ABSTRACT
Plant productivity is limited by the scarcity of the essential micronutrient iron particularly in alkaline soils. The root secretion of phenolics has long been recognized as a component of the acidification-reduction strategy to acquire iron (strategy I). However, very little molecular insight into this process was available until recently several research groups independently discovered the important role of coumarins for the growth of Arabidopsis thaliana under Fe-limited conditions. Genome-wide analyses of iron deficiency responses, mutant screening and metabolomics experiments all converged on the finding that the synthesis and root exudation of scopoletin, esculetin and other coumarins is essential for iron uptake from substrates with low iron availability. Here we describe the evidence supporting this conclusion and discuss important questions that now have to be addressed in order to better understand the mechanistic basis of coumarin-dependent iron uptake and its significance within the plant kingdom.
Keywords: Fe homeostasis, Fe uptake, metabolite profiling, phenylpropanoids, root exudation, 2-oxoglutarate Fe(II) oxygenases
Abbreviations and acronyms
- GC-MS
gas chromatography mass spectrometry
- UPLC-ESI-MS/MS
ultra-performance liquid chromatography electrospray ionization mass spectrometry
Introduction
Iron (Fe) is an essential nutrient. As a redox-active metal it is involved in many indispensable cellular processes, e.g. photosynthesis and respiration. Fe is very abundant in the earth's crust and life evolved in an Fe-rich environment.1 With rising oxygenation of the atmosphere, however, Fe availability for organisms dropped by several orders of magnitude because Fe oxides have very low solubility. This forced most life forms to evolve mechanisms for the acquisition of scarcely available Fe. Pathogens, for instance, engage in a battle for the Fe reserves of their hosts.2 Plant productivity is limited by Fe particularly on alkaline soils as solubility of Fe oxides is exceedingly low above neutral pH.3 Two different strategies of Fe acquisition by the roots of higher plants have been defined. Non-graminaceous plants employ strategy I consisting of rhizosphere acidification, reduction of Fe(III) and uptake of Fe(II). The strategy II expressed in grasses involves the secretion of Fe(III) chelating phytosiderophores and subsequent uptake of Fe(III)-phytosiderophore complexes.4,5
The secretion of phenolics and possibly other low molecular weight compounds as chelators and/or reductants of Fe(III) has always been regarded as an additional component of strategy I.6,7 Its importance was, for example, demonstrated when the removal of phenolics from the root culture medium resulted in severe Fe deficiency of red clover plants.8 However, while other players of strategy I such as transporters, proton pumps and regulatory proteins have been elucidated in molecular detail,3-5 the nature of root exudation in response to Fe deficiency remained enigmatic until recently. The substantial progress achieved in the past 2 y with respect to root exudates and Fe acquisition is the subject of this review.
Discovery of coumarin secretion as a mechanism of Fe acquisition
A major advance in our understanding of phenolics secretion and its role for plant Fe nutrition was achieved when recently 4 research groups independently and following different approaches arrived at the conclusion that the secretion of coumarins is essential for Fe acquisition from alkaline substrates in A. thaliana. One line of investigation was guided by the observation that several proteins involved in phenylpropanoid metabolism and specifically coumarin biosynthesis show increases in abundance under conditions of Fe deficiency.9 Among them was the 2-oxoglutarate Fe(II) oxygenase Feruloyl-CoA 6´-Hydroxylase (F6′H1), which had in earlier microarray data already been shown to be transcriptionally up-regulated upon Fe limitation.10,11 F6′H1 (At3g13610) encodes an enzyme that is essential for the synthesis of the major coumarins scopoletin and scopolin in A. thaliana.12 Mutant plants defective in F6′H1 contain only trace amounts of scopoletin and its β-D-glucopyranoside scopolin, the major coumarins synthesized by A. thaliana. The product of a paralogous gene, F6′H2, shows the same activity yet does barely contribute to total coumarin biosynthesis, presumably due to a low expression level relative to F6′H1.12
Initial analyses indicated enhanced scopoletin accumulation in Fe-deficient A. thaliana plants but neither scopoletin nor scopolin were detected in root exudates.9 After the up-regulation of phenylpropanoid biosynthesis had been confirmed in an RNA-seq study of the A. thaliana Fe deficiency response, f6´h1 mutant seedlings were tested for Fe acquisition defects. Indeed, on a medium with very low Fe availability (pH 7.0, 10 μM FeCl3) lack of functional F6´H1 resulted in severe Fe deficiency symptoms and a strong reduction in the synthesis of UV-fluorescent compounds presumed to represent phenolics.13 Synthesis of these compounds was stimulated in wild-type seedlings under Fe-deplete conditions. Identity of the fluorescent compounds, however, was not determined in this study.
Phenolics not only accumulated in roots. They were also secreted into the medium as apparent from remaining fluorescence after removal of the roots. Mutants lacking a functional PDR9 gene (ABCG37) encoding an ATP-Binding Cassette transporter appeared to still synthesize phenolics but failed to secrete them as the fluorescence of the medium was strongly reduced. The ABCG37 gene was tested because it equally belongs to a network of genes co-regulated under Fe deficiency.9 In a separate study the exudates of Fe-deficient Brassica napus and A. thaliana roots were analyzed in detail after their crucial role for growth under conditions of Fe deficiency had been confirmed.14 HPLC coupled to ESI-MS/MS enabled the identification of several compounds accumulating in A. thaliana Col-0 wild type roots in Fe-deplete conditions, namely scopoletin, fraxetin, isofraxidin, 5-methoxyscopoletin and several glucosides of scopoletin and derivatives (Fig. 1). The aglycones as well as a small fraction of the scopoletin glucosides were in addition found in the exudates of Fe-deficient roots. Loss of ABCG37, however, suppressed the exudation of coumarins almost completely, which strongly suggested an involvement of this transporter in the secretion of coumarins.
Figure 1.
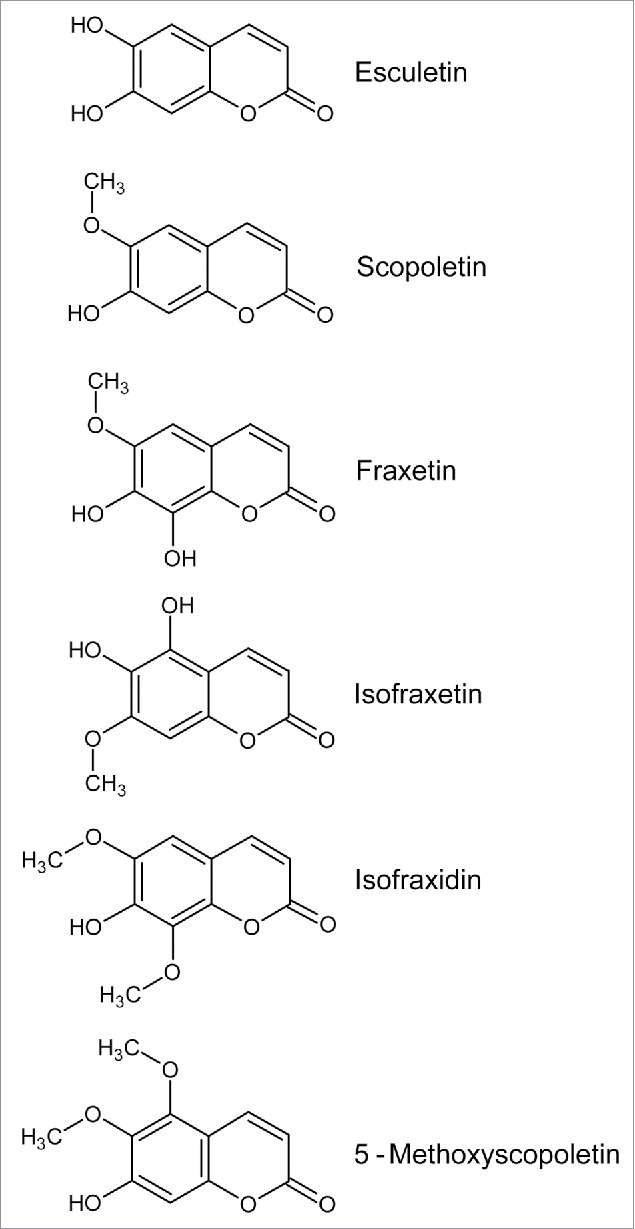
Coumarins detected in the root exudates of A. thaliana plants exposed to conditions of low Fe availability. β-D-glucopyranosides of esculetin, scopoletin and fraxetin (esculin, scopolin and fraxin, respectively) are also reported.
The third approach leading to the discovery of the crucial role of coumarins was a forward genetic screen with a set of A. thaliana T-DNA insertion lines.15 One mutant was isolated that was severely compromised in its ability to grow on alkaline soil (pH 7.2) while growth on pH 5.5 soil or when supplied with extra Fe(III)-EDDHA was unaffected relative to wild-type plants. Sequencing revealed an insertion in the F6´H1 gene as the cause of the phenotype. The accumulation of fluorescent compounds in Fe-deficient roots was detected specifically in the outer cells layers, i.e. epidermis and cortex, and shown to co-localize with F6´H1 promoter activity. Mutant plants did not show the increase in the secretion of fluorescent compounds elicited by Fe deficiency in wild-type plants. Importantly, wild-type root exudates were indeed found to mobilize Fe(III) from Fe hydroxide under alkaline conditions far more efficiently than f6′h1 root exudates. Removal of fluorescent compounds and other phenolics from the exudates by reversed-phase chromatography strongly reduced the Fe-chelating activity, suggesting a direct contribution of these compounds to Fe acquisition. Metabolite profiling of roots and root exudates by UPLC-ESI-MS/MS identified several coumarins as F6’H1-dependent metabolites. These included scopolin, esculin and their respective aglycones scopoletin and esculetin (Fig. 1). Concentrations were very different, with scopolin and scopoletin being the dominant compounds. Expectedly, both aglycones showed higher exudation rates than the conjugates. Also identified were fraxetin and isofraxetin (Fig. 1).
Finally, an unbiased metabolomics analysis using GC-MS and UPLC-ESI-MS/MS revealed a set of metabolites that showed higher abundance in roots of A. thaliana plants exposed to 2 different conditions of Fe deficiency, i.e., a medium not containing Fe or a medium containing Fe but at a pH of 7.8.16 GC-MS analyses found as a major Fe deficiency response increases in the abundance of malate and citrate, 2 organic acids that have been implicated in Fe homeostasis.17 Nearly one hundred features (m/z - retention time pairs) showed robust changes in roots under conditions of Fe deficiency. Among the few compounds that could be unequivocally identified through tandem MS analyses and comparisons with data base entries or authentic standards were scopolin and scopoletin as well as fraxetin and its conjugated form, fraxin (Fig. 1). All four compounds were more abundant in root extracts of Fe-deficient plants. The identification of these coumarins prompted analysis of f6′h1 mutant plants and confirmed the strong growth inhibition under alkaline conditions. Partial rescue of this phenotype by co-cultivation with wild-type plants in hydroponics indicated the importance of root exudation, i.e. an extracellular role of coumarins. Metabolite profiling of root exudates identified scopoletin and esculetin as coumarins secreted in an F6’H1-dependent manner.
Open questions
With the discovery that coumarin secretion by A. thaliana roots is essential for Fe acquisition under alkaline conditions at least 4 major questions arose. First, are there additional phenolic compounds that contribute to Fe acquisition? Second, which other proteins besides F6’H1 and ABCG37 are involved in the synthesis, processing and secretion of coumarins and possibly other phenolics by Fe-deficient root cells? Third, what is the biochemical basis for the coumarin-mediated Fe acquisition under alkaline conditions? Fourth, how widespread is the mechanism in the plant kingdom?
LC-ESI-MS analyses, which cover predominantly compounds of the secondary metabolism,18 revealed that the root metabolic response to Fe deficiency is very complex.14–16 Far more secondary metabolites than currently known are apparently synthesized at higher rates upon Fe limitation. A significant fraction of them can be expected to be secreted since A. thaliana root exudates contain a large number of secondary metabolites.19 Their identification remains a tedious process based on advancing databases,20 the careful analysis of biosynthesis mutants,21 synthesis of reference compounds, and the development of new algorithms that aid in structure prediction.22
Dissection of the coumarin secretion pathway will require the identification of additional proteins. While loss of F6’H1 function reduced scopoletin secretion nearly quantitatively the accumulation of other coumarins was less affected, suggesting the existence of alternative biosynthetic pathways.15,16 For instance, esculetin biosynthesis is not fully understood yet.23 Similarly, abcg37 mutant plants still secrete minor amounts of coumarins indicating that other transporters may contribute.14 Secretion of both conjugates and aglycones raised the question as to which compounds are the actual substrates for ABCG37. A study on the transcription factor Myb72, which is involved in activating Fe deficiency responses24 and induced systemic resistance, provided first answers.25 The Myb72-regulated β-glucosidase gene BGLU42 is required for efficient coumarin secretion. This suggests that glucosides are hydrolyzed by BGLU42 and then secreted as aglycones. Related enzymes likely contribute because loss of BLGU42 does not abolish coumarin release completely. Candidates are BLGU21, 22 and 23. They were purified from A. thaliana roots and upon heterologous expression in insect cells shown to hydrolyze scopolin and esculin.26
Another question concerning further processing relates to the extracellular fate and activity of secreted coumarins. Available evidence strongly suggests a direct role of coumarin secretion for Fe acquisition, as opposed to an indirect role such as toxicity to bacteria competing for soil Fe as mentioned by Fourcroy et al.14 Besides the Fe(III) mobilizing activity and the partial rescue of the f6’h1 mutant by co-cultivation with wild type plants on agar plates,13 or in hydroponic culture,16 the effects of coumarin feeding experiments further support this hypothesis. Exogenous application of esculin, esculetin and scopoletin suppressed Fe deficiency symptoms in f6’h1 seedlings grown axenically in the presence of low Fe.15 However, when tested in vitro, only esculetin was able to mobilize Fe(III) from Fe hydroxide. Neither esculin nor scopoletin showed this activity. The discrepancy between feeding effects and in vitro activity led Schmid et al.15 to postulate extracellular conversion of these coumarins to esculetin via deglucosylation or demethylation, respectively. The structure of esculetin with 2 neighboring hydroxyl groups suggests an Fe(III)-chelating activity similar to classic catechol-type siderophores. The other identified coumarins in the root exudates lack this structural feature, with the exception of fraxetin and isofraxetin (Fig. 1). Originally, the role of phenolic compounds in Fe acquisition had been regarded as being possibly due to an Fe(III) chelating or an Fe(III) reducing capacity.6 Thus, these 2 possible modes of action for coumarins are being discussed. Accordingly, an alternative explanation for the beneficial effect of scopoletin feeding could be that this coumarin itself enhances Fe availability without prior conversion to a catechol-like molecule.
Coumarins have intensively been investigated for a wide range of therapeutic purposes because of reported anti-inflammatory, anti-neurodegenerative, or anti-cancer activities to name a few.27 For the simple coumarins, to which the ones secreted by A. thaliana roots belong, anti-oxidant (e.g., esculetin) or anti-microbial properties (e.g., scopoletin) were reported. In plants scopoletin has been characterized as a phytoalexin.28 Both toxicity and anti-oxidant properties of coumarins could be related to an interaction with Fe because Fe(II) can catalyze the production of reactive oxygen species in Fenton chemistry. Chelation could attenuate ROS production, reduction of Fe(III) could trigger it.29 Testing such interactions of natural compounds with Fe under physiological conditions poses a challenge. For instance, in aqueous solutions Fe(III) is barely soluble especially at neutral or alkaline pH and forms oxo-, hydroxyl- and aquo-complexes. UV-vis spectroscopy in non-aqueous solutions indicated reduction of Fe(III) by all tested coumarins (scopoletin, fraxetin, esculetin) and the formation of complexes with Fe(II).16 The physiological significance of these observations is not clear though. When tested in DMSO, various coumarins reduced Fe(III). In buffered solution reduction was only observed at acidic pH.29 Chelating ability was found for the coumarins with 2 hydroxyl groups in ortho position, i.e., molecules similar to esculetin, not for coumarins with single hydroxyl groups.29 Taken together, the evidence supports an Fe(III) chelating activity of esculetin as one underlying mechanism,15 but clearly much more work is needed to fully understand the strong beneficial effect of coumarin secretion on Fe acquisition under alkaline conditions both biochemically and physiologically. A prerequisite will be comprehensive information on possible conversions of secreted metabolites and their actual concentrations in the rhizosphere. According to available data, the presumably less active or inactive scopoletin is far more abundant than the active esculetin (Figs 1–2).
Figure 2.
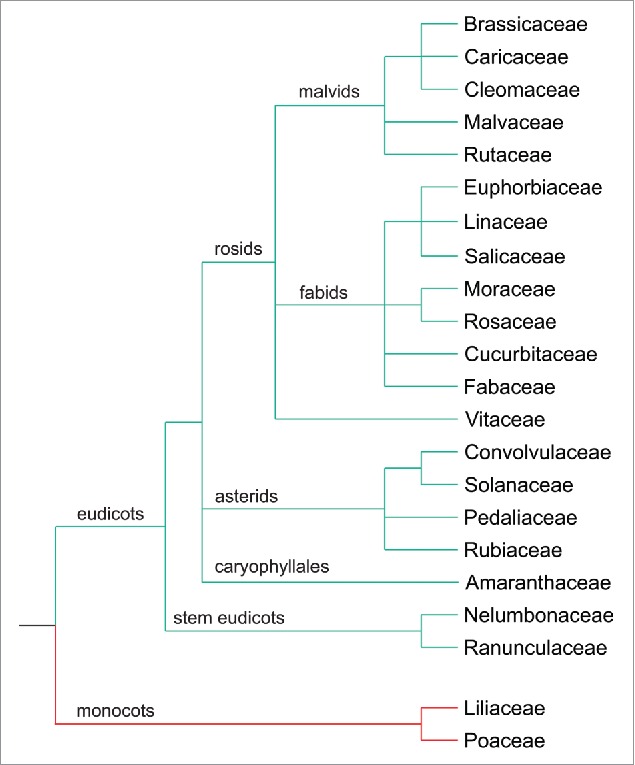
Plant families with or without putative F6´H1 orthologs. A BlastP search was performed with amino acids 151 to 309 of A. thaliana F6’H1 in the NCBI (http:// ncbi.nlm.nih.gov) and Phytozome (http://phytozome.org) databases (maximum target sequences: 5000). Sequences were selected as putative orthologs when a reverse BlastP search yielded AtF6’H1 or AtF6’H2 as the top hit. Species with putative orthologs where then placed into a phylogenetic tree based on the taxonomic classification in NCBI. Shown are families with (green branches) or without (red branches) putative F6’H1 orthologs. Species representing the families are: Arabidopsis thaliana (Brassicaceae), Carica papaya (Caricaceae), Tarenaya hassleriana (Cleomaceae), Gossypium raimondii (Malvaceae), Ruta graveolens (Rutaceae), Manihot esculenta (Euphorbiaceae), Linum usitatissimum (Linaceae), Salix purpurea (Salicaceae), Morus notabilis (Moraceae), Malus domestica (Rosaceae), Cucumis sativus (Cucurbitaceae), Cicer arietinum (Fabaceae), Vitis vinifera (Vitaceae), Ipomoea batatas (Convolvulaceae), Nicotiana sylvestris (Solanaceae), Sesamum indicum (Pedaliaceae), Coffea canephora (Rubiaceae), Spinacia oleracea (Amaranthaceae), Nelumbo nucifera (Nelumbonaceae), Aquilegia coerulea (Ranunculaceae), Lilium longiflorum (Liliaceae), Triticum aestivum (Poaceae).
Finally, how common is this mechanism? Or in other words: how important is this mechanism ecologically? How relevant is it for crop plants? Coumarins are widespread in nature. Most of the more than 1000 different reported coumarins have been isolated from plants.30,31 One way to assess their distribution is to derive an estimate about the prevalence of the required enzymatic activities within the plant kingdom. A key step in the synthesis of coumarins is the ortho-hydroxylation of cinnamates, which channels their use as precursors away from lignin biosynthesis.23 The responsible enzyme for this step in A. thaliana, F6′H1, is a representative of clade DOXC30 according to a recent phylogenetic classification of 2-oxoglutarate Fe(II) oxygenases by Kawai et al.32 Some DOXC clades are present in all land plants, others in vascular plants. Representatives of the latter are for instance involved in the synthesis of gibberellins. DOXC30 is one of the clades found only in certain plant species. Mosses (Physcomitrella patens), lycophytes (Selaginella moellendorffii), gymnosperms (Picea abies) and monocots (Oryza sativa) apparently do not possess DOXC30 members. On the other hand, DOXC30 proteins are present outside of A. thaliana or the Brassicaceae. In order to gain a more detailed overview we performed an analysis similar to the one reported by Shimizu et al.23 Part of the F6′H1 amino acid sequence was used for BlastP searches. We decided to take the stretch from amino acid 151 to amino acid 309, which contains the catalytic triade consisting of Tyr151, Val238 and Phe309 as well as the binding sites for Fe and 2-oxoglutarate. Species with putative F6′H1 orthologs were placed in a phylogenetic tree based on the taxonomic classification in NCBI (http://www.ncbi.nlm.nih.gov/taxonomy). The results indicate absence in monocots and widespread occurrence among dicots (Fig. 2). This finding is consistent with the reported list of orders with putative F6′H1 orthologs, ranging from Vitales, through Malvales, Fabales, and Rosales, to Lamiales.23
While this overview suggests presence of coumarin biosynthesis across the dicots it certainly does not allow to draw direct conclusions as to which plant species express coumarin-dependent Fe acquisition from alkaline low Fe substrates. Fabaceae such as Medicago truncatula likely possess enzymes able to catalyze the ortho-hydroxylation of feruloyl-CoA or other cinnamates. However, according to genome-wide analyses M. truncatula does not respond to Fe deficiency with upregulation of the phenylpropanoid pathway as A. thaliana. Instead, it shows enhanced biosynthesis and secretion of riboflavins. Their functional equivalence to coumarins was demonstrated by the rescue of the f6′h1 mutant via co-cultivation with wild-type M. truncatula plants.13 Furthermore, because occurrence of F6′H1 orthologs is apparently so widespread, their presence or absence cannot be correlated with the ability or inability to grow in alkaline soil. For instance, Gossypium species prefer acidic soil yet carry F6′H1 orthologs. Thus, there is a need for systematic experimental searches to identify the plant species that respond to Fe scarcity by secretion of coumarins.
Disclosure of potential conflicts of interest
No potential conflict of interest were disclosed.
Acknowledgments
Research in the author's laboratory is financially supported by the Deutsche Forschungsgemeinschaft.
References
- 1. Anbar A. Oceans: Elements and evolution. Science 2008; 322:1481-3; PMID:19056967; http://dx.doi.org/ 10.1126/science.1163100 [DOI] [PubMed] [Google Scholar]
- 2. Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science 2012; 338:768-72; PMID:23139325; http://dx.doi.org/ 10.1126/science.1224577 [DOI] [PubMed] [Google Scholar]
- 3. Brumbarova T, Bauer P, Ivanov R. Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci 2015; 20:124-33; PMID:25499025; http://dx.doi.org/ 10.1016/j.tplants.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 4. Kobayashi T, Nishizawa NK. Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 2012; 63:131-52; PMID:22404471; http://dx.doi.org/ 10.1146/annurev-arplant-042811-105522 [DOI] [PubMed] [Google Scholar]
- 5. Thomine S, Vert G. Iron transport in plants: better be safe than sorry. Curr Opin Plant Biol 2013; 16:322-7; PMID:23415557; http://dx.doi.org/ 10.1016/j.pbi.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 6. Römheld V, Marschner H. Mechanism of iron uptake by peanut plants.1.FeIII reduction, chelate splitting, and release of phenolics. Plant Physiol 1983; 71:949-54; PMID:16662934; http://dx.doi.org/ 10.1104/pp.71.4.949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marschner H, Römheld V. Strategies of plants for acquisition of iron. Plant Soil 1994; 165:261-74; http://dx.doi.org/ 10.1007/BF00008069 [DOI] [Google Scholar]
- 8. Jin CW, You GY, He YF, Tang C, Wu P, Zheng SJ. Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol 2007; 144:278-85; PMID:17369430; http://dx.doi.org/ 10.1104/pp.107.095794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lan P, Li W, Wen T, Shiau J, Wu Y, Lin W, Schmidt W. iTRAQ protein profile analysis of Arabidopsis roots reveals new aspects critical for iron homeostasis. Plant Physiol 2011; 155:821-34; PMID:21173025; http://dx.doi.org/ 10.1104/pp.110.169508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colangelo EP, Guerinot ML. The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 2004; 16:3400-12; PMID:15539473; http://dx.doi.org/ 10.1105/tpc.104.024315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang TJ, Lin W-D, Schmidt W. Transcriptional profiling of the Arabidopsis iron deficiency response reveals conserved transition metal homeostasis networks. Plant Physiol 2010; 152:2130-41; PMID:20181752; http://dx.doi.org/ 10.1104/pp.109.152728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kai K, Mizutani M, Kawamura N, Yamamoto R, Tamai M, Yamaguchi H, Sakata K, Shimizu BI. Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana. Plant J 2008; 55:989-99; PMID:18547395; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03568.x [DOI] [PubMed] [Google Scholar]
- 13. Rodríguez-Celma J, Lin W-D, Fu G-M, Abadía J, López-Millán A-F, Schmidt W. Mutually exclusive alterations in secondary metabolism are critical for the uptake of insoluble iron compounds by Arabidopsis and Medicago truncatula. Plant Physiol 2013; 162:1473-85; PMID:23735511; http://dx.doi.org/ 10.1104/pp.113.220426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fourcroy P, Sisó-Terraza P, Sudre D, Savirón M, Reyt G, Gaymard F, Abadía A, Abadia J, Álvarez-Fernández A, Briat J-F. Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol 2014; 201:155-67; PMID:24015802; http://dx.doi.org/ 10.1111/nph.12471 [DOI] [PubMed] [Google Scholar]
- 15. Schmid NB, Giehl RFH, Doll S, Mock H-P, Strehmel N, Scheel D, Kong X, Hider RC, von Wiren N. Feruloyl-CoA 6’-Hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol 2014; 164:160-72; PMID:24246380; http://dx.doi.org/ 10.1104/pp.113.228544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmidt H, Günther C, Weber M, Spörlein C, Loscher S, Böttcher C, Schobert R, Clemens S. Metabolome analysis of Arabidopsis thaliana roots identifies a key metabolic pathway for iron acquisition. PLoS ONE 2014; 9:e102444; PMID:25058345; http://dx.doi.org/ 10.1371/journal.pone.0102444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abadia J, Lopez-Millan AF, Rombola A, Abadia A. Organic acids and Fe deficiency: a review. Plant Soil 2002; 241:75-86; http://dx.doi.org/ 10.1023/A:10160-93317898 [DOI] [Google Scholar]
- 18. Saito K, Matsuda F. Metabolomics for functional genomics, systems biology, and biotechnology. Annu Rev Plant Biol 2010; 61:463-89; PMID:19152489; http://dx.doi.org/ 10.1146/annurev.arplant.043008.092035 [DOI] [PubMed] [Google Scholar]
- 19. Strehmel N, Böttcher C, Schmidt S, Scheel D. Profiling of secondary metabolites in root exudates of Arabidopsis thaliana. Phytochem 2014; 108:35-46; PMID:25457500; http://dx.doi.org/18939963 10.1016/j.phytochem.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 20. Matsuda F, Yonekura-Sakakibara K, Niida R, Kuromori T, Shinozaki K, Saito K. MS/MS spectral tag-based annotation of non-targeted profile of plant secondary metabolites. Plant J 2009; 57:555-77; PMID:18939963; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03705.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Böttcher C, von Roepenack-Lahaye E, Schmidt J, Schmotz C, Neumann S, Scheel D, Clemens S. Metabolome analysis of biosynthetic mutants reveals a diversity of metabolic changes and allows identification of a large number of new compounds in Arabidopsis. Plant Physiol 2008; 147:2107-20; PMID:18552234; http://dx.doi.org/ 10.1104/pp.108.117754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morreel K, Saeys Y, Dima O, Lu F, Peer YV de, Vanholme R, Ralph J, Vanholme B, Boerjan W. Systematic structural characterization of metabolites in Arabidopsis via candidate substrate-product pair networks. Plant Cell 2014; 26:929-45; PMID:24685999; http://dx.doi.org/ 10.1105/tpc.113.122242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shimizu B-I. 2-Oxoglutarate-dependent dioxygenases in the biosynthesis of simple coumarins. Front Plant Sci 2014; 5:549; PMID:25404933; http://dx.doi.org/ 10.3389/fpls.2014.00549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmer CM, Hindt MN, Schmidt H, Clemens S, Guerinot ML. MYB10 and MYB72 are required for growth under iron-limiting conditions. PLoS Genet 2013; 9:e1003953; PMID:24278034; http://dx.doi.org/ 10.1371/journal.pgen.1003953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zamioudis C, Hanson J, Pieterse CMJ. β-Glucosidase BGLU42 is a MYB72-dependent key regulator of rhizobacteria-induced systemic resistance and modulates iron deficiency responses in Arabidopsis roots. New Phytol 2014; 204:368-79; PMID:25138267; http://dx.doi.org/ 10.1111/nph.12980 [DOI] [PubMed] [Google Scholar]
- 26. Ahn YO, Shimizu B, Sakata K, Gantulga D, Zhou Z, Bevan DR, Esen A. Scopolin-hydrolyzing β-glucosidases in roots of Arabidopsis. Plant Cell Physiol 2010; 51:132-43; PMID:19965874; http://dx.doi.org/ 10.1093/pcp/pcp174 [DOI] [PubMed] [Google Scholar]
- 27. Barot KP, Jain SV, Kremer L, Singh S, Ghate MD. Recent advances and therapeutic journey of coumarins: current status and perspectives. Med Chem Res 2015; 24:2771-98; http://dx.doi.org/ 10.1007/s00044-015-1350-8 [DOI] [Google Scholar]
- 28. Gnonlonfin GJB, Sanni A, Brimer L. Scopoletin - a coumarin phytoalexin with medicinal properties. Crit Rev Plant Sci 2012; 31:47-56; http://dx.doi.org/ 10.1080/07352689.2011.616039 [DOI] [Google Scholar]
- 29. Mladenka P, Macakova K, Zatloukalova L, Rehakova Z, Singh BK, Prasad AK, Parmar VS, Jahodar L, Hrdina R, Saso L. In vitro interactions of coumarins with iron. Biochimie 2010; 92:1108-14; PMID:20381579; http://dx.doi.org/ 10.1016/j.biochi.2010.03.025 [DOI] [PubMed] [Google Scholar]
- 30. Murray RD. Coumarins. Nat Prod Rep 1989; 6:591-624; PMID:2699016; http://dx.doi.org/ 10.1039/np9890600591 [DOI] [PubMed] [Google Scholar]
- 31. Hoult JRS, Paya M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen Pharmacol 1996; 27:713-22; PMID:8853310; http://dx.doi.org/ 10.1016/0306-3623(95)02112-4 [DOI] [PubMed] [Google Scholar]
- 32. Kawai Y, Ono E, Mizutani M. Evolution and diversity of the 2-oxoglutarate-dependent dioxygenase superfamily in plants. Plant J 2014; 78:328-43; PMID:24547750; http://dx.doi.org/ 10.1111/tpj.12479 [DOI] [PubMed] [Google Scholar]


