abstract
Signaling mediated by reactive oxygen species (ROS) has emerged as a key component of plants' responses to environmental stress. The ROS-regulated transcription factor ZAT12 was revealed as a negative regulator of iron (Fe) deficiency responses through its direct interaction with the bHLH protein FIT. In the epidermis of the early root differentiation zone, ZAT12 stability depended on the presence of the ZAT12 EAR motif. It was concluded that ZAT12 may be the target of 2 alternative degradation pathways. Here, we present a model aiming to explain the regulatory mechanisms by which ZAT12 could be targeted for degradation and to predict the types of potential regulators involved. In addition to an E3 ubiquitin ligase, we predict 2 critical regulatory factors, namely a protein interacting with the ZAT12 EAR motif and a ROS-responsive regulatory protein.
Keywords: Fe acquisition, FIT, hydrogen peroxide, ROS signaling, ZAT12
In order to adapt to their dynamic surroundings, plants need to efficiently process and respond to a large variety of environmental cues. On cellular level, this response is achieved by rebalancing an array of processes with the help of small molecules that serve as signaling mediators. Reactive oxygen species (ROS) have emerged as key mediators in plant stress responses. The rapid ROS-production and ROS-scavenging reactions allow the precise determination of signal duration, intensity and subcellular location. In addition, secondary ROS production in neighboring cells may be activated, therefore allowing ROS to serve also as long-distance intermediates.1
An increase in ROS production occurs in response to many external stress conditions, such as drought, salinity, temperature stress, nutrient deprivation.2,3 In Arabidopsis (Arabidopsis thaliana), one of the stress-response marker genes induced as a consequence of specific ROS accumulation is ZAT12. It encodes a C2H2 Zn-finger transcription factor required for the upregulation of the ROS signaling-related genes APX1, ZAT7, and WRKY25.4,5 ZAT12 protein was shown to be involved in the response to cold, oxidative and osmotic stress, salinity, and high light.4,6,7
Recently, we showed that ZAT12 links ROS signaling and iron (Fe) acquisition, by directly interacting with the bHLH transcription factor FIT.8 FIT acts as a central transcriptional regulator of the Fe deficiency-induced strategy for Fe acquisition from the soil. This strategy is based on 3 steps: (i) soil acidification, mainly conferred by the H+-ATPase AHA2, (ii) Fe(III) reduction by the FERRIC REDUCTASE-OXIDASE 2 (FRO2), and (iii) subsequent Fe(II) uptake by the IRON-REGULATED TRANSPORTER 1 (IRT1), whereby FIT upregulates AHA2, FRO2 and IRT1 upon Fe starvation.9-11
Fe homeostasis is tightly linked with the formation of ROS. Under excess of free cellular Fe, ROS is generated as a result of the Fenton reaction,12 while under Fe deficiency, a consistently elevated ROS production, probably as a signaling intermediate, was shown to be dependent on FIT activity.8 Consistent with this, FIT was revealed as a target for negative regulation by ZAT12 through direct ZAT12-FIT protein interaction and inhibition of FIT gene expression. It was suggested that ZAT12 can sequester FIT protein molecules, preventing them from activating Fe acquisition.8
Of special interest is to understand the role of ROS, in the form of hydrogen peroxide (H2O2), in the regulation of ZAT12 stability in the epidermis of the early differentiation zone of the root, which is the essential zone for Fe acquisition under deficient Fe supply.11,13-15
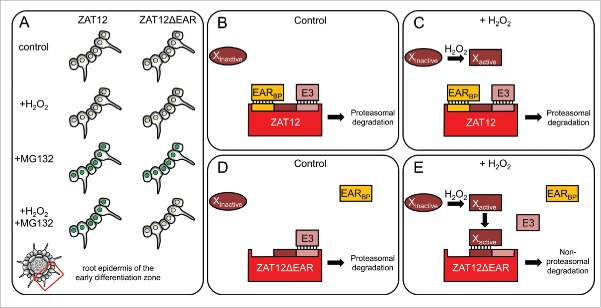
It was found that ZAT12 undergoes proteasome-dependent degradation, which occurs also in the presence of elevated H2O2 levels.8 Deletion analysis showed that the EAR motif, which ZAT12 employs for the interaction with FIT, is essential for this proteasome targeting. ZAT12 proteins without EAR motif are degraded by different pathways, depending on the presence or absence of H2O2 (summarized in Fig. 1A).8 We present here a model aiming to explain these findings and predict the types of potential regulators involved in ZAT12 degradation (Fig. 1B–E).
Figure 1.
Hypothetical model of EAR motif- and H2O2-dependent ZAT12 protein stability regulation in the epidermis of the early root differentiation zone. (A) Protein stability of full-length ZAT12 and ZAT12 lacking the EAR motif (ZAT12ΔEAR) in root epidermis cells of the early differentiation zone. Protein sensitivity to the application of H2O2, proteasome inhibitor MG132, and the combined treatment, is presented as protein accumulation in the nuclei (green circles) of epidermis cells. In the control condition, and whenever no change in protein accumulation was observed, the nuclei are represented as light green circles to reflect the presence of basal protein levels. (B-E) ZAT12 is involved in differential protein-protein interactions either with an EAR motif-binding protein (EARBP) through its EAR motif (yellow) or with an active protein X through an X-binding site (brown). X is an H2O2-dependent activator of non-proteasomal protein degradation. ZAT12 also contains a site for E3 ubiquitin ligase (E3) binding (Indian red). (B, D) Under control conditions characterized by basal H2O2 levels, the protein X remains inactive. ZAT12 exists in 2 different protein complexes, depending on the occupation of its EAR motif, where lack of EARBP binding is mimicked by the lack of EAR motif in ZAT12ΔEAR (D). E3 can bind to ZAT12 and target it for proteasomal degradation. (C, E) Under conditions where enhanced H2O2 levels accumulate, the protein X is activated. It is not able to bind the ZAT12-EARBP complexes due to steric interference caused by EARBP. These complexes can still be targeted to the proteasome by E3 binding. However, activated X can bind EARBP-free ZAT12 and displace E3, leading to a non-proteasomal degradation of ZAT12. The ZAT12ΔEAR form (E) mimics the latter situation.
ZAT12 stability depends on 2 proteins – E3 ubiquitin ligase (E3), for proteasomal targeting, and the protein X, for non-proteasomal targeting. Whether they will bind to ZAT12 or not depends on 2 factors – the interaction of ZAT12 with an EAR motif-binding protein (EARBP), and the presence of a specific ROS signal. EARBP, X and E3 occupy distinct and partially mutually exclusive binding sites on ZAT12. The interaction of EARBP with ZAT12 interferes with X binding, the interaction of X with ZAT12 disturbs E3 binding. Interaction of EARBP with ZAT12 does not influence the formation of E3-ZAT12 complexes in terms of ZAT12 degradation. The role of the ROS signal is to activate the X protein. Thus, in the absence of a specific ROS signal, the cells have basal H2O2 levels (Fig. 1B, D), protein X is inactive and the degradation of ZAT12 is entirely dependent on the E3 protein, irrespective of the EARBP binding. When specific ROS signaling is initiated (Fig. 1C, E), enhanced cellular H2O2 levels activate protein X. Activated X is able to bind ZAT12 when it is not involved in interaction with EARBP. Binding of activated X will prevent E3-ZAT12 interaction and will promote the non-proteasomal degradation of ZAT12. Our model implies that protein X might serve either as a targeting signal for non-proteasomal degradation or it can be a protease itself. The mechanism of X activation could potentially involve protein oxidation which can be either reversible or not.
The reason for the 2 existing ZAT12 degradation mechanisms remains an open question. It has been shown that the stability of the human Iron regulatory protein 2 (IRP2) is also controlled by 2 alternative pathways. Here, the non-proteasomal degradation is the default mechanism under physiological conditions. IRP2 protein overaccumulation, for example upon Fe starvation, leads to saturation of the non-proteasomal pathway and the activation of IRP2 targeting to the proteasome.16 Although in our test conditions we did not observe non-proteasomal degradation of full-length ZAT12 in the epidermis, the non-proteasomal degradation pathway may be relevant if low levels of ZAT12 or specific physiological conditions are present.
ZAT12 emerges as a hub where oxidative stress can be linked to other signaling events in the plant. In the future, it will be interesting to further uncover the mechanisms by which ZAT12 specifically translates ROS signals. For this, it will be crucial to uncover the role of the alternative ZAT12 degradation pathways in regulating ZAT12 activity.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trends Plant Sci 2011; 16:300-9; PMID:21482172; http://dx.doi.org/ 10.1016/j.tplants.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 2.Baxter A, Mittler R, Suzuki N. ROS as key players in plant stress signalling. J Exp Bot 2014; 65:1229-40; PMID:24253197; http://dx.doi.org/ 10.1093/jxb/ert375 [DOI] [PubMed] [Google Scholar]
- 3.Mittler R, Blumwald E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015; 27:64-70; PMID:25604442; http://dx.doi.org/ 10.1105/tpc.114.133090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizhsky L, Davletova S, Liang H, Mittler R. The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J Biol Chem 2004; 279:11736-43; PMID:14722088; http://dx.doi.org/ 10.1074/jbc.M313350200 [DOI] [PubMed] [Google Scholar]
- 5.Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 2005; 17:268-81; PMID:15608336; http://dx.doi.org/ 10.1105/tpc.104.026971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davletova S, Schlauch K, Coutu J, Mittler R. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol 2005; 139:847-56; PMID:16183833; http://dx.doi.org/ 10.1104/pp.105.068254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J 2005; 41:195-211; PMID:15634197; http://dx.doi.org/ 10.1111/j.1365-313X.2004.02288.x [DOI] [PubMed] [Google Scholar]
- 8.Le CTT, Brumbarova T, Ivanov R, Stoof C, Weber E, Mohrbacher J, Fink-Straube C, Bauer P. ZINC FINGER OF ARABIDOPSIS THALIANA12 (ZAT12) Interacts with FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (FIT) Linking Iron Deficiency and Oxidative Stress Responses. Plant Physiol 2015; PMID:26556796; http://dx.doi.org/ 10.1104/pp.15.01589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanov R, Brumbarova T, Bauer P. Fitting into the Harsh Reality: Regulation of Iron Deficiency Responses in Dicotyledonous. Plants Mol Plant 2012; 5:27-42; PMID:21873619; http://dx.doi.org/ 10.1093/mp/ssr065 [DOI] [PubMed] [Google Scholar]
- 10.Brumbarova T, Bauer P, Ivanov R. Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci 2015; 20:124-33; PMID:25499025; http://dx.doi.org/ 10.1016/j.tplants.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 11.Jakoby M, Wang HY, Reidt W, Weisshaar B, Bauer P. FRU (BHLH029) is required for induction of iron mobilization genes in Arabidopsis thaliana. FEBS Lett 2004; 577:528-34; PMID:15556641; http://dx.doi.org/ 10.1016/j.febslet.2004.10.062 [DOI] [PubMed] [Google Scholar]
- 12.Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett 1995; 82–83:969-74; PMID:8597169; http://dx.doi.org/ 10.1016/0378-4274(95)03532-X [DOI] [PubMed] [Google Scholar]
- 13.Ivanov R, Brumbarova T, Blum A, Jantke AM, Fink-Straube C, Bauer P. SORTING NEXIN1 Is Required for Modulating the Trafficking and Stability of the Arabidopsis IRON-REGULATED TRANSPORTER1. Plant Cell 2014; PMID:24596241; http:dx.doi.org/http://dx.doi.org/ 10.1105/tpc.113.116244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blum A, Brumbarova T, Bauer P, Ivanov R. Hormone influence on the spatial regulation of expression in iron-deficient roots. Plant Signal Behav 2014; 9; PMID:24721759; http://dx.doi.org/ 10.4161/psb.28787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 2008; 320:942-5; PMID:18436742; http://dx.doi.org/ 10.1126/science.1153795 [DOI] [PubMed] [Google Scholar]
- 16.Chang AH, Jeong J, Levine RL. Iron regulatory protein 2 turnover through a nonproteasomal pathway. J Biol Chem 2011; 286:23698-707; PMID:21558272; http://dx.doi.org/ 10.1074/jbc.M110.216788 [DOI] [PMC free article] [PubMed] [Google Scholar]