ABSTRACT
microRNA172 (miR172) expression has been shown to have a positive effect on Arabidopsis fruit (siliques) growth. In contrast, over-expression of miR172 has a negative influence on fruit growth in apple, resulting in a dramatic reduction in fruit size. This negative influence is supported by the results of analyzing a transposable element (TE) insertional allele of a MIR172 gene that has reduced expression of the miRNA and is associated with an increase in fruit size. Arabidopsis siliques are a dry fruit derived from ovary tissues, whereas apple is a fleshy pome fruit derived mostly from hypanthium tissues. A model has been developed to explain the contrasting impact of miR172 expression in these two plant species based on the differences in their fruit structure. Transgenic apple plants with extremely high levels of miR172 overexpression produced flowers consisting of carpel tissues only, which failed to produce fruit. By comparison, in tomato, a fleshy berry fruit derived from the ovary, high level over-expression of the same miR172 resulted in carpel-only flowers which developed into parthenocarpic fruit. These results further indicate that the influence of miR172 on fruit growth in different plant species depends on its fruit type.
KEYWORDS: APETALA2, arabidopsis, fruit development, malus x domestica, microRNA172, tomato
microRNA172 (miR172) is conserved in plants and plays important roles in plant development. Plant species have multiple MIR172 genes that can produce conserved mature miR172 sequences in different tissues and at different developmental stages.1-3 The mature miR172 sequences target mRNA of a subfamily of APETALA2 (AP2) genes by sequence complementation and repress expression by inhibiting translation or initiating degradation of the target mRNA.4-7 As members of the AP2 subfamily have different temporal and spatial expression patterns3,8,9 and interact with different proteins,10 the MIR172 gene family potentionally can affect many aspects of plant development.
Early flowering is a commonly observed phenotypic change for overexpression of miR172 in annual plant species,4,6,7,10,11 and in some instances floral organ abnormalities have been observed.5,11 As AP2 governs floral organ development12 and organ size,13 miR172 has the potential to influence fruit growth. In Arabidopsis, a recent study has shown that miR172 positively regulates Arabidopsis silique growth.1 The silique is derived from carpel tissues and its growth is negatively regulated by AP2, and AP2 expression in the silique valve is inhibited by miR172. When miR172 function is repressed in transgenic plants using microRNA mimicry technology, the silique size is reduced. Similarly, silique size is reduced in transgenic plants expressing a modified AP2 gene without a functional miR172-target sequence.1
In contrast with the Arabidopsis silique, apple fruit is mostly derived from the hypanthium that is hypothesized to consist of the fused bases of the sepals, petals and stamens.14 Modern molecular studies have further indicated that sepal bases contribute most to apple flesh formation.15 As sepal development is positively regulated by AP2,16 the reduction of AP2 abundance by over-expression of miR172 would reduce apple fruit growth.
Fifteen MIR172 genes have been predicted (MIR172a-o) from the M. x domestica genome sequence2 and one (MIR172p) from fruit expressed sequence tag (EST) sequences.17 We have shown, in a recent publication, that a 15-fold overexpression of miR172p in transgenic apple plants reduced fruit weight more than 60 fold and in some flowers converted sepal or sepal segments into petals.3 This partial sepal to petal conversion is similar to that reported following miR172 over-expression in tobacco plants.11 We have also identified a TE insertional allele in the MIR172p gene of apple that was associated with a two-fold reduction of the primary transcript level of MIR172p. MIR172p was co-located with one of four fruit-size QTLs and the TE insertional allele was associated with large fruit in the progeny of a controlled cross. Our analyses with the distribution of this allele in the genus Malus indicated that this allele underlies fruit size evolution from wild crabapples to large cultivated apples. Thus, we named the genes as CrabApple Fruit Size and its wild-type and transposon insertion alleles CAFS and cafs, respectively.
When the expression level of miR172p was increased 20–24 fold, transgenic apple plants exhibited more extreme changes in phenotype, including flowers consisting entirely of carpel tissues, with no sepals, petals or stamens.3 Similar floral defects including stigmatic papillae on cauline leaf margins and solitary carpels forming from the axil of cauline leaves have been reported in Arabidopsis plants over-expressing miR172.7 These carpel-only flowers of apple failed to produce fruit after hand pollination.
Having seen how miR172 influences fruit development in examples of a silique and pome fruit, we wanted to understand how miR172 might influence the development of an ovary-derived flesh fruit. To achieve this we transformed tomato (Solanum lycopersicum ‘UC28b’) using an Agrobacterium-mediated transformation protocol18 with the same construct we had used for apple transformation.3 As with apple, two types of altered flowers were observed in tomato (Fig. 1). The first type showed yellowish sepals in one of nine transgenic plants produced, indicating partial sepal to petal conversion and poorly developed anthers (Fig. 1A, C). These floral structures were unable to self-pollinate due to the absence of viable pollen, but went on to develop parthenocarpic seedless fruit (Fig. 1B, D ). The second flower type consisted entirely of carpel tissues, with no sepals, petals or stamens (Fig. 1E) in four of the nine transgenic plants. The ovary was fully formed. However, style and stigma were not fully developed (Fig. 1E). In contrast with apple, these tomato carpel-only flowers developed into parthenocarpic fruit that produced ectopic ovaries inside the fruit (Fig. 1F, G). In some extreme cases, a fruit-in-fruit phenotype was displayed (Fig.1H). Formation of ectopic organs inside the fruit indicated a loss of determinacy in the floral meristem. A similar loss of floral meristem determinacy has been observed in transgenic rice plants over-expressing miR172.5 In addition, the size of the tomato fruit from both types of flowers was reduced 4–5 fold compared with wild-type control fruit. This fruit size reduction is not as dramatic as that observed in apple and may be a result of the lack of seed development because seed number is positively correlated with fruit size in many species.19
Figure 1.
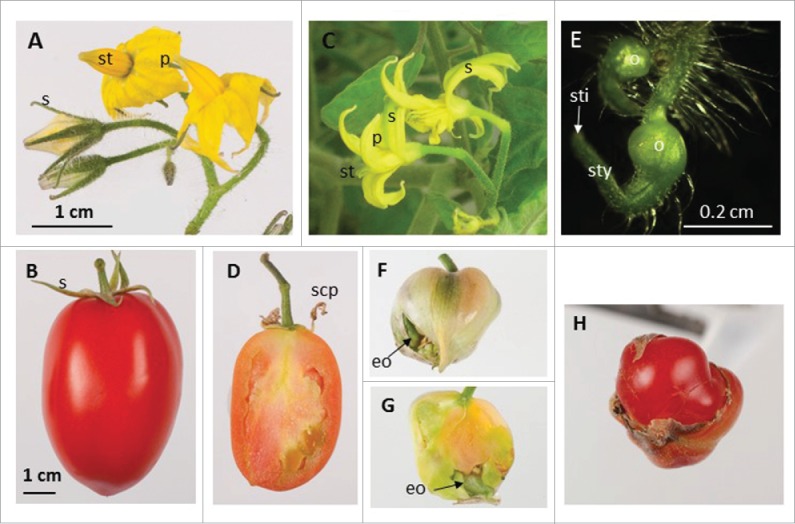
Over-expression of miR172 in transgenic tomato alters flower and fruit development. (A) Wild-type flowers show green sepals (s), yellow petals (p) and yellow stamens (st). (B) Wild-type mature fruit with green sepals attached. (C) Transgenic flowers showing yellowish sepals (s) and underdeveloped stamens (st). (D) Seedless fruit developed from flowers of (C) with dried, sepal-converted petals (scp) attached. (E) Transgenic flowers showing fully formed ovary (o) and incompletely developed style (sty) and stigma (sti). (F) and (G) Seedless fruit developed from flowers of (E) with no sepals attached but with ectopic ovaries (eo) inside the fruit. (H) A fruit-in-fruit developed from flowers of (E). (A) and (C) share the same scale bar, (B), (D), and (F) to (H) share the same scale bar.
The differing impact of miR172 on the development and growth of Arabidopsis, apple and tomato fruit may be explained by the differences in fruit structure (ovary- or non-ovary-derived) and the type of development (fleshy or dry) of the three species. miR172 promotes Arabidopsis silique growth through a double negative effect, by negatively regulating AP2 that would otherwise inhibit the AGAMOUS (AG) and FRUITFUL (FUL)12 required for ovary and silique growth.1,12,16 By contrast miR172 inhibits apple fruit growth through negative regulation of AP2 that is required for sepal development16 with the sepal tissues then contributing to fruit flesh development.3,15 Tomato fruit development is similar to Arabidopsis siliques in terms of the ovary origin, so its fruit growth would require sufficient temporal and spatial miR172 expression to supress AP2 expression and permit appropriate AG and FUL expression. It is possible that overexpression of miR172 promotes fruit development in the absence of fertilization. Tomato also shares some similarities with apple in terms of fruit flesh development, with flesh tissue growth dependent largely on cell division, and cell expansion occurring after fertilization and subsequent stimulation by hormones released from seed. If the effect of miR172 over-expression in promoting tomato fruit growth could not fully compensate for the seed-derived hormone stimulation required for complete fruit growth, this hypothesis would explain why the parthenocarpic tomato fruit were smaller than the seeded control fruit.
So far, the effect of miR172 on fruit development has been analyzed in three species with fruits classified as silique, pome and berry. There are many fruit types in the plant kingdom20 and it is likely that altering miR172 expression affects fruit development in other fruit types, potentially leading to novel fruit phenotypes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
J.X. was supported in part by the China Scholarship Council. The authors acknowledge Tim Holmes for assistance with photography and we thank Kimberley Snowden for commenting on the manuscript.
References
- 1.Ripoll JJ, Bailey LJ, Mai QA, Wu SL, Hon CT, Chapman EJ, et al.. microRNA regulation of fruit growth. Nat Plants 2015; 1; http://dx.doi.org/ 10.1038/nplants.2015.36 [DOI] [PubMed] [Google Scholar]
- 2.Xia R, Zhu H, An YQ, Beers EP, Liu ZR. Apple miRNAs and tasiRNAs with novel regulatory networks. Gen Biol 2012; 13:R47; http://dx.doi.org/ 10.1186/gb-2012-13-6-r47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao JL, Xu J, Cornille A, Tomes S, Karunairetnam S, Luo Z, Bassett H, Whitworth C, Rees-George J, Ranatunga C, et al.. A microRNA allele that emerged prior to apple domestication may underlie fruit size evolution. Plant J 2015; 84:417-27; PMID:26358530; http://dx.doi.org/ 10.1111/tpj.13021 [DOI] [PubMed] [Google Scholar]
- 4.Zhu QH, Helliwell CA. Regulation of flowering time and floral patterning by miR172. J Exp Bot 2011; 62:487-95; PMID:20952628; http://dx.doi.org/ 10.1093/jxb/erq295 [DOI] [PubMed] [Google Scholar]
- 5.Zhu QH, Upadhyaya NM, Gubler F, Helliwell CA. Overexpression of miR172 causes loss of spikelet determinacy and floral organ abnormalities in rice (Oryza sativa). BMC Plant Biol 2009; 9:149; PMID:20017947; http://dx.doi.org/ 10.1186/1471-2229-9-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen XM. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 2004; 303:2022-5; PMID:12893888; http://dx.doi.org/ 10.1126/science.1088060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aukerman MJ, Sakai H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 2003; 15:2730-41; PMID:14555699; http://dx.doi.org/ 10.1105/tpc.016238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang M, Li G, Chen M. The phylogeny and expression pattern of APETALA2-like genes in rice. J Genet Genom 2007; 34:930-8; http://dx.doi.org/ 10.1016/S1673-8527(07)60104-0 [DOI] [PubMed] [Google Scholar]
- 9.Okamuro JK, Caster B, Villarroel R, VanMontagu M, Jofuku KD. The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc Natl Acad Sci USA 1997; 94:7076-81; PMID:9192694; http://dx.doi.org/ 10.1073/pnas.94.13.7076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teotia S, Tang G. To bloom or not to bloom: role of microRNAs in plant flowering. Mol Plant 2015; 8:359-77; PMID:25737467; http://dx.doi.org/ 10.1016/j.molp.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 11.Mlotshwa S, Yang Z, Kim Y, Chen X. Floral patterning defects induced by Arabidopsis APETALA2 and microRNA172 expression in Nicotiana benthamiana. Plant Mol Biol 2006; 61:781-93; PMID:16897492; http://dx.doi.org/ 10.1007/s11103-006-0049-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yant L, Mathieu J, Dinh TT, Ott F, Lanz C, Wollmann H, Chen X, Schmid M. Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 2010; 22:2156-70; PMID:20675573; http://dx.doi.org/ 10.1105/tpc.110.075606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jofuku KD, Omidyar PK, Gee Z, Okamuro JK. Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc Natl Acad Sci USA 2005; 102:3117-22; PMID:15708974; http://dx.doi.org/ 10.1073/pnas.0409893102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pratt C. Apple flower and fruit: morphology and anatomy. Hort Rev 1988; 10:273-308 [Google Scholar]
- 15.Yao JL, Dong YH, Morris BA. Parthenocarpic apple fruit production conferred by transposon insertion mutations in a MADS-box transcription factor. Proc Natl Acad Sci USA 2001; 98:1306-11; PMID:11158635; http://dx.doi.org/ 10.1073/pnas.98.3.1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weigel D, Meyerowitz EM. The ABCs of floral homeotic genes. Cell 1994; 78:203-9; PMID:7913881; http://dx.doi.org/ 10.1016/0092-8674(94)90291-7 [DOI] [PubMed] [Google Scholar]
- 17.Gleave AP, Ampomah-Dwamena C, Berthold S, Supinya D, Karunairetnam S, Bhawana N, et al.. Identification and characterisation of primary microRNAs from apple (Malus domestica cv. Royal Gala) expressed sequence tags. Tree Genet Genom 2008; 4:343-58; http://dx.doi.org/ 10.1007/s11295-007-0113-1 [DOI] [Google Scholar]
- 18.McCormick S. Transformation of tomato with Agrobacterium tumefaciens Plant Tiss Cult Manual B6. The Netherlands: Kluwer, 1991:1-9 [Google Scholar]
- 19.Correa J, Ravest G, Laborie D, Mamani M, Torres E, Munoz C, et al.. Quantitative trait loci for the response to gibberellic acid of berry size and seed mass in tablegrape (Vitis vinifera L.). Austr J Grape Wine Res 2015; 21:496-507; http://dx.doi.org/ 10.1111/ajgw.12141 [DOI] [Google Scholar]
- 20.Spjut RW. A systematic treatment of fruit types. Memoirs New York Botanical Garden 1994; 70:1-182 [Google Scholar]


