high rachis resistance by cell wall thickening [60]. 4CL is an important R gene for both lignin and flavonoid biosynthesis, and was induced in cucumber against powdery mildew [61], cotton against wilt fungus Verticillium dahlia [62], and potato against Phytophthora infestans [30]. Laccase and peroxidase (POD) involved in lignin biosynthesis were up-regulated in our study, emphasizing an increased lignification of cell walls around infection site in R-RIL. In our study, the peroxidase was highly expressed in RP (FPKM = 45.08). The involvement of POD in the defense responses of wheat to Fg infection has been reported [39]. Genes involved in flavonoid biosynthesis like chalcone synthase8 (CHS8), cinnamoyl reductase (CR) and dihydroflavonol 4-reductase (DHFR) were detected only in R-RIL. The disruption of flavonoid pathway significantly reduced flavonoid metabolites [61]. Resistance in wheat to the hemibiotrophic pathogen, Septoria tritici was due to a higher accumulation of CHS transcripts [63]. RR proteins restrict the spread of pathogens. In our study, we detected several PR proteins such as chitinase, peroxidase, and PR1 with higher expressions. Chitinases are very important in plant defense against many fungal pathogens, as they degrade the fungal cell walls, which are primarily made up of chitin. Expression of barley class-II chitinase gene in wheat conferred high level of resistance against F. graminearum under greenhouse and field conditions [64]. Expression of rice chitinase enhanced resistance against Magnaporthe grisea in rice [65], Uncinula necator in Italian ryegrass [66], and Puccinia coronata in grapevine [67].
Mycotoxins produced by Fg, such as trichothecenes, play a major role in pathogenesis, especially DON, a well-known virulence factor. Therefore, the resistance to DON is crucial to confer enhanced FHB resistance [68]. Mutant Fg strains (unable to produce DON) showed reduced FHB severity [69]. In previous studies, it has been reported that DON is converted into less toxic DON-3-O-glucoside (D3G) [70,71]. However, several genes are involved in DON reduction in plants, such as multidrug-resistant protein, multidrug resistance-associated protein, UDP-glycosyltransferase and ABC transporters were detected with higher transcript abundances upon Fg inoculation in wheat cv. Nobeokabouzu-komugi [17]. The accumulation of glutathione S-transferase (TaGSTF5) in wheat resistant cv. Ning7840 upon Fg invasion was significantly higher [72]. Similarly, in our study we found higher expressions of ABC transporter b family member 4, UDP-glycosyltransferase 85a2, UDP-glycosyltransferase 74e1, pleiotropic drug resistance protein 4 and glutathione S-transferase in R-RIL upon pathogen inoculation. Transgenic Arabidopsis and wheat expressing a barley UDP-glucosyltransferase (HvUGT13248) detoxifies deoxynivalenol and provides high levels of resistance to F. graminearum [68,73]. ABC transporter proteins (yeast PDR5 like) confined to plasma membrane confers partial resistance against trichothecenes in wheat by serving as drug efflux pumps [74]. The higher transcript abundances of detoxification genes, clearly explain the reduced levels of DON accumulation in R-RIL, thus contributing to FHB resistance.
QTL-Fhb2 imparts resistance through additive effects of cell wall reinforcement and DON detoxification
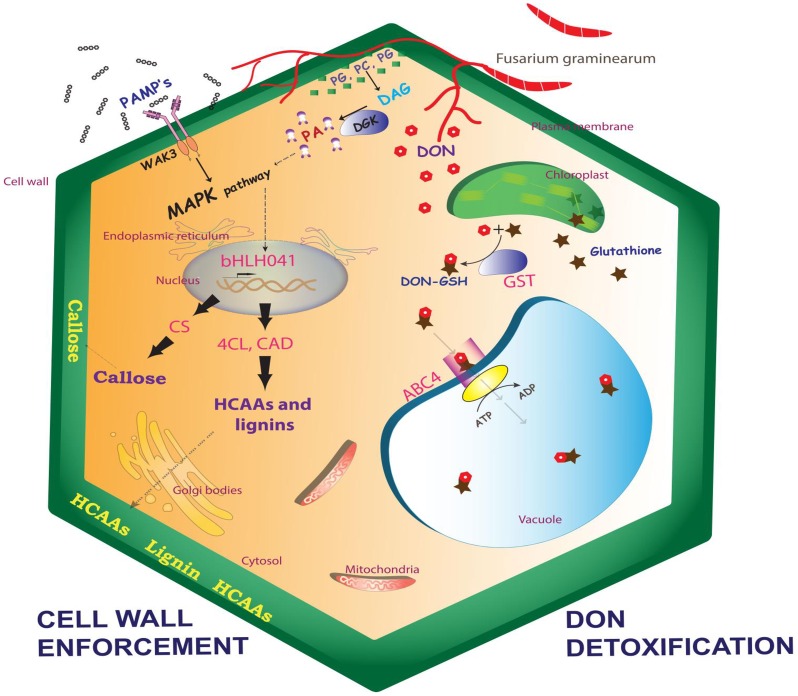
The markers flanking the QTL locus were sequenced and the sequence (http://wheat-urgi.versailles.inra.fr/) within the two flanking markers was retrieved and the potential R genes in that region were identified (Fig 4). QTL-Fhb2 was consistently mapped on chromosome 6BS, conferring high rachis resistance [23,24]. Based on high FC metabolites and transcripts, we identified 4CL, CS, bHLH041, GST, ABC4, and CAD as putative candidate R genes localized within the QTL-Fhb2 region. Based on our study, we propose a hypothetical model for FHB resistance in wheat line carrying resistant alleles of genes in QTL-Fhb2 (Fig 6). The importance of each candidate gene in the model on plant defense is discussed.
Fig 6. Hypothetical model for FHB resistance in wheat line carrying resistant alleles of R genes in QTL-Fhb2.
Based on our findings we propose that the QTL-Fhb2 imparts high rachis resistance through combined effects of cell wall reinforcement and DON detoxification.
4CL, CS and CAD confer resistance through cell wall reinforcement
4-Coumarate CoA: Ligase (4CL)
We detected a higher transcript abundance (FC = 1.23) of 4CL in R-RIL. 4CL is an important enzyme that catalyzes the conversion of coumaric, ferulic, caffeic, and sinapic acids into hydroxycinnamoyl-CoA thiol esters, which further serve as precursors in lignin, flavonoid, polyphenol, coumarin and suberin biosynthesis [75]. Two HCCAs, N-Caffeoylputrescine (FC = 5.03) and feruloyl-2-hydroxyputrescine (FC = 3.31) were in high abundance, showing a higher expression of 4CL to biosynthesize them in R-RIL than in S-NIL. Increased expression of 4CL increased HCCAs accumulation in potato against P. infestans, imparting resistance through thickening of cell walls [28–30,33]. Interestingly, we also detected higher abundances of metabolites and transcripts from lignin and flavonoid pathways, implicating the role of 4CL in the biosynthesis of precursors. Silencing of 4CL reduced lignin content in switch grass [76], and poplar [77]. As 4CL is a hub R gene in the phenylpropanoid pathway leading to the biosynthesis of lignins and flavonoids, we consider this to be a potential candidate R gene conferring high rachis resistance to FHB.
Callose synthase (CS)
Plants restrict the spread of pathogens through deposition of an RR metabolite, such as callose (β-1,3-glucan) to form cell wall appositions or papillae [78]. Papillae are complex structures formed around the invading hyphae, which are produced in-between plasma membrane and the cell wall. The papilla is composed of different classes of compounds such as, phenolics, reactive oxygen species, cell wall proteins and glucans [79]. In our study, we also detected a higher transcript abundance of callose synthase. Callose synthase 5 (CalS5) in Arabidopsis thaliana plays a predominant role in the synthesis of the callose wall and callose plugs, and containment of powdery mildew hyphae in Arabidopsis [80]. Arabidopsis thaliana callose synthase PMR4 expression in barley increased penetration resistance to powdery mildew [81]. Hence, we consider callose synthase as one of the integral candidates in FHB defense.
Cinnamyl alcohol dehydrogenase (CAD)
CAD is a key enzyme in lignin biosynthesis that catalyzes reduction of cinnamaldehydes into cinnamyl alcohols, the last step of monolignol biosynthesis, before oxidative polymerization in the cell wall [82]. Lignin is a complex phenolic polymer which is deposited in the cell walls of many plants. Deposition of lignin (lignification) is known to confer resistance against invading pathogens. Lignification thickens the cell wall and makes it difficult for fungal appressoria to penetrate into the cell [83]. It also makes cell walls more water resistant, and in turn, less accessible to cell wall degrading enzymes [83]. Gene expression profiling and silencing showed monolignol biosynthesis is very important in penetration defense in wheat against powdery mildew invasion [84]. Cinnamyl alcohol dehydrogenase-C and D play a crucial role conferring resistance in Arabidopsis against bacterial pathogen Pseudomonas syringae pv. tomato [85]. AtCAD1 is involved in lignification of elongating stems in Arabidopsis thaliana [86]. CAD was upregulated in NILs containing QTL-Fhb1, upon pathogen invasion [6].
ABC transporter and GST aiding resistance through DON reduction
ABC transporter-4 (ABC-4)
DON inhibits eukaryotic protein synthesis and increases the virulence of F. graminearum by suppressing RR protein and metabolite biosynthesis in plants. A wheat ABC transporter (TaABCC3.1) imparts DON tolerance [87]. A TaABCC (ABC transporter C family) gene within FHB resistant QTL-2DS conferred resistance by reducing DON accumulation [88]. TaABCC gene underlying wheat resistance QTL-Fhb1 imparts FHB resistance [89]. Similarly, in our study we identified higher transcript abundance of ABC transporter b-family member 4 and consider this to play a significant role in rachis resistance, by reducing DON for virulence.
Glutathione S-transferase (GST)
GSTs play an important role in plant resistance against biotic and abiotic stresses [90]. These are dimeric enzymes that catalyze the conjugation of electrophilic molecules to glutathione (GSH) [91]. DON the Fusarium virulence factor upon conjugation with GST is presumably detoxified, thus decreasing the pathogenicity of the fungus [27,92]. GSTs from barley are reported to detoxify DON [70]. Glutathione S-transferase genes were induced in Nicotiana benthamiana upon Colletotrichum destructivum and C. orbiculare infection and a GST gene was implicated in resistance [91]. GSTs were induced in potato upon Phytophthora infestans, wheat upon Erysiphe graminis and Arabidopsis upon Peronospora parasitica infection [93]. The expression of GST gene from Lilium regale conferred resistance to Fusarium oxysporum in tobacco [94]. Similarly, we presume that the GST identified in our study, reduces the virulence of pathogen by detoxification of DON, and in turn, aid in enhancing FHB resistance.
bHLH041: A novel candidate for FHB resistance
The bHLH group of transcription factors shares a basic helix loop helix protein structure. The size of these transcription regulators varies anywhere from 60–100 amino acids and consists of two highly conserved domains [95]. The N-terminal basic domain functions as a DNA-binding domain, and the second basic domain which is separated by a loop, determines the dimerization capacity of a protein [95,96]. These transcription regulators usually bind to a consensus sequence known an E-box (CANNTG) [97]. The role of bHLH transcription regulators in plant response to pathogen attack has been well documented [96,98]. A transcription regulator TabHLH060 was highly expressed in wheat leaves upon invasion by an obligate pathogen Puccinia striiformis f. sp. tritici [96]. In our study, the transcription regulator TabHLH041 was detected only in RP (FPKM = 0.42). Further functional characterization and the identification of its potential downstream targets should increase our understanding about its role in FHB defense reactions.
Our study identified several important R genes localized in the QTL-Fhb2. Even though this QTL was fine mapped it contains several genes with different mechanisms of resistance, but acting cumulatively to impart high level of rachis resistance. These genes should be sequenced to verify if they are functional in R-RIL, but nonfunctional in S-RIL. These polymorphic candidate genes identified here can be used in breeding, following validation of gene resistance functions based on silencing these genes in resistant genotype. The functional alleles of these genes can be used to replace the alleles in susceptible commercial cultivars, if nonfunctional, based on genome editing.
Conclusion
FHB resistance is polygenic in nature; many R genes with major and/or minor effects contribute cumulatively conferring resistance. The mapped FHB resistant QTLs localize several genes governing resistance. We dissected FHB resistant QTL-Fhb2, based on integrated metabolo-transcriptomics approach and putatively identified six resistance genes. These genes confer structural resistance through cell wall reinforcement, reducing the spread of pathogen through rachis within the spike. Several other genes detoxify DON, the virulence factor, and thus reducing the severity of the disease. In conclusion, we report that the wheat resistant QTL-Fhb2 confers high rachis resistance through combined effects of cell wall enforcement and reduction of DON. The candidate genes identified here, upon functional characterization, can pave the way to develop highly FHB resistant genotypes.
Materials and Methods
Genetic background of RILs carrying contrasting alleles of QTL-Fhb2
The recombinant inbred population of 1,440 F2:7 lines were developed using single seed descent method, through a cross between BW-278 (AC Domain*2/Sumai-3 = FHB resistant parent) and AC Foremost (HY320*5/BW553//HY320*6/7424-BW5B4 = FHB susceptible parent) [23]. The resistant parent BW-278 is a descendant of Sumai-3, a Chinese wheat cultivar that exhibits high rachis resistance and is the source of major FHB resistance QTLs. However, BW-278 is known to lack QTL-Fhb1 resistance alleles on chromosome 3BS. The RILs derived segregated for three known FHB resistance QTLs on chromosomes 3BSc, 5A (QTL-Fhb5), and 6B (QTL-Fhb2). The lines were further genotyped using simple sequence repeat (SSR) markers on chromosome 6B (WMC104, WMC397, GWM219), 5A (GWM154, GWM304, WMC415), and 3BS (WMC78, GWM566, WMC527) to select RILs homozygous susceptible for QTL intervals on 3BSc, 5A, and recombinant for the interval on 6B carrying the FHB resistance gene [23]. The seeds of FHB resistant and susceptible RILs carrying contrasting alleles of QTL-Fhb2 were obtained from Dr. Curt McCartney (AAFC, Winnipeg, MA). The highly resistant RIL = PbI-170 (carrying resistance alleles of QTL-Fhb2, R-RIL) and the highly susceptible RIL = QeJ-004 (carrying susceptible alleles of Fhb2, S-RIL) were selected for this study taking into consideration the phenotypic data, both greenhouse and field, provided by Dr. McCartney.
Plant and pathogen production, and inoculation
All experiments were conducted in greenhouse as a randomized complete block design, with two RILs (R-RIL and S-RIL), two inoculations (pathogen and mock as control) and three to five replications over time, depending upon the nature of the experiment. In each pot, 4 seeds were sown at 5 d intervals. Plants were grown at 25 ± 3°C, 70 ± 10% relative humidity and 16 hours of photoperiod throughout the growing period. Plants were irrigated every day and fertilized at 15 d intervals with 20-20-20 NPK, and the trace elements according to the growth stage of the plants [6]. The F. graminearum, isolate Z-3639 was initially cultured on potato dextrose agar (PDA, DIFCO Laboratories Detroit, Michigan, USA) medium to produce mycelia and then in rye-B agar medium with incubation under UV light for the production of macroconidia [99]. Cultures grown for a week were used to prepare macroconidial suspension for inoculations. The final concentration of spore in the suspension was adjusted to 105 ml-1 using sterilized distilled water and 10 μl suspension was inoculated per spikelet [20]. Plants were inoculated at about 50% anthesis (GS = 60–65) and were covered with plastic bags for 48 hours to maintain high humidity in order to facilitate initial infection.
Phenotyping of RILs
Disease severity analysis and fungal biomass quantification were carried out to phenotype RILs, for FHB resistance. For disease severity analysis, a single alternate pair of spikelets, in the middle of a spike of each RIL was inoculated with F. graminearum macroconidial suspension. The total number of spikes inoculated per replication were ten and the total number of replications were five. The number of spikelets diseased were recorded every three days until 21 days post inoculation (dpi) and the proportion of spikelets diseased (PSD) and the area under disease progress curve (AUDPC) was calculated. For fungal biomass quantification, three alternate pairs of spikelets per spike per RIL were inoculated with Fg spore suspension and distilled water, separately. The number of spikes inoculated per replication and the number of total replications were three. 7dpi, six successive pairs of spikelets (three alternate pairs inoculated and three uninoculated) were harvested; the rachis region underlying these six spikelets was collected. The total DNA was extracted from rachis tissues using DNeasy Plant Mini Kit (Qiagen, Germany). The concentration of DNA was normalized to 20 ng/μl and the fungal biomass was quantified using F. graminearum specific Tri6 as target gene and wheat specific Actin as a housekeeping gene [20]. The relative gene copy number of Tri6 was calculated following 2−ΔΔCT method [100].
Sample collection, metabolite extraction, metabolic profiling, and data analysis
For metabolic profiling, the experiment was conducted in a randomized complete block, with two genotypes (R-RIL and S-RIL), two inoculations (pathogen = P and mock = M) and five replications per treatment. Three alternate pairs of spikelets of a spike were inoculated with F. graminearum spore suspension (P) or distilled water (M). 7dpi, six successive pairs of spikelets (three alternate pairs inoculated and three uninoculated) were harvested and the rachis region underlying these six spikelets was separately collected, ground in liquid nitrogen using pre-chilled mortar and pestle. 100 mg of tissue samples were weighed in 2 ml sterilized micro-centrifuge tubes and used for metabolite extraction. Metabolites were extracted using absolute methanol followed by 60% methanol in order to extract polar, semi-polar and non-polar metabolites [101]. The metabolites were analyzed using LC-ESI-LTQ-Orbitrap-MS. Randomization of samples was done to avoid any structural errors associated with the liquid chromatography and high resolution mass spectrometry (LC-HRMS = LC-LTQ-Orbitrap) analysis. The output data files obtained from LC-MS analysis were first converted into mzXML/.cdf and were exported to MZmine2 software for peak deconvolution, peak detection, spectral filtering and normalization of peaks [102].
The abundance of peaks were subjected to paired t-test (comparison of two treatments at a time) to identify treatment significant metabolites. Treatment significant metabolites with P < 0.05 were retained for further analysis. Metabolites with higher abundance in resistant genotype (R-RIL) than in susceptible genotype (S-RIL) were considered as resistance related (RR) metabolites. A RR metabolite based on inoculation (M) was considered as constitutive resistance related constitutive (RRC = RM>SM) metabolite. A metabolite with significantly higher abundance in the pathogen inoculated treatments than in mock inoculated treatments was considered as a pathogenesis related (PR) metabolite, in resistant (PRr = RP>RM) or susceptible (PRs = SP>SM) genotypes. A PRr metabolite in a resistant genotype with abundance greater than that in susceptible pathogen (PRs) inoculated was considered as a resistance related induced metabolite (RRI = (RP>RM) > (SP>SM)) metabolite [6,10]. The resistance metabolites were identified with putative compound names using different databases PlantCyc (http://www.plantcyc.org/), METabolite LINk (METLIN) (https://metlin.scripps.edu), KNApSAcK (http://kanaya.naist.jp/KNApSAcK/) and Kyoto encyclopedia genes and genomes (KEGG) (http://www.genome.jp/kegg/). The putatively identified metabolites were further confirmed based on: i) accurate mass match (with accurate mass error < 5 ppm) [6,35]; ii) fragmentation pattern match [35,38].
Sample collection, RNA extraction, library preparation, Illumina sequencing, and data analysis
For transcriptome analysis, three alternate pairs of spikelets of three spikes per RIL were inoculated with F. graminearum spore suspension and water (control). Each treatment (resistant pathogen = RP, resistant mock = RM, susceptible pathogen = SP, susceptible mock = SM) consisted of three biological replicates. At 48 hpi, the three inoculated and three un-inoculated spikes were harvested, the rachis in inoculated region was harvested, and ground in liquid nitrogen using pre-chilled mortar and pestle. 100 mg of tissue samples were weighed in 2 ml sterilized micro-centrifuge tubes and the total RNA was extracted using Qiagen RNeasy Mini Kit (Qiagen, Germany). Total RNA was quantified using a NanoDrop Spectrophotometer ND-1000 (NanoDrop Technologies, Inc.) and its integrity was assessed using a 2100 Bioanalyzer (Agilent Technologies). Libraries were prepared from 250 ng of total RNA using the TruSeq stranded mRNA sample preparation kit (http://www.illumina.com/products/truseq_stranded_mrna_sample_prep_kit.html), as per the manufacturer’s recommendation. Using the Poly-A selection, mRNA molecules were separated and fragmented, followed by cDNA synthesis, ligation of adapters and cDNA fragments enrichment (PCR) (http://www.illumina.com/applications/sequencing/rna/mrna-seq.html). Libraries were quantified using the Quant-iT™ PicoGreen® dsDNA Assay Kit (Life Technologies) and the Kapa Illumina GA with Revised Primers-SYBR Fast Universal kit (D-Mark). Average size fragment was determined using a 2100 Bioanalyzer (Agilent Technologies). Libraries were sequenced with an Illumina Hiseq 2000 sequencer, with 100 bp paired-end reads (http://support.illumina.com/sequencing/sequencing_instruments/hiseq_2000.html). The Illumina Hiseq paired end raw reads were quality checked using FastQC. The raw reads were processed using ABLT in-house program to filter adapters and low quality bases towards 3'-end. The raw reads obtained were aligned to the reference genome using TopHat [103]. Cufflinks package was used to assemble transcript, estimates their abundances, and tests for differential expression and regulation [104]. The Cuffdiff program in Cufflinks was used to identify differentially expressed transcripts. The transcripts that were not aligned to reference genome were assembled using de novo assembly [39]. The transcripts were differentially classified as up-regulated, down-regulated and neutrally regulated. The transcripts with FC = ±1 were considered as neutrally regulated transcripts. Two treatments were compared at a time: RP vs RM, and SP vs SM, respectively, and further the fold change (FC) was calculated taking into consideration FC coming from resistant and susceptible genotypes to identify up, down, neutrally regulated expression (RP/RM = FC1, SP/SM = FC2, FC = FC1/FC2). The transcripts that were only expressed in one of those treatments compared at a time were provided with FPKM values, and the FPKM values were compared in resistant and susceptible to denote significantly higher expression. To make it clear the transcripts that were detected up-regulated only in resistant or susceptible genotype may be down-regulated or neutrally in other, or may be not significant at P < 0.01, so are not detected in the contrasting genotype in RNA-seq data.
Bioinformatics annotation and methods
The transcripts were annotated using Blast2go program against several databases such as non-redundant protein database, Swiss-Prot, KEGG, etc. for annotation and GO functional classification [39]. The transcripts with P < 0.01 and log2 FC ≥ 1 were further retained. All the transcripts that were set to threshold of P < 0.01 were crosschecked with the preliminary annotation for all treatments. Pathway analysis was done by using KAAS (http://www.genome.jp/tools/kaas/). Arabidopsis thaliana (thale cress) and Oryza sativa japonica (Japanese rice) (RefSeq) were taken as reference. The transcription factors (TFs) were predicted by PlantTFDB webserver [105]. We used the threshold determination as set by the server and the established criteria to identify the candidate, which were considered for predicted TFs and the binding sites. Furthermore, the predictions were used for interpreting the GO annotations.
Expression analysis using quantitative Real-Time PCR (qRT-PCR)
The RNA extracted for transcriptome analysis (RNA-seq) was used for cDNA synthesis. The first strand was synthesized using Affinity Script qPCR cDNA Synthesis Kit (Agilent Technologies, Canada). The qRT-PCR was performed in a reaction volume of 10 μl consisting of 25 ng cDNA, 2 pmole of each primer, Qi-SYBR Green supermix (BioRad, Canada). The reactions were carried out in CF”X384TM Real-Time system (BioRad, ON, Canada). The relative transcript abundance in pathogen treated treatments compared to water (control) treatments were analyzed using 2−ΔΔCT (CT = cycle threshold) [100]. The transcripts, only those were expressed in pathogen treated treatments; RP>SP were analyzed. The relative transcript abundance was represented as FC values; whereas, in RNA-seq data the values are presented as log2FC. The primers used for qRT-PCR analysis were designed using NCBI primer blast software (S4 Table) [106].
Supporting Information
(TIF)
(XLSX)
(XLSX)
The genes within two markers GWM-133 and GWM-644 flanking the QTL-Fhb2 region were taken into account. The list of genes with gene ID, chromosome localization and gene ontology is given.
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank the Ministere de l’Agriculture, des Pecheries et de l’Alimentation du Quebec (MAPAQ), Quebec, Canada for providing grants for the project. DD thanks The Indian Council of Agricultural Research (ICAR), New Delhi for ICAR-International Fellowship, and Innovagrains and McGill University for financial assistance towards his graduate study. He also would like to thank Dr. Prashanth Suravajhala for his timely guidance towards bioinformatics analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors would like to thank the Ministere de l’Agriculture, des Pecheries et de l’Alimentation du Quebec (MAPAQ), Quebec, Canada for providing grants for the project. DD thanks The Indian Council of Agricultural Research (ICAR), New Delhi for ICAR-International Fellowship, and Innovagrains and McGill University for financial assistance towards his graduate study.
References
- 1.Ding L, Xu H, Yi H, Yang L, Kong Z, Zhang L, et al. Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PloS One. 2011;6(4):e19008 10.1371/journal.pone.0019008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin J. Role of cuticle in the defense against plant disease. Annual Review of Phytopathology. 1964;2(1):81–100. [Google Scholar]
- 3.Didi V, Jackson P, Hejátko J. Hormonal regulation of secondary cell wall formation. Journal of Experimental Botany. 2015;66:5015–27. 10.1093/jxb/erv222 [DOI] [PubMed] [Google Scholar]
- 4.González-Lamothe R, Mitchell G, Gattuso M, Diarra MS, Malouin F, Bouarab K. Plant antimicrobial agents and their effects on plant and human pathogens. International Journal of Molecular Sciences. 2009;10(8):3400–19. 10.3390/ijms10083400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balmer D, Flors V, Glauser G, Mauch-Mani B. Metabolomics of cereals under biotic stress: current knowledge and techniques. Frontiers in Plant Science. 2013;4: 10.3389/fpls.2013.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunnaiah R, Kushalappa AC, Duggavathi R, Fox S, Somers DJ. Integrated metabolo-proteomic approach to decipher the mechanisms by which wheat QTL (Fhb1) contributes to resistance against Fusarium graminearum. PloS One. 2012;7(7):e40695 10.1371/journal.pone.0040695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahuja I, Kissen R, Bones AM. Phytoalexins in defense against pathogens. Trends in Plant Science. 2012;17(2):73–90. 10.1016/j.tplants.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 8.Sudisha J, Sharathchandra R, Amruthesh K, Kumar A, Shetty HS. Pathogenesis related proteins in plant defense response. Plant Defence: Biological Control. 2012:379–403. [Google Scholar]
- 9.Boyd LA, Ridout C, O'Sullivan DM, Leach JE, Leung H. Plant–pathogen interactions: disease resistance in modern agriculture. Trends in Genetics. 2013;29(4):233–40. 10.1016/j.tig.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 10.St. Clair DA. Quantitative disease resistance and quantitative resistance loci in breeding. Annual review of phytopathology. 2010;48:247–68. 10.1146/annurev-phyto-080508-081904 [DOI] [PubMed] [Google Scholar]
- 11.Poland JA, Balint-Kurti PJ, Wisser RJ, Pratt RC, Nelson RJ. Shades of gray: the world of quantitative disease resistance. Trends in Plant Science. 2009;14(1):21–9. 10.1016/j.tplants.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 12.Kushalappa AC, Kalenahalli YN, Karre S. Plant innate immune response: Qualitative and Quantitative resistance. Critical Reviews in Plant Sciences. 2016. 10.1080/07352689.2016.1148980 [DOI] [Google Scholar]
- 13.Kushalappa AC, Gunnaiah R. Metabolo-proteomics to discover plant biotic stress resistance genes. Trends in Plant Science. 2013;18(9):522–31. 10.1016/j.tplants.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 14.Holme IB, Wendt T, Holm PB. Intragenesis and cisgenesis as alternatives to transgenic crop development. Plant Biotechnology Journal. 2013;11(4):395–407. 10.1111/pbi.12055 [DOI] [PubMed] [Google Scholar]
- 15.Bai G, Shaner G. Management and resistance in wheat and barley to Fusarium head blight. Annual Review of Phytopathology. 2004;42:135–61. [DOI] [PubMed] [Google Scholar]
- 16.McCartney C, Somers D, Fedak G, DePauw R, Thomas J, Fox S, et al. The evaluation of FHB resistance QTLs introgressed into elite Canadian spring wheat germplasm. Molecular Breeding. 2007;20(3):209–21. [Google Scholar]
- 17.Kosaka A, Manickavelu A, Kajihara D, Nakagawa H, Ban T. Altered gene expression profiles of wheat genotypes against Fusarium head blight. Toxins. 2015;7:604–620 10.3390/toxins7020604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller J, Young J, Sampson D. Deoxynivalenol and Fusarium head blight resistance in spring cereals. Journal of Phytopathology. 1985;113(4):359–67. [Google Scholar]
- 19.McMullen M, Jones R, Gallenberg D. Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Disease. 1997;81(12):1340–8. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Karre S, Dhokane D, Kage U, Hukkeri S, Kushalappa AC. Real-time quantitative PCR based method for the quantification of fungal biomass to discriminate quantitative resistance in barley and wheat genotypes to fusarium head blight. Journal of Cereal Science. 2015;64:16–22. [Google Scholar]
- 21.Buerstmayr H, Ban T, Anderson JA. QTL mapping and marker‐assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breeding. 2009;128(1):1–26. [Google Scholar]
- 22.Buerstmayr H, Lemmens M, Hartl L, Doldi L, Steiner B, Stierschneider M, et al. Molecular mapping of QTLs for fusarium head blight resistance in spring wheat. I. Resistance to fungal spread (Type II resistance). Theoretical and Applied Genetics. 2002;104(1):84–91. [DOI] [PubMed] [Google Scholar]
- 23.Cuthbert PA, Somers DJ, Brulé-Babel A. Mapping of Fhb2 on chromosome 6BS: a gene controlling Fusarium head blight field resistance in bread wheat (Triticum aestivum L.). Theoretical and Applied Genetics. 2007;114(3):429–37. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Gilbert J, Somers D, Fedak G, Procunier J, McKenzie I. Marker assisted selection of Fusarium head blight resistance genes in two doubled haploid populations of wheat. Molecular Breeding. 2003;12(4):309–17. [Google Scholar]
- 25.Mayer KF, Rogers J, Doležel J, Pozniak C, Eversole K, Feuillet C, et al. A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science. 2014;345(6194):1251788 10.1126/science.1251788 [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Pumphrey M, Gill B, Trick H, Zhang J, Dolezel J, et al. Toward positional cloning of Fhb1, a major QTL for Fusarium head blight resistance in wheat. Cereal Research Communications. 2008;36(6):195–201. [Google Scholar]
- 27.Schweiger W, Steiner B, Ametz C, Siegwart G, Wiesenberger G, Berthiller F, et al. Transcriptomic characterization of two major Fusarium resistance quantitative trait loci (QTLs), Fhb1 and Qfhs. ifa‐5A, identifies novel candidate genes. Molecular Plant Pathology. 2013;14(8):772–85. 10.1111/mpp.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yogendra KN, Kumar A, Sarkar K, Li Y, Pushpa D, Mosa KA, et al. Transcription factor StWRKY1 regulates phenylpropanoid metabolites conferring late blight resistance in potato. Journal of Experimental Botany. 2015;66(22):7377–89. 10.1093/jxb/erv434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yogendra KN, Kushalappa AC, Sarmiento F, Rodriguez E, Mosquera T. Metabolomics deciphers quantitative resistance mechanisms in diploid potato clones against late blight. Functional Plant Biology. 2015;42(3):284–98. [DOI] [PubMed] [Google Scholar]
- 30.Yogendra KN, Pushpa D, Mosa KA, Kushalappa AC, Murphy A, Mosquera T. Quantitative resistance in potato leaves to late blight associated with induced hydroxycinnamic acid amides. Functional & Integrative Genomics. 2014;14(2):285–98. [DOI] [PubMed] [Google Scholar]
- 31.Fiehn O. Metabolomics–the link between genotypes and phenotypes. Plant Molecular Biology. 2002;48(1–2):155–71. [PubMed] [Google Scholar]
- 32.Schauer N, Fernie AR. Plant metabolomics: towards biological function and mechanism. Trends in Plant Science. 2006;11(10):508–16. [DOI] [PubMed] [Google Scholar]
- 33.Pushpa D, Yogendra KN, Gunnaiah R, Kushalappa AC, Murphy A. Identification of late blight resistance-related metabolites and genes in potato through nontargeted metabolomics. Plant Molecular Biology Reporter. 2014;32(2):584–95. [Google Scholar]
- 34.Hamzehzarghani H, Paranidharan V, Abu-Nada Y, Kushalappa A, Dion Y, Rioux S, et al. Metabolite profiling coupled with statistical analyses for potential high-throughput screening of quantitative resistance to fusarium head blight in wheat. Canadian Journal of Plant Pathology. 2008;30(1):24–36. [Google Scholar]
- 35.Bollina V, Kumaraswamy GK, Kushalappa AC, Choo TM, Dion Y, Rioux S, et al. Mass spectrometry‐based metabolomics application to identify quantitative resistance‐related metabolites in barley against Fusarium head blight. Molecular Plant Pathology. 2010;11(6):769–82. 10.1111/j.1364-3703.2010.00643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bollina V, Kushalappa AC, Choo TM, Dion Y, Rioux S. Identification of metabolites related to mechanisms of resistance in barley against Fusarium graminearum, based on mass spectrometry. Plant Molecular Biology. 2011;77(4–5):355–70. 10.1007/s11103-011-9815-8 [DOI] [PubMed] [Google Scholar]
- 37.Kumaraswamy G, Kushalappa A, Choo T, Dion Y, Rioux S. Differential metabolic response of barley genotypes, varying in resistance, to trichothecene‐producing and‐nonproducing (tri5−) isolates of Fusarium graminearum. Plant Pathology. 2012;61(3):509–21. [Google Scholar]
- 38.Kumaraswamy GK, Bollina V, Kushalappa AC, Choo TM, Dion Y, Rioux S, et al. Metabolomics technology to phenotype resistance in barley against Gibberella zeae. European Journal of Plant Pathology. 2011;130(1):29–43. [Google Scholar]
- 39.Xiao J, Jin X, Jia X, Wang H, Cao A, Zhao W, et al. Transcriptome-based discovery of pathways and genes related to resistance against Fusarium head blight in wheat landrace Wangshuibai. BMC Genomics. 2013;14(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanubile A, Ferrarini A, Maschietto V, Delledonne M, Marocco A, Bellin D. Functional genomic analysis of constitutive and inducible defense responses to Fusarium verticillioides infection in maize genotypes with contrasting ear rot resistance. BMC Genomics. 2014;15(1):710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golkari S, Gilbert J, Prashar S, Procunier JD. Microarray analysis of Fusarium graminearum‐induced wheat genes: identification of organ‐specific and differentially expressed genes. Plant Biotechnology Journal. 2007;5(1):38–49. [DOI] [PubMed] [Google Scholar]
- 42.Jia H, Cho S, Muehlbauer GJ. Transcriptome analysis of a wheat near-isogenic line pair carrying Fusarium head blight-resistant and-susceptible alleles. Molecular Plant-Microbe Interactions. 2009;22(11):1366–78. 10.1094/MPMI-22-11-1366 [DOI] [PubMed] [Google Scholar]
- 43.Ruelland E, Djafi N, Zachowski A. The phosphoinositide dependent-phospholipase C pathway differentially controls the basal expression of DREB1 and DREB2 genes. Plant Signaling & Behavior. 2013;8(10):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Testerink C, Munnik T. Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends in Plant Science. 2005;10(8):368–75. [DOI] [PubMed] [Google Scholar]
- 45.Raho N, Ramirez L, Lanteri ML, Gonorazky G, Lamattina L, Ten Have A, et al. Phosphatidic acid production in chitosan-elicited tomato cells, via both phospholipase D and phospholipase C/diacylglycerol kinase, requires nitric oxide. Journal of Plant Physiology. 2011;168(6):534–9. 10.1016/j.jplph.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 46.Graça J. Hydroxycinnamates in suberin formation. Phytochemistry Reviews. 2010;9(1):85–91. [Google Scholar]
- 47.Campos L, Lisón P, López-Gresa MP, Rodrigo I, Zacarés L, Conejero V, et al. Transgenic tomato plants overexpressing tyramine N-hydroxycinnamoyltransferase exhibit elevated hydroxycinnamic acid amide levels and enhanced resistance to Pseudomonas syringae. Molecular Plant-Microbe Interactions. 2014;27(10):1159–69. 10.1094/MPMI-04-14-0104-R [DOI] [PubMed] [Google Scholar]
- 48.Cao A, Reid LM, Butrón A, Malvar RA, Souto XC, Santiago R. Role of hydroxycinnamic acids in the infection of maize silks by Fusarium graminearum Schwabe. Molecular Plant-Microbe Interactions. 2011;24(9):1020–6. 10.1094/MPMI-03-11-0079 [DOI] [PubMed] [Google Scholar]
- 49.Ishihara A, Hashimoto Y, Tanaka C, Dubouzet JG, Nakao T, Matsuda F, et al. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. The Plant Journal. 2008;54(3):481–95. 10.1111/j.1365-313X.2008.03441.x [DOI] [PubMed] [Google Scholar]
- 50.Lattanzio V, Lattanzio VM, Cardinali A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Photochemistry: Advances in Research. 2006;661:23–67. [Google Scholar]
- 51.Parvez MM, Tomita-Yokotani K, Fujii Y, Konishi T, Iwashina T. Effects of quercetin and its seven derivatives on the growth of Arabidopsis thaliana and Neurospora crassa. Biochemical Systematics and Ecology. 2004;32(7):631–5. [Google Scholar]
- 52.Treutter D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biology. 2005;7(6):581–91. [DOI] [PubMed] [Google Scholar]
- 53.Walley JW, Kliebenstein DJ, Bostock RM, Dehesh K. Fatty acids and early detection of pathogens. Current Opinion in Plant Biology. 2013;16(4):520–6. 10.1016/j.pbi.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 54.Dedyukhina EG, Chistyakova TI, Mironov AA, Kamzolova SV, Morgunov IG, Vainshtein MB. Arachidonic acid synthesis from biodiesel‐derived waste by Mortierella alpina. European Journal of Lipid Science and Technology. 2014;116(4):429–37. [Google Scholar]
- 55.Pohl CH, Kock JL, Thibane VS. Antifungal free fatty acids: a review. Science against Microbial Pathogens: Current Research and Technological Advances. 2011;1:61–71. [Google Scholar]
- 56.Singh P, Zimmerli L. Lectin receptor kinases in plant innate immunity. Frontiers in Plant Science. 2013;4: 10.3389/fpls.2013.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Afzal AJ, Wood AJ, Lightfoot DA. Plant receptor-like serine threonine kinases: roles in signaling and plant defense. Molecular Plant-Microbe Interactions. 2008;21(5):507–17. 10.1094/MPMI-21-5-0507 [DOI] [PubMed] [Google Scholar]
- 58.Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proceedings of the National Academy of Sciences. 2010;107(20):9452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen X, Shang J, Chen D, Lei C, Zou Y, Zhai W, et al. AB‐lectin receptor kinase gene conferring rice blast resistance. The Plant Journal. 2006;46(5):794–804. [DOI] [PubMed] [Google Scholar]
- 60.Kage U, Karre S, Kushalappa AC, and McCartney C. Identification and characterization of wheat QTL-2DL specific TaACT gene as candidate for resistance against fusarium head blight based on metabolo-genomics approach. Plant Biotechnology Journal. 2016. (Sub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fofana B, Benhamou N, McNally DJ, Labbé C, Séguin A, Bélanger RR. Suppression of induced resistance in cucumber through disruption of the flavonoid pathway. Phytopathology. 2005;95(1):114–23. 10.1094/PHYTO-95-0114 [DOI] [PubMed] [Google Scholar]
- 62.Xu L, Zhu L, Tu L, Liu L, Yuan D, Jin L, et al. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. Journal of Experimental Botany. 2011;62(15):5607–21. 10.1093/jxb/err245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohammadi M, Ramezanpour SS, Navabpour S, Soltanloo H, Kia S, Arabi MK. Study on expression pattern of Chalcone synthase and 1, 3-Glucanase under Septoria tritici treatment in wheat by quantitative real time PCR. American-Eurasian Journal of Agricultural and Environmental Sciences. 2012;12:1431–1436. [Google Scholar]
- 64.Shin S, Mackintosh CA, Lewis J, Heinen SJ, Radmer L, Dill-Macky R, et al. Transgenic wheat expressing a barley class II chitinase gene has enhanced resistance against Fusarium graminearum. Journal of Experimental Botany. 2008;59(9):2371–8. 10.1093/jxb/ern103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishizawa Y, Nishio Z, Nakazono K, Soma M, Nakajima E, Ugaki M, et al. Enhanced resistance to blast (Magnaporthe grisea) in transgenic Japonica rice by constitutive expression of rice chitinase. Theoretical and Applied Genetics. 1999;99(3–4):383–90. 10.1007/s001220051248 [DOI] [PubMed] [Google Scholar]
- 66.Takahashi W, Fujimori M, Miura Y, Komatsu T, Nishizawa Y, Hibi T, et al. Increased resistance to crown rust disease in transgenic Italian ryegrass (Lolium multiflorum Lam.) expressing the rice chitinase gene. Plant Cell Reports. 2005;23(12):811–8. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto T, Iketani H, Ieki H, Nishizawa Y, Notsuka K, Hibi T, et al. Transgenic grapevine plants expressing a rice chitinase with enhanced resistance to fungal pathogens. Plant Cell Reports. 2000;19(7):639–46. [DOI] [PubMed] [Google Scholar]
- 68.Shin S, Torres-Acosta JA, Heinen SJ, McCormick S, Lemmens M, Paris MPK, et al. Transgenic Arabidopsis thaliana expressing a barley UDP-glucosyltransferase exhibit resistance to the mycotoxin deoxynivalenol. Journal of Experimental Botany. 2012;63(13):4731–40. 10.1093/jxb/ers141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gunnaiah R, Kushalappa AC. Metabolomics deciphers the host resistance mechanisms in wheat cultivar Sumai-3, against trichothecene producing and non-producing isolates of Fusarium graminearum. Plant Physiology and Biochemistry. 2014;83:40–50. 10.1016/j.plaphy.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 70.Gardiner SA, Boddu J, Berthiller F, Hametner C, Stupar RM, Adam G, et al. Transcriptome analysis of the barley-deoxynivalenol interaction: evidence for a role of glutathione in deoxynivalenol detoxification. Molecular Plant-Microbe Interactions. 2010;23(7):962–76. 10.1094/MPMI-23-7-0962 [DOI] [PubMed] [Google Scholar]
- 71.Lemmens M, Scholz U, Berthiller F, Dall'Asta C, Koutnik A, Schuhmacher R, et al. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Molecular Plant-Microbe Interactions. 2005;18(12):1318–24. [DOI] [PubMed] [Google Scholar]
- 72.Zhou W, Kolb FL, Riechers DE. Identification of proteins induced or upregulated by Fusarium head blight infection in the spikes of hexaploid wheat (Triticum aestivum). Genome. 2005;48(5):770–80. [DOI] [PubMed] [Google Scholar]
- 73.Li X, Shin S, Heinen S, Dill-Macky R, Berthiller F, Nersesian N, et al. Transgenic wheat expressing a barley UDP-glucosyltransferase detoxifies deoxynivalenol and provides high levels of resistance to Fusarium graminearum. Molecular Plant-Microbe Interactions. 2015;28(11):1237–46. 10.1094/MPMI-03-15-0062-R [DOI] [PubMed] [Google Scholar]
- 74.Ghaffari MR, Mardi M, Ehya F, Karimi Farsad L, Hosseini S, et al. Mapping and expression analysis of a Fusarium head blight resistance gene candidate pleiotropic drug resistance 5 (PDR5) in wheat. Iranian Journal of Biotechnology 2010;8:112–116. [Google Scholar]
- 75.Ehlting J, Büttner D, Wang Q, Douglas CJ, Somssich IE, Kombrink E. Three 4‐coumarate: coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms. The Plant Journal. 1999;19(1):9–20. [DOI] [PubMed] [Google Scholar]
- 76.Xu B, Escamilla‐Treviño LL, Sathitsuksanoh N, Shen Z, Shen H, Percival Zhang YH, et al. Silencing of 4‐coumarate: coenzyme A ligase in switchgrass leads to reduced lignin content and improved fermentable sugar yields for biofuel production. New Phytologist. 2011;192(3):611–25. 10.1111/j.1469-8137.2011.03830.x [DOI] [PubMed] [Google Scholar]
- 77.Voelker SL, Lachenbruch B, Meinzer FC, Jourdes M, Ki C, Patten AM, et al. Antisense down-regulation of 4CL expression alters lignification, tree growth, and saccharification potential of field-grown poplar. Plant Physiology. 2010;154(2):874–86. 10.1104/pp.110.159269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Underwood W. The plant cell wall: a dynamic barrier against pathogen invasion. Frontiers in Plant Science. 2012;3: 10.3389/fpls.2012.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Voigt CA. Callose-mediated resistance to pathogenic intruders in plant defense-related papillae. Frontiers in Plant Science. 2014;5: 10.3389/fpls.2014.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ellinger D, Naumann M, Falter C, Zwikowics C, Jamrow T, Manisseri C, et al. Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiology. 2013;161(3):1433–44. 10.1104/pp.112.211011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blumke A, Somerville SC, Voigt CA. Transient expression of the Arabidopsis thaliana callose synthase PMR4 increases penetration resistance to powdery mildew in barley. Advances in Bioscience and Biotechnology. 2013;4:810–813. [Google Scholar]
- 82.Preisner M, Kulma A, Zebrowski J, Dymińska L, Hanuza J, Arendt M, et al. Manipulating cinnamyl alcohol dehydrogenase (CAD) expression in flax affects fibre composition and properties. BMC Plant Biology. 2014;14(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhuiyan NH, Selvaraj G, Wei Y, King J. Role of lignification in plant defense. Plant Signaling & Behavior. 2009;4(2):158–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhuiyan NH, Selvaraj G, Wei Y, King J. Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. Journal of Experimental Botany. 2009;60(2):509–21. 10.1093/jxb/ern290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tronchet M, Balague C, Kroj T, Jouanin L, Roby D. Cinnamyl alcohol dehydrogenases‐C and D, key enzymes in lignin biosynthesis, play an essential role in disease resistance in Arabidopsis. Molecular Plant Pathology. 2010;11(1):83–92. 10.1111/j.1364-3703.2009.00578.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eudes A, Pollet B, Sibout R, Do C-T, Séguin A, Lapierre C, et al. Evidence for a role of AtCAD 1 in lignification of elongating stems of Arabidopsis thaliana. Planta. 2006;225(1):23–39. 10.1007/s00425-006-0326-9 [DOI] [PubMed] [Google Scholar]
- 87.Walter S, Kahla A, Arunachalam C, Perochon A, Khan MR, Scofield SR, et al. A wheat ABC transporter contributes to both grain formation and mycotoxin tolerance. Journal of Experimental Botany. 2015;66:2583–93. 10.1093/jxb/erv048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Handa H, Namiki N, Xu D, Ban T. Dissecting of the FHB resistance QTL on the short arm of wheat chromosome 2D using a comparative genomic approach: from QTL to candidate gene. Molecular Breeding. 2008;22(1):71–84. [Google Scholar]
- 89.Walter S, Brennan JM, Arunachalam C, Ansari KI, Hu X, Khan MR, et al. Components of the gene network associated with genotype-dependent response of wheat to the Fusarium mycotoxin deoxynivalenol. Functional & Integrative Genomics. 2008;8(4):421–7. [DOI] [PubMed] [Google Scholar]
- 90.Shahrtash M. Plant glutathione S-transferases function during environmental stresses: a review article. Romanian Journal of Biology—Plant Biology. 2013;58(1):19–25. [Google Scholar]
- 91.Dean J, Goodwin P, Hsiang T. Induction of glutathione S-transferase genes of Nicotiana benthamiana following infection by Colletotrichum destructivum and C. orbiculare and involvement of one in resistance. Journal of Experimental Botany. 2005;56(416):1525–33. [DOI] [PubMed] [Google Scholar]
- 92.Kugler KG, Siegwart G, Nussbaumer T, Ametz C, Spannagl M, Steiner B, et al. Quantitative trait loci-dependent analysis of a gene co-expression network associated with Fusarium head blight resistance in bread wheat (Triticum aestivum L.). BMC Genomics. 2013;14(1):728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dean JD, Goodwin PH, Hsiang T. Colletotrichum gloeosporioides infection induces differential expression of glutathione S-transferase genes in Malva pusilla. Functional Plant Biology. 2003;30(7):821–8. [DOI] [PubMed] [Google Scholar]
- 94.Han Q, Chen R, Yang Y, Cui X, Ge F, Chen C, et al. A glutathione S-transferase gene from Lilium regale Wilson confers transgenic tobacco resistance to Fusarium oxysporum. Scientia Horticulturae. 2016;198:370–8. [Google Scholar]
- 95.Alves MS, Dadalto SP, Gonçalves AB, de Souza GB, Barros VA, Fietto LG. Transcription factor functional protein-protein interactions in plant defense responses. Proteomes. 2014;2(1):85–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang F, Lin R, Feng J, Qiu D, Chen W, Xu S. Wheat bHLH transcription factor gene, TabHLH060, enhances susceptibility of transgenic Arabidopsis thaliana to Pseudomonas syringae. Physiological and Molecular Plant Pathology. 2015;90:123–30. [Google Scholar]
- 97.Chaudhary J, Skinner MK. Basic helix-loop-helix proteins can act at the E-box within the serum response element of the c-fos promoter to influence hormone-induced promoter activation in Sertoli cells. Molecular Endocrinology. 1999;13(5):774–86. [DOI] [PubMed] [Google Scholar]
- 98.Malinovsky FG, Batoux M, Schwessinger B, Youn JH, Stransfeld L, Win J, et al. Antagonistic regulation of growth and immunity by the Arabidopsis basic helix-loop-helix transcription factor homolog of brassinosteroid enhanced expression2 interacting with increased leaf inclination1 binding bHLH1. Plant Physiology. 2014;164(3):1443–55. 10.1104/pp.113.234625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Proctor RH, Hohn TM, McCormick SP. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Molecular Plant Microbe Interactions. 1995;8(4):593–601. [DOI] [PubMed] [Google Scholar]
- 100.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 101.De Vos RC, Moco S, Lommen A, Keurentjes JJ, Bino RJ, Hall RD. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nature Protocols. 2007;2(4):778–91. [DOI] [PubMed] [Google Scholar]
- 102.Katajamaa M, Oresic M. Processing methods for differential analysis of LC/MS profile data. BMC Bioinformatics. 2005;6(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–11. 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology. 2010;28(5):511–5. 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jin J, Zhang H, Kong L, Gao G, Luo J. PlantTFDB 3.0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Research. 2013;42: 10.1093/nar/gkt1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(XLSX)
(XLSX)
The genes within two markers GWM-133 and GWM-644 flanking the QTL-Fhb2 region were taken into account. The list of genes with gene ID, chromosome localization and gene ontology is given.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.