Abstract
A significant feature of plant cells is the extensive motility of organelles and the cytosol, which was originally defined as cytoplasmic streaming. We suggested previously that a three-way interaction between plant-specific motor proteins myosin XIs, actin filaments, and the endoplasmic reticulum (ER) was responsible for cytoplasmic streaming.1 Currently, however, there are no reports of molecular components for cytoplasmic streaming other than the actin-myosin-cytoskeleton and ER-related proteins. In the present study, we found that elongated cells of inflorescence stems of Arabidopsis thaliana exhibit vigorous cytoplasmic streaming. Statistical analysis showed that the maximal velocity of plastid movements is 7.26 µm/s, which is much faster than the previously reported velocities of organelles. Surprisingly, the maximal velocity of streaming in the inflorescence stem cells was significantly reduced to 1.11 µm/s in an Arabidopsis mutant, abcb19-101, which lacks ATP BINDING CASSETTE SUBFAMILY B19 (ABCB19) that mediates the polar transport of the phytohormone auxin together with PIN-FORMED (PIN) proteins. Polar auxin transport establishes the auxin concentration gradient essential for plant development and tropisms. Deficiency of ABCB19 activity eventually caused enhanced gravitropic responses of the inflorescence stems and abnormally flexed inflorescence stems. These results suggest that ABCB19-mediated auxin transport plays a role not only in tropism regulation, but also in cytoplasmic streaming.
Keywords: ABC transporter; ABCB19; PGP19; MDR1; Arabidopsis thaliana, cytoplasmic streaming; shoot gravitropism
Abbreviations
- ABC transporter
- ATP-binding cassette transporter
- ABCB19
- ATP BINDING CASSETTE SUBFAMILY B19
- MDR1
- MULTIDRUG RESISTANCE PROTEIN1
- PGP19
- P-GLYCOPROTEIN19
- PIN
- PIN-FORMED
Introduction
Plant tropism has attracted significant attention over the years. Nearly every organ is able to bend in response to a variety of environmental stimuli including gravity and light.2, 3 According to the classical Cholodny–Went model, gravitropic and phototropic curvature of roots and stems occurs by a lateral auxin gradient that promotes differential cell elongation.2 Gravistimulation induces asymmetrical distribution of the major endogenous auxin indol-3-acetic acid in rice and corn coleoptile, which causes differential growth of cells on either side of the organ to establish the organ curvature.4,5
The molecular mechanisms underlying tropisms have been demonstrated in Arabidopsis thaliana. Arabidopsis plants sense gravity in endodermal cells of shoots6 and in columella cells of roots.7 The main components of inducing a lateral auxin redistribution are PIN FORMED (PIN) protein PIN38 and ATP BINDING CASSETTE SUBFAMILY B19 (ABCB19).9 ABCB19 is also known as MULTIDRUG RESISTANCE PROTEIN1 (MDR1) and P-GLYCOPROTEIN 19 (PGP19). ABCB19 has an ability to transport auxin through plasma membrane and interacts with the auxin efflux carrier proteins PINs (PIN1 and PIN2), suggesting a complicated relationship between these transporters and auxin distribution.10 Arabidopsis mutants with defects in the ABCB19 gene showed not only abnormal auxin distribution but also abnormal tropisms: enhanced gravitropism and phototropism in hypocotyl and enhanced gravitropism in roots.9,11-13
In the present study, we found that ABCB19 is required for gravitropic response of inflorescence stems and organelle movements in the stem cells. Cytoplasmic streaming was first described in 1774.14 Unidirectional actin filament bundles and organelle-associated myosin XIs, a plant-specific class of myosin motors, are thought to cause bulk flow in the cell.15,16 Previously, we demonstrated that the endoplasmic reticulum (ER) dynamics are driven primarily by the ER-associated myosin XI-K and that myosin XI deficiency affects organization of the ER network and orientation of the actin filament bundles. We suggested a model whereby dynamic three-way interactions between ER, F-actin, and myosin XIs determine the architecture and movement patterns of the ER thick strands, and cause cytosol hauling traditionally defined as cytoplasmic streaming.1 However, the molecular mechanism and physiological meanings of cytoplasmic streaming are largely unknown.
Results
An inflorescence stem of Arabidopsis comprises radially patterned tissue layers of epidermis, cortex, endodermis, vasculature, and the inner tissues (Fig. 1A and 1B). Each layer has specific functions: the epidermis acts as an interface between the plant and the environmental stresses; the endodermis plays a role of sensing gravity;17 and the vasculature consisting of phloem and xylem functions in the nutrient and water flow. The inner tissues comprise elongated cells with lengths up to 1 mm (Fig. 1C). However, the physiological function of the elongated cells is not known. To investigate the function of these elongated cells, hand-cut vertical sections of the inflorescence stem were prepared and the elongated cells of the inner tissues were observed in the living state. We found that the typical organelles plastids in the elongated cells rapidly streamed along the longitudinal axis of the cells (Fig. 1D). Statistical analysis revealed that the maximal velocity of the plastid movements was 7.26 µm/s (Table 1), which is much higher than the velocities of organelle movements reported before.18,19 For example, the maximal velocity of the ER and the cytosol in petiole cells was reported to be ∼3.5 µm/s.1 These movements of organelles and cytosol correspond to cytoplasmic streaming. Hence, the elongated cells of the inflorescence stems exhibit active cytoplasmic streaming.
Figure 1.
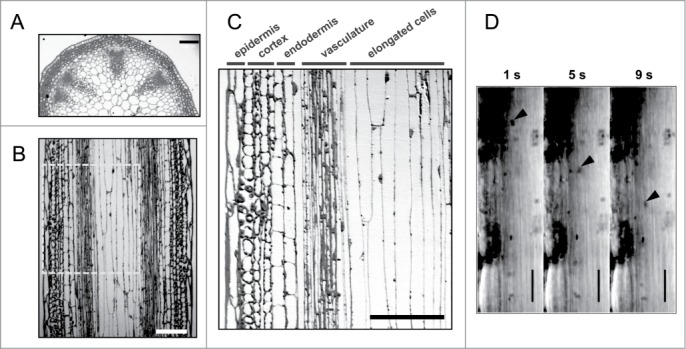
Active cytoplasmic streaming in elongated cells of the inflorescence stem. (A–C) Cross section (A) and transverse section (B and C) of upper part of the inflorescence stem were stained with Toluidine Blue. Bars = 100 μm. Boxed area in (B) was magnified in (C). (D) Time course of the plastid movement in the elongated cells of the inflorescence stem (upper part). Arrow heads indicate the plastids showing maximal velocity in the time-lapse sequence. Bars = 20 μm. See also Supplementary Movie 1.
Table 1.
Maximal velocity of plastids in the fiber cells of abcb19-101. Absolute values and statistical analysis of the data presented in Fig. 2. n, number of data points in the data set; SD, standard deviation; SE, standard error.
| maximal velocities (µm/sec) |
|||||
|---|---|---|---|---|---|
| average | SD | SE | n | P value by t-test (wild type versus fiz1/+) | |
| wild type | 7.26 | 1.07 | 0.38 | 8 | |
| abcb19-101 | 1.11 | 0.58 | 0.14 | 17 | 2.04E-15 |
Quite unexpectedly, we discovered that cytoplasmic steaming was drastically impaired by depletion of ABCB19 (Fig. 2A). The maximal velocity of plastid movements in abcb19-101 was determined to be 1.11 µm/s (Table 1), indicating that a deficiency of ABCB19 caused a dramatic reduction of cytoplasmic streaming activity by up to 85% (Fig. 2B). Moreover, most of the plastids streamed long distances with almost constant velocities in the wild-type cells, whereas they streamed short distances in a discontinuous manner and frequently stopped moving (Supplemental Movie 1). These results suggest that ABCB19 plays a role in regulating cytoplasmic streaming of plant cells and that ABCB19 is related to the actin-myosin XI cytoskeleton. Until now, no components other than the actin-myosin XIs and ER-related proteins19 were reported as being involved in cytoplasmic streaming. Furthermore, despite extensive studies on involvement of ABCB19/MDR1/PGP19 in auxin distribution and tropisms in hypocotyls and roots, no ABCB19 functions at the subcellular levels have been reported. Therefore, the discovery of a role of ABCB19 provides novel insights into the field of cytoplasmic streaming in plants.
Figure 2.
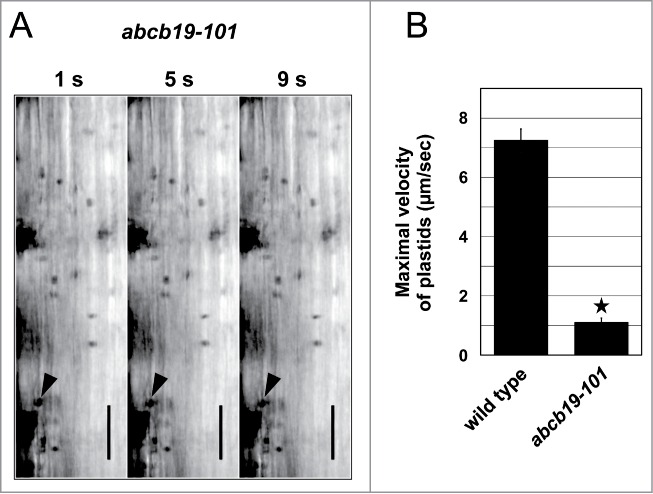
Defects of cytoplasmic streaming in elongated cells of the abcb19 inflorescence stem. (A) Time course of the plastid movement in the elongated cells of the abcb19-101 inflorescence stem (upper part). Arrow heads indicate the plastids showing maximal velocity in the time-lapse sequence. Bars = 20 μm. See also Supplementary Movie 1. (B) Maximal velocity of plastids in the elongated cells of wild type and abcb19-101. Error bars indicate SE. *, P < 0.001 by the Student's t test. See also Table 1.
Next, we examined the gravitropic response of the wild-type and the abcb19-101 mutant by focusing on the inflorescence stems, in which ABCB19 is expressed.20 Plants with growing primary inflorescence stems were placed horizontally for 6 h. In the initial response phase, the stem of the wild-type bent 90° to become vertical (Fig. 3A). Subsequently, the wild-type continued growing vertically. Conversely, abcb19-101 first overshot in one direction up to 120° and then overshot in the other direction (Figure 3A). The precise movements of the inflorescence stems of the wild-type and abcb19-101 are shown in Supplementary Movie 1. Figure 3B shows the superimposed time-lapse images during the course of gravitropic response of inflorescence stems, showing the overshooting stem of abcb19-101. Statistical analysis of gravitropic bending of the stems (Table 2) and kinetics of gravitropism of the stems (Fig. 3C) clearly revealed that a deficiency of ABCB19 caused an enhanced gravitropic response of the inflorescence stems. This result is consistent with the enhanced bending of hypocotyls in response to gravity.11 ABCB19 may be involved in adjusting the bending of the stems in response to gravity. The abcb19-101 plants exhibited a weak dwarf phenotype and kinked gross morphology (Fig. 4A). The organs including the inflorescence stems and pedicels were abnormally flexed (Fig. 4B). These abnormal phenotypes may be due to the enhanced gravitropic organ bending in the mutant.
Figure 3.
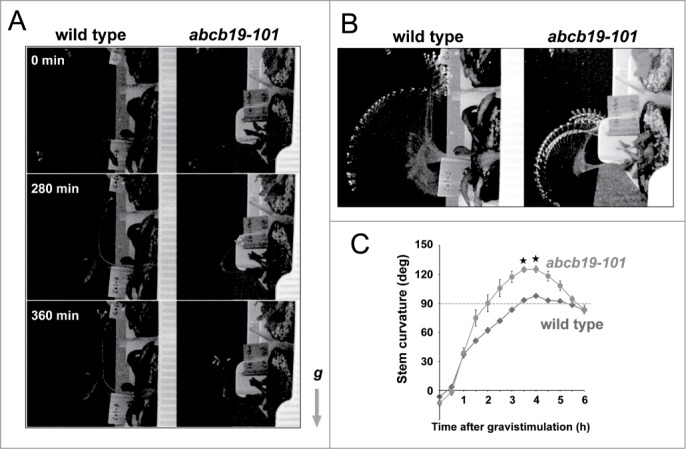
Deficiency of ABCB19 enhances bending of an inflorescence stem in response to gravity stimuli. (A) Time course of gravitropism of inflorescence stem. (B) Maximum intensity projections of time-lapse images during gravitropism of inflorescence stems. Left, wild-type; right, abcb19-101. See also Supplementary Movie 2. (C) Kinetics of gravitropism of inflorescence stems of wild-type and the abcb19-101 mutant after placing the plant horizontally. Error bars indicate SE. *, P < 0.001 (Student's t test). See also Table 2.
Table 2.
Gravitropism of the abcb19-101 inflorescence stem. Absolute values and statistical analysis of the data presented in Fig. 3. n, number of data points in the data set; SD, Standard deviation.
| wild type |
abcb19-101 |
||||||
|---|---|---|---|---|---|---|---|
| curvature (deg) |
curvature (deg) |
||||||
| Time (min) | average | SD | n | average | SD | n | P value by t-test (wild type versus abcb19-101) |
| 0 | −6.5 | 3.1 | 15 | −12.9 | 10.5 | 13 | 9.76E-01 |
| 30 | 3.6 | 3.0 | 15 | −1.7 | 11.2 | 13 | 6.49E-01 |
| 60 | 37.2 | 6.8 | 15 | 38.8 | 18.7 | 13 | 1.52E-01 |
| 90 | 51.4 | 9.2 | 15 | 74.9 | 30.6 | 13 | 5.16E-03 |
| 120 | 62.2 | 11.5 | 15 | 90.2 | 31.8 | 13 | 5.03E-03 |
| 150 | 71.9 | 9.3 | 15 | 105.7 | 32.3 | 13 | 4.73E-03 |
| 180 | 83.4 | 6.4 | 15 | 117.4 | 21.4 | 13 | 4.74E-03 |
| 210 | 93.3 | 4.9 | 15 | 125.0 | 10.4 | 13 | 1.21E-04 |
| 240 | 97.8 | 5.0 | 15 | 125.3 | 12.4 | 13 | 5.73E-04 |
| 270 | 93.1 | 7.3 | 15 | 118.2 | 18.3 | 13 | 6.77E-03 |
| 300 | 92.2 | 6.1 | 15 | 108.2 | 17.6 | 13 | 3.65E-02 |
| 330 | 88.4 | 3.7 | 15 | 94.4 | 11.7 | 13 | 3.50E-01 |
| 360 | 83.0 | 4.1 | 15 | 83.8 | 16.1 | 13 | 5.04E-01 |
Figure 4.
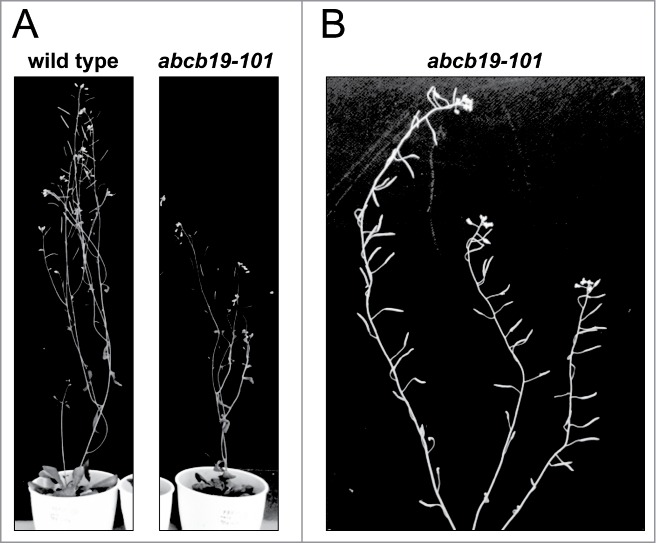
Kinked gross morphology of the abcb19 mutant. (A) Six-week-old plants of wild-type Col-0 and the abcb19-101 mutant grown in a growth chamber. (B) Inflorescence stems of the abcb19-101 mutant (9 weeks old).
Discussion
In the present study, we show that the inflorescence stems of abcb19-101 displays enhanced gravitropic responses as does the abcb19-101 hypocotyls.21 However, this phenotype is inconsistent with the report of impaired gravitropic response of the stem of another abcb19 allele mdr1.13 This inconsistency may be due to the difference of the ecotypes of the A. thaliana plants: the genetic background of abcb19-101 is Col-0, whereas that of mdr1 is WS. The discovery of an involvement of ABCB19 in intracellular movements of organelles is unexpected. This suggests that the auxin transporter ABCB19 is associated with the actin-myosin cytoskeleton.
Cytoplasmic streaming is inhibited by treatment with the endogenous auxin indole-3-acetic acid.22 Other carboxylic acids, including acetic acid, propionic acid, and decanoic acid, have similar effects as indole-3-acetic acid on cytoplasmic streaming22 and affect the organization of actin cytoskeletons in the treated cells. These events are also supported by a report published ∼80 years ago, showing that cytoplasmic streaming was suppressed by treatment with high concentrations of auxin.23 Because abcb19 causes auxin leakage from the inner tissue cells of hypocotyls to the epidermis10 and abcb19 impairs the auxin polar transport in the hypocotyls,12 abcb19 may cause a defect in auxin distribution in inflorescence stems. The abnormal auxin distributions may affect the actin cytoskeleton organization, resulting in impaired cytoplasmic streaming. Another possibility is derived from the hypothesis that the actin cytoskeleton regulates polar localization of PIN proteins by potentially participating in the vesicular cycling of the PIN1 protein.24
Materials and Methods
Plant materials and growth conditions
Arabidopsis thaliana L. (Heynh) accession Columbia-0 (CS60000) was used as the wild type. A T-DNA insertion line (abcb19-101; SALK_033455)25 was obtained from the Arabidopsis Biological Resource Center. The T-DNA insertion was confirmed by PCR with primers abcb19-101F (CTCAGGCAATTGCTCAAGTTC), abcb19-101R (GCAATTGCAATTCTCTGCTTC), and LBa1 (TGGTTCACGTAGTGGGCCATCG). Plants were initially grown on plates of Murashige–Skoog medium containing 1% (w/v) sucrose, 0.5% (w/v) MES-KOH (pH 5.7), and 0.5% (w/v) Gellan gum (Wako), under continuous light at 22°C for 2–3 weeks before being transferred to soil.
Thin sections of the inflorescence stem
Thin sections were prepared as described previously26 with the upper parts (1–3 cm from the top) of the primary inflorescence stem (14 cm height). The sections were stained with 1% Toluidine Blue27 and were observed in a bright field with a light microscope (Axioplan 2 Imaging; Zeiss) using objectives (20 × 0.5 NA and 10 × 0.3 NA).
Hand-cut sections of the inflorescence stem
Vertical sections of the inflorescence stem were prepared as described previously.28 Briefly, double-sided adhesive tape was cut and stuck onto a glass slide. A 4-week-old inflorescence stem segment (less than 1 cm long) was excised from the region 1–2 cm below the apex of the stem and stuck to the tape. The segment was cut longitudinally using a razor blade.
Observation of cytoplasmic streaming in the inflorescence stem
To examine cytoplasmic streaming in the inflorescence stem, the hand-cut vertical sections were observed in a bright field using a light microscopy (LSM 510 META; Zeiss) with a 63 × 1.2 numerical aperture water-immersion objective. The differential interference contrast images were taken at 1-s intervals for 1 min. For estimation of the velocity, moving plastids in four or five randomly selected sequential frames of 8 (wild-type) and 17 (abcb19-101) time-lapse images (143 × 143 µm) were tracked manually using the ImageJ software (http://rsb.info.nih.gov/ij/).
Gravitropism assay of inflorescence stems
Intact plants with 4- to 8-cm primary stems were placed horizontally in non-directional dim light (1 µmol m−2 s−1). Photographs were taken automatically every 10 min by a digital camera (Canon). Stem curvature was defined as the angle formed between the growing direction of the apex and the horizontal base line and was measured on the digital images using the ImageJ software.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
We are grateful to the ABRC for providing seeds of A. thaliana T-DNA insertion mutant. This work was supported by a Specially Promoted Research of Grant-in-Aid for Scientific Research to I.H-.N. (No. 22000014), Grants-in-Aid for Scientific Research to H.U. (Nos. 21200065 and 25440132), and a research fellowship to K.O. (No. 23.2) from the Japan Society for the Promotion of Science (JSPS).
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
References
- 1.Ueda H, Yokota E, Kutsuna N, Shimada T, Tamura K, Shimmen T, Hasezawa S, Dolja VV, Hara-Nishimura I. Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc Natl Acad Sci USA 2010; 107: 6894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Went FW, Thimann KV. Phytohormones New York: MacMillan, 1937. [Google Scholar]
- 3.Evans ML. Gravitropism: interaction of sensitivity modulation and effector redistribution. Plant Physiol 1991; 95: 1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godbolé R, Michalke W, Nick P, Hertel R. Cytoskeletal drugs and gravity-induced lateral auxin transport in rice coleoptiles. Plant Biol 2000; 2: 176-81. [Google Scholar]
- 5.Iino M. Mediation of tropisms by lateral translocation of endogenous indole‐3‐acetic acid in maize coleoptiles. Plant Cell Environ 1991; 14: 279-86. [Google Scholar]
- 6.Fukaki H, Wysocka-Diller J, Kato T, Fujisawa H, Benfey PN, Tasaka M. Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J 1998; 14: 425-30. [DOI] [PubMed] [Google Scholar]
- 7.Blancaflor EB, Fasano JM, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol 1998; 116: 213-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002; 415: 806-9. [DOI] [PubMed] [Google Scholar]
- 9.Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS. Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 2003; 423: 999-1002. [DOI] [PubMed] [Google Scholar]
- 10.Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, Cheng Y, Bouchard R, Adamec J, et al.. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 2007; 19: 131-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagashima A, Uehara Y, Sakai T. The ABC subfamily B auxin transporter AtABCB19 is involved in the inhibitory effects of N-1-naphthyphthalamic acid on the phototropic and gravitropic responses of Arabidopsis hypocotyls. Plant Cell Physiol 2008; 49: 1250-5. [DOI] [PubMed] [Google Scholar]
- 12.Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, Titapiwatanakun B, Ennis M, Kaiserli E, Lee OR, et al.. phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol 2011; 9: e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar P, Millar KDL, Kiss JZ. Inflorescence stems of the mdr1mutant display altered gravitropism and phototropism. Environ Exp Bot 2011; 70: 244-50. [Google Scholar]
- 14.Corti B. Osservazioni microscopiche sulla tremella e sulla circolazione del fluido in una pianta acquajuola. 1774. [Google Scholar]
- 15.Shimmen T, Yokota E. Cytoplasmic streaming in plants. Curr Opin Cell Biol 2004; 16: 68-72. [DOI] [PubMed] [Google Scholar]
- 16.Shimmen T. The sliding theory of cytoplasmic streaming: fifty years of progress. J Plant Res 2007; 120: 31-43. [DOI] [PubMed] [Google Scholar]
- 17.Hashiguchi Y, Tasaka M, Morita MT. Mechanism of higher plant gravity sensing. Am J Bot 2013; 100: 91-100. [DOI] [PubMed] [Google Scholar]
- 18.Tominaga M, Kimura A, Yokota E, Haraguchi T, Shimmen T, Yamamoto K, Nakano A, Ito K. Cytoplasmic streaming velocity as a plant size determinant. Dev Cell 2013; 27: 345-52. [DOI] [PubMed] [Google Scholar]
- 19.Stefano G, Renna L, Brandizzi F. The endoplasmic reticulum exerts control over organelle streaming during cell expansion. J Cell Sci 2014; 127: 947-53. [DOI] [PubMed] [Google Scholar]
- 20.Noh B, Murphy AS, Spalding EP. Multidrug resistance -like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 2001; 13: 2441-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagashima A, Suzuki G, Uehara Y, Saji K, Furukawa T, Koshiba T, Sekimoto M, Fujioka S, Kuroha T, Kojima M, et al.. Phytochromes and cryptochromes regulate the differential growth of Arabidopsis hypocotyls in both a PGP19-dependent and a PGP19-independent manner. Plant J 2008; 53: 516-29. [DOI] [PubMed] [Google Scholar]
- 22.Tominaga M, Sonobe S, Shimmen T. Mechanism of inhibition of cytoplasmic streaming by auxin in root hair cells of Hydrocharis. Plant Cell Physiol 1998; 39: 1342-9. [Google Scholar]
- 23.Thimann KV, Sweeney BM. The effect of auxins upon protoplasmic streaming. J Gen Physiol 1937; 21: 123-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muday GK, Peer WA, Murphy AS. Vesicular cycling mechanisms that control auxin transport polarity. Trends Plant Sci 2003; 8: 301-4. [DOI] [PubMed] [Google Scholar]
- 25.Lin R, Wang H. Two homologous ATP-binding cassette transporter proteins, AtMDR1 and AtPGP1, regulate Arabidopsis photomorphogenesis and root development by mediating polar auxin transport. Plant Physiol 2005; 138: 949-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirakawa M, Ueda H, Koumoto Y, Fuji K, Nishiyama C, Kohchi T, Hara-Nishimura I, Shimada T. CONTINUOUS VASCULAR RING (COV1) is a trans-Golgi network-localized membrane protein required for Golgi morphology and vacuolar protein sorting. Plant Cell Physiol 2014; 55: 764-72. [DOI] [PubMed] [Google Scholar]
- 27.Shimada T, Koumoto Y, Hara-Nishimura I. Evaluation of defective endosomal trafficking to the vacuole by monitoring seed storage proteins in Arabidopsis thaliana. Methods Mol Biol 2014; 1209: 131-42. [DOI] [PubMed] [Google Scholar]
- 28.Saito C, Morita MT, Kato T, Tasaka M. Amyloplasts and vacuolar membrane dynamics in the living graviperceptive cell of the Arabidopsis inflorescence stem. Plant Cell 2005; 17: 548-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


