Abstract
Salicylic acid (SA), is a plant hormone with multifunction that is involved in plant growth, development and the acquisition of stress tolerance. Hydrogen sulfide (H2S) is emerging similar functions, but crosstalk between SA and H2S in the acquisition of heat tolerance is not clear. Our recent study firstly reported that SA treatment enhanced the activity of L-cysteine desulfhydrase (L-DES), a key enzyme in H2S biosynthesis, followed by induced endogenous H2S accumulation, which in turn improved the heat tolerance of maize seedlings.1 In addition, NaHS, a H2S donor, enhanced SA-induced heat tolerance, while its biosynthesis inhibitor DL-propargylglycine (PAG) and scavenger hydroxylamine (HT) weakened SA-induced heat tolerance. Also, NaHS had no significant effect on SA accumulation and its biosynthesis enzymes phenylalanine ammonia lyase (PAL) and benzoic-acid-2-hydroxylase (BA2H) activities, as well as significant difference was not observed in NaHS-induced heat tolerance of maize seedlings by SA biosynthesis inhibitors paclobutrazol (PAC) and 2-aminoindan-2-phosph- onic acid (AIP) treatment.1 Further study displayed that SA induced osmolytes (proline, betaine and trehalose) accumulation and enhancement in activity of antioxidant system in maize seedlings. These results showed that antioxidant system and osmolyte play a synergistic role in SA and H2S crosstalk-induced heat tolerance of maize seedlings.
Keywords: antioxidant system, heat tolerance, maize seedlings, osmolyte, salicylic acid, signal crosstalk, sodium hydrosulfide
Salicylic acid (SA), a plant hormone with multifunction that is involved in plant growth, development and the acquisition of stress tolerance, is mainly synthesized through the phenylalanine (Phe) route localized in the cytoplasm, phenylalanine ammonia lyase (PAL) and benzoic-acid-2-hydroxylase (BA2H) are key enzymes in SA biosynthesis in plants.2-5 SA has long been considered as an important endogenous immune signal in the induction of disease resistance response in plants.3,6
In addition, SA has also functions in responses to abiotic stress factors such as heavy metal toxicity, salt, drought, chilling and heat.3-4 Spraying potato plants with an acetyl SA can improve the resistance of plants to heat stress in a concentration manner.2 In addition, treatment with SA led to an increase in the level of endogenous H2O2 by reduction in catalase activity, similar to heat hardening at 45°C for 1 h.7 In maize, treatment of seeds with SA not only could markedly enhanced chilling tolerance, but also heat tolerance, and the acquisition of this tolerance is closely relative to enhancement in the activity of antioxidant system.8 Our previous work firstly found that exogenous SA enhanced the activity of L-cysteine desulfhydrase (L-DES), a key enzyme in H2S biosynthesis, followed by induced endogenous H2S accumulation, which in turn improved the heat tolerance of maize seedlings, NaHS (H2S donor) enhanced SA-induced heat tolerance, indicated that H2S might be a downstream signal molecule in SA-induced heat tolerance of maize seedlings,1 but the mechanism of SA and H2S interaction-induced heat tolerance is not completely clear.
Hydrogen sulfide (H2S) has recently been identified as a third endogenous gaseous transmitter after nitric oxide (NO) and reactive oxygen species (ROS).9 In plants, L-cysteine desulfhydrase (L-DES, E.C. 4.4.1.1.) is considered to be a key enzyme in H2S biosynthesis.9,10 Recently, many positive effects of H2S is being emerged in multiple physiological processes, including seed germination, organogenesis, stomata movement, osmotic stress, salt stress, chilling stress, oxidative stress and heavy metal stress.11-17 Our previous research results also showed that NaHS treatment can improve heat tolerance of tobacco cells, maize and wheat seedlings.1,18-24
Enhancement in antioxidant defense system and osmotic adjustment are common mechanisms of plants adapt to adverse environments including heat stress by scavenging reactive oxygen species (ROS); buffering redox; stabilizing protein, nucleic acid and biomembrane; regulating acidity in cytoplasm.25,26 Further study showed that SA and NaHS treatment alone or in combination significantly increased the activity of antioxidant enzymes superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), catalase (CAT, SA treatment alone inhibited CT activity) and guaiacol peroxidase (GPX), and the content of antioxidants ascorbic acid (AsA) and glutathione (GSH), as well as the accumulation of endogenous osmolytes such as proline, betaine and trehalose in maize seedlings (Fig. 1). In addition, these effects induced by SA were enhanced by NaHS treatment (Fig. 1). These data indicated that antioxidant system and osmolyte exert a very important role in SA and H2S interaction-induced heat tolerance of maize seedlings.
Figure 1.
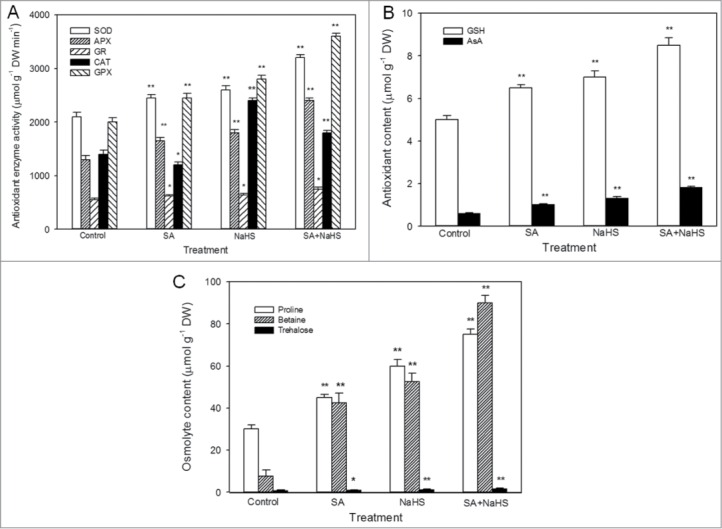
Effects of SA pretreatment on the accumulation of osmolyte (A), antioxidant enzyme activity (B) and antioxidant content (C) in maize seedlings under normal culture conditions. Error bars represent standard error and each data in figure represents the mean ± SE of 3 experiments, and asterisk and double asterisks indicate significant difference (P < 0.05) and very significant difference (P < 0.01) from the control without SA or NaHS treatment, respectively.
In conclusion, pretreatment of maize seedlings with SA significantly stimulated an increase in L-DES activity in maize seedlings, followed by induced endogenous H2S accumulation, which in turn increased the heat tolerance of maize seedlings (Fig. 2).1 In addition, SA-induced heat tolerance was enhanced by NaHS, weakened by PAG and HT (Fig. 2),1 suggesting that crosstalk between SA and H2S exist in the heat tolerance of maize seedlings. Further research showed that SA and H2S interaction induced an increase in the activity of antioxidant system and the accumulation of osmolyte in maize seedlings, implied that antioxidant system and osmolyte play a synergistic role in SA and H2S crosstalk-induced heat tolerance of maize seedlings (Fig. 2). However, plant heat tolerance is involved in crosstalk among Ca2+, H2O2, NO, H2S and plant hormones, which needs to be further investigated in the future.
Figure 2.
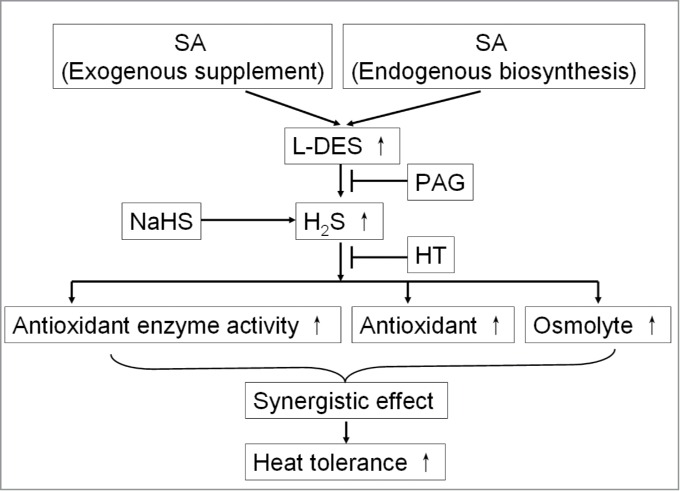
A possible model of crosstalk between SA and H2S in the acquisition of heat tolerance in plants. Arrows (→) indicate positive effects, arrows (↑) represent increase in index, blunt line (├) indicates negative effect. H2S, hydrogen sulfide; HT, hydroxylamine; L-DES, L-cysteine desulfhydrase; PAG, DL-propargylglycine; SA, salicylic acid (adapted from Li et al.1).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We appreciate the reviewers and editors for their exceptionally helpful comments on the article.
Funding
This research is supported by National Natural Science Foundation of China (31360057).
References
- 1.Li Z-G, Xie L-R, Li X-J. Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J Plant Physiol 2015; 177:121-7; PMID:25727780; http://dx.doi.org/ 10.1016/j.jplph.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 2.Szalai G, Horgosi S, Soós V, Majláth I, Balázs E, Janda T. Salicylic acid treatment of pea seeds induces its de novo synthesis. J Plant Physiol 2011; 168:213-9; PMID:20933297; http://dx.doi.org/ 10.1016/j.jplph.2010.07.029 [DOI] [PubMed] [Google Scholar]
- 3.Pál M, Szalai G, Kovács V, Gondor OK, Janda T. Salicylic acid-mediated abiotic stress tolerance In: Salicylic acid. Hayat S, Ahmad A, Alyemeni MN, editors. LLC: Springer Science+Business Media; 2013; pp183-247. [Google Scholar]
- 4.Janda M, Ruelland E. Magical mystery tour: Salicylic acid signaling. Environ Exp Bot 2014; 114:117-28; http://dx.doi.org/ 10.1016/j.envexpbot.2014.07.003 [DOI] [Google Scholar]
- 5.Miura K, Tada Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci 2014; 5:4; PMID:24478784; http://dx.doi.org/ 10.3389/fpls.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar D. Salicylic acid signaling in disease resistance. Plant Sci 2014; 228:127-34; PMID:25438793; http://dx.doi.org/ 10.1016/j.plantsci.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 7.Dat JF, Foyer CH, Scott IM. Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol 1998; 118: 455-61; PMID:9847121; http://dx.doi.org/ 10.1104/pp.118.4.1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du CK, Li ZG, Gong M. The adaptations to heat and chilling stresses and relation to antioxidant enzymes of maize seedlings induced by salicylic acid. Plant Physiol Commun 2005; 41:19-22 [Google Scholar]
- 9.García-Mata C, Lamattina L. Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci 2013; 201/202:66-73; PMID:23352403; http://dx.doi.org/ 10.1016/j.plantsci.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 10.Lisjak M, Teklic T, Wilson ID, Whiteman M, Hancock JT. Hydrogen sulfide: Environmental factor or signalling molecule? Plant Cell Environ 2013; 36:1607-16; PMID:23347018; http://dx.doi.org/ 10.1111/pce.12073 [DOI] [PubMed] [Google Scholar]
- 11.Li ZG, Gong M, Liu P. Hydrogen sulfide is a mediator in H2O2-induced seed germination in Jatropha Curcas. Acta Physiol Plant 2012; 34:2207-13; http://dx.doi.org/ 10.1007/s11738-012-1021-z [DOI] [Google Scholar]
- 12.Fang T, Cao ZY, Li JL, Shen WB, Huang LQ. Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol Biochem 2014; 76:44-51; PMID:24463534; http://dx.doi.org/ 10.1016/j.plaphy.2013.12.024 [DOI] [PubMed] [Google Scholar]
- 13.Lisjak M, Teklić T, Wilson ID, Wood M, Whiteman M, Hancock JT. Hydrogen sulfide effects on stomatal apertures. Plant Signal Behav 2011; 6:1444-6; PMID:21904118; http://dx.doi.org/ 10.4161/psb.6.10.17104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christou A, Manganaris GA, Papadopoulos I, Fotopouls V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot 2013; 64:1953-66; PMID:23567865; http://dx.doi.org/ 10.1093/jxb/ert055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu PN, Wang WJ, Hou LX, Liu X. Hydrogen sulfide is involved in the chilling stress response in Vitis vinifera L. Acta Soc Bot Pol 2013; 82:295-302; http://dx.doi.org/ 10.5586/asbp.2013.031 [DOI] [Google Scholar]
- 16.Zhang H, Hua SL, Zhang ZJ, Hua LY, Jiang CX, Wei ZJ, Liu JA, Wang HL, Jiang ST.. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharv Biol Technol 2011; 60:251-7; http://dx.doi.org/ 10.1016/j.postharvbio.2011.01.006 [DOI] [Google Scholar]
- 17.Chen J, Wang WH, Wu FH, You CY, Liu WT, Dong XK, He JX, Zheng HL.. Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 2013; 362:301-18; http://dx.doi.org/ 10.1007/s11104-012-1275-7 [DOI] [Google Scholar]
- 18.Li Z-G, Gong M, Xie H, Yang L, Li J. Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci 2012; 185/186:185-9; PMID:22325880; http://dx.doi.org/ 10.1016/j.plantsci.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 19.Li Z-G, Ding X-J, Du P-F. Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J Plant Physiol 2013; 170:741-7; PMID:23523123; http://dx.doi.org/ 10.1016/j.jplph.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 20.Li Z-G, Yang S-Z, Long W-B, Yang G-X, Shen Z-Z. Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ 2013; 36:1564-72; PMID:23489239; http://dx.doi.org/ 10.1111/pce.12092 [DOI] [PubMed] [Google Scholar]
- 21.Li Z-G, Luo L-J, Zhu L-P. Involvement of trehalose in hydrogen sulfide donor sodium hydrosulfide-induced the acquisition of heat tolerance in maize (Zea mays L.) seedlings. Bot Stud 2014; 55:20; http://dx.doi.org/ 10.1186/1999-3110-55-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z-G, Yi X-Y, Li Y-T. Effect of pretreatment with hydrogen sulfide donor sodium hydrosulfide on heat tolerance in relation to antioxidant system in maize (Zea mays) seedlings. Biologia 2014; 69:1001-9 [Google Scholar]
- 23.Wu DH, Li YL, Xia X, Pu ZP, Liao JM, Huang K, Li ZG.. Hydrogen sulfide donor sodium hydrosulfide pretreatment improved multiple resistance abilities of wheat to high temperature and drought stress. J Yunnan Normal Univ 2013; 33:29-35 [Google Scholar]
- 24.Li Z-G, Zhu L-J. Hydrogen sulfide donor sodium hydrosulfide-induced accumulation of betaine is involved in the acquisition of heat tolerance in maize seedlings. Braz J Bot 2015; 38(1):31-8; http://dx.doi.org/ 10.1007/s40415-014-0106-x [DOI] [Google Scholar]
- 25.Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 2009; 11:861-905; PMID:19239350; http://dx.doi.org/ 10.1089/ars.2008.2177 [DOI] [PubMed] [Google Scholar]
- 26.Li Z-G, Yuan L-X, Wang Q-L, Ding Z-L, Dong C-Y. Combined action of antioxidant defense system and osmolytes in chilling shock-induced chilling tolerance in Jatropha curcas seedlings. Acta Physiol Plant 2013; 35:2127-36; http://dx.doi.org/ 10.1007/s11738-013-1249-2 [DOI] [Google Scholar]


