Abstract
Background
Ghana is affected by regular cholera epidemics and an annual average of 3,066 cases since 2000. In 2014, Ghana experienced one of its largest cholera outbreaks within a decade with more than 20,000 notified infections. In order to attribute this rise in cases to a newly emerging strain or to multiple simultaneous outbreaks involving multi-clonal strains, outbreak isolates were characterized, subtyped and compared to previous epidemics in 2011 and 2012.
Methodology/Principal Findings
Serotypes, biotypes, antibiotic susceptibilities were determined for 92 Vibrio cholerae isolates collected in 2011, 2012 and 2014 from Southern Ghana. For a subgroup of 45 isolates pulsed-field gel electrophoresis, multilocus sequence typing and multilocus-variable tandem repeat analysis (MLVA) were performed. Eighty-nine isolates (97%) were identified as ctxB (classical type) positive V. cholerae O1 biotype El Tor and three (3%) isolates were cholera toxin negative non-O1/non-O139 V. cholerae. Among the selected isolates only sulfamethoxazole/trimethoprim resistance was detectable in 2011, while 95% of all 2014 isolates showed resistance towards sulfamethoxazole/trimethoprim, ampicillin and reduced susceptibility to ciprofloxacin. MLVA achieved the highest subtype discrimination, revealing 22 genotypes with one major outbreak cluster in each of the three outbreak years. Apart from those clusters genetically distant genotypes circulate during each annual epidemic.
Conclusions/Significance
This analysis suggests different endemic reservoirs of V. cholerae in Ghana with distinct annual outbreak clusters accompanied by the occurrence of genetically distant genotypes. Preventive measures for cholera transmission should focus on aquatic reservoirs. Rapidly emerging multidrug resistance must be monitored closely.
Author Summary
The bacterium Vibrio cholerae is mainly transmitted faecal-orally via human-to-human contact or via environmental water sources in which V. cholerae is able to persist. West Africa, including Ghana, is regularly affected by Cholera epidemics, in particular during rainy seasons. In 2014, Ghana experienced an exceptionally large outbreak with over 20,000 cases, which raised questions about newly emerging V. cholerae strains in this region. In this study, we described the duration, the geographical spread and demographics of the outbreak using data from the National Ghanaian Surveillance system. Further, we characterized outbreak isolates from the outbreak years 2011, 2012 and 2014 by three different subtyping methods. These analyses revealed strains with different genetic background and increasing antibiotic resistance circulating during each outbreak year. These data suggest that V. cholerae has an endemic reservoir in the environment and selection pressure results in a highly heterogeneous population of V. cholerae with a few strains evolving into pathogenic clones during each outbreak period. Public health authorities must be vigilant to prevent cholera transmission through aquatic reservoirs, particularly within urban agglomerations during the start of the rainy season. The rapidly emerging antibiotic resistance has to be monitored closely.
Introduction
The World Health Organization (WHO) estimates that 3–5 million annual cases of cholera occur worldwide, resulting in 100,000–120,000 deaths [1]. Since its introduction to Africa during the current seventh cholera pandemic in 1970, Vibrio cholerae caused regular vast epidemics across the continent with cumulative 3,762,902 case-notifications to WHO by 2013 [2]. In 2013 alone, 22 African countries reported 56,329 cholera cases. However, these numbers are considered to be substantially underestimated due to poorly functioning national epidemiological and laboratory surveillance systems, which are not able to detect the majority of mild disease presentations. Furthermore, systematic underreporting is common to avoid economic and political damage [3,4].
The first cholera case has been reported from Ghana in 1970 [5]. With 90% of the population at risk for cholera transmission, Ghana always ranks among the most affected countries on the African continent [4]. Ghana notified an annual average of 3,066 (range: 50–10,628) cholera cases between 2000 and 2013 with an overall case fatality rate of 1.7% [2]. In the year 2014, Ghana experienced an exceptionally large cholera outbreak with 28,975 infections notified to the World Health Organization between June and November [6].
Causes of this sudden increase in case numbers might be very diverse and calls for investigation in a timely manner so as to implement preventive measures accordingly. This recent epidemic might be explained by the extrinsic introduction of a new V. cholerae strain into a susceptible population, as seen for cholera outbreaks in Zimbabwe and Haiti in the years 2009 and 2011 respectively [7,8]. Alternatively, multiple, endemic, genetically non-related strains might be responsible for concomitant epidemics, as reported from Kenya [9]. In the latter scenario V. cholerae might persist in aquatic reservoirs and regular epidemics are triggered by low hygienic conditions and climatic factors such as increased rainfall or flooding [10–12]. In recent years, a number of molecular typing tools have been developed to elucidate the source and evolution of V. cholerae outbreaks [13].
This study aims to describe the 2014 cholera epidemic in Ghana and uses molecular subtyping techniques to detect responsible newly emerging or multi-clonal strains, which are then compared to strains that circulated during the 2011 and 2012 epidemics. Results will advise public health authorities whether to focus on monitoring of endemic environmental reservoirs or rather on surveillance of mobile populations, with cross-border epidemiological collaborations to prevent importation of V. cholerae.
Methods
Epidemiological description of the 2014 outbreak
Within the Ghana Health Service, the Disease Surveillance Service supported by the National Public Health & Reference Laboratory (NPHRL) conducts cholera surveillance in Ghana. A case of cholera is defined according to the WHO standard case definition: If cholera is not known to be present in the area, a case of cholera is considered in a patient ≥5 years with severe dehydration or death from acute watery diarrhea, while during a cholera epidemic every patient aged ≥5 years with acute watery diarrhea and/or vomiting is considered as a case.
Standardized line lists with suspected cholera cases are provided on a weekly basis by the District Health Management Teams to the Central Disease Surveillance Service in Accra, which collates information on name, place of residence, sex, age, disease onset, disease outcome and hospitalization of cases. A small subset of specimens is usually tested in peripheral laboratories by various methods and results are notified in the line lists. For laboratory confirmation, district and regional laboratories are encouraged to send suspected cholera stool samples to the NPHRL, where culture and serotype identification is performed.
For the purposes of this study, all suspected cholera cases in the surveillance database from six Southern Ghanaian regions (Greater Accra, Central, Western, Eastern, Ashanti, Volta) from the year 2014 were extracted and described by sex, age and place of residence. Continuous variables were summarized as means with standard deviation (median with interquartile range for non-normally distributed variables) and dichotomous or categorical variables were summarized as proportions/percentages. Missing values were excluded from the analysis, thus the denominators for some comparisons differ.
Surveillance data were computerized using Excel (Microsoft, USA) and statistical analysis was performed with Stata v. 12.1 (Statacorp, Texas, USA). For spatio-temporal visualisation case data were imported into Arc GIS 10.0 (ESRI: ArcGis Desktop: Release 10.2011). Suspected cases were linked to the respective notifying districts. For the spatio-temporal visualisation, case data/district were then aggregated in six temporal groups, each covering five outbreak weeks.
Characterization of isolates and molecular epidemiology
Identification of V. cholerae isolates
All V. cholerae isolates, which were sent to the NPHRL from the Greater Accra-, Central-, Ashanti- and Volta regions, from the years 2011 (n = 18) and 2012 (n = 12), in addition to 62 randomly selected isolates from the year 2014 (out of 261 isolates), were chosen for further analysis. Samples were randomly selected by district and time, reflecting the spatial-temporal distribution within the outbreak. All 92 isolates were initially cultured on Thiosulfate-citrate-bile salts-sucrose (TCBS) agar and subcultured on Tryptic Soy Agar (TSA). DNA was extracted out of a suspension of V. cholerae cells, which had been incubated for 10 min at 100°C. After centrifugation, the supernatant, containing the DNA, was preserved at -20°C until further processed. V. cholerae isolates were confirmed by species-specific PCR and tested for the presence of cholera toxin (ctxAB) by PCR as described before [14,15]. For the year 2013 50 cholera cases have been reported from Ghana, but no isolates were send for confirmation to the NPHRL. For further analyses the isolates were transported to the national reference laboratory at the ISCIII in Madrid, Spain.
Phenotyping
The isolates were serotyped with polyvalent O1 antiserum and monovalent Inaba and Ogawa antisera (Denka Seiken, Tokyo, Japan). Biotypes were determined by (i) susceptibility to polymyxin B, (ii) haemolysis of sheep erythrocytes and (iii) the Voges-Proskauer test, which measured the production of acetylmethylcarbinol.
Antimicrobial susceptibility testing was performed with the Kirby-Bauer disk diffusion method on Mueller-Hinton agar. The Escherichia coli reference strain ATCC 25922 served as a control. Isolates were tested against 7 antimicrobial drugs as follows: ampicillin (10 μg), ciprofloxacin (5 μg), chloramphenicol (30 μg), gentamycin (30 μg), nalidixic acid (30 μg), sulfamethoxazole/trimethoprim (1.25 μg + 23.75 μg) and tetracycline (30 μg)(all Oxoid, Basingstoke, United Kingdom). Diameters were interpreted according to the 2015 Clinical and Laboratory Standards Institute (CLSI) guidelines as resistant, susceptible or intermediate (http://www.clsi.org). Intermediate isolates will be referred to as reduced susceptible in the following. When no interpretive criteria for V. cholerae were available, breakpoints for Enterobacteriaceae according to the 2015 European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines were applied (http://www.eucast.org).
Genotyping
The type of the cholera toxin-encoding gene (ctxB) was determined by the mismatch amplification mutation PCR as described elsewhere [16]. From all isolates identified within a two-week period in a specific district, one isolate was randomly selected for further molecular characterization, resulting in a subset of 45 isolates. The geographical origin of these isolates is shown in the supplement (S1 Fig).
Pulse-field gel electrophoresis (PFGE) was conducted according to the PulseNet protocol for V. cholerae, using a single restriction enzyme digestion with NotI as described before [17]. PFGE banding patterns and dendrogram were analyzed with Bionumerics version 6.6 (Applied Math, Texas, USA). The definition of a PFGE cluster was based on a similarity cutoff of 95% (Dice coefficient, represented by UPGMA, 1.0% optimization and 1.5% tolerance). For each detected PFGE pulsotype, one isolate was chosen for Multilocus Sequence Typing (MLST), following the published protocol for 7 loci as previously described [18]. Newly identified alleles and sequence types were submitted to the public cholera database for assignment to new sequence types (http://pubmlst.org/vcholerae/).
Multilocus variable-number tandem-repeat (VNTR) analysis (MLVA) was carried out by amplification of 5 loci using primers and PCR conditions as described in previous studies [19]. The purified PCR products were sequenced in both directions and sequence data were analyzed using the DNASTAR Lasergene version 7.0 software. MLVA types were assigned by combining numbers of repeat units of each locus in the order VC0147, VC0436-7, VC1650, VCA0171, VCA0283. A minimum spanning tree was generated using Bionumerics version 6.6 (Applied Math, Texas, USA) based on the categorical coefficient. Clonal complexes were defined as isolates connected through a chain of single-locus variants.
Results
Epidemiology of the 2014 outbreak
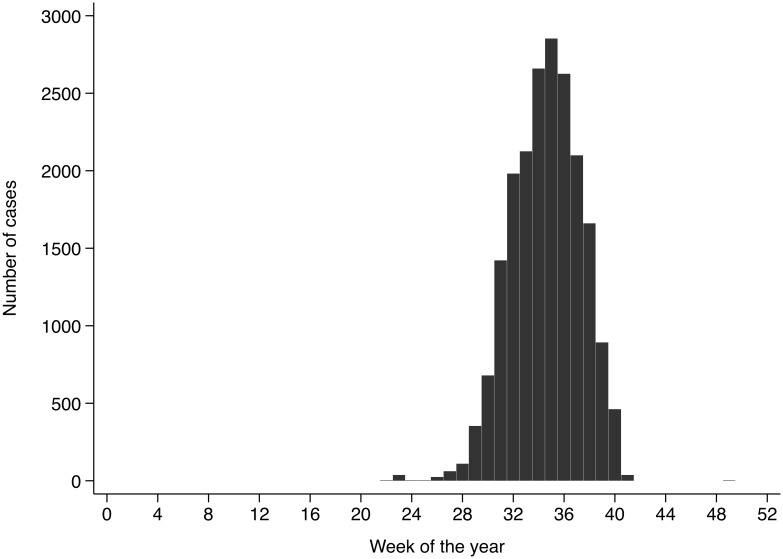
A total of 20,185 cases of cholera were reported to the Ghanaian Disease Surveillance Center from the Ashanti-, Central-, Eastern-, Greater Accra-, Western- and Volta regions in 2014. The date of disease onset was reported for 20,120 cases, the remaining 65 cases were removed from the dataset for all other analyses. The earliest reported onset date was the 20th of May 2014 and the latest was the 11th of December 2014, with a peak number of 2,853 cases in the 35th calendar week (25–31 August; Fig 1). Age was reported for 19,863 cases and distributed with a median age of 26 years and an interquartile range (IQR) of 20–35 years. The median age of cases did not change during the course of the outbreak. The majority of cases was male (58.4%; n = 11,796), and median age was not markedly different between males (26 years; IQR 20–35) and females (25 years; IQR 19–35). The case fatality rate (CFR) was 0.8% (165 deaths) with a higher median age among deceased of 34 years (IQR 24–47).
Fig 1. Weekly notification of suspected cholera cases.
The Disease Surveillance Service of the Ghana Health Service reports 20,120 cholera cases according to the WHO case definition between May 2014 and December 2014 with a peak number of 2,853 cases in the 35th calendar week (25–31 August).
Laboratory testing was performed in regional laboratories with rapid diagnostic tests from different suppliers for 496 out of 20,120 (2.5%) suspected cases with a positivity rate of 53% (264/496).
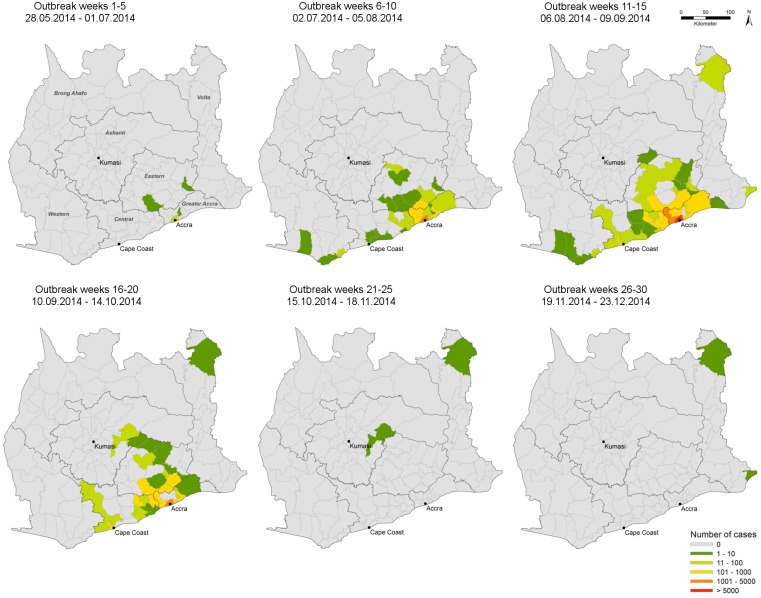
Spatio-temporal analysis traced back the first cases during the first five weeks of the outbreak to four districts in the Eastern and Greater Accra regions (Fig 2). During the peak of the outbreak (outbreak week 6–20) the majority of cases were centred around the city of Accra, spreading in northward-direction to the Ashanti region and to the East along the coast. During the last 10 weeks of the outbreak, only three districts within the Ashanti and the Volta region reported cases.
Fig 2. Spatial and temporal location of suspected cholera cases (Mai 2014-December 2014; n = 20,120).
As notified to the Disease Surveillance Service of the Ghana Health Service according to the WHO case definition suspected cholera cases are plotted by district by 5-week period panels. The figure was produced with Arc GIS 10.0 (ESRI: ArcGis Desktop: Release 10.2011).
Antimicrobial susceptibility
Antimicrobial susceptibility testing identified mono-resistance towards sulfamethoxazole/trimethoprim in 100% (18/18) of isolates from 2011 (Table 1). The resistance pattern changed in 2012 with 75% (9/12) of isolates expressing resistance against sulfamethoxazole/trimethoprim, nalidixic acid in combination to reduced susceptibility against ampicillin and ciprofloxacin. The same resistance profile was detected in 95% (59/62) of isolates in 2014. No resistance against chloramphenicol, gentamycin or tetracycline was observed for any of the isolates.
Table 1. Antimicrobial resistance for each antibiotic (A) and resistance profile (B) of Vibrio cholerae isolates, by year of disease onset (n = 92).
| A. | |||
| Resistance for each antibiotic [n (%)] | |||
| Antibiotic | 2011 (n = 18) | 2012 (n = 12) | 2014 (n = 62) |
| SxT | 18 (100.0) | 10 (83.3) | 60 (96.8) |
| Nal | 0 (0.0) | 11 (91.7) | 62 (100.0) |
| Cip | 0 (0.0) | 11 (91.7) | 61 (98.4) |
| Amp | 0 (0.0) | 11 (91.7) | 59 (95.2) |
| B. | |||
| Resistance profile [n (%)] | |||
| Antibiotics | 2011 (n = 18) | 2012 (n = 12) | 2014 (n = 62) |
| SxT | 18 (100.0) | 1 (8.3) | 0 (0.0) |
| Nal | 0 (0.0) | 0 (0.0) | 1 (1.6) |
| Nal+Cip | 0 (0.0) | 0(0.0) | 1 (1.6) |
| Nal+Cip+Amp | 0 (0.0) | 2 (16.7) | 0 (0.0) |
| Nal+Cip+SxT | 0 (0.0) | 0 (0.0) | 1 (1.6) |
| Nal+Cip+Amp+SxT | 0 (0.0) | 9 (75.0) | 59 (95.2) |
Amp, ampicillin; Cip, ciprofloxacin; Nal, nalidixic acid; SxT, sulfamethoxazole/trimethoprim; no antimicrobial resistance was found for chloramphenicol, gentamycin and tetracycline.
Genotyping
The selected 92 V. cholerae isolates consisted of 88 (95.7%) V. cholerae O1 biotype El Tor serotype Ogawa, 1 (1.1%) V. cholerae O1 biotype EL Tor serotype Inaba and 3 (3.3%) non-01/non-O139 V. cholerae. Apart from the non-O1/non-O139 V. cholerae strains with no cholera toxin production, all isolates produced the classical type cholera toxin.
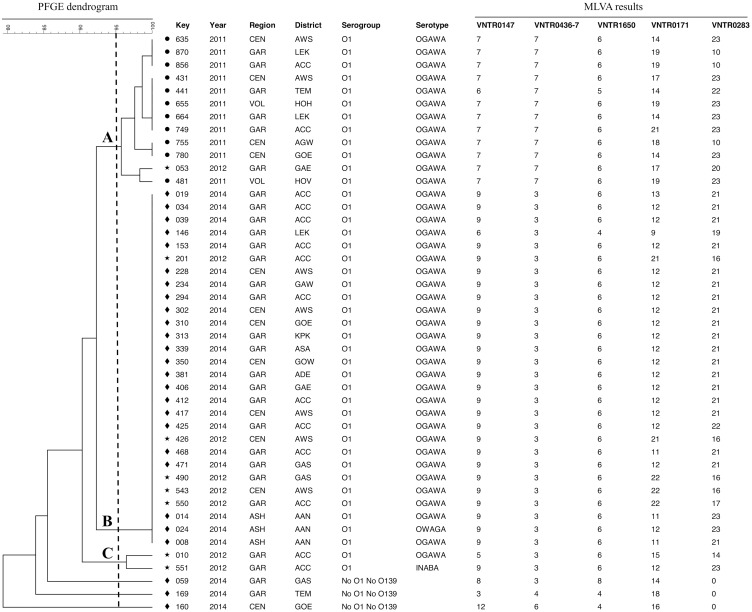
Out of these 92 isolates, 11 isolates from 2011, eight isolates from 2012 and 26 isolates from 2014 were genotyped by MLST, PFGE and MLVA (S1 Fig). All V. cholerae O1 belonged to the sequence type (ST) 69, while the three non-O1/non-O139 isolates presented with new alleles (isolate 059: metE allele 108; isolate 160: metE allele 109; and isolate 169: metE allele 110 and pyrC allele 85) and could not be allocated to any known ST. Theses strains were assigned new ST numbers (isolate 059: ST211, isolate 160: ST212 and isolate 169: ST213; corresponding to IDs 196, 197 and 198 in the V. cholerae pubmlst database: http://pubmlst.org/vcholerae/), which are closest related to the already known ST40 and ST39. As shown in Fig 3, using a cutoff of 95%, PFGE divided all strains into three main clusters and 11 pulsotypes (Fig 3). All 2011 isolates clustered together (cluster A) as 4 different pulsotypes. Isolates from 2012 belonged to 4 different pulsotypes dispersed in clusters A, B and C. All V. cholerae O1 from 2014 showed the same pulsotype and were grouped in cluster B. Finally, the non-O1/non-O139 isolates collected in 2014 showed different pulsotypes and were grouped outside clusters A, B or C. Neither regional nor district specific pulsotypes had been detected.
Fig 3. Pulse-field gel electrophoresis (PFGE) dendrogram for Vibrio cholerae isolates (n = 45).
The three clusters A, B and C (bold letters) are based on a similarity cut-off of 95% (Dotted line; Dice coefficient, represented by UPGMA, 1.0% optimization and 1.5% tolerance). The geographical location, year of disease onset, serogroup, serotype and multilocus variable-number tandem-repeat (VNTR) analysis (MLVA) results are given for each V. cholerae isolate. Regional three-letter codes: ASH, Ashanti region; CEN, Central region; GAR, Greater Accra region; VOL, Volta region. District three-letter codes: AAN, Asante Akim North; ACC, Accra; ADE, Adentan; AGW, Agona-Swedru; AWS, Awutu-Senya; ASA, Ashaiman; GAE, Ga East; GAS, Ga South; GAW, Ga West; GOE, Gomoa East; GOW, Gomoa West; HOH, Hohoe; HOV, Ho; KPK, Kpone-Katamanso; LEK, Ledzekuku-Krowor; TEM, Tema.
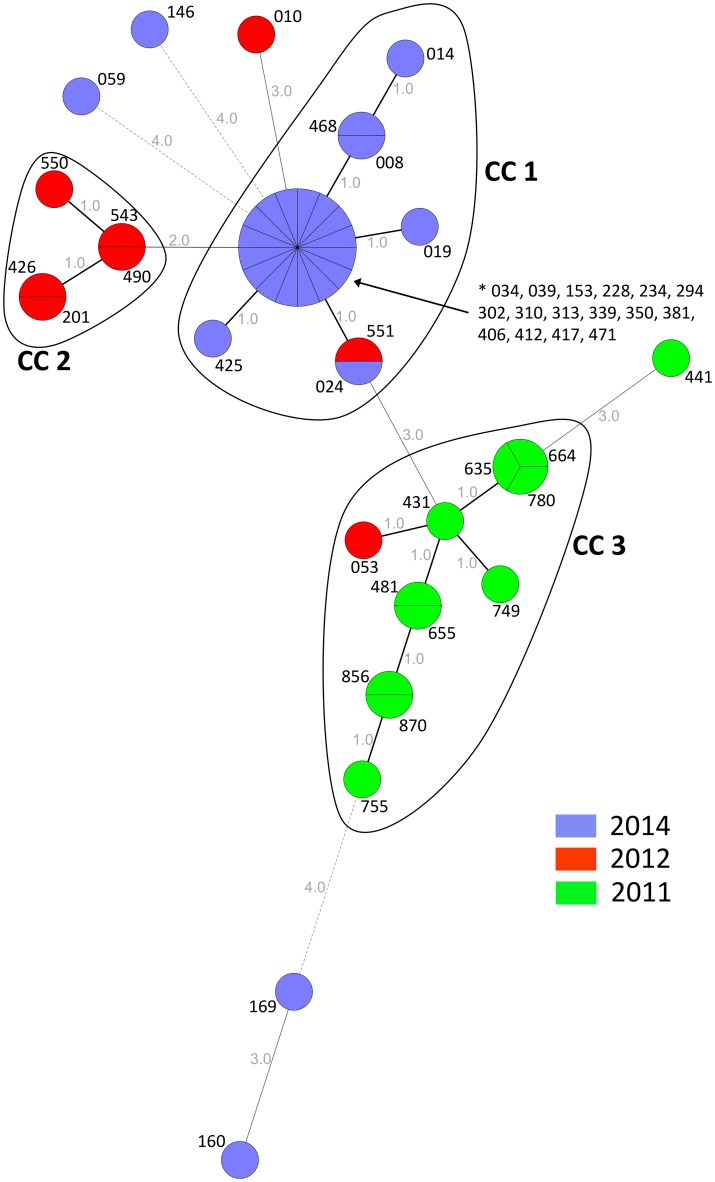
MLVA differentiated the isolates into three clonal complexes (CC1-CC3) and 22 genotypes (Fig 4). The majority of 22 out of 26 isolates from 2014 clustered within CC1 together with one isolate from 2012. Distinct from the CC1 were the three non-O1/non-O139 and one O1 V. cholerae isolate (Isolate 146) from Ledzekuku-Krowor District (Greater Accra Region). For 2012, five out of eight isolates grouped together in CC2, while the other three isolates were genetically distinct, within or close to CC1 and CC3. Similarly, 10 of the 11 isolates from 2011 were classified into CC3, and one strain clustered outside with three alleles difference to the other CC3 strains. Analogous to the PFGE distribution, subtypes did not cluster by region or by district.
Fig 4. Minimum spanning tree of multilocus variable-number tandem-repeat (VNTR) analysis (MLVA) for Vibrio cholerae isolates (n = 45) by year of disease onset.
Clonal complexes (CC 1, CC 2, CC 3) were defined as isolates connected through a chain of single-locus variants. Grey figures indicate the number of different alleles. Three-digit codes present the laboratory isolate number.
Discussion
This study investigated the largest cholera outbreak in Ghana in more than a decade caused by Vibrio cholerae O1 biotype El Tor carrying the classical cholera toxin. The current seventh pandemic is caused by El Tor biotype, which almost completely replaced the classical biotype of previous pandemics [20]. A recent sweep originated from Asia, in which the classical cholera toxin gene selectively replaced the El Tor toxin gene, as currently observed in the Ghanaian outbreak [21,22]. It has been suggested that the recent emergence of a classical ctxB variant in the predominant global El Tor linage is associated with increased clinical virulence, linked with a possible increase in cholera toxin production [23].
Despite the high number of cases, the fatality rate remained below the WHO target of 1% during the 2014 outbreak [1], in contrast to reported numbers from other major cholera outbreaks on the African continent, such as from Kenya (CFR = 2.3%) or Zimbabwe (CFR = 4.7%) [9,24]. In line with the Ghanaian data, older age is a risk factor for mortality as reported from other outbreaks [25]. However, case fatality data from hospital based mortality figures have to be interpreted with caution, because in resource limited settings, many deaths may occur before patients are able to reach the hospital and might have escaped notification/reporting [4].
Ghana has been classified as a cholera endemic country based on epidemiological data, but this has not yet been confirmed using molecular genotyping methods [4]. While surveillance system data suggest a spread from a single source within the capital Accra to surrounding regions, molecular subtyping data hints to the existence of co-circulating V. cholerae strains distinct from the major outbreak strain.
Previous outbreak investigations have demonstrated the need to distinguish between clonal and multi-clonal outbreaks in order to focus prevention measures [11]. As shown in previous studies MLVA proves to be more discriminatory than PFGE to distinguish V. cholerae O1 strains [17,26–28]. Using this technique, both multi-clonal and clonal outbreaks have been reported from Africa. An outbreak in Guinea in 2012 was ascribed to a single V. cholerae clone, probably imported by fishermen from Sierra Leone. The closest known relative was a strain isolated in Bangladesh [29]. In contrast, a genotyping study in Kenya traced the 2009/2010 cholera outbreak to multiple genetic lineages [9]. The present study suggests annual dominant MLVA types, which circulated in Ghana during the years 2011, 2012 and 2014, with minor MLVA types occurring during the same outbreak period. The detection of different distinct genotypes among annual outbreak strain collections between 1970 and 2006 in Ghana and Nigeria supports these findings [14,30]. Outside of Africa, similar co-circulating genotypes have been observed in Thailand between 2007 and 2010 [31]. These data suggest that in Ghana V. cholerae has perhaps an endemic reservoir in the environment and selection pressure results in a highly heterogeneous population of V. cholerae with a few strains evolving into pathogenic clones [32,33], however the present study is not able to proof this hypothesis and a recurrent annual introduction of strains, although unlikely, cannot be ruled out.
The onset of the 2014 outbreak in Ghana coincided with the start of the rainy season in May. Environmental studies conducted in Haiti have demonstrated a seasonal correlation between Vibrio onset and increases in precipitation and water temperature [34]. This is consistent with observations from countries with endemic V. cholerae O1, where the seasonal rise in water temperatures or the onset of the rainy season serve as triggers for the proliferation of V. cholerae O1 in the environment, preceding seasonal cholera epidemics [34]. It has been shown that long-term survival of a particular genotype may be attained in watery environments or in humans with no signs of cholera [35]. V. cholerae infections might occur when people come into close contact with such aquatic reservoirs, particularly during rainy seasons, natural disasters, such as floods or cyclones, but also during droughts, when water sources are over-exploited and polluted [11]. Consequently, cholera outbreaks may be triggered predominantly in large densely populated urban areas, as shown for Abidjan, Cotonou, Douala or Lomé [36–39].
However, no perennial environmental reservoir of toxigenic V. cholerae O1 has yet been identified in West Africa, which could be attributed to the lack of appropriate studies [11]. With environmental cholera sources being suspected in Ghana, enhanced monitoring of aquatic reservoirs and drinking water should be taken into consideration, particularly within urban agglomerations. People involved in any kind of water related activities, such as fishing, may be targeted by prevention measures.
This study identified three non-O1/non-O139 V. cholerae strains, which are naturally present in aquatic ecosystems, often non-pathogenic or associated with only mild disease [23]. However, depending on virulence factors they may cause severe diarrhoea, similar to pandemic V. cholerae O1 strains [23]. In this study none of those strains carried the cholera toxin. Due to the non-specific case definition it remains unclear whether these strains caused the watery diarrhea or those patients were co-infected with other enteric pathogens.
Of serious concern is the increase of antimicrobial resistances between 2011 and 2014, as it has been shown that effective antibiotics shorten the duration of diarrhoea, reduce the volume of stool losses by up to 50% and reduce the duration of shedding of viable organisms in stool from several days to 1–2 days [40]. Multidrug resistance (MDR), defined as resistance to at least three classes of antimicrobial agents normally effective against V. cholerae, was detected in 95% of 2014 isolates, while no MDR isolates were present in 2011. In a study on Ghanaian isolates from 2006 31% of V. cholerae were reported to be MDR [30]. Resistance to ampicillin, ciprofloxacin, nalidix acid, and sulfamethoxazole/trimethoprim have all been described before from various countries worldwide, including Ghana and other regions of the African continent [24,30,41–44]. An increase in the minimal inhibitory concentrations to quinolones has been noticed since the 1980s and has become common in endemic areas being associated with treatment failures [40]. Therefore, careful and regular laboratory monitoring of the antibiotic sensitivity of circulating environmental and outbreak strains is recommended in all settings. Isolates within this study were not screened for macrolides resistance and Extended-spectrum β-lactamases (ESBL). However, ESBL producing V. cholerae strains are rarely reported. Nevertheless, they have been detected sporadically in Ghana in 2006 and South Africa in 2009 [24,30].
This study has some limitations. The surveillance database is based on a non-specific case definition. Only about half of all specimens tested in peripheral hospitals were positive for V. cholerae. Whether this is due to the case definition itself or rather due to sampling, transport or storage conditions remains uncertain. However, WHO estimates that the official reported cholera cases represent 5–10% of the actual disease burden [4]. The database is restricted to six Southern Ghanaian regions around the outbreak epicentre (Greater Accra, Central, Western, Eastern, Ashanti, Volta) for which data quality was adequate for the analysis. The V. cholerae isolates originate from the same Southern regions. Therefore, results cannot be generalized to the whole of Ghana and outbreak numbers differ from official WHO numbers.
This study uses well-established subtyping methods, such as MLST, PFGE and MLVA. Future studies should consider Whole Genome Sequencing approaches in order to place West African V.cholerae isolates into the regional and global phylogenetic framework.
Conclusion
The Ghanaian Cholera outbreak in 2014 was caused by Vibrio cholerae O1 biotype El Tor carrying the classical cholera toxin. Molecular subtyping data of three outbreak years illustrate major annual outbreak clusters with co-circulating genetically distant genotypes, which might hint to an endemic reservoir of V. cholerae in Ghana. Public health authorities must be vigilant and take steps to prevent cholera transmission through aquatic reservoirs, particularly within urban agglomerations during the start of the rainy season. Considering the rapidly emerging multidrug resistance among V. cholerae isolates, laboratories are encouraged to monitor antimicrobial susceptibility closely.
Supporting Information
From all isolates identified within a two-week period in a specific district, one isolate was randomly selected for multilocus sequence typing (MLST), Pulse-field gel electrophoresis (PFGE) and multilocus variable-number tandem-repeat (VNTR) analysis (MLVA), resulting in a subset of 45 isolates. The figure was produced with Arc GIS 10.0 (ESRI: ArcGis Desktop: Release 10.2011).
(TIF)
(TXT)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the German Center for Infection Research (Deutsches Zentrum für Infektionsforschung, DZIF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Cholera Cholera-Fact Sheet no. 107. World Health Organization; 2014. [Google Scholar]
- 2.World Health Organization. Cholera [every year since 2000]. Wkly Epidemiol Rec.
- 3.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet. 2012. June 30;379(9835):2466–76. 10.1016/S0140-6736(12)60436-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali M, Lopez AL, You YA, Kim YE, Sah B, Maskery B, et al. The global burden of cholera. Bull World Health Organ. 2012. March 1;90(3):209–18A. 10.2471/BLT.11.093427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pobee J, Grant F. Case Report of Cholera. Ghana Med J. 1970;306–9. [Google Scholar]
- 6.ProMED-mail. Cholera, diarrhea & dysentery update (74): Americas, Africa, Asia [Internet]. ProMED-mail. 2014. p. 20141016.2863793. Available from: http://www.promedmail.org/
- 7.Chin C-S, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, et al. The origin of the Haitian cholera outbreak strain. N Engl J Med. 2011. January 6;364(1):33–42. 10.1056/NEJMoa1012928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers K. Zimbabwe’s battle against cholera. Lancet. 2009. March 21;373(9668):993–4. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed AA, Oundo J, Kariuki SM, Boga HI, Sharif SK, Akhwale W, et al. Molecular epidemiology of geographically dispersed Vibrio cholerae, Kenya, January 2009-May 2010. Emerg Infect Dis. 2012;18(6):925–31. 10.3201/eid1806.111774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Constantin de Magny G, Guégan J-F, Petit M, Cazelles B. Regional-scale climate-variability synchrony of cholera epidemics in West Africa. BMC Infect Dis. 2007. January;7:20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rebaudet S, Sudre B, Faucher B, Piarroux R. Cholera in coastal Africa: a systematic review of its heterogeneous environmental determinants. J Infect Dis. 2013. November 1;208(Suppl. 1):S98–106. 10.1093/infdis/jit202 [DOI] [PubMed] [Google Scholar]
- 12.Codeço CT. Endemic and epidemic dynamics of cholera: the role of the aquatic reservoir. BMC Infect Dis. 2001. January;1:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De R, Ghosh JB, Sen Gupta S, Takeda Y, Nair GB. The Role of Vibrio cholerae genotyping in Africa. J Infect Dis. 2013;208(Suppl. 1):S98–106. [DOI] [PubMed] [Google Scholar]
- 14.Tarr CL, Patel JS, Puhr ND, Sowers EG, Bopp C a., Strockbine N a. Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J Clin Microbiol. 2007;45(1):134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipp EK, Rivera ING, Gil AI, Espeland EM, Choopun N, Louis VR, et al. Direct Detection of Vibrio cholerae and ctxA in Peruvian Coastal Water and Plankton by PCR Direct Detection of Vibrio cholerae and ctxA in Peruvian Coastal Water and Plankton by PCR. 2003;69(6):3676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naha A, Pazhani GP, Ganguly M, Ghosh S, Ramamurthy T, Nandy RK, et al. Development and evaluation of a PCR assay for tracking the emergence and dissemination of Haitian variant ctxB in Vibrio cholerae O1 strains isolated from Kolkata, India. J Clin Microbiol. 2012;50(5):1733–6. 10.1128/JCM.00387-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper KLF, Luey CKY, Bird M, Terajima J, Nair GB, Kam KM, et al. Development and validation of a PulseNet standardized pulsed-field gel electrophoresis protocol for subtyping of Vibrio cholerae. Foodborne Pathog Dis. 2006. January;3(1):51–8. [DOI] [PubMed] [Google Scholar]
- 18.Octavia S, Salim A, Kurniawan J, Lam C, Leung Q, Ahsan S, et al. Population structure and evolution of non-O1/non-O139 Vibrio cholerae by multilocus sequence typing. PLoS One. 2013. January;8(6):e65342 10.1371/journal.pone.0065342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh R, Nair GB, Tang L, Morris JG, Sharma NC, Ballal M, et al. Epidemiological study of Vibrio cholerae using variable number of tandem repeats. FEMS Microbiol Lett. 2008;288(2):196–201. 10.1111/j.1574-6968.2008.01352.x [DOI] [PubMed] [Google Scholar]
- 20.Kaper JB, Morris JG, Levine MM. Cholera. Clin Microbiol Rev. 1995. January;8(1):48–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raychoudhuri A, Patra T, Ghosh K, Ramamurthy T, Nandy RK, Takeda Y, et al. Classical ctxB in Vibrio cholerae O1, Kolkata, India. Emerg Infect Dis. 2009. January;15(1):131–2. 10.3201/eid1501.080543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutreja A, Kim DW, Thomson NR, Connor TR, Lee JH, Kariuki S, et al. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature. 2011. September 22;477(7365):462–5. 10.1038/nature10392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F, Du P, Li B, Ke C, Chen A, Chen J, et al. Distribution of virulence-associated genes and genetic relationships in non-O1/O139 Vibrio cholerae aquatic isolates from China. Appl Environ Microbiol. 2014;80(16):4987–92. 10.1128/AEM.01021-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ismail H, Smith AM, Tau NP, Sooka A, Keddy KH. Cholera outbreak in South Africa, 2008–2009: Laboratory analysis of Vibrio cholerae O1 strains. J Infect Dis. 2013;208(SUPPL. 1):S39–45. 10.1093/infdis/jit200 [DOI] [PubMed] [Google Scholar]
- 25.Page A-L, Ciglenecki I, Jasmin ER, Desvignes L, Grandesso F, Polonsky J, et al. Geographic distribution and mortality risk factors during the cholera outbreak in a rural region of Haiti, 2010–2011. PLoS Negl Trop Dis. 2015. March;9(3):e0003605 10.1371/journal.pntd.0003605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arakawa E, Murase T, Matsushita S, Shimada T, Yamai S, Ito T, et al. Pulsed-field gel electrophoresis-based molecular comparison of Vibrio cholerae O1 isolates from domestic and imported cases of cholera in Japan. J Clin Microbiol. 2000. January;38(1):424–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cameron DN, Khambaty FM, Wachsmuth IK, Tauxe R V, Barrett TJ. Molecular characterization of Vibrio cholerae O1 strains by pulsed-field gel electrophoresis. J Clin Microbiol. 1994. July;32(7):1685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danin-Poleg Y, Cohen LA, Gancz H, Broza YY, Goldshmidt H, Malul E, et al. Vibrio cholerae strain typing and phylogeny study based on simple sequence repeats. J Clin Microbiol. 2007. March;45(3):736–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebaudet S, Mengel MA, Koivogui L, Moore S, Mutreja A, Kande Y, et al. Deciphering the Origin of the 2012 Cholera Epidemic in Guinea by integrating epidemiological and molecular analyses. PLoS Negl Trop Dis. 2014;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson CC, Freitas FS, Marin MA, Fonseca EL, Okeke IN, Vicente ACP. Vibrio cholerae O1 lineages driving cholera outbreaks during seventh cholera pandemic in Ghana. Infect Genet Evol. 2011. December;11(8):1951–6. 10.1016/j.meegid.2011.08.020 [DOI] [PubMed] [Google Scholar]
- 31.Okada K, Roobthaisong A, Nakagawa I, Hamada S, Chantaroj S. Genotypic and PFGE/MLVA analyses of Vibrio cholerae O1: Geographical spread and temporal changes during the 2007–2010 cholera outbreaks in Thailand. PLoS One. 2012;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, et al. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc Natl Acad Sci U S A. 2009. September 8;106(36):15442–7. 10.1073/pnas.0907787106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faruque SM, Chowdhury N, Kamruzzaman M, Dziejman M, Rahman MH, Sack DA, et al. Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc Natl Acad Sci U S A. 2004. February 17;101(7):2123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alam MT, Weppelmann T a., Longini I, De Rochars VMB, Morris JG, Ali A. Increased Isolation Frequency of Toxigenic Vibrio cholerae O1 from Environmental Monitoring Sites in Haiti. PLoS One. 2015;10(4):e0124098 10.1371/journal.pone.0124098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson EJ, Harris JB, Morris JG, Calderwood SB, Camilli A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol. 2009. October;7(10):693–702. 10.1038/nrmicro2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowenhaupt E, Huq A, Colwell RR, Adingra A, Epstein PR. Rapid detection of Vibrio cholerae O1 in West Africa. Lancet. 1998. January 3;351(9095):34. [DOI] [PubMed] [Google Scholar]
- 37.Gbary AR, Dossou JP, Sossou RA, Mongbo V, Massougbodji A. Epidemiologic and medico-clinical aspects of the cholera outbreak in the Littoral department of Benin in 2008 [in French]. Med Trop (Mars). 2011. April;71(2):157–61. [PubMed] [Google Scholar]
- 38.Akoachere J-FTK, Mbuntcha CKP. Water sources as reservoirs of Vibrio cholerae O1 and non-O1 strains in Bepanda, Douala (Cameroon): relationship between isolation and physico-chemical factors. BMC Infect Dis. 2014. January;14(1):421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Essoya LD, Gessner BD, Kossi B, Tsidi T, Ibrahim ND, Anoumou D, et al. National surveillance data on the epidemiology of cholera in Togo. J Infect Dis. 2013;208(SUPPL. 1):115–9. [DOI] [PubMed] [Google Scholar]
- 40.Leibovici-Weissman Y, Neuberger A, Bitterman R, Sinclair D, Salam MA, Paul M. Antimicrobial drugs for treating cholera. Cochrane database Syst Rev. 2014. January;6:CD008625 10.1002/14651858.CD008625.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marin MA, Fonseca EL, Andrade BN, Cabral AC, Vicente ACP. Worldwide occurrence of integrative conjugative element encoding multidrug resistance determinants in epidemic Vibrio cholerae O1. PLoS One. 2014. January;9(9):e108728 10.1371/journal.pone.0108728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marin MA, Thompson CC, Freitas FS, Fonseca EL, Aboderin AO, Zailani SB, et al. Cholera Outbreaks in Nigeria are associated with multidrug resistant atypical El Tor and Non-O1/Non-O139 Vibrio cholerae. PLoS Negl Trop Dis. 2013;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahmud ZH, Islam S, Zaman RU, Akter M, Talukder KA, Bardhan PK, et al. Phenotypic and genotypic characteristics of Vibrio cholerae O1 isolated from the Sierra Leone cholera outbreak in 2012. Trans R Soc Trop Med Hyg. 2014. November;108(11):715–20. 10.1093/trstmh/tru137 [DOI] [PubMed] [Google Scholar]
- 44.Quilici ML, Massenet D, Gake B, Bwalki B, Olson DM. Vibrio cholerae O1 variant with reduced susceptibility to ciprofloxacin, Western Africa. Emerg Infect Dis. 2010. November;16(11):1804–5. 10.3201/eid1611.100568 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
From all isolates identified within a two-week period in a specific district, one isolate was randomly selected for multilocus sequence typing (MLST), Pulse-field gel electrophoresis (PFGE) and multilocus variable-number tandem-repeat (VNTR) analysis (MLVA), resulting in a subset of 45 isolates. The figure was produced with Arc GIS 10.0 (ESRI: ArcGis Desktop: Release 10.2011).
(TIF)
(TXT)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.