Abstract
Mechanical wounding of Arabidopsis thaliana leaves results in modifications of most membrane lipids within 6 hours. Here, we discuss the lipid changes, their underlying biochemistry, and possible relationships among activated pathways. New evidence is presented supporting the role of the processive galactosylating enzyme SENSITIVE TO FREEZING2 in the wounding response.
Keywords: Arabidopsis thaliana, glycerolipids, leaf wounding, lipidomics, oxidized lipids, sterol derivatives
Abbreviations
- ASG
Acyl sterol glucoside
- acMGDG
Acylated monogalactosyldiacylglycerol
- acPG
Acylated phosphatidylglycerol
- DAD1
Defective in anther dehiscence 1
- DAG
Diacylglycerol
- DGDG
Digalactosyldiacylglycerol
- DGMG
Digalactosylmonoacylglycerol
- dnOPDA
Dinor-oxophytodienoyl
- GL
Galactolipid
- Glc
Glucoside
- GlcCer
Glycosylceramide
- GIPC
Glycosylinositolphosphoceramide
- LPC
Lysophosphatidylcholine
- LPE
Lysophosphatidyl-ethanolamine
- MGDG
Monogalatosyldiacylglyerol
- oxDGDG
Oxidized digalactosyl- diacylglycerol
- oxMGDG
Oxidized monogalactosyldiacylglycerol
- OPDA
Oxophytodienoyl
- PA
Phosphatidic acid
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PG
Phosphatidylglycerol
- PG
Phosphatidylglycerol
- PI
Phosphatidylinositol
- PS
Phosphatidylserine
- PLA2
Phospholipase A2
- PLD
Phospholipase D
- PL
Phospholipid
- PDAT1
Phospholipid:diacylglycerol acyltransferase 1
- SFR2
SENSITIVE TO FREEZING 2
- SG
Sterol glucoside
- SQDG
Sulfoquinovosyldiacylglycerol
- TeGDG
Tetra-galactosyldiacylglycerol
- TAG
Triacylglycerol
- TrGDG
Tri-galactosyldiacylglycerol.
Wounding (i.e., mechanical damage) of plant tissues can be caused by wind, rain, or hail or can occur during insect attack, grazing, or pathogen infection. In the laboratory, mechanical wounding of plants provides a controlled experimental treatment to elicit chemical responses, including changes in gene expression, protein expression, and metabolite composition. Changes in lipids occur rapidly, with many examples of changes observed in 5 min or less.1-4.
In a recent study,5 complex lipids were analyzed in leaves of unwounded plants and mechanically wounded plants, sampled 45 min and 6 h after wounding, with each treatment replicated 31 times. The levels of 254 out of the total of 264 complex lipids detected changed significantly in response to mechanical wounding. Here, the observed changes in complex lipids in wound response are examined further and considered in relation to known and proposed lipid metabolic pathways.
Overview
Vu et al.5 found that, after wounding, the total levels of membrane and membrane-derived lipids dropped. The mass spectral intensity of all measured leaf lipids fell 6% from the level in unwounded plants at 45 min after wounding and a further 22% at 6 h after wounding (Fig. 1A). The drop in total lipid level was mainly due to decreases in the levels of normal-chain, diacyl polar membrane lipids including phospholipids (PLs), sulfolipid (sulfoquinovosyldiacylglycerol, SQDG), and galactolipids (GLs) (Fig. 1A and B). On the other hand, many less abundant lipids increased (Fig. 1A, C and D). Lipids with increased levels include molecular species with oxidized fatty acyl chains, head-group acylated membrane lipids, monoacyl polar lipids, phosphatidic acids, oligogalactolipids, sterol glucosides, acyl sterol glucosides, and triacylglycerols. Additionally there were subtle increases in lipid unsaturation in PC and PA. In the following sections, we detail and discuss these changes and discuss possibilities for the underlying biochemistry (Fig. 2).
Figure 1.
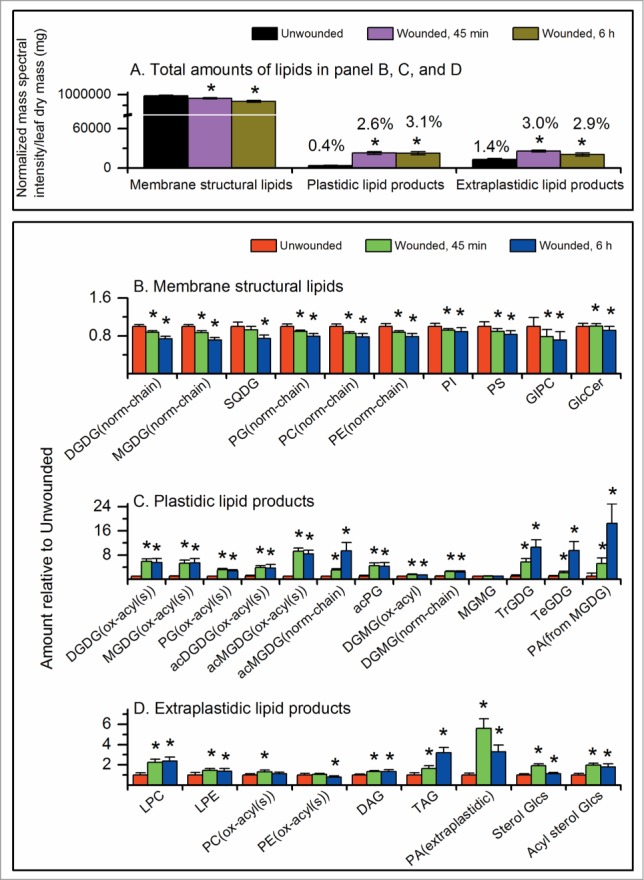
Changes in leaf lipid classes following pressure wounding on leaves of wild-type Arabidopsis thaliana across the mid-vein with a hemostat. Leaves were harvested from unwounded or wounded plants 45 min and 6 h after wounding.5 This figure is based on data in ref. 5. (A) Normalized mass spectral intensity for lipids in the panels (B, C, and D). 1 unit of normalized mass spectral signal equals the signal obtained from 1 pmol of internal standard. Values over the bars indicate the percent of the total lipids represented by the bar. (B) Amount of structural (membrane) lipid classes after wounding relative to unwounded level for each class. (C) Amounts of minor plastidic lipid classes relative to the unwounded level for each class. (D) Amounts of minor extraplastidic lipid classes relative to the unwounded level for each class. * indicates significant changes compared to unwounded samples, T-test, P < 0.001, n = 31. Error bars are standard deviation.
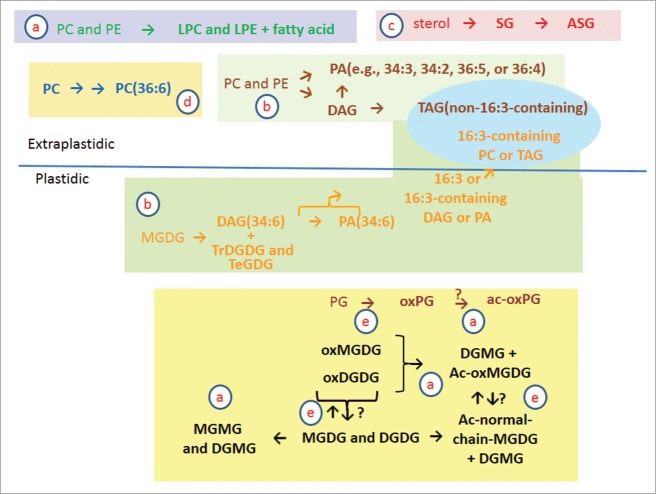
Figure 2.
Proposed reactions of membrane lipids in the wounding response in Arabidopsis leaves. (a) Lipid head-group acylation and lipolytic reactions producing monoacyl lipid molecular species; (b) galactosylation of lipids and other reactions producing DAG, PA, and TAG; (c) formation of sterol glucosides and acyl sterol glucosides; (d) increase of fully desaturated PC and PA fractions during the wounding response; and e. oxidation of lipids in the wounding response. See text for details.
Glycerolipid head-group acylation and lipolytic reactions producing monoacyl lipid molecular species
Fatty acylation of the lipid head group of monogalatosyldiacylglyerol (MGDG) was originally described by Heinz.6 MGDG acylation occurs when a fatty acyl chain is transferred from digalactosyldiacylglycerol (DGDG) to the 6-position on the galactose of MGDG,7,8 producing an acylated MGDG and digalactosylmonoacylglycerol (DGMG) (Fig. 2, block a). More recently, oxidized acylated MGDGs have been identified in Arabidopsis leaves following bacterial infection or wounding; the most common molecular species is an MGDG with oxidized fatty acids in the 1- and 2- positions (oxophytodienoyl/dinor-oxophytodienoyl; OPDA/dnOPDA), acylated on galactose with OPDA. This species was given the common name “Arabidopside E”.9,10 It is now apparent that a wide variety of acylated MGDG molecular species and DGMGs are produced upon wounding of Arabidopsis leaves (Fig. 1C and D).5,8,11-13 Additionally phosphatidylglycerol (PG) with an acyl chain on the terminal glycerol and small amounts of head-group acylated DGDGs are also produced in Arabidopsis leaves when they are wounded (Fig. 1C).5,11,13 DGMG may also be produced by the acylhydrolases, such as DAD1 and DAD1-like enzymes, localized in chloroplasts and capable of acting on galactolipids.14
In addition to DGMG, mono-acyl glycerolipids produced in response to wounding include lysophosphatidylcholines, which increase more than 2 fold at 45 min after wounding, and lysophosphatidylethanolamines (Fig. 1D). These may be formed from PC and PE by the action of acylhydrolases such as the patatin-like PLA2s (e.g., refs. 15-16).
Galactosylation of galactolipids and other reactions producing DAG, PA, and TAG
In response to freezing, tri- and tetra-galactosyldiacylglycerol (TrGDG and TeGDG) are formed by the processive addition of galactose moieties from available MGDG molecular species, forming first DGDG, and then TrGDG and TeGDG by the enzyme SENSITIVE TO FREEZING 2 (SFR2) (Fig. 2, block b).17,18 The side product, DAG, can be converted to TAG by an acyltransferase.17 DAG that has accumulated in the chloroplast envelopes during non-freezing conditions can enter PL metabolism, possibly through action of a DAG kinase.19-20 Following wounding, the tight co-occurrence of PA(34:6) with TrGDG(34:6) and TeGDG(34:6), and parallel reduction of MGDG(34:6),5 is consistent with this pathway of processive galactosylation and PA(34:6) as a by-product.
Because previous data suggested that the presence of SFR2 is only critical to plant survival following freezing,21-22 the role of SFR2 in wounding was confirmed using an Arabidopsis line lacking SFR2, sfr2–3.17 The data are included as Supplemental Table 1. Data extracted from this data set and shown in Figure 3 demonstrate that galactosylation during the wounding response is dependent on the presence of SFR2. In the absence of SFR2, TrGDG and TeGDG are not formed, and PA(34:6) is much reduced. Additionally, TAG(18:3/34:6), a plastid-derived molecular species containing 16:3 in the “34:6” moiety, is not formed during wounding in sfr2–3 (Fig. 3D). These data indicate that wounding produces a measurable activation of SFR2 in leaves of wild-type plants, and confirms that PA is correlated with SFR2 activity, adding to the known effects of SFR2. Further investigation of the physical characteristics associated with the wounding suggested that tissue desiccation occurred around the wounding site. If SFR2 is responding to the desiccation, this would be consistent with observed production of oligogalactolipids during desiccation of the resurrection plant.23
Figure 3.
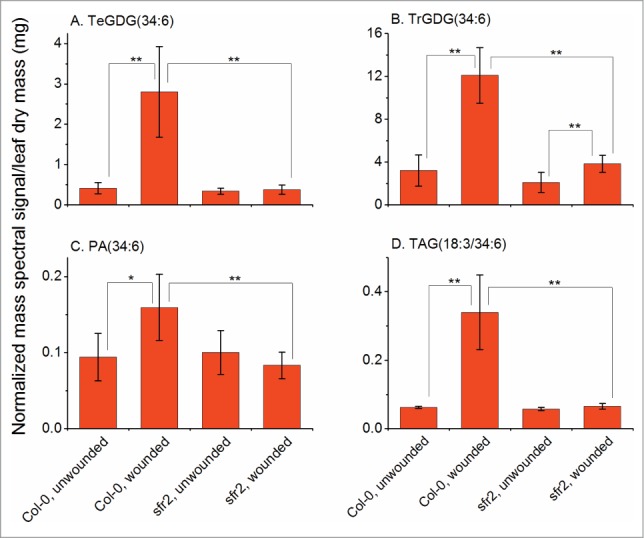
Formation of TrGDG, TeGDG, PA(34:6), and 16:3-containing TAG is abolished in sfr2–3 (SALK_106253) plants. Leaves were harvested from unwounded or wounded plants 45 min after wounding as in ref. 5. In the indicated lipids, “34:6” is composed of 18:3 and 16:3 fatty acyl chains. 1 unit of normalized mass spectral signal equals the signal obtained from 1 pmol of internal standard. ** indicates a significant change at P < 0.01, * indicates a significant change at P < 0.05, T-test, n = 6. Error bars are standard deviation.
Small increases in DAG levels are detectable during the leaf's response to wounding (Fig. 1D). Like PA, DAG can accumulate after wounding as a result of multiple reactions, including SFR2 acting on MGDG and phospholipase C acting on phospholipids. Production of PA via DAG by a phospholipase C-diacylglycerol kinase pathway has been detected in Arabidopsis in low-temperature stress.24 PA is also formed from DAG by DAG kinase in plant-fungus interaction.25 Formation of PA via DAG and DAG kinase was also shown to play roles in abscisic acid response and plant tolerance to drought and salt stresses.26 Both DAG derived from extraplastidic phospholipids and DAG formed from MGDG are converted to triacylglycerols (TAGs) during the wounding response. This is likely to occur by the action of PDAT1.20 Indeed, TAG levels increase over a prolonged period after wounding (Fig. 1D). The formation of TAG was recently demonstrated to be an essential intermediate in the delivery of plastidic fatty acids to peroxisomal β-oxidation, to maintain membrane lipid homeostasis in leaves.20
In addition to the SFR2 and phospholipase C-diacylglycerol kinase pathways, PA can be produced by hydrolysis of phospholipids by phospholipase D (PLD) during wounding stress.4 About half of the PA formed in the first 15 min after wounding can be attributed to the action of PLDα1.4 PA species formed by PLD contain DAG moieties characteristic of extraplastidic phospholipids (e.g., 34:3, 34:2, 36:5, and 36:4), distinguishing them from the PA(34:6) derived from MGDG. Additionally, PAs derived from extraplastidic PLs and PA(34:6) from MGDG are formed with different kinetics; specifically, the maximal increase in PA-containing fatty acids from extraplastidic lipids occurs more quickly than maximal formation of PA(34:6) (Fig. 1C and D).
Formation of sterol glucosides and acyl sterol glucosides
Vu et al.5 identified increases in sterol glucosides and acyl sterol glucosides upon wounding (Fig. 1 and Fig. 2, block c). Sterol glucosides probably are formed by UDP-Glc:sterol glycosyltransferase(s).27 Acyl sterol glucosides are likely to be formed by acylation of sterol glucosides, but acylating enzymes acting on sterol glucosides are not identified.
Increase of fully desaturated PC and PA fractions during the wounding response
One of the more subtle changes detected in response to wounding was an increase in the unsaturation of the PC and PA pools, with fully desaturated 34:6 and 36:6 species particularly increased. “34:6” represents an 18:3/16:3 acyl chain combination, while “36:6” represents an 18:3/18:3 combination. Unsaturation indices and percentage of the PA and PC classes that can be attributed to these fully desaturated species are shown in Table 1. In response to wounding, the unsaturation of PC and PA increases significantly. In particular, the proportion of PC(36:6) and PA(36:6) species as a% of the total head group class increases at 45 min after wounding, and the proportion of PC(34:6), and PA(34:6), species increases strongly at 6 h after wounding. The increase in PC(36:6) and PA(36:6) may result from an increased amount of desaturase activity per PC molecule due to a decrease in the size of the PC pool (Fig. 2, block d). However, given that SFR2 and other activities are forming PL precursors from the highly desaturated plastid pool of MGDG, the formation of PC and PA species containing 16:3 likely arises from transfer of 16:3 from the plastids. The identity of the molecule transferred is not clear, but the strong incorporation of 16:3 into PA could suggest that PA or intact DAG is the transferred species.
Table 1.
PA and PC unsaturation is increased in wounded plants. In particular, this is due to increases in 36:6 and 34:6 molecular species. The unsaturation index is a measure of the average unsaturation of the fatty acyl chains in each class. The unsaturation index of a lipid species is calculated by multiplying the number of double bonds by the fraction of that lipid in the lipid class divided by the number of acyl chains in the lipid (i.e., 2 for diacyl lipids). The unsaturation index of the class is the total of the unsaturation index of all lipid species in that class. The PA and PC species as percent of the total indicate the percentage of that class composed on the indicated lipid species. One-way ANOVA was performed to compare the unsaturation indices or lipid percentages among the three treatments. If a significant difference (p < 0.001; n = 31) was detected for a lipid, a Tukey post-hoc test was performed to identify the differing treatments. * indicates that the value in the wounded sample is significantly increased compared to the unwounded sample.
| Unwounded | 45 min after wounding | 6 h after wounding | |
|---|---|---|---|
| Unsaturation index (n = 31) | |||
| PA class | 1.60 ± 0.04 | 1.69* ± 0.01 | 1.66* ± 0.02 |
| PC class | 1.83 ± 0.02 | 1.86* ± 0.02 | 1.87* ± 0.01 |
| PA species as percent of total PA | |||
| PA(36:6) | 5.1 ± 0.8 | 6.6* ± 0.5 | 5.8* ± 0.6 |
| PA(34:6) | 0.3 ± 0.3 | 0.3 ± 0.1 | 1.6* ± 0.4 |
| PC species as percent of total PC | |||
| PC(36:6) | 10.7 ± 0.8 | 12.0* ± 0.8 | 11.9* ± 0.9 |
| PC(34:6) | 0.2 ± 0.0 | 0.3* ± 0.0 | 0.5* ± 0.1 |
Oxidation of lipids in the wounding response
While oxidation of extraplastidic phospholipids, such as PC and PE, during wounding is minimal (Fig. 1D),5,8,12 plastidic lipids including PG, MGDG, DGDG, and acylated MGDG are converted to a plethora of oxidized molecular species during wounding (Fig. 2, block e).
In response to wounding, total oxidized DGDG and MGDG of leaves increased 5 to 6-fold at 45 min after wounding.5 Oxidized membrane lipids described by Vu et al.,5,12 include those potentially produced by established pathways, such as OPDA, and numerous molecular species whose structures and biosynthetic pathways are less clearly delineated. Chemical formulas for detected molecular species include those consistent with the presence of hydroxy fatty acids, such as 18:2-O (hydroxylated 18:2) and 18:3-O (hydroxylated 18:3), ketols (such as 16:3–2O and 18:3–2O), and phytoprostanes (e.g., 18:4–2O and 18:4–3O).5,12 The two most abundant oxidized MGDG species are Arabidopside A, also known as MGDG(OPDA/dnOPDA) and MGDG(34:8–2O), and Arabidopside B, also known as MGDG(diOPDA) and MGDG(36:8–2O), which are increased ∼100 and ∼50 fold at 45 min after wounding, respectively.28 The most abundant oxidized DGDG, Arabidopside D, also known as DGDG(diOPDA) and DGDG(36:8–2O), increased ∼45 fold at 45 min compared to its level in unwounded plants.29 Oxidized acylated MGDG species are also formed quickly and in relatively high amounts. Arabidopside E, MGDG(OPDA/dnOPDA) acylated on galactose with OPDA, and also known as acMGDG(18:4-O/34:8–2O), is increased over 200-fold at 45 min after wounding. The formation of OPDA-containing galactolipids seems to be restricted to Arabidopsis and related species.30 However, other plant species produce membrane lipids with oxidatively modified acyl chains, such as colneleic acid-containing PtdIns in potato tubers; etherolenic acid-containing DAGs (linolipin A and B) and etherolenic acid containing DGDGs (linolipin C and D) in flax leaves upon responding to freezing-thawing.31-33
In earlier work, a more resolved time-course experiment demonstrated that MGDG(OPDA/dnOPDA), MGDG(OPDA/OPDA), and DGDG(OPDA/OPDA) were formed faster than their counterparts with a single oxylipin chain.34 Nilsson et al.35 demonstrated that oxidation of trienoic fatty acids to OPDA and dnOPDA occurs on intact galactolipid molecules and does not involve hydrolysis of the acyl chains from the backbone. Together, these data suggest that lipoxygenase acts shortly after wounding and is more likely to oxidize both chains on a galactolipid than a single chain.
Figure 4 shows levels of 4 types of acMGDG: acMGDG with 3 normal chains, acMGDG with 1 out of 3 fatty acyl chains oxidized, acMGDG with 2 out of 3 fatty acyl chains oxidized, and acMGDG with 3 oxidized chains. The fully oxidized acMGDG pool is induced most quickly, and to the highest final level: 87 fold at 45 min, with a drop at 6 h. Other acMGDG pools with a mixture of oxidized and normal chains (or all normal chains) were produced more slowly, and levels were higher at 6 h after wounding than at 45 min. This again suggests that oxidation happens quickly after wounding, that there is a preference for multiple oxidation of acyl chains on a single molecule, and that oxidized chains are cleared by replacement with non-oxidized chains at later times.
Figure 4.
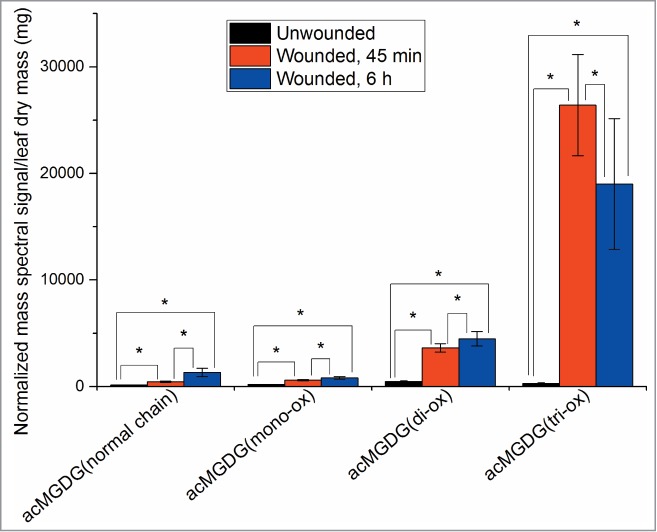
Levels of 4 types of acMGDG as a function of oxidation. This figure is based on data in Vu et al.5 acMGDG with 3 oxidized chains, acMGDG with 2 out of 3 fatty acyl chains oxidized, acMGDG with 1 out of 3 fatty acyl chains oxidized, and acMGDG with 3 normal chains are shown. 1 unit of normalized mass spectral signal equals the signal obtained from 1 pmol of internal standard. Levels are compared to the level in unwounded plants. The total of the categorized acMGDG accounts for 71% of acMGDG at 45 min and 6 h after wounding; 29% of acMGDG cannot be readily categorized, due to ambiguity in acyl chain identification. * indicates a significant change, T-test, P < 0.001, n = 31. Error bars are standard deviation.
Conclusion and Perspectives
The data presented here and in Vu et al.5 demonstrate that membrane lipids undergo extensive, diverse modifications during wounding. These changes raise important questions concerning the function of the modifications. Wounding of plant tissues can be caused by abiotic stresses, such as wind, rain, and hail or biotic stresses, such as insect attack, grazing, or pathogen infection. Our recent studies indicate that distinctive types of ac-lipids are produced in plants responding to different stresses, such as freezing, wounding, and pathogen infection.12 In Arabidopsis, the acMGDGs Arabidopsides E and G have antimicrobial functions and are proposed to be reserves for quick release of oxylipins in response to stress. On the other hand, ac-MGDGs acylated with un-oxidized, normal fatty acids (e.g., 16:0) are not oxylipin reserves and likely have other functions. However, the cellular and physiological functions are virtually unknown for most of the wound-induced lipid metabolites and modifications. Potentially, they could be involved in protecting cells from oxidative damage, provide stress pattern recognition and/or cell signaling. The identification of the compounds and the deduced metabolic pathways for their production will facilitate greatly functional investigations.
It is also noted that our data on whole leaves do not provide informations about potentially different levels of responsiveness to wounding in various leaf cells. Such differences might be due to differences in cell types or physical locations relative to the wounding sites. Toward this end, application of methodology with spatial resolution capacity, such as mass spectrometry imaging (reviewed by Horn & Chapman),36 is likely provide to novel insights that promote functional studies of wound-induced membrane lipids.
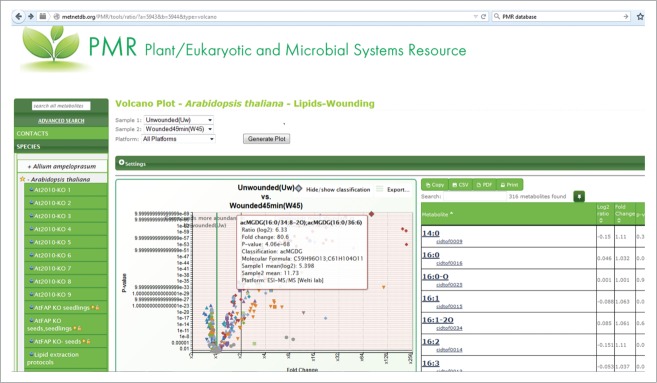
Data and metadata from this work and the work of Vu et al.5 are available in the metabolomics-focused database, Plant/Eukaryotic and Microbial Systems Resource (PMR; http://www.metnetdb.org/PMR/).37 In PMR, the data on lipid changes during leaf wounding in Arabidopsis can be plotted interactively and otherwise explored further. A screenshot from PMR showing an interactive volcano plot of the wounding data is provided in Figure 5. The experiment is publically accessible at http://www.metnetdb.org/PMR/experiments/?expid=243.
Figure 5.
Screenshot of Arabidopsis wounding data volcano plot at Plant/Eukaryotic and Microbial Systems Resource (http://www.metnetdb.org/PMR/). Mouse-over of any point in the plot provides information about the metabolite and its change in response to wounding. Data include levels of fatty acyl chains in complex lipids as well as levels of intact complex lipid species.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank the reviewer for excellent suggestions. The authors are grateful to Mary Roth and Pamela Tamura for their technical assistance. The lipid analyses described in this work were performed at the Kansas Lipidomics Research Center (KLRC).
Funding
The work was supported by National Science Foundation MCB 0920663 and MCB 1413036. Instrument acquisition at KLRC was supported by National Science Foundation (EPS 0236913, DBI 0521587, DBI1228622), Kansas Technology Enterprise Corporation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of National Institute of Health (P20GM103418), and Kansas State University. Contribution 15–373-J from the Kansas Agricultural Experiment Station.
References
- 1.Glauser G, Grata E, Dubugnon L, Rudaz S, Farmer EE, Wolfender J-L. Spatial and temporal dynamics of jasmonate synthesis and accumulation in Arabidopsis in response to wounding. J Biol Chem 2008; 283:16400-07; PMID:18400744; http://dx.doi.org/ 10.1074/jbc.M801760200 [DOI] [PubMed] [Google Scholar]
- 2.Koo AJ, Gao X, Jones AD, Howe GA. A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J 2009; 59:974-86; PMID:19473329; http://dx.doi.org/ 10.1111/j.1365-313X.2009.03924.x [DOI] [PubMed] [Google Scholar]
- 3.Chauvin A, Caldelari D, Wolfender JL, Farmer EE. Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: a role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol 2013; 197:566-75; PMID:23171345; http://dx.doi.org/ 10.1111/nph.12029 [DOI] [PubMed] [Google Scholar]
- 4.Zien CA, Wang C, Wang X, Welti R. In-vivo substrates and the contribution of the common phospholipase D, PLDα, to wound-induced metabolism of lipids in Arabidopsis. Biochim Biophys Acta 2001; 1530:236-48; PMID:11239826; http://dx.doi.org/ 10.1016/S1388-1981(01)00091-9 [DOI] [PubMed] [Google Scholar]
- 5.Vu HS, Shiva S, Roth MR, Tamura P, Zheng L, Li M, Sarowar S, Honey S, McEllhiney D, Hinkes P, et al.. Lipid changes after leaf wounding in Arabidopsis thaliana: Expanded lipidomic data form the basis for lipid co-occurrence analysis. Plant J 2014; 80:728-43; PMID:25200898; http://dx.doi.org/ 10.1111/tpj.12659 [DOI] [PubMed] [Google Scholar]
- 6.Heinz E. Acylgalactosyl diglyceride from leaf homogenates. Biochim Biophys Acta 1967; 144:321-32; PMID:6064609; http://dx.doi.org/ 10.1016/0005-2760(67)90161-0 [DOI] [PubMed] [Google Scholar]
- 7.Heinz E. On the enzymatic formation of acylgalactosyl diglyceride. Biochim Biophys Acta 1967; 144:333-44; PMID:6064610; http://dx.doi.org/ 10.1016/0005-2760(67)90162-2 [DOI] [PubMed] [Google Scholar]
- 8.Vu HS, Roth MR, Tamura P, Samarakoon T, Shiva S, Honey S, Lowe K, Schmelz EA, Williams TD, Welti R. Head-group acylation of monogalactosyldiacylglycerol is a common stress response, and the acyl-galactose acyl composition varies with the plant species and applied stress. Physiol Plant 2014; 150:517-28; PMID:24286212; http://dx.doi.org/ 10.1111/ppl.12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson MX, Hamberg M, Kourtchenko O, Brunnström A, McPhail KL, Gerwick WH, Göbel C, Feussner I, Ellerström M. Oxylipin profiling of the hypersensitive response in Arabidopsis thaliana. Formation of a novel oxo-phytodienoic acid-containing galactolipid Arabidopside E. J Biol Chem 2006; 281:31528-37; PMID:16923817; http://dx.doi.org/ 10.1074/jbc.M604820200 [DOI] [PubMed] [Google Scholar]
- 10.Kourtchenko O, Andersson MX, Hamberg M, Brunnström A, Göbel C, McPhail KL, Gerwick WH, Feussner I, Ellerström M. (Oxo-phytodienoic acid-containing galactolipids in Arabidopsis: jasmonate signaling dependence. Plant Physiol 2007; 145:1658-69; PMID:17951463; http://dx.doi.org/ 10.1104/pp.107.104752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim A, Schütz AL, Galano JM, Herrfurth C, Feussner K, Durand T, Brodhun F, Feussner I. The alphabet of galactolipids in Arabidopsis thaliana. Front Plant Sci 2011; 2:95; PMID:22639619; http://dx.doi.org/ 10.3389/fpls.2011.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vu HS, Tamura P, Galeva NA, Chaturvedi R, Williams TD, Wang X, Shah J, Welti R. Direct infusion mass spectrometry of oxylipin-containing Arabidopsis thaliana membrane lipids reveals varied patterns in different stress responses. Plant Physiol 2012; 158:324-39; PMID:22086419; http://dx.doi.org/ 10.1104/pp.111.190280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilsson AK, Johansson ON, Fahlberg P, Steinhart F, Gustavsson MB, Ellerström M, Andersson MX. Formation of oxidized phosphatidylinositol and 12-oxo-phytodienoic acid containing acylated phosphatidylglycerol during the hypersensitive response in Arabidopsis. Phytochem 2014; 101:65-75; PMID:24559746; http://dx.doi.org/ 10.1016/j.phytochem.2014.01.020 [DOI] [PubMed] [Google Scholar]
- 14.Seo YS, Kim EY, Kim JH, Kim WT. 2009. Enzymatic characterization of class I DAD1-like acylhydrolase members targeted to chloroplast in Arabidopsis. FEBS Lett 2009; 583:2301-07; PMID:19527719; http://dx.doi.org/ 10.1016/j.febslet.2009.06.021 [DOI] [PubMed] [Google Scholar]
- 15.Yang W, Zheng Y, Bahn SC, Pan X, Li M, Vu HS, Roth MR, Scheu B, Welti R, Wang X. Patatin-related phospholipase A pPLAIIα modulates oxylipin formation and water loss in Arabidopsis thaliana. Mol Plant 2012; 5:452-60; PMID:22259021; http://dx.doi.org/ 10.1093/mp/ssr118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Bahn SC, Guo L, Musgrave W, Berg H, Welti R, Wang X. Patatin-related phospholipase pPLAIIIβ-induced changes in lipid metabolism alter cellulose content and cell elongation in Arabidopsis. Plant Cell 2011; 23:1107-23; PMID:21447788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moellering ER, Muthan B, Benning C. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 2010; 330:226-28; PMID:20798281; http://dx.doi.org/ 10.1126/science.1191803 [DOI] [PubMed] [Google Scholar]
- 18.Roston RL, Wang K, Kuhn LA, Benning C. Structural determinants allowing transferase activity in SENSITIVE TO FREEZING 2, classified as a family I glycosyl hydrolase. J Biol Chem 2014; 289:26089-106; PMID:25100720; http://dx.doi.org/ 10.1074/jbc.M114.576694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthan B, Roston RL, Froehlich JE, Benning C Probing Arabidopsis chloroplast diacylglycerol pools by selectively targeting bacterial diacylglycerol kinase to suborganellar membranes. Plant Physiol 2013; 163:61-74; PMID:23839866; http://dx.doi.org/ 10.1104/pp.113.222513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan J, Yan C, Roston R, Shanklin J, Xu C. Arabidopsis lipins, PDAT1 acyltransferases, and SDP1 triacylglycerol lipase synergistically direct fatty acids toward β-oxidation, thereby maintaining membrane lipid homeostasis. Plant Cell 2014; 26:4119-34; PMID:25293755; http://dx.doi.org/ 10.1105/tpc.114.130377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorlby G, Fourrier N, Warren G. The SENSITIVE TO FREEZING2 gene, required for freezing tolerance in Arabidopsis thaliana, encodes a β-glucosidase. Plant Cell 2004; 16:2192-203; PMID:15258268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fourrier N, Bédard J, Lopez-Juez E, Barbrook A, Bowyer J, Jarvis P, Warren G, Thorlby G. A role for SENSITIVE TO FREEZING2 in protecting chloroplasts against freeze-induced damage in Arabidopsis. Plant J 2008; 55:734-45; PMID:18466306; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03549.x [DOI] [PubMed] [Google Scholar]
- 23.Gasulla F, Vom Dorp K, Dombrink I, Zähringer U, Gisch N, Dörmann P, Bartels D. The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: a comparative approach. Plant J 2013; 75:726-41; PMID:23672245; http://dx.doi.org/ 10.1111/tpj.12241 [DOI] [PubMed] [Google Scholar]
- 24.Arisz SA, van Wijk R, Roels W, Zhu JK, Haring MA, Munnik T. Rapid phosphatidic acid accumulation in response to low temperature stress in Arabidopsis is generated through diacylglycerol kinase. Front Plant Sci 2013; 4:1; PMID:23346092; http://dx.doi.org/ 10.3389/fpls.2013.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Jong CF, Laxalt AM, Bargmann BOR, De Wit PJGM, Joosten MHAJ, Munnik T. Phosphatidic acid accumulation is an early response in the Cf-4/Avr4 interaction. Plant J 2004; 39:1-12 [DOI] [PubMed] [Google Scholar]
- 26.Peters C, Li M, Narashimhan R, Roth R, Welti R, Wang X. Nonspecific phospholipase C NPC4 promotes responses to abscisic acid and tolerance to hyperosmotic stress in Arabidopsis. Plant Cell 2010; 22:2642-59; PMID:20699393; http://dx.doi.org/ 10.1105/tpc.109.071720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeBolt S, Scheible WR, Schrick K, Auer M, Beisson F, Bischoff V, Bouvier-Navé P, Carroll A, Hematy K, Li Y, et al.. Mutations in UDP-glucose:sterol glucosyltransferase in Arabidopsis cause transparent testa phenotype and suberization defect in seeds. Plant Physiol 2009; 151:78-87; PMID:19641030; http://dx.doi.org/ 10.1104/pp.109.140582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hisamatsu Y, Goto N, Hasegawa K, Shigemori H. Arabidopsides A and B, two new oxylipins from Arabidopsis thaliana. Tetrahedron Lett 2003; 44:5553-6; PMID:1584495915844959 [Google Scholar]
- 29.Hisamatsu Y, Goto N, Sekiguchi M, Hasegawa K, Shigemori H. Oxylipins arabidopsides C and D from Arabidopsis thaliana. J Nat Prod 2005; 68:600-3 [DOI] [PubMed] [Google Scholar]
- 30.Böttcher C, Weiler EW. Cyclo-oxylipin-galactolipids in plants: occurrence and dynamics. Planta 2007; 226:629-37; PMID:17404756; http://dx.doi.org/ 10.1007/s00425-007-0511-5 [DOI] [PubMed] [Google Scholar]
- 31.Fauconnier ML, Williams TD, Marlier M, Welti R.. Potato tuber phospholipids containing colneleic acid in the 2-position. FEBS Lett 2003; 538:155-158; PMID:12633870; http://dx.doi.org/ 10.1016/S0014-5793(03)00171-6 [DOI] [PubMed] [Google Scholar]
- 32.Chechetkin IR, Mukhitova FK, Blufard AS, Yarin AY, Antsygina LL, Grechkin AN. Unprecedented pathogen-inducible complex linolipins A and B. FEBS J 2009; 276:4463-72; PMID:19645727; http://dx.doi.org/ 10.1111/j.1742-4658.2009.07153.x [DOI] [PubMed] [Google Scholar]
- 33.Chechetkin IR, Blufard AS, Khairutdinov BI, Mukhitova FK, Gorina SS, Yarin AY, Antsygina LL, Grechkin AN. Isolation and structure elucidation of linolipins C and D, complex oxylipins from flax leaves. Phytochemistry 2013; 96:110-6; PMID:24042063; http://dx.doi.org/ 10.1016/j.phytochem.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 34.Buseman CM, Tamura P, Sparks AA, Baughman EJ, Maatta S, Zhao J, Roth MR, Esch SW, Shah J, Williams TD, et al.. Wounding stimulates the accumulation of glycerolipids containing oxophytodienoic acid and dinor-oxophytodienoic acid in Arabidopsis leaves. Plant Physiol 2006; 142:28-39; PMID:16844834; http://dx.doi.org/ 10.1104/pp.106.082115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsson AK, Fahlberg P, Ellerström M, Andersson MX. Oxo-phytodienoic acid (OPDA) is formed on fatty acids esterified to galactolipids after tissue disruption in Arabidopsis thaliana. FEBS Lett 2012; 586:2483-87; PMID:22728240; http://dx.doi.org/ 10.1016/j.febslet.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 36.Horn P, Chapman KD. Lipidomics in situ: insights into plant lipid metabolism from high resolution spatial maps of metabolites. Prog Lipid Res 2014; 54:32-52; PMID:24480404; http://dx.doi.org/ 10.1016/j.plipres.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 37.Hur M, Campbell AA, Almeida-de-Macedo M, Li L, Ransom N, Jose A, Crispin M, Nikolau B, Wurtele ES. A global approach to analysis and interpretation of metabolic data for plant natural product discovery. Nat Prod Rep 2013; 30:565-83; PMID:23447050; http://dx.doi.org/ 10.1039/c3np20111b [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.