Abstract
Systemic acquired resistance (SAR) is a form of broad-spectrum disease resistance that is induced in response to primary infection and that protects uninfected portions of the plant against secondary infections by related or unrelated pathogens. SAR is associated with an increase in chemical signals that operate in a collective manner to confer protection against secondary infections. These include, the phytohormone salicylic acid (SA), glycerol-3-phosphate (G3P), azelaic acid (AzA) and more recently identified signals nitric oxide (NO) and reactive oxygen species (ROS). NO, ROS, AzA and G3P function in the same branch of the SAR pathway, and in parallel to the SA-regulated branch. NO and ROS function upstream of AzA/G3P and different reactive oxygen species functions in an additive manner to mediate chemical cleavage of the C9 double bond on C18 unsaturated fatty acids to generate AzA. The parallel and additive functioning of various chemical signals provides important new insights in the overlapping pathways leading to SAR.
Keywords: free radicals, glycerol-3-phosphate, nitric oxide, plant defense, reactive oxgyen species, systemic acquired resistance
Systemic acquired resistance (SAR) is a highly desirable form of resistance that protects plants against a broad-spectrum of pathogens. SAR involves the generation of signals at the site of primary infection, which enhances the ability of distal uninfected portions of a plant to counter subsequent secondary infections by related or unrelated pathogens. A number of SAR-associated signals or proteins have been identified, especially in the last 5 y (reviewed in1–4). These include, salicylic acid (SA), which is long known to be essential for SAR,5 the methylated derivative of SA (MeSA),6 azelaic acid (C9 dicarboxylic acid, AzA7), and glycerol-3-phosphate (phosphorylated sugar derivative, G3P;8–11 Fig. 1). Recently we showed that the free radicals, nitric oxide (NO) and reactive oxygen species (ROS) are also required for normal SAR11. Although the SAR involvement of these radicals was previously suggested,12,13 we established their absolute requirement by evaluating SAR in genetic mutants that do not accumulate detectable levels of these molecules, and wild type plants treated with exogenous NO donors or ROS. Using a combination of genetic and pharmacological approaches we also established the relationships among many of these structurally unrelated chemical inducers of SAR.10,11
Figure 1.
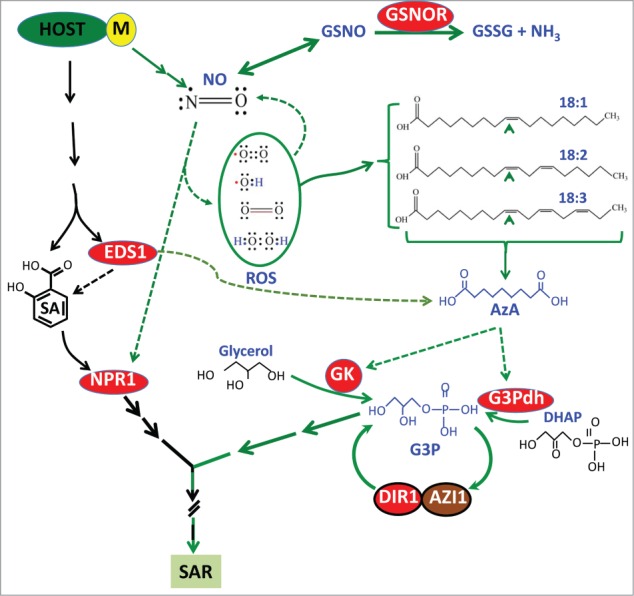
A simplified model illustrating chemical- and protein-based signaling during SAR. Inoculation of avirulent pathogen (designated by M; microbe) leads to the accumulation of salicylic acid (SA) and nitric oxide (NO). Cellular NO levels can be regulated via the storage molecule, GSNO (S-nitrosoglutathione), which can be reduced to GSSG (glutathione disulfide) and NH3 by GSNOR (S-nitrosoglutathione reductase). NO acts upstream of reactive oxygen species (ROS), which comprises of different species including superoxide radicals, singlet oxygen, hydroxyl radicals, and hydrogen peroxide. These act in an additive manner to catalyze the oxidation of C9 double bond on C18 unsaturated fatty acids (FAs). NO and ROS operate in a feedback loop. Oxidation of C18 FAs generates AzA, which triggers the biosynthesis of G3P by upregulating genes encoding the G3P biosynthetic enzymes, glycerol kinase (GK) and G3P dehdrogenase (G3Pdh). G3P and the lipid transfer proteins DIR1 and AZI1 operate in a feedback loop, since azi1 and dir1 mutants cannot accumulate pathogen-responsive G3P and DIR1 and AZI1 proteins do not accumulate in G3P biosynthetic mutants. DIR1 and AZ11 interact with self and each other. The SA and NO/ROS pathways cross-talk at several levels, including the nitrosylation of NPR1/TGA, key positive regulators of SAR. In addition, EDS1, which can act in parallel to SA,20 positively regulates biosynthesis of SA as well as AzA.
In Arabidopsis, there are at least 2 routes for pathogen-responsive NO biosynthesis/accumulation; one via the reduction of nitrate by nitrate reductases (NIA1 and NIA2), and the other via an unknown mechanism by the GTPase, NO associated protein 1 (AtNOA1).14–17 Notably, only the noa1 nia1 or noa1 nia2 double mutants are fully compromised in pathogen-responsive NO accumulation and SAR. This suggests that the NOA1 and NIA activities are partially redundant for SAR-associated NO accumulation and that the 2 NIA isoforms are functionally non-redudant because SAR is lost in both noa1 nia1 and noa1 nia2 plants.11 The constitutive NO biosynthesis, and thereby the constitutive defense signaling in the ssi2 (Suppressor of SA Insensitivity) mutant plants can also be shut down by the noa1 nia1/2 double mutations.14 Like the ssi2 mutation, mutations in NOX1 (NO overproducing) or GSNOR1 (S-Nitrosoglutathione Reductase) also result in constitutive NO accumulation.11 This correlates well with the increased NOA1 protein levels in the nox1 and gsnor1 mutant plants (Fig. 2A). Surprisingly, unlike ssi2, which shows constitutive resistance against virulent bacterial pathogen,18-21 both nox1 and gsnor1 show compromised resistance (Fig. 2B).22,23 These differences could be because ssi2, nox1 and gsnor1 plants either accumulate different levels of NO, or accumulate NO in different subcompartments that produces a different effect. As in vertebrates,24 the signaling role of NO and ROS in plant SAR is concentration dependent. Thus, excessive or too little accumulation of NO and ROS, both equally abrogate SAR. For example, mutations in GSNOR1 or NOX1 result in constitutive elevation of NO levels, and both gsnor1 and nox1 plants are compromised in SAR.11 Likewise, localized applications of high concentrations of NO or ROS are ineffective in inducing SAR. A previous report of enhanced SAR in GSNOR1-silenced plants could be attributable to the partial reduction (versus complete loss in gsnor1 mutant) of GSNOR1 activity in these plants that allows NO accumulation at levels that are biologically stimulatory for SAR.25
Figure 2.
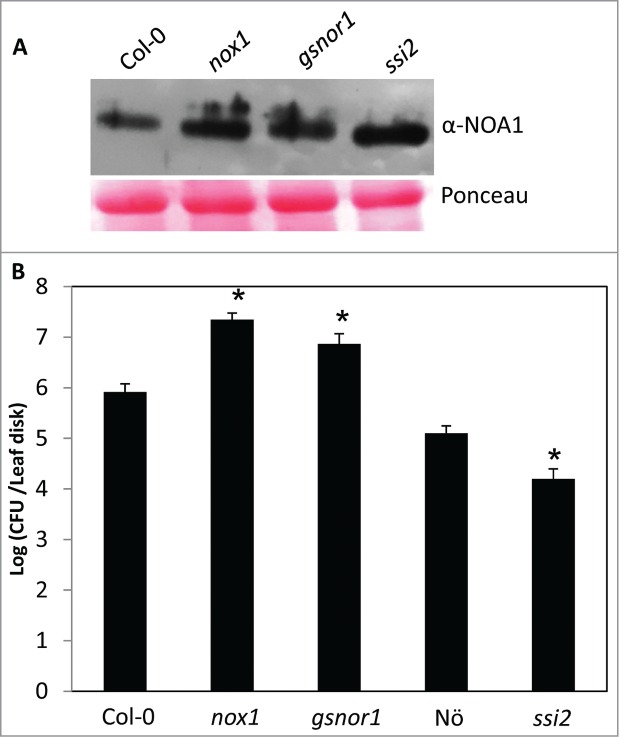
NOA1 levels and pathogen resistance in ssi2, gsnor and nox1 plants. (A) Protein immunoblot showing NOA1 levels in indicated genotypes. Ponceau-S staining of the immunoblot was used as the loading control. (B) Growth of virulent Pseudomonas syringae DC3000 bacteria on indicated genotypes. The error bars indicate SD (n = 4). Asterisks indicate data statistically significant from wild-type (Columbia, Col-0; Nössen, Nö P<0.003). The experiment was repeated twice with similar results.
The ROS radicals clearly function downstream of NO in SAR since NO application did not confer SAR in the RBOH (Respiratory Burst Oxidase Homologs) mutants, rbohD and rbohF26 These mutants are unable to accumulate superoxide radicals in response to pathogen infection.11,27 On the other hand, ROS was able to restore SAR in the NO deficient noa1 nia2 plants. The rbohD and rbohF mutants also showed reduced accumulation of NO, suggesting that superoxide radicals are critical for pathogen-induced NO accumulation. ROS also appears to be required for the hydrolysis of C18 unsaturated fatty acids (FAs), that generates the SAR inducer AzA. All three C18 unstaturated FAs oleic acid (18:1), linoleic acid (18:2), and linolenic acid (18:3), contain a double bond between C9 and C10, the hydrolysis of which can generate the C9 dicarboxylic acid AzA via its immediate precursor 9-oxononanoic acid ONA (monocarboxylic acid). This is consistent with the fact that exogenous application of either 18:1 or 18:2 can induce AzA biosynthesis and confer SAR.7 The FAs that serve as precursors for AzA are in turn obtained from galactolipids, which constitute ∼80% of total plant lipids and are primarily present in the plastids10,28,29 Intriguingly, AzA likely quickly complexes with other lipids since only a small portion of exogenous AzA remains in its free form.10,29 The identity and SAR-associated functions of AzA-associated lipids remain unknown.
AzA confers SAR by inducing the biosynthesis of G3P.10 Consequently, plants defective in the G3P synthesizing (encode G3P dehydrogenase, G3Pdh30 or glycerol kinase, GK31) enzymes, are AzA insensitive.10 Likewise, both mutants are unable to induce wild type-like levels of G3P and exogenous application of G3P is able to restore SAR in these mutants.10 Thus, the NO→ROS→AzA→G3P pathway likely forms one important branch of SAR, with C18 Δ9 FAs on galactolipids playing an important role in generating AzA.10,11,29 The NO→G3P pathway appears to be distinct from the SA pathway since exogenous SA was unable to restore SAR in mutants defective in NO, ROS, or G3P biosynthesis. Conversely, NO/ROS were unable to confer SAR on mutants unable to synthesize SA or mediate SA signaling.11 Consistent with these results, NO and SA deficient mutants accumulated normal levels of SA and NO, respectively.11 However, several studies suggest crosstalk between the SA and NO→G3P branches of SAR, which could fine-balance the induction of this intricate form of immunity. For example, gsnor1 plants accumulate elevated levels of NO, but significantly reduced SA levels in response to pathogen,22 NO S-nitrosylates NPR1 and TGA1, 2 key components of the SA pathway,32,33 and more recently another important component of the SA pathway, EDS1 (Enhanced Disease Susceptibility 1) was shown to regulate the biosynthesis of ONA/AzA.34 The parallel signaling of the NO→G3P and SA pathways provide multiple points of co-regulation of these pathways, which likely facilitates a more optimal regulatory control of systemic immunity.
Experimental Procedures
Plant growth conditions and pathogen inoculation
Plants were grown in MTPS 144 Conviron (Winnipeg, MB, Canada) walk-in-chambers at 22°C, 65% relative humidity and 14 hour photoperiod. The photon flux density of the day period was 106.9 μmoles m−2 s−1 and was measured using a digital light meter (Phytotronic Inc., Earth city, MO). The ssi2, gsnor1 and nox1 plants used in this study are described earlier.11,18–21,35 For bacterial inoculations with Pseudomonas syringae DC 3000, the bacterial cultures were grown overnight in King's B medium containing rifampicin and/or kanamycin. The cells were washed and suspended in 10 mM MgCl2. The bacterial suspension (105 CFU ml−1) was injected into the abaxial surface of the leaf using a needle-less syringe. Three discs from the inoculated leaves were collected at 0 and 3 d post inoculation and homogenized in 10 mM MgCl2. The extract was diluted and appropriate dilutions were plated on King's B medium.
Protein extraction and Immunoblot analysis
Proteins were extracted in buffer containing 50 mM Tris-HCl, pH7.5, 10% glycerol, 150 mM NaCl, 10mM MgCl2, 5 mM EDTA, 5 mM DTT, and 1 X protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Protein concentration was measured by the Bio-RAD protein assay (Bio-Rad, CA). For Ponceau-S staining, PVDF membranes were incubated in Ponceau-S solution (40% methanol (v/v), 15% acetic acid (v/v), 0.25% Ponceau-S). The membranes were destained using deionized water. Proteins (30–50 μg) were fractionated on a 10% SDS-PAGE gel and subjected to immunoblot analysis using α-NOA1 antibody. Immunoblots were developed using ECL detection kit (Roche).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Work in AK and PK laboratories is funded by the National Science Foundation (MCB#0421914, IOS#051909) and Kentucky Soybean Board.
References
- 1. Wendehenne D, Gao Q-M, Kachroo A, Kachroo P. Free radical-mediated systemic immunity in plants. Curr Opin Plant Biol 2014; 20:127-34; PMID:24929297; http://dx.doi.org/ 10.1016/j.pbi.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 2. Shah J, Zeier J. Long-distance communication and signal amplification in systemic acquired resistance. Front Plant Sci 2013; 4:30; PMID:23440336; http://dx.doi.org/ 10.3389/fpls.2013.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kachroo A, Robin GP. Systemic signaling during plant defense. Curr Opin Plant Biol 2013; 16:527-33; PMID:23870750; http://dx.doi.org/ 10.1016/j.pbi.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 4. Gao Q-M, Kachroo A, Kachroo P. Chemical inducers of systemic immunity in plants. J Exp Bot 2014; 65:1849-55; PMID:24591049; http://dx.doi.org/ 10.1093/jxb/eru010 [DOI] [PubMed] [Google Scholar]
- 5. Gaffney T, Friedrich L, Vernooij B, Negmtto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 1993; 261:754-6; PMID:17757215; http://dx.doi.org/ 10.1126/science.261.5122.754 [DOI] [PubMed] [Google Scholar]
- 6. Park S-W, Kaimoyo E, Kumar D, Mosher S, Klessig D. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 2007; 318:113-6; PMID:17916738; http://dx.doi.org/ 10.1126/science.1147113 [DOI] [PubMed] [Google Scholar]
- 7. Jung HW, Tschaplinkski TJ, Wang L, Glazebrook J, Greenberg JT. Priming in systemic plant immunity. Science 2009; 324:89-91; PMID:19342588; http://dx.doi.org/ 10.1126/science.1170025 [DOI] [PubMed] [Google Scholar]
- 8. Chanda B, Xia Y, Mandal M, Yu K, Sekine K, Gao Q-M, Selote D, Hu Y, Stromberg A, Navarre D., et al. (2011) Glycerol-3-phosphate, a critical mobile inducer of systemic immunity in plants. Nature Genetics 2011; 43:421-7; PMID:21441932; http://dx.doi.org/ 10.1038/ng.798 [DOI] [PubMed] [Google Scholar]
- 9. Mandal M, Chanda B, Xia Y, Yu K, Sekine K, Gao Q-M, Selote D, Kachroo A, Kachroo P. Glycerol-3-phosphate and systemic immunity. Plant Signal Behavior 2011; 6:1871-4; PMID:22067992; http://dx.doi.org/ 10.4161/psb.6.11.17901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu K, Soares JM, Mandal MK, Wang C, Chanda B, Gifford AN, Fowler JS, Navarre D, Kachroo A, Kachroo P. A feedback regulatory loop between G3P and lipid transfer proteins DIR1 and AZI1 mediates azelaic-acid-induced systemic immunity. Cell Reports 2013; 3:1266-78; PMID:23602565; http://dx.doi.org/ 10.1016/j.celrep.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 11. Wang C, El-Shetehy M, Shine MB, Yu K, Navarre D, Wendehenne D, Kachroo A, Kachroo P. Free radicals mediate systemic acquired resistance. Cell Reports 2014; 7:348-55; PMID:24726369; http://dx.doi.org/ 10.1016/j.celrep.2014.03.032 [DOI] [PubMed] [Google Scholar]
- 12. Alvarez ME, Pennell RI, Meijer P-J, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen Intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 1998; 92:773-84; PMID:9529253; http://dx.doi.org/ 10.1016/S0092-8674(00)81405-1 [DOI] [PubMed] [Google Scholar]
- 13. Song F, Goodman RM. Activity of nitric oxide is dependent on, but is partially required for function of, salicylic acid in the signaling pathway in tobacco systemic acquired resistance. Mol Plant-Microbe Interact 2001; 14:1458-62; PMID:11768542; http://dx.doi.org/ 10.1094/MPMI.2001.14.12.1458 [DOI] [PubMed] [Google Scholar]
- 14. Mandal MK, Chandra-Shekara AC, Jeong R-D, Yu K, Zhu S, Chanda B, Navarre D, Kachroo A, Kachroo P. Oleic acid-dependent modulation of NITRIC OXIDE ASSOCIATED 1 protein levels regulates nitric oxide-mediated defense signaling in arabidopsis. Plant Cell 2012; 24:1654-74; PMID:22492810; http://dx.doi.org/ 10.1105/tpc.112.096768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moreau M, Lee GI, Wang Y, Crane BR, Klessig DF. AtNOS/AtNOA1 is a functional arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J Biol Chem 2008; 283:32957-67; PMID:18801746; http://dx.doi.org/ 10.1074/jbc.M804838200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo FQ, Okamoto M, Crawford NM. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 2003; 302:100-3; PMID:14526079; http://dx.doi.org/ 10.1126/science.1086770 [DOI] [PubMed] [Google Scholar]
- 17. Crawford NM. Mechanism of nitric oxide synthesis in plants. J Exp Bot 2006; 57:471-8; PMID:16356941; http://dx.doi.org/ 10.1093/jxb/erj050 [DOI] [PubMed] [Google Scholar]
- 18. Kachroo P, Venugopal SC, Navarre DA, Lapchyk L, Kachroo A. Role of salicylic acid and fatty acid desaturation pathways in ssi2-mediated signaling. Plant Physiol 2005; 139:1717-35; PMID:16306139; http://dx.doi.org/ 10.1104/pp.105.071662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kachroo A, Lapchyk L, Fukushigae H, Hildebrand D, Klessig D, Kachroo P. Plastidial fatty acid signaling modulates salicylic acid- and jasmonic acid-mediated defense pathways in the arabidopsis ssi2 mutant. Plant Cell 2003; 12:2952-65; PMID:14615603; http://dx.doi.org/ 10.1105/tpc.017301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Venugopal SC, Jeong R-D, Mandal M, Zhu S, Chandra-Shekara AC, Xia Y, Hersh M, Stromberg AJ, Navarre D, Kachroo A., et al. ENHANCED DISEASE SUSCEPTIBILITY 1 and SALICYLIC ACID act redundantly to regulate resistance gene expression and low oleate-induced defense signaling. PLOS Genet 2009; 5:e1000545; PMID:19578402; http://dx.doi.org/ 10.1371/journal.pgen.1000545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xia Y, Gao Q-M, Yu K, Lapchyk L, Navarre D, Hildebrand D, Kachroo A, Kachroo P. An intact cuticle in distal tissues is essential for the induction of systemic acquired resistance in plants. Cell Host Microbe 2009; 5:151-65; PMID:19218086; http://dx.doi.org/ 10.1016/j.chom.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 22. Feechan A, Kwon E, Yun B-W, Wang Y, Pallas JA, Loake GJ. A central role for S-nitrosothiols in plant disease resistance. Proc Natl Acad Sci USA 2005; 102: 8054-9; PMID:15911759; http://dx.doi.org/ 10.1073/pnas.0501456102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kneeshaw S, Gelineau S, Tada Y, Loake GJ, Spoel SH. Selective protein denitrosylation activity of thioredoxin-h5 modulates plant immunity. Mol Cell 2014; 56:153-62; PMID:25201412; http://dx.doi.org/ 10.1016/j.molcel.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 24. Wink DA, Hines HB, Cheng RYS, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA: Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol 2011; 89: 873-91; PMID:21233414; http://dx.doi.org/ 10.1189/jlb.1010550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rustérucci C, Espunya MC, Díaz M. Chabannes M, Martínez MC. S-nitrosoglutathione reductase affords protection against pathogens in arabidopsis, both locally and systemically. Plant Physiol 2001; 143:1282-92; PMID:17277089; http://dx.doi.org/ 10.1104/pp.106.091686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sagi M, Fluhr R. (2006). Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 2006; 141:336-40; PMID:16760484; http://dx.doi.org/ 10.1104/pp.106.078089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Torres MA, Dangl JL, Jones JDG. Arabidopsis gp91phox homo- logues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 2002; 99:517-22; PMID:11756663; http://dx.doi.org/ 10.1073/pnas.012452499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zoeller M, Stingl N, Krischke M, Fekete A, Waller S-B, Mueller MJ. Lipid profiling of the arabidopsis hypersensitive response reveals specific lipid peroxidation and fragmentation processes: biogenesis of pimelic and azelaic acid. Plant Physiol 2012; 160:365-8; PMID:22822212; http://dx.doi.org/ 10.1104/pp.112.202846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao Q-M, Yu K, Xia Y, Shine MB, Navarre D, Kachroo A, Kachroo P. Mono- and di-galactosyldiacylglycerol function non-redundantly to regulate systemic required resistance in plants. Cell Rep 2014; 9:1681–91; PMID:25466253 [DOI] [PubMed] [Google Scholar]
- 30. Kachroo A, Venugopal SC, Lapchyk L, Falcone D, Hildebrand D, Kachroo P. Oleic acid levels regulated by glycerolipid metabolism modulate defense gene expression in arabidopsis. Proc Natl Acad Sci USA 2004; 101:5152-7; PMID:15044700; http://dx.doi.org/ 10.1073/pnas.0401315101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang L, Li J, Zhao T, Xiao F, Tang X, Thilmony R, He SY, Zhou J-M. Interplay of the arabidopsis nonhost resistance gene NHO1 with bacterial virulence. Proc Natl Acad Sci USA 2003; 100:3915-24; PMID:12626746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindermayr C, Sell S, Müller B, Leister D, Durner J. Redox regulation of the NPR1-TGA1 system of arabidopsis thaliana by nitric oxide. Plant Cell 2010; 22:2894-907; PMID:20716698; http://dx.doi.org/ 10.1105/tpc.109.066464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, Zuo J, Dong X. Plant immunity requires conformational changes of NPR1 via S-nitrosylation of NPR1. Science 2008; 321:952-6; PMID:18635760; http://dx.doi.org/ 10.1126/science.1156970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wittek F, Hoffmann T, Kanawati B, Bichlmeier M, Knappe C, Wenig M, Schmitt-Kopplin P, Parker JE, Schwab W, Vlot AC. Arabidopsis ENHANCED DISEASE SUSCEPTIBILITY 1 promotes systemic acquired resistance via azelaic acid and its precursor 9-oxo-nonanoic acid. J Exp Bot 65:5919-31; PMID:25114016; http://dx.doi.org/ 10.1093/jxb/eru331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. He Y, Tang R-H, Stevens RD, Cook CW, Ahn SM, Jing L, Yang Z, Chen L, Guo F, Fiorani F., et al. Nitric oxide represses the arabidopsis floral transition. Science 2004; 305:1968-71; PMID:15448272; http://dx.doi.org/ 10.1126/science.1098837 [DOI] [PubMed] [Google Scholar]


