Abstract
Mitogen-activated protein kinase (MAPK) cascades play a fundamental role in signaling of plant immunity and mediate elicitation of cell death. Xanthomonas spp. manipulate plant signaling by using a type III secretion system to deliver effector proteins into host cells. We examined the ability of 33 Xanthomonas effectors to inhibit cell death induced by overexpression of components of MAPK cascades in Nicotiana benthamiana plants. Five effectors inhibited cell death induced by overexpression of MAPKKKα and MEK2, but not of MAP3Kϵ. In addition, expression of AvrBs1 in yeast suppressed activation of the high osmolarity glycerol MAPK pathway, suggesting that the target of this effector is conserved in eukaryotic organisms. These results indicate that Xanthomonas employs several type III effectors to suppress immunity-associated cell death mediated by MAPK cascades.
Keywords: cell death, hypersensitive response, MAP kinase, plant immunity, type III secreted effectors, Xanthomonas, yeast
Plants have developed complex recognition systems and signaling pathways to defend themself against microbial pathogens. A first layer of plant immune responses confers resistance to a broad range of microorganisms and relies on the recognition of conserved microbial molecules known as microbe- or pathogen-associated molecular patterns (MAMPs or PAMPs).1 These responses are collectively referred to as PAMP-triggered immunity (PTI). A second layer of plant immunity, which is effective against host-adapted pathogens, is activated by the specific recognition of pathogen effector proteins and is termed effector-triggered immunity (ETI). ETI is usually associated with the hypersensitive response (HR), a rapid cell death in the infected plant tissues.2 Mitogen-activated protein kinase (MAPK) cascades play a fundamental role in signaling pathways of both layers of plant immunity.3 The MAP3Kα and MAP3Kϵ MAPKKKs are involved in signaling pathways mediating the development of ETI-associated HR in N. benthamiana plants.4,5 Moreover, MAP3Kα and MAP3Kϵ were shown to be required for tomato disease resistance to bacterial pathogens.4,5 The MEK2 MAPKK was reported to be a key regulator of the HR elicited by multiple R/avr gene pairs in N. benthamiana plants and to act downstream of both MAP3Kα and MAP3Kϵ, and upstream of the SIPK and WIPK MAPKs.4-8 Interestingly, N. benthamiana SIPK and WIPK and their Arabidopsis thaliana homologs MPK6 and MPK3, respectively, are also important regulators of PAMP-triggered immunity (PTI).9,10 Similarly, the Arabidopsis thaliana MKK4 and MKK5, which act upstream of MPK6 and MPK3, were shown to be involved in the activation of PTI.9
Xanthomonas is a genus of plant pathogenic bacteria that cause disease in hundreds of plant crops.11 The ability of most Xanthomonas spp to cause infection and colonize their hosts largely depends on a type III secretion (T3S) system.11 This secretion apparatus translocates a suite of effecter proteins directly into the cytosol of the host cell.12 Inside the host cell, effector proteins contribute to pathogenesis by suppressing immune responses and manipulating host metabolism and hormone signaling.13-17 Several effectors of Xanthomonas and other phytopathogenic bacteria were reported to suppress cell death caused by activation of immune responses. For example, the XopQ, XopN, XopZ and XopX effectors of Xanthomonas oryzae were shown to suppress cell death induced by recognition of damage-associated molecular patterns (DAMPs) in rice.18 The AvrBsT and XopQ effectors of Xanthomonas euvesicatoria were reported to attenuate ETI-mediated cell death in resistant cultivars of pepper and tomato plants.19,20 Several effectors of Pseudomonas syringae were found to suppress the HR-like cell death induced by the effector HopPsyA in tobacco and Arabidopsis.21 In this study, we performed a functional screen to identify Xanthomonas type III effectors that suppress cell death induced by overexpression of components of immunity-associated MAP kinase cascades.
We hypothesized that certain Xanthomonas effectors interfere with plant immunity by targeting components of MAP kinase cascades or other downstream signal proteins that are involved in the elicitation of the HR. To test this hypothesis, we screened a group of T3S effectors of Xanthomonas euvesicatoria and Xanthomonas perforans bacteria for their ability to suppress cell death induced by overexpression of known regulators of cell-death signaling associated with plant immunity. These included MAP3Kα,4 the kinase domain of MAP3Kϵ (MAP3KϵKD)5 or a constitutively active form of MEK2 (MEK2DD).8 Thirty-3 effectors were transiently co-expressed via Agrobacterium in N. benthamiana leaves with each one of the cell death inducers or an empty vector (Table 1). Expression of the effectors was driven by the CaMV 35S promoter, while that of MAP3Kα, MAP3KϵKD, and MEK2DD was under the control of an estradiol-inducible system.22 Leaves were visually monitored for the development of cell death during 7 days after estradiol application. Expression of certain effectors with an empty vector caused chlorosis (yellowing of the leaf tissue without apparent necrotic damage) or various degrees of cell death (necrotic damage) in the infiltrated area (Table 1). These phenotypes can be ascribed either to the virulence activity of the effectors in plant cells, or to their recognition by the plant surveillance system. As shown in Fig. 1A and Table 1, the XopE1, XopF2, XopH, XopI, XopM, XopQ, XopV, AvrBs1 and AvrXv4 effectors partially or fully inhibited cell death triggered by at least one of the cell death inducers. To quantify the inhibition of cell death for these 9 effectors, ion leakage from the infiltrated areas was measured at 36 h after estradiol application (Fig. 1B). A significant reduction in ion leakage induced by expression of MEK2DD and MAP3Kα was observed in leaves expressing XopE1, XopM, XopQ, AvrBs1 and AvrXv4, but not other effectors (Fig. 1B). Ion leakage induced by MAP3KϵKD was not affected by any of the tested effectors (Fig. 1B).
Table 1.
Inhibition of cell death by Xanthomonas type III effectors. N. benthamiana leaves were co-inoculated with Agrobacterium strains expressing the indicated effector protein from Xcv strain 85-10 and either MAP3Kα, MAP3KϵKD, MEK2DD or an empty vector control (EV). Scores of +++, ++ and + indicate the appearance of cell death at 3, 5 and 7 days post-inoculation, respectively. A minus sign (−) indicates that no cell death was observed up to 7 days after inoculation. C indicates the appearance of leaf chlorosis in the infiltrated area 5 days after inoculation.
| Type III effector | EV | MAP3Kα | MAP3KϵKD | MEK2DD |
|---|---|---|---|---|
| Empty | − | +++ | +++ | +++ |
| XopB | ++ | +++ | +++ | +++ |
| XopC | − | +++ | +++ | +++ |
| XopD | C | +++ | +++ | +++ |
| XopE1 | C | + | +++ | ++ |
| XopE2 | C | +++ | +++ | +++ |
| XopF1 | − | +++ | +++ | +++ |
| XopF2 | + | ++ | ++ | +++ |
| XopG | − | +++ | +++ | +++ |
| XopH | C | ++ | ++ | +++ |
| XopI | − | +++ | ++ | +++ |
| XopJ | C | +++ | +++ | +++ |
| XopK | − | +++ | +++ | +++ |
| XopL | + | ++ | +++ | +++ |
| XopM | C | + | +++ | ++ |
| XopN | ++ | +++ | +++ | +++ |
| XopO | − | +++ | +++ | +++ |
| XopQ | C | C | +++ | + |
| XopR | − | +++ | +++ | +++ |
| XopS | C | +++ | +++ | +++ |
| XopV | C | ++ | +++ | +++ |
| XopX | ++ | +++ | +++ | +++ |
| XopZ | − | +++ | +++ | +++ |
| XopAD | − | +++ | +++ | +++ |
| XopAE | − | +++ | +++ | +++ |
| XopAK | − | +++ | +++ | +++ |
| Ecf | C | +++ | +++ | +++ |
| AvrBs1 | − | − | +++ | + |
| AvrBs2 | − | +++ | +++ | +++ |
| AvrRxo1 | + | +++ | +++ | +++ |
| AvrBsTa | +++ | +++ | +++ | +++ |
| AvrBs3b | C | +++ | +++ | +++ |
| AvrXv3c | − | +++ | +++ | +++ |
| AvrXv4c | C | + | +++ | ++ |
Effector derived from the Xcv 75–3 strain.
Effector derived from the Xcv 116 strain.
Effector derived from the Xcv 91–118 strain.
Figure 1.
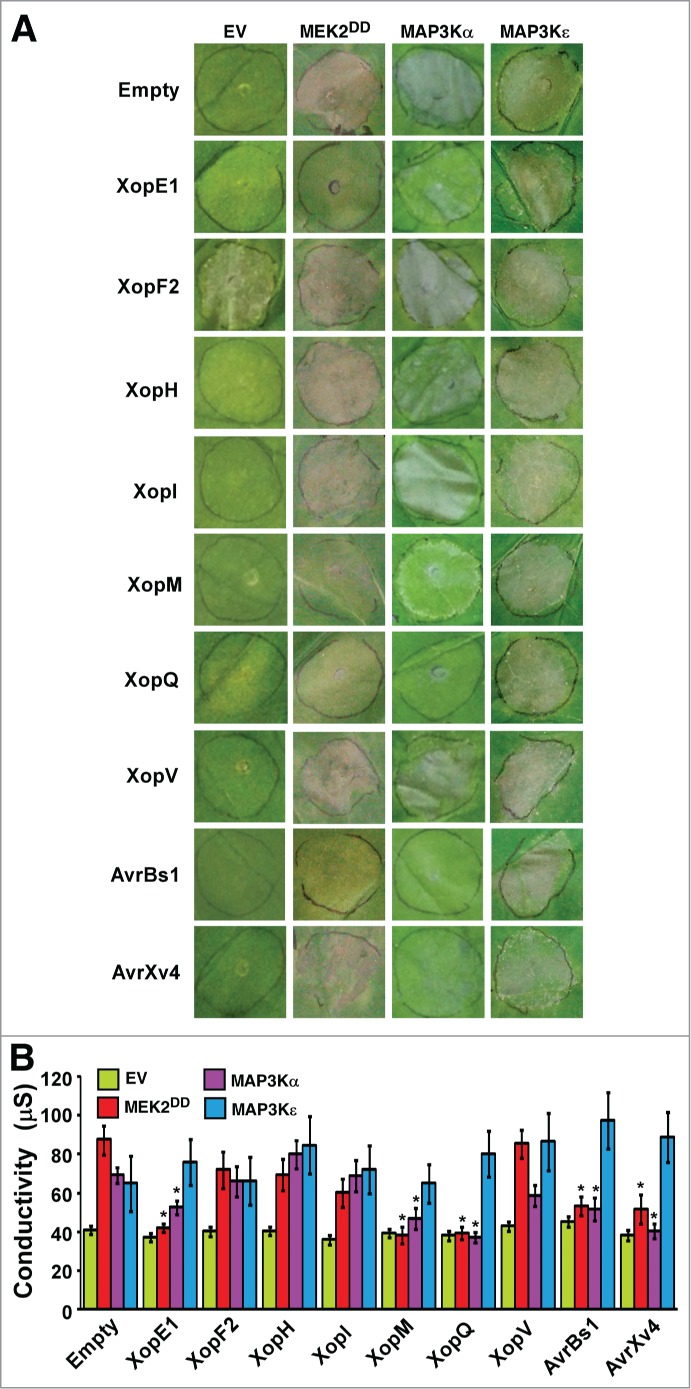
Inhibition of cell death by Xanthomonas type III effectors. An empty vector (EV), a constitutively active form of MEK2 (MEK2DD), MAP3Kα or the kinase domain of MAP3Kϵ (MAP3KϵKD), driven by the estradiol-inducible XVE expression system, were co-expressed via Agrobacterium in N. benthamiana leaves with the indicated effector protein driven by the CaMV 35S promoter. Expression of MEK2DD, MAP3Kα and MAP3KϵKD was induced by 17β-estradiol at 24 h after agro-infiltration. (A). Pictures of the infiltrated leaves at 5 days after 17β-estradiol treatment. (B). Quantification of cell death in leaves by measuring electrolyte leakage at 36 h after 17β-estradiol treatment. Values are the mean conductivity ± SE for leaf samples from at least 10 plants. Asterisks indicate a significant difference (Student's t test, P < 0.05) as compared to the empty vector control.
Based on the assumption that MAP kinase cascades are conserved in eukaryotic organisms, we used a heterologous yeast system to examine if the effectors that suppressed cell death in plants target a conserved signaling components. To address this question, we tested the ability of the effectors to inhibit activation of the yeast high osmolarity glycerol (HOG) MAPK pathway.23 To this aim, we monitored activation by osmotic stress of a lacZ reporter driven by the 8xCRE HOG responsive element in the presence of XopE1, XopM, XopQ, AvrBs1 or AvrXv4.24 Expression of the effectors was first induced in yeast cultures containing the HOG-responsive reporter and 1 M sorbitol was then added to activate the HOG pathway. Following 1 h incubation, cultures were subjected to a β-galactosidase assay to monitor the activation of the reporter. As shown in Fig. 2, expression of AvrBs1, but not that of the other effectors, significantly attenuated the activation of the HOG reporter by approximately 70%. These results suggest that AvrBs1 directly targets a component of the MAPK cascade or a downstream signaling protein, which is conserved in plants and yeast.
Figure 2.
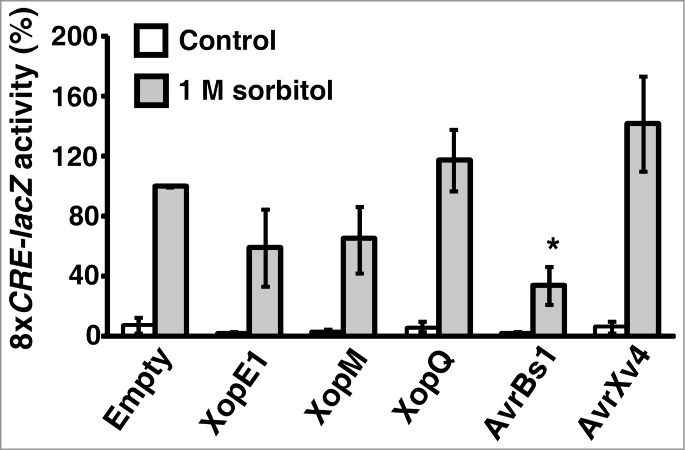
AvrBs1 attenuates activation of the yeast HOG pathway. Activation of an 8xCRE-regulated lacZ reporter monitored upon incubation of yeast strains containing an empty vector or the indicated effectors with or without 1 M sorbitol. Activity was normalized to that of yeast containing an empty vector and treated with sorbitol. Values represent the means ±SE of the relative activation 1 h after sorbitol addition in 3 biological repeats. Asterisks indicate a significant difference (Student's t test, P < 0.05) as compared to yeast containing an empty vector and treated with sorbitol.
MAPK cascades play a central role in immune signaling and activation of the ETI-associated HR cell death. Therefore, components of these cascades or their downstream proteins represent convenient targets for bacterial effectors. Indeed, effectors of multiple pathogens were reported to manipulate MAPK signaling: for example, the PexRD2 effector of Phytophthora infestans was reported to directly target MAPKKKϵ to promote disease in N. benthamiana plants.25 In addition, the Pseudomonas syringae effectors HopF2 and HopAI1 suppress PTI by inhibiting MAP kinase cascades: HopF2 inactivates Arabidopsis MKK5 by ADP-ribosylation and HopAI1 dephosphorylates Arabidopsis MPK3 and MPK6 inhibiting their kinase activity.26,27 In this study, we identified 5 Xanthomonas effectors that significantly inhibit cell death induced by MAPKKKα and a constitutively active form of MEK2. However, none of the 33 tested effectors suppressed the cell death induced by MAPKKKϵ, suggesting that the target of the 5 cell death suppressors probably participate in signaling rather than in the execution of cell death. We utilized a heterologous MAPK reporter system in yeast to examine whether the target of these effectors is conserved in other eukaryotic organisms. Among the 5 effectors that inhibited cell death, AvrBs1 was able to suppress activation of the HOG MAPK pathway in yeast. This is in agreement with previous findings showing that expression of AvrBs1 in yeast causes sensitivity to NaCl and sorbitol that are known inducers of the HOG pathway.28
In conclusion, our screen identified 5 Xanthomonas effectors that suppressed cell death induced by components of immunity-associated MAP kinase cascades. Furthermore we found that AvrBs1 suppresses activation of the HOG MAPK pathway in yeast, suggesting that this effector targets a signaling component that is conserved in eukaryotic organisms.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by the Israel Science Foundation (ISF; grant no. 326/10 to G.S.) and by the United States-Israel Binational Agricultural Research and Development Fund (BARD; grant no. IS-4510–12C to G.S. and G.B.M.).
References
- 1.Bigeard J, Colcombet J, Hirt H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol Plant 2015; 8:521-139; PMID:25744358; http://dx.doi.org/ 10.1016/j.molp.2014.12.022 [DOI] [PubMed] [Google Scholar]
- 2.Mur LA, Kenton P, Lloyd AJ, Ougham H, Prats E. The hypersensitive response; the centenary is upon us but how much do we know? J Exp Bot 2008; 59:501-20; PMID:18079135; http://dx.doi.org/ 10.1093/jxb/erm239 [DOI] [PubMed] [Google Scholar]
- 3.Meng X, Zhang S. MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol 2013; 51:245-66; PMID:23663002; http://dx.doi.org/ 10.1146/annurev-phyto-082712-102314 [DOI] [PubMed] [Google Scholar]
- 4.del Pozo O, Pedley KF, Martin GB. MAPKKKα is a positive regulator of cell death associated with both plant immunity and disease. EMBO J 2004; 23:3072-82; PMID:15272302; http://dx.doi.org/ 10.1038/sj.emboj.7600283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melech-Bonfil S, Sessa G. Tomato MAPKKKε is a positive regulator of cell-death signaling networks associated with plant immunity. Plant J 2010; 64:379-91; PMID:21049563; http://dx.doi.org/ 10.1111/j.1365-313x.2010.04333.x [DOI] [PubMed] [Google Scholar]
- 6.Oh CS, Martin GB. Tomato 14-3-3 protein TFT7 interacts with a MAP kinase kinase to regulate immunity-associated programmed cell death mediated by diverse disease resistance proteins. J Biol Chem 2011; 286:14129-36; PMID:21378171; http://dx.doi.org/ 10.1074/jbc.m111.225086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekengren SK, Liu Y, Schiff M, Dinesh-Kumar SP, Martin GB. Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J 2003; 36:905-17; PMID:14675454; http://dx.doi.org/ 10.1046/j.1365-313x.2003.01944.x [DOI] [PubMed] [Google Scholar]
- 8.Yang KY, Liu Y, Zhang S. Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci USA 2001; 98:741-6; PMID:11209069; http://dx.doi.org/ 10.1073/pnas.98.2.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002; 415:977-83; PMID:11875555; http://dx.doi.org/ 10.1038/415977a [DOI] [PubMed] [Google Scholar]
- 10.Segonzac C, Feike D, Gimenez-Ibanez S, Hann DR, Zipfel C, Rathjen JP. Hierarchy and roles of pathogen-associated molecular pattern-induced responses in Nicotiana benthamiana. Plant Physiol 2011; 156:687-99; PMID:21478366; http://dx.doi.org/ 10.1104/pp.110.171249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan RP, Vorholter FJ, Potnis N, Jones JB, Van Sluys MA, Bogdanove AJ, Dow JM. Pathogenomics of Xanthomonas: understanding bacterium-plant interactions. Nat Rev Microbiol 2011; 9:344-55; PMID:21478901; http://dx.doi.org/ 10.1038/nrmicro2558 [DOI] [PubMed] [Google Scholar]
- 12.Buttner D, He SY. Type III protein secretion in plant pathogenic bacteria. Plant Physiol 2009; 150:1656-64; PMID:19458111; http://dx.doi.org/ 10.1104/pp.109.139089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marois E, Van den Ackerveken G, Bonas U. The Xanthomonas type III effector protein AvrBs3 modulates plant gene expression and induces cell hypertrophy in the susceptible host. Mol Plant Microbe Interact 2002; 15:637-46; PMID:12118879; http://dx.doi.org/ 10.1094/mpmi.2002.15.7.637 [DOI] [PubMed] [Google Scholar]
- 14.Sonnewald S, Priller JP, Schuster J, Glickmann E, Hajirezaei MR, Siebig S, Mudgett MB, Sonnewald U. Regulation of cell wall-bound invertase in pepper leaves by Xanthomonas campestris pv. vesicatoria type three effectors. PLoS One 2012; 7:e51763; PMID:23272161; http://dx.doi.org/ 10.1371/journal.pone.0051763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JG, Stork W, Mudgett MB. Xanthomonas type III effector XopD desumoylates tomato transcription factor SlERF4 to suppress ethylene responses and promote pathogen growth. Cell Host Microbe 2013; 13:143-54; PMID:23414755; http://dx.doi.org/ 10.1016/j.chom.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verdier V, Triplett LR, Hummel AW, Corral R, Cernadas RA, Schmidt CL, Bogdanove AJ, Leach JE. Transcription activator-like (TAL) effectors targeting OsSWEET genes enhance virulence on diverse rice (Oryza sativa) varieties when expressed individually in a TAL effector-deficient strain of Xanthomonas oryzae. New Phytol 2012; 196:1197-207; PMID:23078195; http://dx.doi.org/ 10.1111/j.1469-8137.2012.04367.x [DOI] [PubMed] [Google Scholar]
- 17.White FF, Potnis N, Jones JB, Koebnik R. The type III effectors of Xanthomonas. Mol Plant Pathol 2009; 10:749-66; PMID:19849782; http://dx.doi.org/ 10.1111/j.1364-3703.2009.00590.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha D, Gupta MK, Patel HK, Ranjan A, Sonti RV. Cell wall degrading enzyme induced rice innate immune responses are suppressed by the type 3 secretion system effectors XopN, XopQ, XopX and XopZ of Xanthomonas oryzae pv. oryzae. PLoS One 2013; 8:e75867; PMID:24086651; http://dx.doi.org/ 10.1371/journal.pone.0075867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szczesny R, Buttner D, Escolar L, Schulze S, Seiferth A, Bonas U. Suppression of the AvrBs1-specific hypersensitive response by the YopJ effector homolog AvrBsT from Xanthomonas depends on a SNF1-related kinase. New Phytol 2010; 187:1058-74; PMID:20609114; http://dx.doi.org/ 10.1111/j.1469-8137.2010.03346.x [DOI] [PubMed] [Google Scholar]
- 20.Teper D, Salomon D, Sunitha S, Kim JG, Mudgett MB, Sessa G. Xanthomonas euvesicatoria type III effector XopQ interacts with tomato and pepper 14-3-3 isoforms to suppress effector-triggered immunity. Plant J 2014; 77:297-309; PMID:24279912; http://dx.doi.org/ 10.1111/tpj.12391 [DOI] [PubMed] [Google Scholar]
- 21.Jamir Y, Guo M, Oh HS, Petnicki-Ocwieja T, Chen S, Tang X, Dickman MB, Collmer A, Alfano JR. Identification of Pseudomonas syringae type III effectors that can suppress programmed cell death in plants and yeast. Plant J 2004; 37:554-65; PMID:14756767; http://dx.doi.org/ 10.1046/j.1365-313x.2003.01982.x [DOI] [PubMed] [Google Scholar]
- 22.Zuo J, Niu QW, Chua NH. An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 2000; 24:265-73. PMID:11069700; http://dx.doi.org/ 10.1046/j.1365-313x.2000.00868.x [DOI] [PubMed] [Google Scholar]
- 23.Saito H, Tatebayashi K. Regulation of the osmoregulatory HOG MAPK cascade in yeast. J Biochem 2004; 136:267-72; PMID:15598881; http://dx.doi.org/ 10.1093/jb/mvh135 [DOI] [PubMed] [Google Scholar]
- 24.Tatebayashi K, Yamamoto K, Tanaka K, Tomida T, Maruoka T, Kasukawa E, Saito H. Adaptor functions of Cdc42, Ste50, and Sho1 in the yeast osmoregulatory HOG MAPK pathway. EMBO J 2006; 25:3033-44; PMID:16778768; http://dx.doi.org/ 10.1038/sj.emboj.7601192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King SR, McLellan H, Boevink PC, Armstrong MR, Bukharova T, Sukarta O, Win J, Kamoun S, Birch PR, Banfield MJ. Phytophthora infestans RXLR effector PexRD2 interacts with host MAPKKKε to suppress plant immune signaling. Plant Cell 2014; 26:1345-59; PMID:24632534; http://dx.doi.org/ 10.1105/tpc.113.120055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J et al.. A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 2007; 1:175-85; PMID:18005697; http://dx.doi.org/ 10.1016/j.chom.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Li J, Hou S, Wang X, Li Y, Ren D, Chen S, Tang X, Zhou JM. A Pseudomonas syringae ADP-ribosyltransferase inhibits Arabidopsis mitogen-activated protein kinase kinases. Plant Cell 2010; 22:2033-44; PMID:20571112; http://dx.doi.org/ 10.1105/tpc.110.075697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salomon D, Dar D, Sreeramulu S, Sessa G. Expression of Xanthomonas campestris pv. vesicatoria type III effectors in yeast affects cell growth and viability. Mol Plant Microbe Interact 2011; 24:305-14; PMID:21062109; http://dx.doi.org/ 10.1094/mpmi-09-10-0196 [DOI] [PubMed] [Google Scholar]


