Abstract
Photosystem II (PSII) is highly susceptible to photoinhibition caused by environmental stimuli such as high light; therefore plants have evolved multifaceted mechanisms to efficiently protect PSII from photodamage. We previously published data suggesting that Maintenance of PSII under High light 1 (MPH1, encoded by AT5G07020), a PSII-associated proline-rich protein found in land plants, participates in the maintenance of normal PSII activity under photoinhibitory stress. Here we provide additional evidence for the role of MPH1 in protecting PSII against photooxidative damage. Two Arabidopsis thaliana mutants lacking a functional MPH1 gene suffer from severe photoinhibition relative to the wild-type plants under high irradiance light. The mph1 mutants exhibit significantly decreased PSII quantum yield and electron transport rate after exposure to photoinhibitory light. The mutants also display drastically elevated photodamage to PSII reaction center proteins after high-light treatment. These data add further evidence that MPH1 is involved in PSII photoprotection in Arabidopsis. MPH1 homologs are found across phylogenetically diverse land plants but are not detected in algae or prokaryotes. Taken together, these results suggest that MPH1 protein began to play a role in protecting PSII against excess light following the transition from aquatic to terrestrial conditions.
Keywords: high light, land plants, Photosystem II, photoinhibition, PSII damage, PSII repair cycle, thylakoid membranes
Abbreviations
- PSII
photosystem II
- Fo
minimum fluorescence yield in dark-adapted state
- Fm
maximum fluorescence yield in dark-adapted state
- Fv/Fm
maximum photochemical efficiency of PSII
- qE
energy-dependent quenching
- qI
photoinhibitory quenching
- ETR
electron transport rate
- Y(PSII)
effective PSII quantum yield
- ROS
reactive oxygen species.
Introduction
Photosystem II (PSII) is an evolutionarily conserved multi-subunit pigment-protein complex embedded in the thylakoid membranes of photosynthetic prokaryotes and chloroplasts.1,2 The complex is the primary reaction center of photosynthesis functioning as a light-driven water:plastoquinone oxidoreductase capable of splitting water to yield molecular oxygen and reduced plastoquinone.3 An inherent feature of PSII from cyanobacteria to land plants is its high vulnerability to light-mediated photooxidative damage.1,4 Plants have therefore evolved a network of adaptive and photoprotective mechanisms to deal with photoinhibitory light by regulating light harvesting and dissipating the light energy absorbed by photosynthetic pigments, respectively.5-7 Despite these regulatory mechanisms, PSII is prone to damage by sunlight at all light intensities.3,8 Plants employ an elaborate repair system that involves disassembly and reassembly of PSII complexes to degrade and replace damaged proteins, primarily the reaction center D1 subunit within PSII.9-11 A suite of auxiliary protein factors have been reported to facilitate this damage-repair cycle of PSII,3,9,12 yet new protein factors participating in the process await to be identified, and there remains many proteins of unknown function in chloroplast.13-16
To identify novel proteins with roles in the damage-repair cycle of PSII, we took advantage of results from the Chloroplast 2010 Project (http://www.plastid.msu.edu) and recently identified a PSII-associated integral thylakoid membrane protein, Maintenance of PSII under High light 1 (MPH1), which plays roles in the protection and stabilization of PSII under high-light stress.17 Lack of MPH1 renders PSII susceptible to photoinhibition. After exposure to high irradiance light, steady-state levels of PSII supercomplexes, dimers and monomers are universally reduced in mph1 mutants. These reductions appear to be restricted to core subunits of PSII (D1, D2, CP43, and CP47) as the amounts of the PSII oxygen-evolving complex protein PsbO and the PSII light-harvesting complex protein Lhcb2 remained unaltered. The decrease of PSII reaction center D1 protein is more pronounced in mph1 mutants relative to the wild type in the presence of lincomycin than in its absence during the course of photoinhibitory irradiance. MPH1 co-purifies primarily with monomeric PSII complex under normal growth light conditions and with dimeric PSII complexes (PSII dimers and supercomplexes) under high light. These data are consistent with the specific interaction of MPH1 with PSII core complexes and the presence of MPH1 in all thylakoid subfractions (grana core- and grana margin-membranes and stroma lamellae).17
Here we provide further evidence for high-light-induced defects in PSII photochemistry in the absence of MPH1 protein. Data are presented showing light intensity dependence of chlorophyll fluorescence parameter the electron transport rate (ETR), kinetics of induction and relaxation of quantum yield of PSII [Y(PSII)], and increased levels of carbonylated thylakoid membrane proteins. The results further demonstrate that lower photosynthetic efficiency observed in mph1 mutants is attributed to more extensive damage to PSII proteins following high light. These data, combined with the phylogenetic analysis of MPH1 and its homologs, suggest that MPH1 is of importance in protecting PSII against photodamage in response to excess light in land plants.
Results
Photochemical efficiency of PSII is markedly decreased in mph1 mutants following a shift to high light
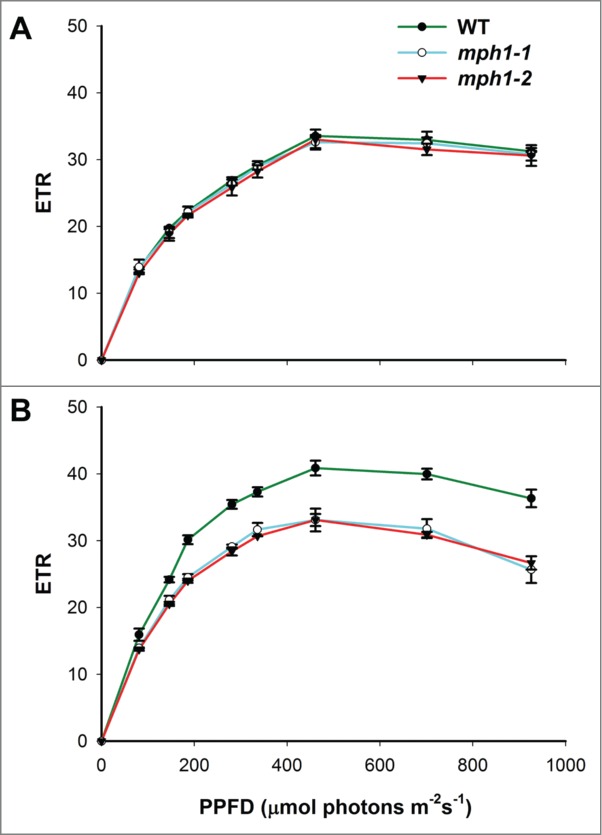
Previous characterization of 2 independent mph1 mutants revealed lower maximum photochemical efficiency of PSII (Fv/Fm) and lower energy-dependent quenching (qE) and higher photoinhibitory quenching (qI) and minimum fluorescence (Fo) than the wild type after high-light treatment.17 The data suggest that MPH1 protein is required for maintaining normal PSII function under high-light irradiance. To further test this hypothesis, we determined the light-response curves of the electron transport rate (ETR) of PSII before and after a 3 h high-light treatment (1000 μmol photons m−2 s−1). As shown in Figure 1A, there were no differences in ETR in mph1 mutants and the wild type at normal growth light (100 μmol photons m−2 s−1). This is consistent with previous results indicate that MPH1 is dispensable for optimal PSII function under ‘normal’ laboratory growth light conditions.17 In contrast, after high-light treatment, the ETR was significantly reduced in mph1 mutants compared to the wild type (Fig. 1B), as was also observed for Fv/Fm.17
Figure 1.
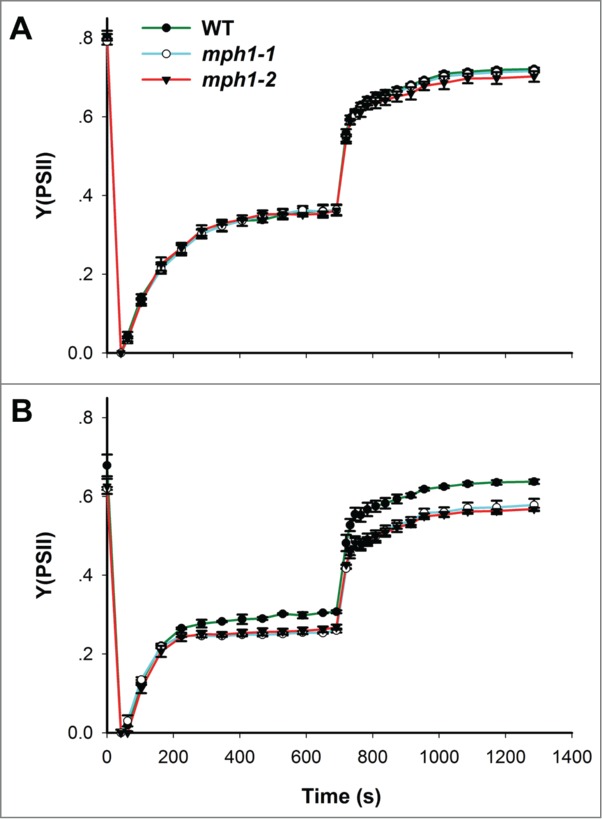
Light-response curves of ETR in mph1 mutants and wild-type plants before (A) and after (B) 3 h high-light treatment. Four-week-old plants that dark-adapted for 30 min were used for measurements of chlorophyll fluorescence parameters. Values are means±SE (n = 5).
To further characterize the defects in PSII lacking MPH1 protein, we monitored the induction and relaxation kinetics of Y(PSII) before and after 3 h high-light treatment. Induction of Y(PSII) occurred during the 735 seconds exposure to actinic light (531 μmol photons m−2 s−1) and relaxation of Y(PSII) occurred after actinic light was turned off. As shown in Figure 2A, before high light treatment, mph1 mutants had kinetics of induction and relaxation of Y(PSII) identical to the wild type. In contrast, after exposure to high light, mph1 mutants had much lower Y(PSII) than the wild type throughout either the 735 seconds induction or the 600 seconds relaxation (Fig. 2B). This is consistent with the previous hypothesis that MPH1 is involved in maintenance of PSII reaction center under photoinhibitory conditions.17 The data from the time course of Y(PSII) kinetics further demonstrated that PSII in mph1 mutants is less efficient than the wild type under high-light stress.
Figure 2.
Kinetics of time-course induction and relaxation of Y(PSII) before (A) and after (B) 3 h high-light treatment. Forty seconds after initial determination of Fo and Fm, actinic light (531 μmol photons m−2 s−1) was switched on for 715 seconds. After termination of actinic light, relaxation was monitored for 600 seconds. Values are means ±SE (n = 5).
Elevated levels of damaged PSII proteins in mph1 mutants following high light
We hypothesized that the marked reduction of PSII activity in mph1 mutant under high light was due to more severe PSII photodamage. Previous in vitro photoinhibitory assays with or without lincomycin collectively suggested that severe photoinhibition observed in mph1 mutants is the result of accelerated rate of damage to PSII.17 To evaluate photodamage directly, the levels of oxidized proteins, caused by reactive oxygen species (ROS), were analyzed. Thylakoid membranes were isolated from dark-treated, growth-light and high-light illuminated plants. As shown in Figure 3, no differences in the amounts of carbonylated proteins were detected when comparing mph1 mutants and the wild type either in the dark or under growth light. In contrast, after exposure to high light PSII proteins were found to be more extensively carbonylated in mph1 mutants than in the wild type, suggesting that mph1 mutants suffer from more photooxidative damage than the wild type. This is consistent with the hypothesis that MPH1 plays a role in the protection of PSII reaction center against photodamage under high-light stress.17
Figure 3.
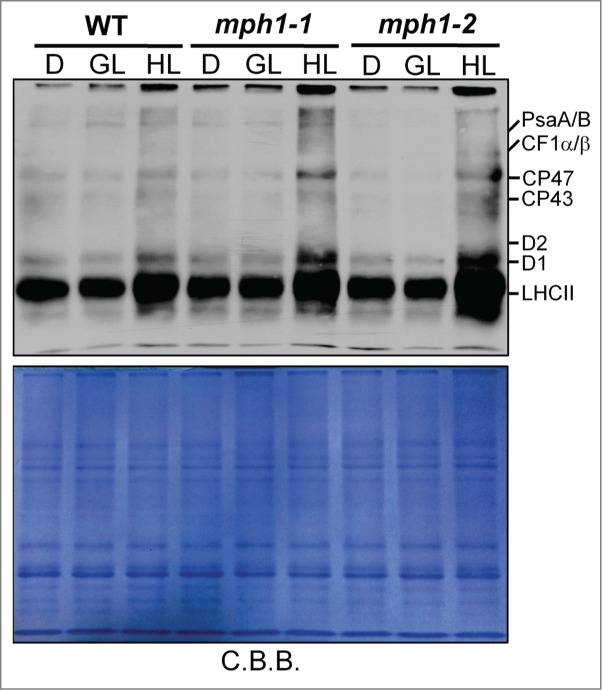
Analysis of oxidation of thylakoid membrane proteins in mph1 mutants and wild-type plants. Leaves were harvested from plants after 16 h dark period (D), at growth light (GL) or after 3 h high-light treatment (HL). Thylakoids containing 2μg chlorophyll were loaded into each lane and carbonylated proteins in thylakoids were immunodetected using OxyBlotTM (Millipore). Shown below is the reference gel stained with Coomassie brilliant blue (C.B.B.).
Phylogenetic analysis of MPH1 protein and its homologs in other land plants
MPH1 Homologs were found in the genomes of numerous land plants, for example, the eudicots castor bean (Ricinus communis), poplar (Populus trichocarpa), soybean (Glycine max) and tomato (Solanum lycopersicum) and, the monocots sorghum (Sorghum bicolor), maize (Zea mays) and rice (Oryza sativa), and the moss Physcomitrella patens (Fig. 4). However, MPH1 homologs are not detected either in algae or cyanobacteria,17 suggesting broad conservation of MPH1 function in land plants. The sessile life style of land plants necessities that they have to cope with changeable environmental conditions in their habitats, such as incident high light or fluctuating light, which require land plants to more rapidly and efficiently protect themselves from damages to photosynthetic machinery under conditions of photooxidative stress.
Figure 4.
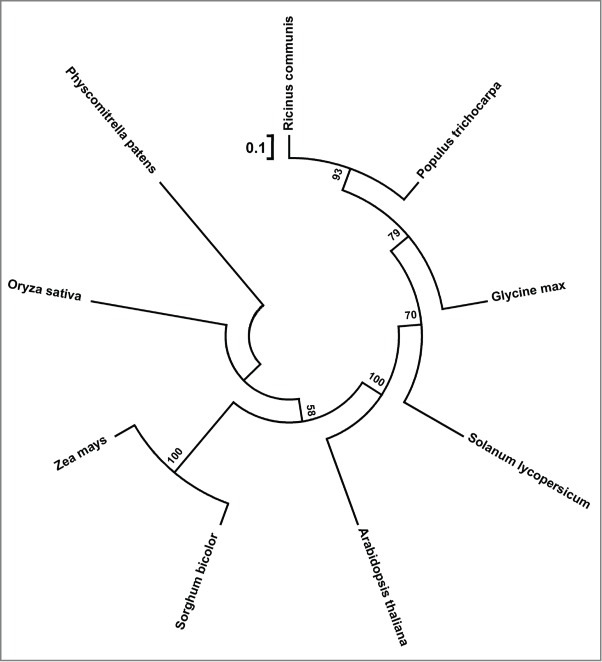
Phylogenetic analysis of MPH1 and Its homologs in other land plants. Full-length homologous amino acid sequences were aligned on Jalview website using MUSCLE program (http://www.jalview.org). The phylogenetic tree was constructed using the neighbor joining method in MEGA version 5 (http://www.megasoftware.net). Percentages over 50% from 1000 bootstrap replicates are shown. Bar = 0.1 amino acid substitutions. The accession numbers for MPH1 and homologs were as described previously in reference 17.
Conclusions
This work provides addition support for the hypothesis that MPH1 participates in maintaining proper PSII function under high-light stress by protecting PSII from photooxidative damage in land plants. Further work to screen for the direct interacting partners of MPH1 would aid in a more detailed understanding of the role of MPH1 in the regulation of PSII damage-repair cycle. The recent characterization of MPH1 and other auxiliary protein factors in PSII turnover, organization and assembly indicate that, in spite of decades of research on PSII, chloroplast proteins of unknown function still need to be identified and the regulatory network of functional PSII are await to be completed.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Funding
This work was supported by the US. National Science Foundation Arabidopsis 2010 Project Grants MCB-0519740 and MCB-124400.
References
- 1.Komenda J, Sobotka R, Nixon PJ. Assembling and maintaining the photosystem II complex in chloroplasts and cyanobacteria. Curr Opin Plant Biol 2012; 15:245-51; PMID:22386092; http://dx.doi.org/ 10.1016/j.pbi.2012.01.017 [DOI] [PubMed] [Google Scholar]
- 2.Pagliano C, Saracco G, Barber J. Structural, functional and auxiliary proteins of photosystem II. Photosynth Res 2013; 116:167-88; PMID:23417641; http://dx.doi.org/ 10.1007/s11120-013-9803-8 [DOI] [PubMed] [Google Scholar]
- 3.Nixon PJ, Michoux F, Yu J, Boehm M, Komenda J. Recent advances in understanding the assembly and repair of photosystem II. Ann Bot 2010; 106:1-16; PMID:20338950; http://dx.doi.org/ 10.1093/aob/mcq059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nath K, Jajoo A, Poudyal RS, Timilsina R, Park YS, Aro E-M, Nam HG, Lee CH. Towards a critical understanding of the photosystem II repair mechanism and its regulation during stress conditions. FEBS Lett 2013; 587:3372-81; PMID:24056074; http://dx.doi.org/ 10.1016/j.febslet.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 5.Nickelsen J, Rengstl B. Photosystem II assembly: from cyanobacteria to plants. Annu Rev Plant Biol 2013; 64:609-35; PMID:23451783; http://dx.doi.org/ 10.1146/annurev-arplant-050312-120124 [DOI] [PubMed] [Google Scholar]
- 6.Ruban AV, Johnson MP, Duffy CDP. The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 2012; 1817:167-81; PMID:21569757; http://dx.doi.org/ 10.1016/j.bbabio.2011.04.007 [DOI] [PubMed] [Google Scholar]
- 7.Takahashi S, Badger MR. Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 2011; 16:53-60; PMID:21050798; http://dx.doi.org/ 10.1016/j.tplants.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama Y, Murata N. Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl Microbiol Biotechnol 2014; 98:8777-96; PMID:25139449; http://dx.doi.org/ 10.1007/s00253-014-6020-0 [DOI] [PubMed] [Google Scholar]
- 9.Mulo P, Sirpiö S, Suorsa M, Aro E-M. Auxiliary proteins involved in the assembly and sustenance of photosystem II. Photosynth Res 2008; 98:489-501; PMID:18618287; http://dx.doi.org/ 10.1007/s11120-008-9320-3 [DOI] [PubMed] [Google Scholar]
- 10.Chi W, Sun X, Zhang L. The roles of chloroplast proteases in the biogenesis and maintenance of photosystem II. Biochim Biophys Acta 2012; 1817:239-46; PMID:21645493; http://dx.doi.org/ 10.1016/j.bbabio.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 11.Yu F, Fu A, Aluru M, Park S, Xu Y, Liu H, Liu X, Foudree A, Nambogga M, Rodermel SR. Variegation mutants and mechanisms of chloroplast biogenesis. Plant Cell Environ 2007; 30:350-65; PMID:17263779; http://dx.doi.org/ 10.1111/j.1365-3040.2006.01630.x [DOI] [PubMed] [Google Scholar]
- 12.Järvi S, Suorsa M, Aro E-M. Photosystem II repair in plant chloroplasts — regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim Biophys Acta 2015; 1847(9):900-9; PMID:25615587; http://dx.doi.org/ 10.1016/j.bbabio.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 13.Ajjawi I, Lu Y, Savage LJ, Bell SM, Last RL. Large-scale reverse genetics in Arabidopsis: case studies from the Chloroplast 2010 Project. Plant Physiol 2010; 15:529-40; PMID:19906890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Savage LJ, Larson MD, Wilkerson CG, Last RL. Chloroplast 2010: a database for large-scale phenotypic screening of Arabidopsis mutants. Plant Physiol 2011; 155:1589-600; PMID:21224340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savage LJ, Imre KM, Hall DA, Last RL. Analysis of essential Arabidopsis nuclear genes encoding plastid-targeted proteins. PLoS ONE 2013; 8:e73291; PMID:24023856; http://dx.doi.org/ 10.1371/journal.pone.0073291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Hall DA, Last RL. A small zinc finger thylakoid protein plays a role in maintenance of photosystem II in Arabidopsis thaliana. Plant Cell 2011; 23:1861-75; PMID:21586683; http://dx.doi.org/ 10.1105/tpc.111.085456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Last R. A land plant-specific thylakoid membrane protein contributes to photosystem II maintenance in Arabidopsis thaliana. Plant J 2015; 82:731-43; PMID:25846821; http://dx.doi.org/ 10.1111/tpj.12845 [DOI] [PubMed] [Google Scholar]


