ABSTRACT
In tomato, the ovary is covered with a thin, electron-dense and uniform cuticle. The first 10 d after anthesis are critical in the cutinisation of the outer epidermal wall. During this period, singular cytoplasmic domains have been identified in the epidermal cells which seem to be involved in lipid biosynthesis. Moreover, the inner side of the procuticle showed a globular structure with vesicle-like particles of different size that seemed to migrate from the cytoplasm to the procuticle. These electron-dense particles are postulated to play an important role in early cutin synthesis.
KEYWORDS: Cuticle, cutin, tomato fruit, ultrastructure
The plant cuticle is a membrane that covers the outer epidermal cell wall of aerial organs such as leaves, stems, flowers and fruits.1 Its chief component is cutin, an aliphatic matrix that is deposited on and intertwined with polysaccharides derived from the cell wall. Using tomato fruit as a model, significant information on cuticle biochemistry and biophysics together with the identification of several genes involved in its biosynthesis has been gained over the last years; however, the microscopic/ultrastructural scenario of cuticle deposition throughout development has not been analyzed in depth until recently.2 At anthesis, the cuticle of the ovary appeared as a very narrow and well-defined electron-dense layer, whereas a few days later a globular and irregular inner surface can be observed. This thin electron-dense layer can be considered the procuticle, an early cutin layer, and has been interpreted as the result of a progressive arrangement and assembly of lipid material.2,3 This procuticle slowly increased its thickness until the onset of cell expansion, around 9–10 d after anthesis (daa), which coincided with a period of significant lipid deposition4 with an 11-fold increase in cuticle thickness and changes in its mechanical properties.5
Before this sudden change in cuticle appearance, some singular features were observed in both the cuticle and the non-cutinised fraction of the outer cell wall. These features were more abundant during 7–9 daa, but they could be detected during the whole cell division period. Fig. 1 shows Transmission Electron Microscopy (TEM) images of tomato fruit epidermis highlighting some of these characteristics. At these stages, cytoplasmic domains in close contact with the epidermal cell wall can be detected (Figs. 1A-B). In these regions, the plasma membrane appeared very wavy and disorganized. These domains contained globular electron-dense structures of variable size, much like the ones that are detected in the upper part of the cell wall in close proximity with the inner surface of the procuticle (Figs. 1C-D). Similar nanostructures had been previously observed in other species such as Eucalyptus sp.,6 Clivia miniata,3 Utricularia sandersonii3 and Hakea suaveolens7 and have been located on the cell wall region close to the procuticle and on the outer surface of the plasma membrane, suggesting a migration from the epidermal cell to the procuticle. Their content, lipid, protein and/or polysaccharide, as well as their origin is currently unknown although they have been considered in the past as procutin precursors.3,7,8 These globular structures may contain lipid material that, in a subsequent step, would be released to the growing epidermal cell wall acquiring the appearance of small lipid electron-dense globules (Figs. 1C-D). Thus, the irregular shape of the inner side of the procuticle would probably be consequence of the progressive molecular arrangement and assembly of cutin molecular domains.
Figure 1.
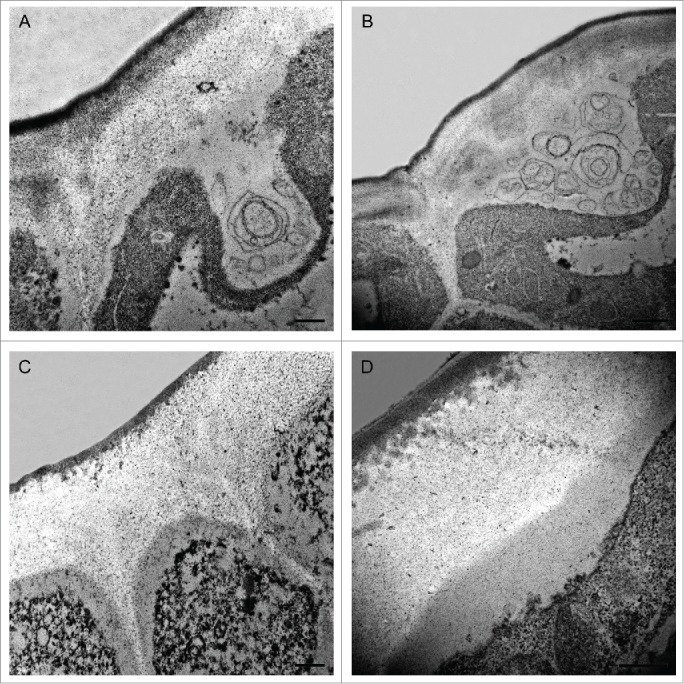
Transmission Electron Microscopy pictures of epidermal cross-sections of ‘Cascada’ fruits at early stages of development. (A,B) 7daa; (C) 8daa; (D) 9daa. Bars (A,C,D) 0.5 μm, (B) 1 μm. daa, days after anthesis.
On the other hand, some membranous systems are observed in these cytoplasmic regions which, at high magnification, seem to contain some granular or fibrillar material (Figs. 1A-B). Special cytoplasmic domains, termed lipotubuloids, have also been observed in Ornithogalum umbellatum ovary epidermis, which are enriched in endoplasmic reticulum, lipid bodies and mitochondria.9 The membranous systems herein observed are different but that lipid biosynthesis has been demonstrated to occur in these lipotubuloids10 and they were postulated as sites of cutin monomer synthesis9 might indicate a common role. In recent years, a role for fatty acids as signals regulating organ growth during the cell division period has been postulated11 and the importance of extracellular lipid composition for signaling suggested.12 Therefore, this massive lipid synthesis and extrusion during early stages of growth could be involved in processes other than cuticle deposition.
Finally, it should be noted that these singular ultrastructures have been observed for first time in tomato ovary epidermis and cuticle. The fact that tomato fruit has a common reticulate and amorphous cuticle very different to the polylaminate cuticle present in other species, where these morphological singularities have been previously described,3 could indicate the existence of a common pathway of early cuticle synthesis. More efforts will be necessary to elucidate the nature and function of these micro and nanostructures. They may have a transient but critical role in cuticle deposition. Perhaps “the devil is in the details.”
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grant AGL2012-32613 from the Plan Nacional de I+D, Ministry of Science and Innovation (MICINN), Spain.
References
- 1.Domínguez E, Heredia-Guerrero JA, Heredia A. Plant cutin genesis: unanswered questions. Trends Plant Sci 2015; 20:551-55; PMID: 26115781; http://dx.doi.org/26668335 10.1016/j.tplants.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 2.Segado P, Domínguez E, Heredia A. Ultrastructure of the epidermal cell wall and cuticle of tomato fruit (Solanum lycopersicum L.) during development. Plant Physiol 2016; 170:935-46; PMID:26668335; http://dx.doi.org/ 10.1104/pp.15.01725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeffree CE. The fine structure of the plant cuticle In Riederer M, Müller C, eds, Biology of the plant cuticle. 2006. Blackwell Publishing, Oxford, UK, pp 11-125 [Google Scholar]
- 4.Domínguez E, López-Casado G, Cuartero J, Heredia A. Development of fruit cuticle in cherry tomato (Solanumlycopersicum). Funct Plant Biol 2008; 35:403-11; http://dx.doi.org/ 10.1071/FP08018 [DOI] [PubMed] [Google Scholar]
- 5.España L, Heredia-Guerrero, Segado P, Benítez JJ, Heredia A, Domínguez E. Biomechanical properties of tomato fruit cuticle during development are modulated by changes in the relative amount of their components. New Phytol 2014; 202: 790-802; PMID:24571168; http://dx.doi.org/24627134 10.1111/nph.12727 [DOI] [PubMed] [Google Scholar]
- 6.Hallam ND. Leaf wax fine structure and ontogeny in Eucalyptus demonstrated by means of a specialized fixation technique. J Microsc 1970; 92:137-44; http://dx.doi.org/ 10.1111/j.1365-2818.1970.tb02245.x [DOI] [Google Scholar]
- 7.Heide-Jørgensen HS. The xeromorphic leaves of Hakea suaveolens R.Br. II. Structure of epidermal cells, cuticle development and ectodesmata. Bot Tidsskr 1978; 72:227-44 [Google Scholar]
- 8.Frey-Wyssling A, Mühlenthaler A. Ultrastructural plant cytology. 1965. Elsevier, Amsterdam, The Netherlands [Google Scholar]
- 9.Kwiatkowska M, Wojtczak A, Popłońska K, Polit JT, Stępiński D, Domίnguez E, Heredia A. Cutinsomes and lipotubuloids appear to participate in cuticle formation in Ornithogalum umbellatum ovary epidermis: EM-immunogold research. Protoplasma 2014; 251:1151-61; PMID:24627134; http://dx.doi.org/ 10.1007/s00709-014-0623-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwiatkowska M. The incorporation of 3H-palmitic acid into Ornithogalum umbellatum lipotubuloids, which are a cytoplasmic domain rich in lipid bodies and microtubules. Light and EM autoradiography. Acta Soc Bot Pol 2004; 73:181-6; http://dx.doi.org/ 10.5586/asbp.2004.024 [DOI] [Google Scholar]
- 11.Anastasiou E, Kenz S, Gerstung M, MacLean D, Timmer J, Fleck C, Lenhard M. Control of plant organ size by KLUH/CYP78A5-dependent intercellular signaling. Dev Cell 2007; 13:843-56; PMID:18061566; http://dx.doi.org/ 10.1016/j.devcel.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 12.Kamata N, Okada H, Komeda Y, Takahashi T. Mutations in epidermis-specific HD-ZIP IV genes affect floral organ identity in Arabidopsis thaliana. Plant J 2013; 75: 430-440; PMID:23590515; http://dx.doi.org/ 10.1111/tpj.12211 [DOI] [PubMed] [Google Scholar]


