Abstract
Melatonin and serotonin are indoleamines first identified as neurotransmitters in vertebrates; they have now been found to be ubiquitously present across all forms of life. Both melatonin and serotonin were discovered in plants several years after their discovery in mammals, but their presence has now been confirmed in almost all plant families. The mechanisms of action of melatonin and serotonin are still poorly defined. Melatonin and serotonin possess important roles in plant growth and development, including functions in chronoregulation and modulation of reproductive development, control of root and shoot organogenesis, maintenance of plant tissues, delay of senescence, and responses to biotic and abiotic stresses. This review focuses on the roles of melatonin and serotonin as a novel class of plant growth regulators. Their roles in reproductive and vegetative plant growth will be examined including an overview of current hypotheses and knowledge regarding their mechanisms of action in specific responses.
Keywords: development, indoleamine, melatonin, mechanism of action, organogenesis, plant growth regulator, phytohormone, reproductive growth, serotonin, vegetative growth
Abbreviations
- 5HT
5-hydroxytryptamine
- AADC
aromatic L-amino acid decarboxylase
- AFMK
N1-acetyl-N2-formyl-5-methoxykynuramine
- ASMT
acetyl serotonin methyltransferase
- AXR3
indole-3-acetic acid inducible 17
- CBF
C-repeat-binding factor
- COMT
caffeic acid-O-methyltransferase
- DREB1
drought response element binding 1 factor
- p-CPA
p-chlorophenylalanine
- HIOMT
hydroxyindole-O-methyltransferase
- MEL
N-acetyl-5-methoxytryptamine
- 5-MIAA
5-methoxyindole -3-acetic acid
- 5-ML
5-methoxytryptophol
- 5-MT
5-methoxytryptamine
- PCIB
p-chlorophenoxy isobutyric acid
- PGR
plant growth regulator
- ROS
reactive oxygen species
- SAG12
senescence associated gene 12
- SEN4
senescence 4
- SNAT
serotonin N-acetyltransferase
- T-5-H
tryptamine-5-hydroxylase
- TDC
tryptophan decarboxylase
- TIBA
triiodobenzoic acid
- TPH
tryptophan hydroxylase.
Introduction
The indoleamines melatonin (MEL; N-acetyl-5-methoxytryptamine) and serotonin (5HT; 5-hydroxytryptamine) are ancient chemicals that were presumably part of the life cycles of the first prokaryotic life forms on Earth millions of years ago where they functioned as powerful antioxidants to combat the increasingly oxygen rich atmosphere.1,2 MEL, in particular, is an extremely powerful reducing agent capable of potentially detoxifying 10 reactive oxygen species for every unit of MEL.1,3-6 Modern complex organisms including plants and animals have finely tuned and regulated systems for sustaining life in which these compounds play pivotal roles.2,7-14 This review examines the functions of 5HT and MEL on vegetative and reproductive growth patterns in plants including interactions between indoleamines and other known plant growth regulators.
Discovery
Both 5HT and MEL evolved in prokaryotes but were first discovered in mammals. MEL was discovered in 1958 and the structure elucidated the same year.15,16 Likewise, 5HT was discovered and identified in animals in the 1930s.3,6,15-17 In animals MEL is a hormone produced by the pineal gland, while 5HT is commonly produced throughout the nervous system,18-20 though extra-pineal locations of biosynthesis have now been established.20-23 MEL plays important roles as a signal for darkness involved in sleep and regulation of circadian rhythms while 5HT is a critical signaling molecule in the nervous system of animals.2,12
In plants, 5HT was first discovered in the medicinal herb cowhage (Mucuna pruriens) in 195417,24; MEL was not identified in plants until 1995.25,26 These initial reports focused on the presence of 5HT and MEL in food and medicinal crops, and in particular, the effect of the presence of these compounds may have after human consumption.24-26 MEL has now been detected in almost all plant families and in some cases at very high concentrations,27,28 while 5HT has been analyzed in fewer but a still significant numbers of plant families.9,29,30 Despite their widespread presence, the functional significance of MEL and 5HT in the life of a plant is less clearly defined.
Biosynthesis
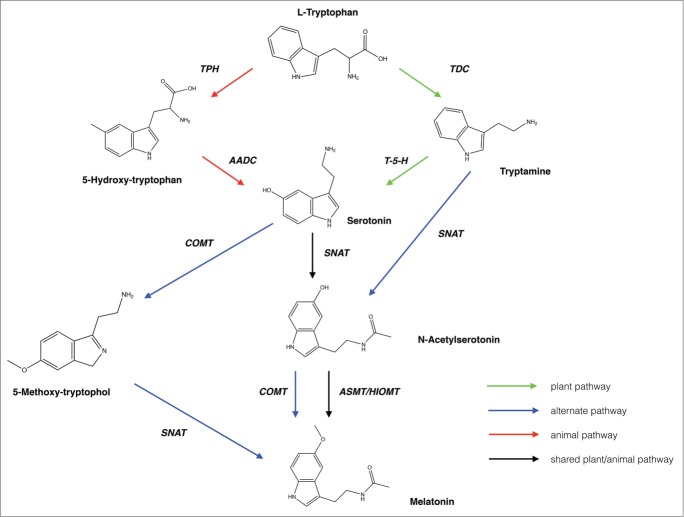
In plants, as in animals, MEL and 5HT are produced from the precursor amino acid tryptophan via two primary pathways, though several potential minor deviations from these pathways have been reported (Fig. 1).31,32 Production of 5HT proceeds via the production of 5-hydroxy-tryptophan by tryptophan hydroxylase (TPH) in animals and it is then converted to 5HT by aromatic acid decarboxylase (AADC).30 In plants 5HT is produced via tryptamine which is catalytically produced by tryptophan decarboxylase (TDC) and is later hydroxylated to form 5HT by tryptamine-5-hydroxylase.33,34 At this point the animal and plant pathways merge such that N-acetyl-serotonin is produced by serotonin N-acetyltransferase (SNAT) which is then converted to MEL by acetyl-serotonin methyltransferase (ASMT) also known as hydroxyindole-O-methyltransferase (HIOMT) in both plants and animals.35-38 TDC in plants and SNAT in animals are generally considered to be the rate limiting steps in these pathways. For example transgenic studies in rice have found that up-regulation of TDC can increase endogenous 5HT and MEL concentrations; however limited literature has also suggested that ASMT may be a more important in determining the amount of melatonin produced in some plant species.34,39,40 Recently, a caffeic acid O-methyltransferase (COMT) was found to convert N-acetylserotonin to MEL in plants32,37,41 This suggests that alternate routes and enzymes may be involved in this process. Additionally, SNAT has been shown to directly metabolize tryptamine to N-acetylserotonin though these alternate routes are generally considered to be more minor players in melatonin synthesis. These may be more important in plant species in which sequences with significant homologies to the known SNAT enzymes are not present.32 In contrast to the well-defined biosynthetic pathways for MEL and 5HT, the catabolic routes of MEL and 5HT are poorly defined in plants. The information from dinoflagellates suggests that they can be degraded via hydroxylation, dealkylation, oxidative pyrrole ring cleavage and deacetylation reactions to breakdown products including 5-methoxytryptamine (5-MT), 5-methoxytryptophol (5-ML), 5-methoxyindole-3-acetic acid (5-MIAA) and N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK); however, these have yet to be demonstrated in higher plants.42-44 Additionally, though much research has been done to investigate the presence and activity of MEL in plants, new analytical technologies have led to the identification of many possible isomers of MEL, which have not been studied in depth.45
Physiological roles
A significant amount of literature has been published related to the activity of MEL in plants, with many fewer investigations examining the role of 5HT. MEL is an important compound in the protection of plants against stresses which were recently reviewed by several authors42,46,47 and include biotic stresses such as pathogen attack and diverse abiotic stresses including drought, freezing, osmotic, heat, heavy metal, UV and oxidative stresses; 5HT has some similar actions in some plant species.42,47-64 Recently, the application of 5HT was shown to result in significant reduction in browning of fruit tissues.65 Perhaps more intriguing is the implication of these compounds as a novel class of plant growth regulators with an increasing body of literature suggesting that the balance between 5HT and MEL may be homologous to that seen between cytokinins and auxins, whose synergistic/antagonistic relationships are widely exploited in tissue culture for determining the direction of desired morphological patterning (Fig. 3).66,67
Regulation of plant growth
In plants there are several universally accepted classes of plant growth regulators (PGRs) which are considered essential to plant growth, development, communication and responses to environmental conditions. These PGRs include: auxins, cytokinins, gibberellins, ethylene, jasmonic acid, brassinosteroids, salicylic and abscisic acid.68-75 Of these, auxins and cytokinins are considered to be the most powerful in determining the outcome of morphological patterns with auxin gradients serving essential functions in maintaining apical dominance, cell elongation, and promotion of rooting among other functions; the cytokinins act to promote cell division and play a major roles in organ development, germination, senescence and maintenance of circadian rhythms.76-78 Complementary and (or) antagonistic responses between the 2 are critical in determining the final growth pattern. For example, during embryo development the inhibition of cytokinin signaling leads to establishment of embryonic root stem cells, and in mature plants disruption of the auxin gradient in roots by cytokinins leads to lateral root formation.79-84 Recent literature, however, suggests that this traditional view of PGRs may be incomplete as there is significant cross talk between these pathways and many classes of compounds have now been found to further modulate these responses and a rise or fall in these compounds can have varying but often significant effects on the growth pattern.68,70,74 Antioxidants such as ascorbic acid, flavonoids, polyamines and brassinosteroids also have been implicated in the regulation of plant growth.75,85-87 The intricacy and interconnectedness of plant growth signaling pathways seem to have increased based on recent findings, leading to a higher number of unknowns regarding their functions. This suggests that perhaps the current perceptions of the core importance of the auxin/cytokinin balance needs to be revisited, with a new concept of a dynamic and complex web of interactions taking its place. This may require new balancing acts working in symphony to produce the diverse plant growth patterns observed in nature. Of these plant growth regulators, perhaps the class of compounds with some of the most intriguing effects are the indoleamines, MEL and 5HT.67,85-87
Melatonin and root organogenesis
The relationship between MEL and regulation of plant growth has been most consistently described in model systems of rooting. One of the first reports of this activity was published by Murch et al. (88), who found that application of the auxin action and transport inhibitors p-chlorophenoxyisobutyric acid (PCIB) and 2,3,5-triiodobenzoic acid (TIBA), respectively, led to a reduction in MEL content of St. John's wort (Hypericum perforatum) explants. This was simultaneously associated with a decrease in survival and rooting which was not completely recovered by addition of exogenous auxin. Since then the root promoting effect of MEL has been reported in numerous species including Brassica juncea, Arabidopsis thaliana, Lupinus albus, Cucumis sativus, Oryza sativa and Prunus species among others (Table 1).48,62,63,89-94 The root promoting effect of MEL appears to be strongest in promoting rooting structures other than primary roots including reports of enhanced lateral and adventitious rooting being the most common. In particular, the production of lateral roots is an area of strong interest where interactions between diverse PGRs have been implicated.63,70,90,92,94 In some cases MEL displays a concurrent inhibition of primary root production or elongation.89,93,95 Equally prominent appears to be the dose dependent manner in which this occurs; low levels of MEL increase plant root growth with increasing concentrations until a maximal response is achieved after which the highest levels of MEL become inhibitory. While this maximal concentration varies greatly across plant species with 10 µM being inhibitory in some species such as cherry rootstocks (Prunus avium x Prunus cerasus) and concentrations as high as 500 µM maintaining a promoting effect in others.91,92 In part, these concentration ranges are also impacted by the mode of application. In cases where MEL is administered in a greenhouse during watering, the same exposure is ineffective in plants grown in tissue culture where MEL is included in the culture medium; this accounts for some of this significant variability. The stability of MEL after light exposure is also a significant concern, which is rarely addressed. Plants supplemented with MEL in clear culture boxes are likely to be exposed to a lower overall concentration of MEL due to light-mediated degradation than in plants exposed in black plastic pots. Without the analysis of MEL in cultures post-exposure it is hard to confirm exposure concentrations. Another possible explanation for the wide range of results is the phenomenon of gating as observed in Arabidopsis for IAA activity.96 This effect may lead to responses only being observed if exogenous MEL is applied at particular times of day or at specific phases in the circadian rhythm or season as was observed by Kolar et al.97 Additionally, the potential for growth regulating activity to be the result of MEL catabolites has been proposed.98 In one such study on etiolated cotyledons of Lupinus albus, the effect of a weak auxin, 5-methoxyindole-3-acetic, a MEL catabolite identified in animals, was tested and found not to be active; this suggests that the activity of MEL was not due to its homology to auxin, but instead was a result of MEL-specific responses.98 It is also possible that degradation of MEL is irrelevant and the plant response is triggered immediately after exposure where the variations in plant responses are due to normal endogenous levels of indoleamines.
Table 2.
Effects of melatonin and serotonin on reproductive growth.
| Effect | Concentration range | Species reported | Mechanism of action/interaction with known pathways | Reference(s) |
|---|---|---|---|---|
| Melatonin | ||||
| Protection of reproductive tissues during development | Datura metel, Malus domestica, edible seed plants, Prunus avium | Antioxidant activity | 36,91,92,130,131 | |
| Modification of flowering time | 100 - 500 uM | Chenopodium rubrum, Oryza sativa | Interaction with calcium/calmodulin signaling | 97,125,126 |
| Promotion post-harvest ripening | Solanum lycopersicum | Interaction with ethylene signaling pathways | 142 | |
| Promotion of seed growth and germination | 1 uM | Glycine max, Cucumbis sativus, Zea mays, Vigna radiata, Brassica oleracea rubrum, Phacellia tanacetifolia | Interaction with gibberellin and absicic acid systems, antioxidant, modification of calcium signaling pathways related genes | 53,55,62,63,105,127-129 |
| Decreased grain yield | Oryza sativa | 126 | ||
| Regulation reproductive development | Oryza sativa, Hypericum perforatum | Modification of related gene expression patterns including, modification of calcium distribution and signaling cascades | 67,127,139 | |
| Serotonin | ||||
| Protection of reproductive tissues during development | Datura metel, Hypericum perforatum | Antioxidant activity | 67,131 | |
| Stimulation pollen germination | Hippeastrum hybridum, Hypericum perforatum | Calcium distribution and signaling | 67,140 |
Table 1.
Effects of melatonin and serotonin on vegetative growth.
| Effect | Concentration range | Species | Proposed mechanism of action | Reference |
|---|---|---|---|---|
| Melatonin | ||||
| Delayed leaf senescence | 20 µM – 10 mM | Malus sp., Arabidopsis thaliana, Hordeum vulgare, Oryza sativa | Antioxidant activity, inhibition chlorophyll degradation, upregulation ascorbic acid and glutathione pathways, down-regulation of senescence related genes, modification of photosynthesis and sugar metabolism, modification of nitrogen metabolism, decreased protein degradation | 58,106-109,121,127 |
| Increased number of lateral roots | 50 – 500 uM | Arabidopsis thaliana, Lupinus albus, Cucumis sativus | Modification of expression levels of transcription factors, cell wall and peroxidase-related genes | 62,63,90,94,156 |
| Increased root elongation | 0.05 – 100 uM | Virginia radiata, Prunus sp, Lupinus albus, rice, Arabidopsis thaliana, Helianthus annus | Antioxidant activity, increased carbohydrate concentration, interaction with calcium/calmodulin signaling cascades | 48,52,90-93,158 |
| Increased root number | 0.1 – 200 uM | Brassica juncea, Hypericum perforatum, Prunus rootstocks, Punica granatum | Interaction with 5HT and auxin | 8,31,88,89,91,92,159 |
| Increased incidence somatic embryogenesis | 100 uM | Coffea canephora | Interaction with calcium signaling cascades | 102 |
| Enhanced microspore development | Hypericum perforatum | Modification of calcium distribution | 8 | |
| Increased plant biomass | 0.05 – 10 µM | Prunus avium x Prunus cerasus, Oryza sativa, Arabidopsis thaliana | Enhancement of carbohydrate and proline metabolism | 48,91,93,160 |
| Increased number adventitious roots | 0.5–100 µM | Lupinus albus, Oryza sativa, Prunus rootstocks | 90,92,93,161 | |
| Increased seedling growth | 10 µM | Oryza sativa, Lupinus albus, Brassica olereacea rubrum, Zea mays | Modification of calicium/calmodulin signaling pathways, enhancement of sugar metabolism, biosynthesis, hydrolysis and transport, enhanced photosynthesis and starch catabolism. | 53,95,98,101,115,126 |
| Inhibition of seedling growth | 100 µM | Zea mays | Inhibition of sugar metabolism, starch accumulation, reduced sucrose biosynthesis, hydrolysis and transport | 101 |
| Increased shoot production | 5.71 µM – 1.16 mg/L | Punica granatum, Vaccinium corymbosum, Mimosa pudica | Interaction with calcium signaling pathways | 114,159,162 |
| Inhibition of primary root formation | high concentration | Brassica juncea, Oryza sativa, Lupinus albus, Arabidopsis thaliana | 48,89,93,115 | |
| Modification of stem and leaf branching patterns | Oryza sativa, Solanum lycopersicum | Interaction with auxin signaling genes | 103,104 | |
| Chronoregulator | 10 uM | Eichhornia crassipes, vitis vinifera, Prunus avium, Ulva sp, Chara australis, Chenopodium rubrum, Oryza sativa | Antioxidant activity, support of photosynthetic apparatus, light signaling | 44,97,110,121,123,125,133,163 |
| Increased hypocotyl elongation | 5–40 uM | Helianthus annus, Lupinus albus | 52,115 | |
| Promotion coleoptile growth | Phalaris canariensis, Avena sativa, Triticum aestivum, Hordeum vulgare | Interaction with polyamines and auxin, antioxidant activity | 95 | |
| Promotion of germination | 100–500 M | Cucumis sativus | Antioxidant | 129 |
| Serotonin | ||||
| Increased incidence somatic embryogenesis | 100 uM | Coffea canephora | Interaction with calcium signaling cascades | 102 |
| Increased shoot production | 100–200 uM | Hypericum perforatum, Mimosa pudica | Interaction with MEL, auxin, and calcium signaling pathways | 88,114 |
| Delayed senescence | 0.8 µM | Oryza sativa | Antioxidant | 116 |
| Increased root length | 0.8 M – 20 uM | Hordeum vulgare, Helianthus annus | 52,112 | |
| Increased root weight | 0.8 µM | Hordeum vulgare | 112 | |
| Increased coleoptile weight | 0.8 µM | Hordeum vulgare | 112 | |
| Increased mitotic index | 0.8 µM | Hordeum vulgare | 112 | |
| Increased lateral root formation | 10–160 uM | Arabidopsis thaliana | Inhibition of auxin activity and transport, modification of auxin gene expression | 111 |
| Increased adventitious root formation | > 160 uM | Arabidopsis thaliana | Inhibition of auxin activity and transport, modification of auxin gene expression | 111 |
| Inhibition of primary root formation | 10–160 uM | Arabidopsis thaliana | Inhibition of auxin activity and transport, modification of auxin gene expression | 111 |
| Increased hypocotyl elongation | 5–15 uM | Helianthus annus | 52 | |
| Induction of root formation | Juglans nigra x regia | 113 | ||
| Promotion germination | Hippeastrum hybridium | 164 |
Melatonin and shoot organogensis
MEL may also be implicated as a modulator of shoot production, with increased exposure leading to expanded seedling growth and shoot number. This is reminiscent of the decreasing gradient effects seen in some species in response to auxin, suggesting the involvement of MEL transport, though the receptors or transport proteins for MEL have yet to be identified in plants.99 These responses are also likely the result of interaction of MEL with other plant growth regulators and the pathways they modulate. This is seen for antioxidants such as glutathione and ascorbic acid which by modulation of the reducing environment in plant cells are able to alter plant responses via reactive oxygen species (ROS)-mediated interaction with established plant growth regulatory pathways.86,100 The interaction with the cytokinin or 5HT responses may also be a causal factor. A recent study on Zea mays has also suggested a direct interaction with sugar metabolism.101 This publication reported that at low levels (10 µM) MEL acted to enhance gene expression of photosynthesis, sucrose biosynthesis and hydrolysis-related genes, to increase starch catabolism during the night, to elevate sucrose-related enzyme activity and to promote photosynthetic rates. This was coupled to a rise in the expression of sugar transporters SUT1 and SUT2 as well as higher levels of hexokinase activity and sugar metabolites which resulted in elevated levels of available sugars for plant growth.101 Interestingly, when the authors increased exogenously applied MEL concentrations to 100 µM, the reverse effect was seen with reduced sucrose metabolism, transport and gene expression, increased starch accumulation in photosynthesizing tissues and lower photosynthetic rates.101
Somatic embryogenesis is induced by MEL in coffee plants in culture (Coffea canephora) though whether this effect is a result of a balance between 5HT and MEL concentrations, or is due to an interaction with other signaling cascades is not reported.102 Additionally, an effect of MEL on leaf and branching structures has been reported in both rice and tomato.103,104 A general increase in total biomass due to MEL treatment has been reported, though this may just be a side effect of an increased production of roots, shoots or leaves.91,93,105 As a whole, these studies suggest that not only is MEL able to interact with signaling and other plant growth regulatory cascades, it also has a function in nutrient cycling.
The ability of MEL to delay senescence has also been widely examined with much more insight now being available into the potential mechanisms of action.58,106-109 An interesting aspect of MEL is its ability to promote formation of new tissues while maintaining aging tissues. Metabolic and genetic factors have been identified through which MEL accomplishes this maintenance. From a metabolic perspective, the antioxidant capacity of MEL is able to maintain the photosynthetic process, preventing chlorophyll degradation and increasing efficiency of photosystem II as well as regulating sugar and nitrogen metabolism and preventing protein degradation.106-108,110 At the genetic level in particular, 3 senescence-related genes may be involved: indole-3-acetic acid inducible 17 (AXR3), senescence associated gene 12 (SAG 12) and senescence 4 (SEN4) suggesting a potential interaction with auxin signaling.109 In apple, autophagy-related genes involved in age-related protein degradation have been found to be suppressed by MEL possibly also contributing metabolically to this mechanism.106
Serotonin in root organogensis
5HT activity was described by Pelagio-Flores et al. (111) in an A. thaliana model system. Application of 10–160 µM 5HT to A. thaliana disrupted auxin transport and subsequently the auxin gradient in roots, resulting in an inhibition of primary root growth. The redirected auxin stimulated already-developed lateral root primordia through modification of auxin-related gene expression.111 At concentrations greater than 160 µM, 5HT inhibited both lateral and primary rooting leading to the production of adventitious roots; this activity occurred independently of auxin-related loci, suggesting there is a 5HT specific response stimulated by exogenous application of this biogenic amine.111 5HT-mediated modification of rooting has also been reported in Hypercium perforatum, Hordeum vulgare, Juglans nigra x Juglans regia, and Helianthus annus among others.52,88,112,113
Serotonin in shoot organogenesis
The cytokinin-like effects of 5HT are not limited to the antagonistic relationships with auxin during root formation. 5HT has also been found to enhance shoot formation and somatic embryogenesis in culture. Murch et al. (88) observed that an increase in endogenous 5HT levels correlated with an enhanced shoot production in H. perforatum. Similarly, the application of inhibitors of human 5HT transporters decreased the number of shoots produced in cultures, while addition of an inhibitor preventing conversion of 5HT to MEL, (p-chlorophenyl alanine; p-CPA) led to an increase in endogenous 5HT concentrations along with inhibition of auxin-induced rooting and promotion of shoot production. More recently, Ramakrishna et al. (102,114) found that the addition of 5HT in Caffea canephora and Mimosa pudica caused a rise in somatic embryogenesis and shoot multiplication, respectively. Addition of p-CPA or fluoxetine induced a reduction in somatic embryogenesis, supporting the results obtained by Murch et al. (88). Additionally, the inclusion of calcium enhanced shoot multiplication, while the addition of calcium chelators or calcium channel inhibitors inhibited the effects of both somatic embryogenesis and shoot multiplication. This suggests that the calcium concentration and signaling are intricately linked to the indoleamine response in plants.102,114 Similar to experiments performed on MEL in Lupinus albus and several other species including Avena sativa and Hordeum vulgare , 5HT was found to enhance hypocotyl elongation in Helianthus annus.52,95,115 5HT has also been found to delay senescence in Oryza sativa due to its antioxidant capacity; however, the effect does not appear to be as prevalent as seen for MEL.116
Melatonin and 5HT in Flowering, Reproduction and Chronoregulation
In mammals, MEL is intricately linked to circadian rhythms and is most commonly known as a signal of darkness, and is in fact readily available as a natural health product for the promotion of sleep. There is less evidence for MEL as a signal of darkness in plants. Nevertheless, MEL content has been found to vary in some species of plant seasonally and also on a daily basis suggesting that even if it is not a primary chronoregulator in plants, it plays a role in timing both chronoregulatory processes and reproductive development (Table 2).36,44 Additionally in several cases, this rhythm was maintained even when plants were kept in the dark, confirming endogenous maintenance of this system.117,118 One possibility is that the MEL content is synchronized, with photosynthetic activity with daylight as the photosynthetic process producing large quantities of dangerous free radicals and reactive oxygen species.4,43,119,120 As MEL is a potent antioxidant, its presence is extremely favorable during periods of intense photosynthesis to combat the high levels of free radicals and reactive oxygen species produced thereby maintaining the integrity of chloroplasts and the surrounding cellular environment.110,121,122 To date, several species of algae have been reported in which the indoleamine content fluctuates repeatedly and predictably including Eichhornia crassipes and Ulva which had peaks of indoleamines in association with the light/dark cycle and Chara australis which showed a circadian rhythm of auxin and MEL.44,123,124 Regulation of flowering time is best illustrated to date by a report published by Kolar et al. (97) who examined flowering time in the short-day plant Chenopodium rubrum. Application of MEL at specific time points, before darkness or in the first half of the dark period, led to a significant decrease in flowering. Interestingly, a previous study had shown that MEL levels varied with time of day in C. rubrum, thereby supporting the potential endogenous effect of MEL in the regulation of flowering.125 More recently, the modification of flowering time and grain yield was reported in a transgenic strain of rice which produced increased MEL due to transformation with SNAT, suggesting that further investigation may prove this response to be more common than previously thought.126
Exogenous application of MEL has promoted seed growth and germination in diverse plants including soybean (Glycine max), cucumber (Cucumis sativus), corn (Zea mays), mung bean (Vigna radiata), red cabbage (Brassica oleracea rubrum) and purple tansy (Phacelia tanacetifolia).53,55,62,63,105,127-129 Compared to the function of MEL in other reproductive applications, there is a large body of knowledge as to the function of MEL in promoting seed growth and germination. Byeon et al. (127) found that MEL modifies expression of germination and growth-related genes, including those involved in cell wall growth and expansion, as well as genes related to calcium signaling cascades. Also, Wei et al. (105) found that in soybean MEL interacts with gibberellin and abscisic acid pathways to promote germination. Others have ascribed the promotion to direct antioxidant effects of MEL and its ability to enhance other cellular antioxidant pathways.53,128,129
Reproductive organs in plants house some of the most important but most vulnerable tissues produced during the life of a plant. Due to periods of both prolonged dormancy and rapid growth and development, the oxidative environment in these tissues can change rapidly, which without protection may lead to poor reproductive success.130,131 As powerful antioxidants, MEL and 5HT have been implicated as protective chemicals during these processes. The accumulation of high levels of MEL and 5HT concentrations in developing flowers and seeds has been reported in various species including Devil's trumpet (Datura metel), St. John's wort (Hypericum perforatum), apple (Malus domestica), grape (Vitis vinifera) and cherry (Prunus avium), with levels of each varying with the developmental stage. Reports of MEL and 5HT content of plants across different tissue types consistently36,67,131-134 demonstrate that concentrations are significantly higher in reproductive tissues than in vegetative tissues. This phenomenon spans many plant families including the Solanaceae, Rubiaceae, Fabacieae, Poaceae and Clusiaceae.135-138 In fact, seeds are a particularly rich source of MEL and 5HT, with many species having been reported to have significant concentrations.7,9,130 Additionally, though there is an overall increase in indoleamine concentration in these reproductive tissues, there is a differential accumulation of MEL and 5HT depending on developmental stage. A study quantifying MEL and 5HT content of Merlot grapes found significant differences across the growing season. While 5HT was detected in none of the grapes collected during prelag phases, it was detected at levels of 8–10 µg/g in 30–35% of grapes during veraison. In contrast, MEL was identified in 23% of the prelag phase grapes with approximate concentrations of 100 µg/g with an increase in concentration with progressive developmental stages to a maximum of 45% of grapes containing detectable MEL in purple grapes. This differential pattern of MEL and 5HT concentration suggests that, in addition to their protective effects, MEL and 5HT may act as signals for reproductive development.134 This idea is supported by similar results seen in devil's trumpet, where MEL concentration increased progressively for the first 10 d after antethesis, at which point the ovule reached maturity, and by studies in apple which found seasonal peaks in MEL and 5HT content that coincided with periods of significant growth and development of the apple fruit. It is worth noting, however, that periods of intense growth and development also generally coincide with periods of high production of reactive oxygen species suggesting that the function of MEL in reproductive tissues may serve a dual purpose.36,131 In both rice and apple MEL has been found to be a modulator of the expression of genes implicated in reproductive development suggesting complex mechanisms of regulation.36,127,139
Though information on the activity of 5HT in plant reproduction is limited, the existing literature suggests that it has a strong effect on pollen germination both in culture and in vivo. In St. John's wort the shifts from the tetrad to uninucleate phase microspore cultures was accompanied by a shift in indoleamine concentration with 5HT steeply increasing and MEL dropping off, again suggesting that the balance of MEL and 5HT at the correct time points is important in maintaining proper plant reproductive development.67,140
Proposed Mechanisms of Action of MEL and 5HT
Though many possible mechanisms of action have been proposed over the years for the action of MEL and 5HT in plant growth, development and reproduction, these hypotheses can be summarized into the following categories: a) interaction with auxin or other phytohormone signaling cascades, b) interaction with calcium/calmodulin signaling cascades, c) direct antioxidant activity and upregulation of other cellular antioxidant processes, d) modification of gene expression including primary metabolic networks including nitrogen, sugar and carbon metabolism, and e) presence of MEL/5HT specific receptors inducing specialized signaling cascades (Fig. 2). In view of several recent reviews of these hypotheses with regards to MEL.7,42,47 the present article will attempt a brief discussion of the importance of these mechanisms in plant growth and development functions with particular emphasis on the potential role of 5HT in these processes.
It is now a well-accepted principle of plant signaling that the phytohormonal networks are intricately interconnected and an action by any particular PGR can have wide reaching effects. It is therefore, logical to expect that MEL and 5HT will interact with other plant growth regulators. Due to their significant effects on rooting and organogenesis, it is not surprising that MEL and 5HT are capable of interacting with auxin gradients, gene expression and signaling networks. The interaction between 5HT and auxin signaling leading to modifications in rooting is evident from the study of 5HT-mediated disruption of the ubiquitin-proteasome cascade that normally results from auxin signaling.111 Additionally extensive studies with plant tissue culture have found that the addition of auxin inhibitors has significant effects on MEL and 5HT concentrations resulting in modification of growth patterns (Table 1). Reports of coinciding maxima of IAA, MEL and 5HT in Chara 117 further suggest that in addition to MEL and 5HT being able to effect auxin activity, there exists the possibility that one of the many auxin biosynthetic pathways 141 work in tandem with the poorly understood indoleamine biosynthetic pathways to produce the observed circadian effects. Other examples of hormonal interaction include improved ripening of tomato fruits with MEL treatment,142 which not surprisingly was attributed to an interaction with ethylene signaling pathways. Research into the promotion of seed growth and germination has led to elucidation of further interactions between MEL and gibberellins and abscisic acid, two classes of PGRs, which are essential to seed germination.105
The second hypothesis involves MEL and 5HT-induced calcium signaling cascades and associated downstream responses. Plants use calcium as an essential signaling molecule143 and also to maintain osmotic gradients for stabilizing their cell walls and membranes. These cascades serve diverse functions and have been implicated in pathogen response, pollen tube growth and abiotic stress responses that represent extremely complex networks for signaling. Like auxin, the presence of calcium gradients in cells may also constitute a signal making this a broad hypothesis.144 Research using calcium channel inhibitors, calcium chelators and co-administration of calcium with MEL or 5HT has shown that plant responses to treatment with MEL or 5HT are enhanced by increasing calcium concentrations and can be inhibited by decreased calcium availability, or inhibiting the function of calcium signaling enzymes and transporters. A study by Jones et al. (145) found that the addition of the calcium channel inhibitor (S)-Bay K8644 led to a change in cell polarity accompanied by a steep increase in auxin concentration,145 5HT and MEL concentrations leading to reduced regeneration in Echinacea purpurea. Similarly, a study in Coffea canephora102 found that somatic embryogenesis was enhanced by the addition of 100 µM MEL or 5HT and that inclusion of either calcium or calcium ionophore led to a further enhancement in this activity. The function of calcium in this system was then confirmed by inclusion of a calcium chelator, and a calcium channel inhibitor; in both cases somatic embryogenesis was significantly inhibited. Interestingly, 5HT was found to be distributed throughout the cultures and somatic embryos and was slightly more effective than MEL alone, although both showed the same interaction with calcium. Calcium signaling has also been implicated in the reproductive development of H. perforatum. Experiments by Murch et al. (67) suggest that calcium gradients may be involved in signaling the transition from tetrad to uninucleate microspore phases associated with a shift from a high 5HT environment to a high MEL environment.67 These results strongly support an important link between calcium and MEL and 5HT action; however, specific calcium receptors or channels that respond to these compounds have not yet been identified.
One of the most promising hypotheses of the function of MEL and 5HT in plants is based on their role as antioxidants. ROS are increasingly being acknowledged as important signaling compounds.86,100,146 To date ROS have been implicated in a multitude of physiological responses such as pathogen attack, herbivory and wounding, and UV exposure as well as playing a role in seed dormancy, and plant growth and development.147-152 In these responses, ROS interact with phytohormone networks and other signaling cascades including calcium, protein phosphorylation and nitric oxide cascades, as well as modifying transcriptional regulation.147,148,153,154 Miller et al. (150) found that ROS-mediated signaling cascades in A. thaliana travel in a self-propagating wave over long distances, at a rate of 8.4 cm/min by modifying the oxidative environment of cells. As MEL and 5HT possess strong antioxidant potential they may modify ROS signaling cascades, leading to interruption of these long distance signals and resulting in dramatic effects on cellular environments and responses.
The interaction between ROS signaling and calcium and calmodulin-dependent signaling cascades suggests that the MEL and 5HT-mediated responses are likely due to a combination of factors and one single hypothesis may not explain the diversity of actions of these compounds. Another less complex implication of the presence of MEL and 5HT in tissues as an antioxidant is their ability to protect cells and cellular components from damage by ROS.65 In chloroplasts and mitochondria, which are derived from the ancient organisms in which MEL and 5HT are thought to have first evolved, MEL may play an important role in neutralizing ROS produced during the photosynthetic and cellular respiration processes. Such protection of the apparatuses and pigments in vital organelles is crucial for enhancing the efficiency and life span of the tissues.1,3,110 For instance, the protection of chlorophyll by MEL directly and through modulation of senescence-related gene expression is a means by which MEL mediates a delay in leaf senescence.36 Additionally, work in an ancestral relative of land plant, Chara australis, has found a direct interaction between MEL application and reduced production of ROS species at the cellular level by using fluorescent probes and examining trans-membrane potential differences; these findings have suggested that the natural circadian rhythms of these compounds produced in Chara species may help to regulate ROS levels.124,155
The ability of MEL to modify gene expression offers an additional insight into its mode of action and interactions with other pathways. MEL has been shown to differentially regulate expression of genes associated with the antioxidant enzymes including ascorbate-glutathione, auxin-related genes, development-associated genes and senescence-associated genes in plant cells.106,107,127,132 Recent differential expression analyses of transcriptomes of plants exposed to MEL have found that the expression of more than 1300 genes are modified by MEL in A. thaliana and over 300 in cucumber. In a transgenic strain of rice expressing a sheep SNAT and producing increased MEL, over 400 genes were found to be differentially expressed. These included genes involved in cell wall structure, nitrogen metabolism, carbon and sugar metabolism, calcium signaling and transcription factors including F-box and leucine repeat proteins. This adds a further level of complexity to the signaling networks influenced by MEL.59,127,156 Furthermore specific research has started to uncover the mechanisms behind this regulation with a C-repeat-binding factors (CBFs)/drought response element binding 1 factors (DREB1s) as well as WRKY, MYB and bHLH transcription factors being implicated in mediation of these responses.48,132,157 Though large-scale transcriptomics analysis has not been undertaken to examine the transcriptional effects of 5HT on plant tissues, differential expression of a large number of genes is plausible due to the many similarities and relationships in MEL and 5HT responses.
MEL and 5HT receptors can explain and facilitate understanding of multiple functions these molecules display in plant physiology. The presence of MEL and 5HT specific receptors in animals is well documented and such receptors are likely present in plants (see refs 40,115 for further discussion of putative receptors). The observation that 5HT and MEL function independently to control auxin activity in Arabdiopsis111 also suggests receptor-mediated modes of action for MEL and 5HT. Unfortunately, despite extensive information available on these receptors in animals, searches for homologous gene sequences in plants have yielded no results. It is likely that a specific receptor is indeed present in plants for these indoleamines due to the specific responses they induce. It is also possible that MEL and 5HT function through interaction with receptors used for other networks such as auxins due to their similarity in structure.
Figure 1.
Proposed biosynthetic pathways for melatonin and serotonin in plants and animals. AADC: aromatic L-amino acid decarboxylase; ASMT: acetylserotonin methyltransferase; COMT: caffeic acid-O-methyltransferase; HIOMT: hydroxyindole-O-methyltransferase; T-5-H: Tryptamine-5-hydroxylase; TDC: tryptophan decarboxylase; TPH: tryptophan hydroxylase; SNAT: serotonin N-acetyltransferase. Green arrows indicate an established biosynthetic pathway in plants, red arrows an established biosynthetic pathway in animals, black a step shared by both the animal and plant established pathways and blue arrows indicate a newly proposed alternate step in the biosynthetic pathway.
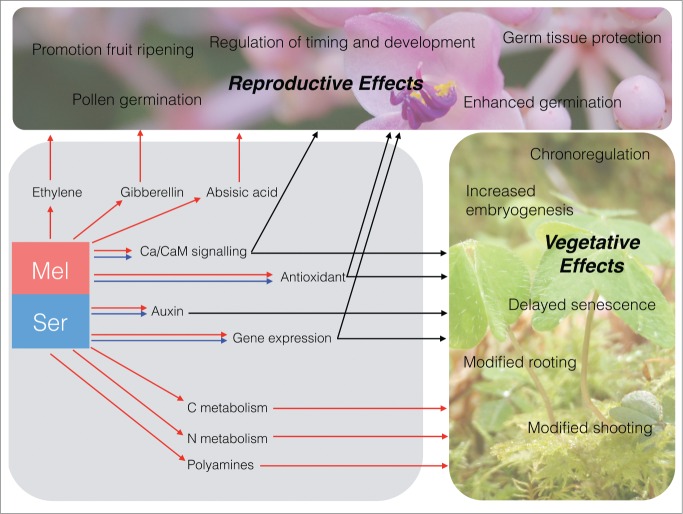
Figure 2.
Summary of mechanism of action of melatonin and serotonin effects on reproductive and vegetative growth and development. Red arrows indicate a melatonin pathway, blue arrows indicate a serotonin pathway and black arrows indicate metabolism of both melatonin and serotonin.
Figure 3.
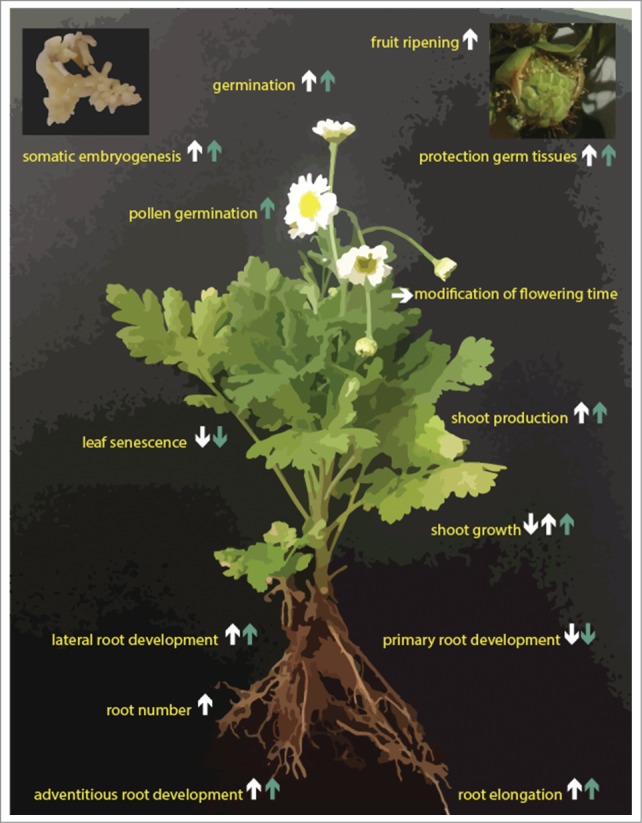
Overview of the primary physiological roles of melatonin and serotonin. White arrows indicate melatonin action, blue arrows serotonin action and direction indicates enhancement (up), inhibition (down) or other modulatory effects (sideways), in some cases multiple arrows are present due to varying reports, or concentration-dependent effects.
Conclusions
MEL and 5HT are indoleamines first identified as neurotransmitter signaling molecules in mammals but are ubiquitous across all forms of life. In plants, MEL and 5HT play important roles in plant growth and development by modulating shoot and root organogenesis, embryogenesis, germination and flowering time and may act as developmental cues in reproductive and vegetative development and aging. Both MEL and 5HT interact with already established plant signaling networks, in particular with auxin signaling, and their involvement in many more metabolic pathways has been suggested. A singular mode of action has not been identified for MEL or 5HT and further research is required to identify their putative receptors. However, the analyses of MEL and 5HT induced and modulated responses indicate that it is more likely that 5HT and MEL function through modulation of calcium and ROS signaling cascades, modulation of gene expression and interaction with other PGRs mediated by specific receptors. These distinct and highly regulated pathways are strong evidence for these compounds as a new class of plant growth regulator as minor changes in the regulatory web, as demonstrated by studies presented in this review, lead to specific and significant changes in gene expression, as well as plant growth patterns and morphology.
As MEL is produced from 5HT via NAS, there is also likely a finely regulated balance between 5HT and MEL in plant cells, which defines the resulting plant change occurring in an extremely tuned response. The biosynthesis of MEL and 5HT has been found to be regulated at almost all steps in the biosynthetic pathway from competition for carbon in the form of tryptophan and tryptamine from other biosynthetic pathways such as auxin biosynthesis, to the feedback inhibition of SNAT. Taken as a whole, the existing research suggests that not only are MEL and 5HT intricately linked with existing plant growth regulatory networks and signaling cascades, they participate in basic nutrient pathways including nitrogen and sugar metabolism. In contrast with acknowledged interactions in plants such as the cytokinin/auxin balance, the dual effect observed for the indoleamines is unique as instead of compounds with significantly different structures produced from separate pathways, MEL and 5HT are differentiated only by the presence of an acetyl and a methoxy group and are produced from a single pathway. This may allow plants to more finely tune this balance as only one pathway, and possibly even only one enzyme need be effected to shift this balance. This is in contrast to many and complex steps, which are required to maintain or shift the auxin/cytokinin balance.
Though research continues to increase into this intriguing and ancient class of PGRs there are many questions still to be answered with regards to the roles MEL and 5HT in plant growth and development and the diverse mechanisms by which they are accomplished. A continually-growing body of knowledge on the roles of MEL and 5HT in plants and acceptance of these compounds as plant growth regulators will have far-reaching conclusions, both for basic plant science and applied technologies such as plant cell culture, crop improvement, cryopreservation and conservation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
We thank the National Sciences and Engineering Research Council (NSERC) and the Gosling Research Institute for Plant Preservation (GRIPP) for supporting research on indoleamine mediated plant responses.
References
- 1.Tan D-X, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes-Broto L, Reiter RJ. The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol Rev Camb Philo Soc 2010; 85:607-623 [DOI] [PubMed] [Google Scholar]
- 2.Olivier B. Serotonin: a never-ending story. Eur J Pharmacol 2015; 753:2-18; PMID:25446560; http://dx.doi.org/ 10.1016/j.ejphar.2014.10.031 [DOI] [PubMed] [Google Scholar]
- 3.Tan D-X, Zheng X, Kong J, Manchester L, Hardeland R, Kim S, Xu X, Reiter R. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. Int J Mol Soc 2014; 15:15858-90; PMID:25207599; http://dx.doi.org/ 10.3390/ijms150915858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen J, Than NN, Koch D, Poeggeler B, Laatsch H, Hardeland R. Interactions of melatonin and its metabolites with the ABTS cation radical: extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J Pineal Res 2006; 41:374-81; PMID:17014695; http://dx.doi.org/ 10.1111/j.1600-079X.2006.00379.x [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Naranjo IM, Moya LM, Cantos-Villar E, Garcia-Parrilla CM. Comparative evaluation of the antioxidant activity of melatonin and related indoles. J Food Comp Anal 2012; 28:16-22; http://dx.doi.org/ 10.1016/j.jfca.2012.07.001 [DOI] [Google Scholar]
- 6.Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull 2001; 56:413-424; PMID:11750787; http://dx.doi.org/ 10.1016/S0361-9230(01)00614-1 [DOI] [PubMed] [Google Scholar]
- 7.Arnao MB. Phytomelatonin: discovery, content, and role in plants. Adv Bot 2014; 2014:1-11; http://dx.doi.org/ 10.1155/2014/815769 [DOI] [Google Scholar]
- 8.Murch SJ, Saxena PK. Melatonin: a potential regulator of plant growth and development? In Vitro Cell Dev Biol - Plant 2002; 38:531-6; http://dx.doi.org/ 10.1079/IVP2002333 [DOI] [Google Scholar]
- 9.Ramakrishna A, Giridhar P, Ravishankar GA. Phytoserotonin: a review. Plant Signal Behav 2011; 6:800-9; PMID:21617371; http://dx.doi.org/ 10.4161/psb.6.6.15242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardeland R. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors 2009; 35:183-92; PMID:19449447; http://dx.doi.org/ 10.1002/biof.23 [DOI] [PubMed] [Google Scholar]
- 11.Szczepanik M. Melatonin and its influence on immune system. J Physiol Pharmacol 2007; 58 Suppl 6:115-24; PMID:18212405 [PubMed] [Google Scholar]
- 12.Singh M, Jadhav HR. Melatonin: functions and ligands. Drug Discov Today 2014; 19:1410-8; PMID:24792719; http://dx.doi.org/ 10.1016/j.drudis.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 13.Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod 1998; 3:13-22; PMID:9509985; http://dx.doi.org/ 10.1530/ror.0.0030013 [DOI] [PubMed] [Google Scholar]
- 14.Rios ERV, Venâncio ET, Rocha NFM, Woods DJ, Vasconcelos S, Macedo D, Sousa FCF de, Fonteles MM de F. Melatonin: pharmacological aspects and clinical trends. Int J Neurosci 2010; 120:583-90; PMID:20707632; http://dx.doi.org/ 10.3109/00207454.2010.492921 [DOI] [PubMed] [Google Scholar]
- 15.Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Am Chem Soc 1958; 80:2587-7; http://dx.doi.org/ 10.1021/ja01543a060 [DOI] [Google Scholar]
- 16.Lerner AB, Case JD, Mori W, Wright MR. Melatonin in peripheral nerve. Nature 1959; 183:1821-1; PMID:14415934; http://dx.doi.org/ 10.1038/1831821a0 [DOI] [PubMed] [Google Scholar]
- 17.Whitaker-Azmitia PM. The discovery of serotonin and its role in neuroscience. Neuropharmacology 1999; 21:S2-S8 [DOI] [PubMed] [Google Scholar]
- 18.Bubenik GA. Review: gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci 2002; 47:2336-2348; PMID:12395907; http://dx.doi.org/ 10.1023/A:1020107915919 [DOI] [PubMed] [Google Scholar]
- 19.Hung ASM, Tsui TYM, Lam JCY, Wai MSM, Chan WM, Yew DT. Serotonin and its receptors in the human CNS with new findings - a mini review. Curr Med Chem 2011; 18:5281-8; PMID:22087825; http://dx.doi.org/ 10.2174/092986711798184253 [DOI] [PubMed] [Google Scholar]
- 20.Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan D-X, Reiter RJ. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 2014; 71:2997-3025; PMID:24554058; http://dx.doi.org/ 10.1007/s00018-014-1579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, García-Corzo L, López LC, Reiter RJ, Acuña-Castroviejo D. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res 2012; 52:217-27; PMID:21884551; http://dx.doi.org/ 10.1111/j.1600-079X.2011.00931.x [DOI] [PubMed] [Google Scholar]
- 22.Yaakob NS, Chinkwo KA, Chetty N, Coupar IM, Irving HR. Distribution of 5-HT3, 5-HT4, and 5-HT7 receptors along the human colon. J Neurogastroenterol Motil 2015; 21:361-9; PMID:26130632; http://dx.doi.org/ 10.5056/jnm14157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 2015; 277:32-48; PMID:25078296; http://dx.doi.org/ 10.1016/j.bbr.2014.07.027 [DOI] [PubMed] [Google Scholar]
- 24.Bowden K, Brown BG, Batty JE. 5-Hydroxytryptamine: its occurrence in cowhage. Nature 1954; 174:925-6; PMID:13214042; http://dx.doi.org/ 10.1038/174925a0 [DOI] [PubMed] [Google Scholar]
- 25.Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J Pineal Res 1995; 18:28-31; PMID:7776176; http://dx.doi.org/ 10.1111/j.1600-079X.1995.tb00136.x [DOI] [PubMed] [Google Scholar]
- 26.Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int 1995; 35:627-34; PMID:7773197 [PubMed] [Google Scholar]
- 27.Paredes SD, Korkmaz A, Manchester LC, Tan D-X, Reiter RJ. Phytomelatonin: a review. J Exp Bot 2009; 60:57-69; PMID:19033551; http://dx.doi.org/ 10.1093/jxb/ern284 [DOI] [PubMed] [Google Scholar]
- 28.Reiter RJ, Tan D-X, Manchester LC, Simopoulos AP, Maldonado MD, Flores LJ, Terron MP. Melatonin in edible plants (phytomelatonin): identification, concentrations, bioavailability and proposed functions. World Rev Nut Diet Basel 2007; 97:211-230 [DOI] [PubMed] [Google Scholar]
- 29.Huang X, Mazza G. Application of LC and LC-MS to the analysis of melatonin and serotonin in edible plants. Crit Rev Food Sci Nutr 2011; 51:269-84; PMID:21432696; http://dx.doi.org/ 10.1080/10408398.2010.529193 [DOI] [PubMed] [Google Scholar]
- 30.Bajwa V, Murch SJ, Saxena PK. Melatonin rich plants: production, significance in agriculture and human health In: Production of Biomass and Bioactive Compounds Using Bioreactor Technology. Dordrecht: Springer Netherlands; 2014; 445-68. [Google Scholar]
- 31.Murch SJ, Krishnaraj S, Saxena PK. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John's wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep 2000; 19:698-704; http://dx.doi.org/ 10.1007/s002990000206 [DOI] [PubMed] [Google Scholar]
- 32.Byeon Y, Lee HY, Lee K, Back K. Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. J Pineal Res 2014; 57:219-27; PMID:25039887; http://dx.doi.org/ 10.1111/jpi.12160 [DOI] [PubMed] [Google Scholar]
- 33.Kang S, Kang K, Lee K, Back K. Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta 2007; 227:263-72; PMID:17763868; http://dx.doi.org/ 10.1007/s00425-007-0614-z [DOI] [PubMed] [Google Scholar]
- 34.Park S, Byeon Y, Back K. Transcriptional suppression of tryptamine 5-hydroxylase, a terminal serotonin biosynthetic gene, induces melatonin biosynthesis in rice (Oryza sativa L.). J Pineal Res 2013; 55:131-7; PMID:23521226; http://dx.doi.org/ 10.1111/jpi.12053 [DOI] [PubMed] [Google Scholar]
- 35.Park S, Byeon Y, Lee HY, Kim Y-S, Ahn T, Back K. Cloning and characterization of a serotonin N-acetyltransferase from a gymnosperm, loblolly pine (Pinus taeda). J Pineal Res 2014; 57:348-55; PMID:25208036; http://dx.doi.org/ 10.1111/jpi.12174 [DOI] [PubMed] [Google Scholar]
- 36.Lei Q, Wang L, Tan D-X, Zhao Y, Zheng X-D, Chen H, Li Q-T, Zuo B-X, Kong J. Identification of genes for melatonin synthetic enzymes in “Red Fuji” apple (Malus domestica Borkh. cv. Red) and their expression and melatonin production during fruit development. J Pineal Res 2013; 55:443-51; PMID:24102635 [DOI] [PubMed] [Google Scholar]
- 37.Park S, Byeon Y, Back K. Functional analyses of three ASMT gene family members in rice plants. J Pineal Res 2013; 55:409-415; PMID:24033370 [DOI] [PubMed] [Google Scholar]
- 38.Champney TH, Holtorf AP, Steger RW, Reiter RJ. Concurrent determination of enzymatic activities and substrate concentrations in the melatonin synthetic pathway within the same rat pineal gland. J Neurosci Res 1984; 11:59-66; PMID:6708134; http://dx.doi.org/ 10.1002/jnr.490110107 [DOI] [PubMed] [Google Scholar]
- 39.Byeon Y, Park S, Lee HY, Kim Y-S, Back K. Elevated production of melatonin in transgenic rice seeds expressing rice tryptophan decarboxylase. J Pineal Res 2014; 56:275-82; PMID:24433490; http://dx.doi.org/ 10.1111/jpi.12120 [DOI] [PubMed] [Google Scholar]
- 40.Kang K, Lee K, Park S, Byeon Y. Molecular cloning of rice serotonin N-acetyltransferase, the penultimate gene in plant melatonin biosynthesis. J Pineal Res 2013; 55:7-13; PMID:22998587; http://dx.doi.org/ 10.1111/jpi.12011 [DOI] [PubMed] [Google Scholar]
- 41.Lee HY, Byeon Y, Lee K, Lee H-J, Back K. Cloning of Arabidopsis serotonin N-acetyltransferase and its role with caffeic acid O-methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localizations. J Pineal Res 2014; 57:418-26; PMID:25250906; http://dx.doi.org/ 10.1111/jpi.12181 [DOI] [PubMed] [Google Scholar]
- 42.Hardeland R. Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions. J Exp Bot 2015; 66:627-46; PMID:25240067; http://dx.doi.org/ 10.1093/jxb/eru386 [DOI] [PubMed] [Google Scholar]
- 43.Tan D-X, Reiter RJ, Manchester LC, Yan M-T, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem 2002; 2:181-97; PMID:11899100; http://dx.doi.org/ 10.2174/1568026023394443 [DOI] [PubMed] [Google Scholar]
- 44.Tan D-X, Manchester LC, Di Mascio P, Martinez GR, Prado FM, Reiter RJ. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: importance for phytoremediation. 2007; 21:1724-9; PMID:17314136 [DOI] [PubMed] [Google Scholar]
- 45.Tan DX, Hardeland R, Manchester LC. Emergence of naturally occurring melatonin isomers and their proposed nomenclature. J Pineal Res 2012; 53:113-21; PMID:22332602; http://dx.doi.org/ 10.1111/j.1600-079X.2012.00979.x [DOI] [PubMed] [Google Scholar]
- 46.Arnao MB, Hernández-Ruiz J. Functions of melatonin in plants: a review. J Pineal Res 2015; 59:133-50; PMID:26094813; http://dx.doi.org/ 10.1111/jpi.12253 [DOI] [PubMed] [Google Scholar]
- 47.Reiter R, Tan D-X, Zhou Z, Cruz M, Fuentes-Broto L, Galano A. Phytomelatonin: assisting plants to survive and thrive. Molecules 2015; 20:7396-437; PMID:25911967; http://dx.doi.org/ 10.3390/molecules20047396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bajwa VS, Shukla MR, Sherif SM, Murch SJ, Saxena PK. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J Pineal Res 2014; 56:238-45; PMID:24350934; http://dx.doi.org/ 10.1111/jpi.12115 [DOI] [PubMed] [Google Scholar]
- 49.Arnao MB, Ruiz JH. Chemical stress by different agents affects the melatonin content of barley roots. J Pineal Res 2009; 46:295-99; PMID:19196434; http://dx.doi.org/ 10.1111/j.1600-079X.2008.00660.x [DOI] [PubMed] [Google Scholar]
- 50.Arnao MB, Hernández-Ruiz J. Melatonin: plant growth regulator and/or biostimulator during stress? Trend Plant Sci 2014; 19:789-97; PMID:25156541; http://dx.doi.org/ 10.1016/j.tplants.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 51.García JJ, López-Pingarrón L, Almeida-Souza P, Tres A, Escudero P, García-Gil FA, Tan D-X, Reiter RJ, Ramírez JM, Bernal-Pérez M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J Pineal Res 2014; 56:225-37; PMID:24571249; http://dx.doi.org/ 10.1111/jpi.12128 [DOI] [PubMed] [Google Scholar]
- 52.Mukherjee S, David A, Yadav S, Baluška F, Bhatla SC. Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol Plant 2014; 152:714-28; PMID:24799301; http://dx.doi.org/ 10.1111/ppl.12218 [DOI] [PubMed] [Google Scholar]
- 53.Posmyk MM, Kuran H, Marciniak K, Janas KM. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J Pineal Res 2008; 45:24-31; PMID:18205729; http://dx.doi.org/ 10.1111/j.1600-079X.2007.00552.x [DOI] [PubMed] [Google Scholar]
- 54.Uchendu EE, Shukla MR, Reed BM, Saxena PK. An efficient method for cryopreservation of St. John's wort and tobacco: role of melatonin. Acta Hortic 2014; 1039:233-41 [Google Scholar]
- 55.Tiryaki I, Keles H. Reversal of the inhibitory effect of light and high temperature on germination of Phacelia tanacetifolia seeds by melatonin. J Pineal Res 2012; 52:332-9; PMID:22225610; http://dx.doi.org/ 10.1111/j.1600-079X.2011.00947.x [DOI] [PubMed] [Google Scholar]
- 56.Uchendu EE, Brown DW, Saxena PK. Cryopreservation of shoot tips and cotyledons of the north american ginseng (Panax quinquefolius l.). Cryo Lett 2011; 32:463-72 [PubMed] [Google Scholar]
- 57.Uchendu EE, Shukla MR, Reed BM, Saxena PK. Melatonin enhances the recovery of cryopreserved shoot tips of American elm (Ulmus americana L.). J Pineal Res 2013; 55:435-42; PMID:24117864 [DOI] [PubMed] [Google Scholar]
- 58.Wang P, Sun X, Li C, Wei Z, Liang D, Ma F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J Pineal Res 2012; 54:292-302; PMID:23106234; http://dx.doi.org/ 10.1111/jpi.12017 [DOI] [PubMed] [Google Scholar]
- 59.Weeda S, Zhang N, Zhao X, Ndip G, Guo Y, Buck GA, Fu C, Ren S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLOS One 2014; 9:e93462; PMID:24682084; http://dx.doi.org/ 10.1371/journal.pone.0093462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin L, Wang P, Li M, Ke X, Li C, Liang D, Wu S, Ma X, Li C, Zou Y, Ma F. Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J Pineal Res 2013; 54:426-34; PMID:23356947 [DOI] [PubMed] [Google Scholar]
- 61.Zhang N, Sun Q, Zhang H, Cao Y, Weeda S, Ren S, Guo Y-D. Roles of melatonin in abiotic stress resistance in plants. J Exp Bot 2015; 66:647-56; PMID:25124318; http://dx.doi.org/ 10.1093/jxb/eru336 [DOI] [PubMed] [Google Scholar]
- 62.Zhang H-J, Zhang N, Yang R-C, Wang L, Sun Q-Q, Li D-B, Cao Y-Y, Weeda S, Zhao B, Ren S, Guo Y-D. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J Pineal Res 2014; 57:269-79; PMID:25112973; http://dx.doi.org/ 10.1111/jpi.12167 [DOI] [PubMed] [Google Scholar]
- 63.Zhang N, Zhao B, Zhang HJ, Weeda S. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J Pineal Res 2013; 54:15-23; PMID:22747917 [DOI] [PubMed] [Google Scholar]
- 64.Zhao Y, Qi L-W, Wang W-M, Saxena PK, Liu C-Z. Melatonin improves the survival of cryopreserved callus of Rhodiola crenulata. J Pineal Res 2011; 50:83-8; PMID:21073518; http://dx.doi.org/ 10.1111/j.1600-079X.2010.00817.x [DOI] [PubMed] [Google Scholar]
- 65.Bajwa VS, Shukla MR, Sherif SM, J MS, Saxena PK. Identification and characterization of serotonin as an anti-browning compound of apple and pear. Postharvest Biol Technol 2015; 110:183-9; http://dx.doi.org/ 10.1016/j.postharvbio.2015.08.018 [DOI] [Google Scholar]
- 66.Murch SJ, Saxena PK. Role of indoleamines in regulation of morphogenesis in in vitro cultures of St. John's wort (Hypericum perforatum L.). Acta Hortic 2004; 629:425-32 [Google Scholar]
- 67.Murch SJ, Saxena PK. Mammalian neurohormones: potential significance in reproductive physiology of St. John's wort (Hypericum perforatum L.)? Naturwissenschaften 2002; 89:555-60; PMID:12536277 [DOI] [PubMed] [Google Scholar]
- 68.Song S, Qi T, Wasternack C, Xie D. Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr Opin Plant Biol 2014; 21:112-9; PMID:25064075; http://dx.doi.org/ 10.1016/j.pbi.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 69.Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 2006; 7:847-59; PMID:16990790; http://dx.doi.org/ 10.1038/nrm2020 [DOI] [PubMed] [Google Scholar]
- 70.Pacurar DI, Perrone I, Bellini C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol Plant 2014; 151:83-96; PMID:24547793; http://dx.doi.org/ 10.1111/ppl.12171 [DOI] [PubMed] [Google Scholar]
- 71.Bar M, Ori N. Leaf development and morphogenesis. Development 2014; 141:4219-30; PMID:25371359; http://dx.doi.org/ 10.1242/dev.106195 [DOI] [PubMed] [Google Scholar]
- 72.Gantait S, Sinniah UR, Ali MN, Sahu NC. Gibberellins - a multifaceted hormone in plant growth regulatory network. Curr Protein Pept Sci 2015; 16:406-12; PMID:25824386; http://dx.doi.org/ 10.2174/1389203716666150330125439 [DOI] [PubMed] [Google Scholar]
- 73.Parthier B. Jasmonates, New regulators of plant growth and development: many facts and few hypotheses on their actions. Bot Acta 2014; 104:446-54; http://dx.doi.org/ 10.1111/j.1438-8677.1991.tb00257.x [DOI] [Google Scholar]
- 74.Santner A, Calderon-Villalobos LIA, Estelle M. Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 2009; 5:301-7; PMID:19377456; http://dx.doi.org/ 10.1038/nchembio.165 [DOI] [PubMed] [Google Scholar]
- 75.Mandava NB. Plant growth-promoting brassinosteroids. Ann Rev Plant Physiol Plant Mol Biol 1988; 39:23-52; http://dx.doi.org/ 10.1146/annurev.pp.39.060188.000323 [DOI] [Google Scholar]
- 76.Enders TA, Strader LC. Auxin activity: past, present, and future. Am J Bot 2015; 102:180-96; PMID:25667071; http://dx.doi.org/ 10.3732/ajb.1400285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Werner T, Schmülling T. Cytokinin action in plant development. Curr Opin Plant Biol 2009; 12:527-38; PMID:19740698; http://dx.doi.org/ 10.1016/j.pbi.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 78.Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 1957; 11:118-30; PMID:13486467 [PubMed] [Google Scholar]
- 79.Perilli S, Moubayidin L, Sabatini S. The molecular basis of cytokinin function. Curr Opin Plant Biol 2010; 13:21-26; PMID:19850510; http://dx.doi.org/ 10.1016/j.pbi.2009.09.018 [DOI] [PubMed] [Google Scholar]
- 80.Laplaze L, Benkova E, Casimiro I, Maes L, Vanneste S, Swarup R, Weijers D, Calvo V, Parizot B, Herrera-Rodriguez MB, Offringa R, Graham N, Doumas P, Frimi J, Bogusz D, Beeckman T, Bennett M. Cytokinins act directly on lateral root founder cells to inhibit root initiation. 2007; 19:3889-900; PMID:18065686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lavenus J, Goh T, Roberts I, Guyomarch S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L. Lateral root development in Arabidopsis: fifty shades of auxin. Trend Plant Sci 2013; 18:450-8; PMID:23701908; http://dx.doi.org/ 10.1016/j.tplants.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 82.Marhavý P, Bielach A, Abas L, Abuzeineh A. Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev Cell 2011; 21:796-804; PMID:21962902; http://dx.doi.org/ 10.1016/j.devcel.2011.08.014 [DOI] [PubMed] [Google Scholar]
- 83.Moubayidin L, Di Mambro R, Sabatini S. Cytokinin–auxin crosstalk. Trend Plant Sci 2009; 14:557-62; PMID:19734082; http://dx.doi.org/ 10.1016/j.tplants.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 84.Shkolnik-Inbar D, Bar-Zvi D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 2010; 22:3560-73; PMID:21097710; http://dx.doi.org/ 10.1105/tpc.110.074641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peer WA, Murphy AS. Flavonoids and auxin transport: modulators or regulators? Trend Plant Sci 2007; 12:556-63; PMID:18198522; http://dx.doi.org/ 10.1016/j.tplants.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 86.Gallie DR. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J Exp Bot 2013; 64:433-43; PMID:23162122; http://dx.doi.org/ 10.1093/jxb/ers330 [DOI] [PubMed] [Google Scholar]
- 87.Couée I, Hummel I, Sulmon C, Gouesbet G, Amrani El A. Involvement of polyamines in root development. Plant Cell Tiss Organ Cult 2004; 76:1-10; http://dx.doi.org/ 10.1023/A:1025895731017 [DOI] [Google Scholar]
- 88.Murch SJ, Campbell SSB, Saxena PK. The role of serotonin and melatonin in plant morphogenesis: regulation of auxin-induced root organogenesis in in vitro-cultured explants of St. John's wort (Hypericum perforatum L.). In Vitro Cell Dev Biol - Plant 2001; 37:786-93; http://dx.doi.org/ 10.1007/s11627-001-0130-y [DOI] [Google Scholar]
- 89.Chen Q, Qi W, Reiter RJ, Wei W, Wang B. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J Plant Physiol 2009; 166:324-328; PMID:18706737; http://dx.doi.org/ 10.1016/j.jplph.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 90.Arnao MB, Hernández-Ruiz J. Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J Pineal Res 2007; 42:147-52; PMID:17286746; http://dx.doi.org/ 10.1111/j.1600-079X.2006.00396.x [DOI] [PubMed] [Google Scholar]
- 91.Sarropoulou V, Dimassi-Theriou K, Therios I, Koukourikou-Petridou M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium × Prunus cerasus). Plant Physiol Biochem 2012; 61:162-8; PMID:23127522; http://dx.doi.org/ 10.1016/j.plaphy.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 92.Sarropoulou VN, Therios IN, Dimassi-Theriou KN. Melatonin promotes adventitious root regeneration in in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunus cerasus L.), Gisela 6 (P. cerasus × P. canescens), and MxM 60 (P. avium × P. mahaleb). J Pineal Res 2012; 52:38-46; PMID:21749439; http://dx.doi.org/ 10.1111/j.1600-079X.2011.00914.x [DOI] [PubMed] [Google Scholar]
- 93.Park S, Back K. Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. J Pineal Res 2012; 53:385-89; PMID:22640001; http://dx.doi.org/ 10.1111/j.1600-079X.2012.01008.x [DOI] [PubMed] [Google Scholar]
- 94.Koyama FC, Carvalho TLG, Alves E, da Silva HB, de Azevedo MF, Hemerly AS, Garcia CRS. The structurally related auxin and melatonin tryptophan-derivatives and their roles in Arabidopsis thaliana and in the human malaria parasite Plasmodium falciparum. J Eukaryot Microbiol 2013; 60:646-51; PMID:24102716; http://dx.doi.org/ 10.1111/jeu.12080 [DOI] [PubMed] [Google Scholar]
- 95.Hernández-Ruiz J, Cano A, Arnao MB. Melatonin acts as a growth-stimulating compound in some monocot species. J Pineal Res 2005; 39:137-42; PMID:16098090; http://dx.doi.org/ 10.1111/j.1600-079X.2005.00226.x [DOI] [PubMed] [Google Scholar]
- 96.Covington MF, Harmer SL. The circadian clock regulates auxin signaling and responses in Arabidopsis. PLoS Biol 2007; 5:e222; PMID:17683202; http://dx.doi.org/ 10.1371/journal.pbio.0050222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kolar J, Johnson CH, Machackova I. Exogenously applied melatonin (N-acetyl-5-methoxytryptamine) affects flowering of the short-day plant Chenopodium rubrum. Physiol Plant 2003; 118:605-12; http://dx.doi.org/ 10.1034/j.1399-3054.2003.00114.x [DOI] [Google Scholar]
- 98.Hernández-Ruiz J, Arnao MB. Melatonin stimulates the expansion of etiolated lupin cotyledons. Plant Growth Reg 2008; 55:29-34; http://dx.doi.org/ 10.1007/s10725-008-9254-y [DOI] [Google Scholar]
- 99.Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Ann Bot 2005; 95:707-35; PMID:15749753; http://dx.doi.org/ 10.1093/aob/mci083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suzuki N, Mittler R. Reactive oxygen species-dependent wound responses in animals and plants. Free Radic Biol Med 2012; 53:2269-76; PMID:23085520; http://dx.doi.org/ 10.1016/j.freeradbiomed.2012.10.538 [DOI] [PubMed] [Google Scholar]
- 101.Zhao H, Su T, Huo L, Wei H, Jiang Y, Xu L, Ma F. Unveiling the mechanism of melatonin impacts on maize seedling growth: sugar metabolism as a case. J Pineal Res 2015; 59:255-66; PMID: 26122919; http://dx.doi.org/ 10.1111/jpi.12258; in press [DOI] [PubMed] [Google Scholar]
- 102.Ramakrishna A, Giridhar P, Jobin M, Paulose CS, Ravishankar GA. Indoleamines and calcium enhance somatic embryogenesis in Coffea canephora P ex Fr. Plant Cell Tiss Organ Cult 2011; 108:267-78; http://dx.doi.org/ 10.1007/s11240-011-0039-z [DOI] [Google Scholar]
- 103.Okazaki M, Higuchi K, Aouini A, Ezura H. Lowering intercellular melatonin levels by transgenic analysis of indoleamine 2,3-dioxygenase from rice in tomato plants. J Pineal Res 2010; 49:239-47; PMID:20609074; http://dx.doi.org/ 10.1111/j.1600-079X.2010.00788.x [DOI] [PubMed] [Google Scholar]
- 104.Wang L, Zhao Y, Reiter RJ, He C, Liu G, Lei Q, Zuo B, Zheng X-D, Li Q, Kong J. Changes in melatonin levels in transgenic “Micro-Tom” tomato overexpressing ovine AANAT and ovine HIOMT genes. J Pineal Res 2013; 56:134-42; PMID:24138427; http://dx.doi.org/ 10.1111/jpi.12105 [DOI] [PubMed] [Google Scholar]
- 105.Wei W, Li Q-T, Chu Y-N, Reiter RJ, Yu X-M, Zhu D-H, Zhang W-K, Ma B, Lin Q, Zhang J-S, et al.. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot 2015; 66:695-707; PMID:25297548; http://dx.doi.org/ 10.1093/jxb/eru392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang P, Sun X, Chang C, Feng F, Liang D, Cheng L, Ma F. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J Pineal Res 2013; 55:424-34; PMID:24103092; http://dx.doi.org/ 10.1111/jpi.12069 [DOI] [PubMed] [Google Scholar]
- 107.Wang P, Yin L, Liang D, Li C, Ma F, Yue Z. Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate-glutathione cycle. J Pineal Res 2012; 53:11-20; PMID:21988707; http://dx.doi.org/ 10.1111/j.1600-079X.2011.00966.x [DOI] [PubMed] [Google Scholar]
- 108.Arnao MB, Hernández-Ruiz J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J Pineal Res 2009; 46:58-63; PMID:18691358; http://dx.doi.org/ 10.1111/j.1600-079X.2008.00625.x [DOI] [PubMed] [Google Scholar]
- 109.Shi H, Reiter RJ, Tan D-X, Chan Z. INDOLE-3-ACETIC ACID INDUCIBLE 17 positively modulates natural leaf senescence through melatonin-mediated pathway in Arabidopsis. J Pineal Res 2014; 58:26-33; PMID:25324183; http://dx.doi.org/ 10.1111/jpi.12188 [DOI] [PubMed] [Google Scholar]
- 110.Lazár D, Murch SJ, Beilby MJ, Khazaaly Al S. Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Signal Behav 2013; 8:e23279; PMID:23299331; http://dx.doi.org/ 10.4161/psb.23279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pelagio-Flores R, Ortíz-Castro R, Méndez-Bravo A, Macías-Rodríguez L, López-Bucio J. Serotonin, a tryptophan-derived signal conserved in plants and animals, regulates root system architecture probably acting as a natural auxin inhibitor in Arabidopsis thaliana. Plant Cell Physiol 2011; 52:490-508; PMID:21252298; http://dx.doi.org/ 10.1093/pcp/pcr006 [DOI] [PubMed] [Google Scholar]
- 112.Csaba G, P l K . Effects of insulin, triiodothyronine, and serotonin on plant seed development. Protoplasma 1982; 110:20-2; http://dx.doi.org/ 10.1007/BF01314677 [DOI] [Google Scholar]
- 113.Gatineau F, Fouché JG, Kevers C, Hausman JF, Gaspar T. Quantitative variations of indolyl compounds including IAA, IAA-aspartate and serotonin in walnut microcuttings during root induction. Biol Plant 1997; 39:131-7; http://dx.doi.org/ 10.1023/A:1000377511120 [DOI] [Google Scholar]
- 114.Ramakrishna A, Girishar P, Ravishankar GA. Indoleamines and calcium channels influence morphogenesis in in vitro cultures of Mimosa pudica L. Plant Signal Behav 2009; 4:1136; PMID:20514228; http://dx.doi.org/ 10.4161/psb.4.12.10101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hernández-Ruiz J, Cano A, Arnao MB. Melatonin: a growth-stimulating compound present in lupin tissues. Planta 2004; 220:140-4; http://dx.doi.org/ 10.1007/s00425-004-1317-3 [DOI] [PubMed] [Google Scholar]
- 116.Kang K, Kim Y-S, Park S, Back K. Senescence-induced serotonin biosynthesis and its role in delaying senescence in rice leaves. Plant Physiol 2009; 150:1380-93; PMID:19439571; http://dx.doi.org/ 10.1104/pp.109.138552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beilby MJ, Turi CE, Baker TC, Tymm F, Murch SJ. Circadian changes in endogenous concentrations of indole-3-acetic acid, melatonin, serotonin, abscisic acid and jasmonic acid in Characeae (Chara australis Brown). Plant Sig Behav 2015; PMID:26382914; doi:10.1080/15592324.2015.1082697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hardeland R, Balzer I, Poeggeler B, Fuhrberg B, Una H, Behrmann G, Wolf R, Meyer TJ, Reiter RJ. On the primary functions of melatonin in evolution: mediation of photoperiodic signals in a unicell, photooxidation, and scavenging of free radicals. J Pineal Res 1995; 18:104-11; PMID:7629689; http://dx.doi.org/ 10.1111/j.1600-079X.1995.tb00147.x [DOI] [PubMed] [Google Scholar]
- 119.Reiter RJ, Poeggeler B, Tan D-X, Chen L-D, Manchester LC, Guerrero JM. Antioxidant capacity of melatonin: a novel action not requiring a receptor. Neuroendocrinol Lett 1993; 15:103-16 [Google Scholar]
- 120.Tan D-X, Manchester LC, Terron MP, Flores LJ, Tamura H, Reiter RJ. Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT 3 melatonin membrane receptor: hypothesis and significance. J Pineal Res 2007; 43:317-20; PMID:17910598; http://dx.doi.org/ 10.1111/j.1600-079X.2007.00513.x [DOI] [PubMed] [Google Scholar]
- 121.Byeon Y, Park S, Kim Y-S, Park D-H, Lee S, Back K. Light-regulated melatonin biosynthesis in rice during the senescence process in detached leaves. J Pineal Res 2012; 53:107-11; PMID:22289080; http://dx.doi.org/ 10.1111/j.1600-079X.2012.00976.x [DOI] [PubMed] [Google Scholar]
- 122.Roopin M, Yacobi YZ, Levy O. Occurrence, diel patterns, and the influence of melatonin on the photosynthetic performance of cultured Symbiodinium. J Pineal Res 2013; 55:89-100; PMID:23496383; http://dx.doi.org/ 10.1111/jpi.12046 [DOI] [PubMed] [Google Scholar]
- 123.Tal O, Haim A, Harel O, Gerchman Y. Melatonin as an antioxidant and its semi-lunar rhythm in green macroalga Ulva sp. J Exp Bot 2011; 62:1903-10; PMID:21220782; http://dx.doi.org/ 10.1093/jxb/erq378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beilby MJ, Turi CE, Murch SJ. Systems biology analysis of changes in potential across plasma membrane: physiological implications In: Rhythms in Plants. Cham: Springer International Publishing; 2015. pp. 343-66. [Google Scholar]
- 125.Wolf K, Kolář J, Witters E, van Dongen W, van Onckelen H, Macháčková I. Daily profile of melatonin levels in Chenopodium rubrum L. depends on photoperiod. J Plant Physiol 2001; 158:1491-3; http://dx.doi.org/ 10.1078/0176-1617-00561 [DOI] [Google Scholar]
- 126.Byeon Y, Back K. An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J Pineal Res 2014; 56:408-14; PMID:24571270; http://dx.doi.org/ 10.1111/jpi.12129 [DOI] [PubMed] [Google Scholar]
- 127.Byeon Y, Park S, Kim Y-S, Back K. Microarray analysis of genes differentially expressed in melatonin-rich transgenic rice expressing a sheep serotonin N-acetyltransferase. J Pineal Res 2013; 55:357-63; PMID:23889160 [DOI] [PubMed] [Google Scholar]
- 128.Janas KM, Posmyk MM. Melatonin, an underestimated natural substance with great potential for agricultural application. Acta Physiol Plant 2013; 35:3285-92; http://dx.doi.org/ 10.1007/s11738-013-1372-0 [DOI] [Google Scholar]
- 129.Posmyk MM, Bałabusta M, Wieczorek M, Sliwinska E, Janas KM. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J Pineal Res 2009; 46:214-23; PMID:19141087; http://dx.doi.org/ 10.1111/j.1600-079X.2008.00652.x [DOI] [PubMed] [Google Scholar]
- 130.Manchester LC, Tan DX, Reiter RJ, Park W, Monis K. High levels of melatonin in the seeds of edible plants: possible function in germ tissue protection. Life Sci 2000; 67:3023-29; PMID:11125839; http://dx.doi.org/ 10.1016/S0024-3205(00)00896-1 [DOI] [PubMed] [Google Scholar]
- 131.Murch SJ, Alan AR, Cao J, Saxena PK. Melatonin and serotonin in flowers and fruits of Datura metel L. J Pineal Res 2009; 47:277-83; PMID:19732299; http://dx.doi.org/ 10.1111/j.1600-079X.2009.00711.x [DOI] [PubMed] [Google Scholar]
- 132.Shi H, Jiang C, Ye T, Tan D-X, Reiter RJ, Zhang H, Liu R, Chan Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J Exp Bot 2015:66:681-94; PMID:25225478; http://dx.doi.org/ 10.1093/jxb/eru373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao Y, Tan D-X, Lei Q, Chen H, Wang L, Li Q-T, Gao Y, Kong J. Melatonin and its potential biological functions in the fruits of sweet cherry. J Pineal Res 2013; 55:79-88; PMID:23480341; http://dx.doi.org/ 10.1111/jpi.12044 [DOI] [PubMed] [Google Scholar]
- 134.Murch SJ, Hall BA, Le CH, Saxena PK. Changes in the levels of indoleamine phytochemicals during véraison and ripening of wine grapes. J Pineal Res 2010; 49:95-100; PMID:20536685 [DOI] [PubMed] [Google Scholar]
- 135.Ramakrishna A, Giridhar P, Sankar KU. Endogenous profiles of indoleamines: serotonin and melatonin in different tissues of Coffea canephora P ex Fr. as analyzed by HPLC and LC-MS-ESI. Acta Physiol Plant 2012; 34:393-96; http://dx.doi.org/ 10.1007/s11738-011-0829-2 [DOI] [Google Scholar]
- 136.Murch SJ, Simmons CB, Saxena PK. Melatonin in feverfew and other medicinal plants. The Lancet 1997; 350:1598-9; PMID:9393344; http://dx.doi.org/ 10.1016/S0140-6736(05)64014-7 [DOI] [PubMed] [Google Scholar]
- 137.Hernández-Ruiz J, Arnao MB. Distribution of melatonin in different zones of lupin and barley plants at different ages in the presence and absence of light. J Agric Food Chem 2008; 56:10567-73; PMID:18975965; http://dx.doi.org/ 10.1021/jf8022063 [DOI] [PubMed] [Google Scholar]
- 138.Okazaki M, Ezura H. Profiling of melatonin in the model tomato (Solanum lycopersicum L.) cultivar Micro-Tom. J Pineal Res 2009; 46:338-43; PMID:19317796; http://dx.doi.org/ 10.1111/j.1600-079X.2009.00668.x [DOI] [PubMed] [Google Scholar]
- 139.Park S, Le T, Byeon Y, Kim YS. Transient induction of melatonin biosynthesis in rice (Oryza sativa L.) during the reproductive stage. J Pineal Res 2013; 55:40-45; PMID:23110463; http://dx.doi.org/ 10.1111/jpi.12021 [DOI] [PubMed] [Google Scholar]
- 140.Roshchina VV, Melnikova EV. Allelopathy and plant reproductive cells: participation of acetylcholine and histamine in signaling in the interactions of pollen and pistil. Allelopathy J 1998; 7:171-82 [Google Scholar]
- 141.Ljung K. Auxin metabolism and homeostasis during plant development. Development 2013; 140:943-50; PMID:23404103; http://dx.doi.org/ 10.1242/dev.086363 [DOI] [PubMed] [Google Scholar]
- 142.Sun Q, Zhang N, Wang J, Zhang H, Li D, Shi J, Li R, Weeda S, Zhao B, Ren S, Guo Y-D. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J Exp Bot 2015; 66:657-68; PMID:25147270; http://dx.doi.org/ 10.1093/jxb/eru332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ranty B, Cotelle V, Galaud J-P, Mazars C. Nuclear calcium signaling and its involvement in transcriptional regulation in plants. Adv Exp Med Biol 2012; 740:1123-43; PMID:22453986; http://dx.doi.org/ 10.1007/978-94-007-2888-2_51 [DOI] [PubMed] [Google Scholar]
- 144.Batistic O, Kudla J. Analysis of calcium signaling pathways in plants. Biochim Biophysia Acta-Gen Sub 2012; 1820:1283-93; PMID:22061997; http://dx.doi.org/ 10.1016/j.bbagen.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 145.Jones AMP, Cao J, O'Brien R, J MS, Saxena PK. The mode of action of thidiazuron: auxins, indoleamines, and ion channels in the regeneration of Echinacea purpurea L. Plant Cell Rep 2007; 26:1481-90; PMID:17483954; http://dx.doi.org/ 10.1007/s00299-007-0357-0 [DOI] [PubMed] [Google Scholar]
- 146.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trend Plant Sci 2011; 16:300-9; PMID:21482172; http://dx.doi.org/ 10.1016/j.tplants.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 147.Wrzaczek M, Brosché M, Kangasjärvi J. ROS signaling loops — production, perception, regulation. Curr Opin Plant Biol 2013; 16:575-82; PMID:23876676; http://dx.doi.org/ 10.1016/j.pbi.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 148.Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010; 143:606-16; PMID:21074051; http://dx.doi.org/ 10.1016/j.cell.2010.10.020 [DOI] [PubMed] [Google Scholar]
- 149.Leymarie J, Vitkauskaité G, Hoang HH, Gendreau E, Chazoule V, Meimoun P, Corbineau F, El-Maarouf-Bouteau H, Bailly C. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol 2012; 53:96-106; PMID:21937678; http://dx.doi.org/ 10.1093/pcp/pcr129 [DOI] [PubMed] [Google Scholar]
- 150.Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2009; 2:ra45; PMID:19690331 [DOI] [PubMed] [Google Scholar]
- 151.Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JD, Shirasu K, Menke F, Jones A, Zipfel C. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell 2014; 54:43-55; PMID:24630626; http://dx.doi.org/ 10.1016/j.molcel.2014.02.021 [DOI] [PubMed] [Google Scholar]
- 152.Kerchev PI, Fenton B, Foyer CH, Hancock RD. Plant responses to insect herbivory: interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant Cell Environ 2011; 35:441-53; PMID:21752032; http://dx.doi.org/ 10.1111/j.1365-3040.2011.02399.x [DOI] [PubMed] [Google Scholar]
- 153.Steinhorst L, Kudla J. Calcium and reactive oxygen species rule the waves of signaling. Plant Physiol 2013; 163:471-85; PMID:23898042; http://dx.doi.org/ 10.1104/pp.113.222950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Takahashi F, Mizoguchi T, Yoshida R, Ichimura K, Shinozaki K. Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol Cell 2011; 41:649-60; PMID:21419340; http://dx.doi.org/ 10.1016/j.molcel.2011.02.029 [DOI] [PubMed] [Google Scholar]
- 155.Beilby MJ, Khazaaly Al S, Bisson MA. Salinity-induced noise in membrane potential of Characeae Chara australis: effect of exogenous melatonin. J Membrane Biol 2014; 248:93-102; PMID:25378124; http://dx.doi.org/ 10.1007/s00232-014-9746-9 [DOI] [PubMed] [Google Scholar]
- 156.Zhang N, Zhang H-J, Zhao B, Sun Q-Q, Cao Y-Y, Li R, Wu X-X, Weeda S, Li L, Ren S, Reiter RJ, Guo Y-D. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J Pineal Res 2013; 56:39-50; PMID:24102657; http://dx.doi.org/ 10.1111/jpi.12095 [DOI] [PubMed] [Google Scholar]
- 157.Shi H, Qian Y, Tan D-X, Reiter RJ, He C. Melatonin induces the transcripts of CBF/DREB1s and their involvement of both abiotic and biotic stresses in Arabidopsis. J Pineal Res 2015; in press [DOI] [PubMed] [Google Scholar]
- 158.Szafrańska K, Glińska S, Janas KM. Ameliorative effect of melatonin on meristematic cells of chilled and re-warmed Vigna radiata roots. Biol Plant 2012; 57:91-6; http://dx.doi.org/ 10.1007/s10535-012-0253-5 [DOI] [Google Scholar]
- 159.Sarrou E, Therios I, Dimassi-Theriou K. Melatonin and other factors that promote rooting and sprouting of shoot cuttings in Punica granatum cv. Wonderful. Turk J Bot 2014; 38:293-301; http://dx.doi.org/ 10.3906/bot-1302-55 [DOI] [Google Scholar]
- 160.Zuo B, Zheng X, He P, Wang L, Lei Q, Feng C, Zhou J, Li Q, Han Z, Kong J. Overexpression of MzASMTimproves melatonin production and enhances drought tolerance in transgenic plants. J Pineal Res 2014; 57:408-17; PMID:25250844; http://dx.doi.org/ 10.1111/jpi.12180 [DOI] [PubMed] [Google Scholar]
- 161.Byeon Y, Lee HY, Lee K, Back K. A rice chloroplast transit peptide sequence does not alter the cytoplasmic localization of sheep serotonin N-acetyltransferase expressed in transgenic rice plants. J Pineal Res 2014; 57:147-54; PMID:24920304; http://dx.doi.org/ 10.1111/jpi.12151 [DOI] [PubMed] [Google Scholar]
- 162.Litwinczuk W, Wadas-Boron M. Development of highbush blueberry (Vaccinium corymbosum hort. non L.) in vitro shoot cultures under the influence of melatonin. Acta Sci Pol Hort Cult 2009; 8:3-12 [Google Scholar]
- 163.Boccalandro HE, González CV, Wunderlin DA, Silva MF. Melatonin levels, determined by LC-ESI-MS/MS, fluctuate during the day/night cycle in Vitis vinifera cv Malbec: evidence of its antioxidant role in fruits. J Pineal Res 2011; 51:226-32; PMID:21605162; http://dx.doi.org/ 10.1111/j.1600-079X.2011.00884.x [DOI] [PubMed] [Google Scholar]
- 164.Roshchina VV, Roshchina VV. Neurotransmitters in Plant Life. Science Publishers, Inc; 2001 [Google Scholar]