Abstract
AtPTPA is a critical regulator for the holoenzyme assembling of protein phosphatase 2A (PP2A) in Arabidopsis. Characterization of AtPTPA improves our understanding of the function and regulation of PP2A in eukaryotes. Further analysis of AtPTPA-overexpressing plants indicates that AtPTPA increases PP2A activity by promoting PP2A's AC dimer formation, thereby holoenzyme assembling. Plant hormone abscisic acid (ABA) reduces PP2A enzyme activity by negatively affects PP2A's AC dimer formation. Therefore, AtPTPA is a positive factor that promotes PP2A holoenzyme assembly, and ABA is a negative factor that prevents PP2A holoenzyme assembly.
Keywords: Arabidopsis, protein phosphatase 2A, PP2A holoenzyme assembly, PP2Ac methylation
Protein phosphatase 2A (PP2A) is an important regulator for plant growth and development, and is essential for proper response to biotic and abiotic stresses in plants.1 PP2A is made of 3 subunits: the scaffolding subunit A, the regulatory subunit B, and the catalytic subunit C.2 We recently reported the functional characterization of AtPTPA, a critical regulator for PP2A activity in Arabidopsis.3 AtPTPA exerts its action on PP2A through binding to PP2A's AC dimer, and the action of AtPTPA in changing the conformation of the C subunits (i.e. PP2Ac) is critical for PP2A holoenzyme formation and maintenance of PP2A activity.3 Reduced AtPTPA in Arabidopsis (e.g. to 10% of wild-type level) leads to dramatic loss of PP2Ac methylation and reduced PP2A activity,3 indicating a dependency of PP2A activity for AtPTPA. To test if overexpression of AtPTPA in plants would increase PP2A activity, we analyzed PP2A enzyme activity using plant cellular extracts from GFP-AtPTPA overexpressing plants and wild-type plants. The results showed that the PP2A activity was increased by 50% in GFP-AtPTPA overexpressing plants in comparing to that in wild-type plants (Fig. 1A), indicating that overexpression of AtPTPA in plants indeed increases PP2A activity.
Figure 1.
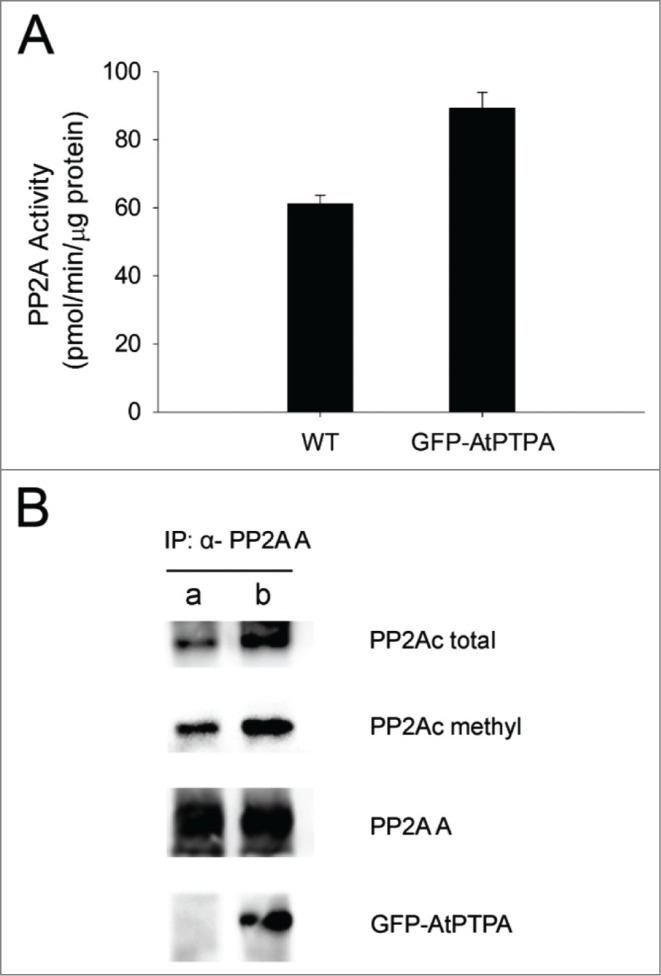
Overexpression of AtPTPA increases PP2A activity by promoting PP2A holoenzyme assembly. (A) PP2A activity is higher in GFP-AtPTPA overexpressing plants in comparing to wild-type plants. Bar indicates standard deviation. The method for PP2A enzyme assay was described in Chen et al.3 (B) PP2A-A antibodies bring down more PP2A-C subunits from cellular extracts of GFP-AtPTPA overexpressing plants than that from wild-type plants. Lane a, immunoprecipitated cellular proteins from wild-type plants; lane b, immunoprecipitated cellular proteins from GFP-AtPTPA overexpressing plants. Equal amount of total proteins from immunoprecipitation was loaded in a and b. Different antibodies were used in the Western blots and these antibodies recognize PP2A-C subunits (PP2Ac total), methylated PP2Ac (PP2Ac methyl), PP2A-A subunits (PP2A A), and GFP-AtPTPA (GFP-AtPTPA fusion protein), respectively.
To study how the PP2A activity was increased in GFP-AtPTPA overexpressing plants, we examined the abundances of methylated and total PP2Ac in GFP-AtPTPA overexpressing plants. We found that both methylated and total PP2Ac were not changed in these plants (data not shown). However, when we used PP2A-A antibodies to perform immunoprecipitation experiments to examine the AC dimer formation in GFP-AtPTPA overexpressing plants and wild-type plants, we found that both total PP2Ac and methylated PP2Ac were pulled-down at higher amounts in GFP-AtPTPA overexpressing plants than in wild-type plants (Fig. 1B). Under normal conditions, more than 90% of PP2Ac is methylated in order to ensure high PP2A activities in plants.3 While the methylated PP2Ac in GFP-AtPTPA overexpressing plants is similar to wild-type level, our data suggest that more AC dimers are found in GFP-AtPTPA overexpressing plants, therefore more AC dimer are available for PP2A holoenzyme assembly, which explains why PP2A activity was increased in GFP-AtPTPA overexpression plants.
ABA was previously reported to quickly down-regulate PP2A activity in plants,4 yet nothing was known about how ABA regulates PP2A at the molecular level. The half-life of PP2A catalytic subunit is about 2 hours in yeast5 and even longer in mammalian cells,6 therefore the increased turnover of catalytic subunits by ABA might be the reason for decreased PP2A activity after ABA treatment. To test this possibility, we performed ABA inhibition experiments. Our results indicated that ABA could reduce PP2A activity within one hour (Fig. 2A), but the steady-state levels of PP2Ac and methylated PP2Ac were both not affected by the ABA treatment (right column, Fig. 2B), indicating that ABA does not increase the turnover rate of PP2Ac. Interestingly, the ABA induced reduction of PP2A activity was not as dramatic as previously reported.7 This is possibly due to different substrates that were used in the PP2A activity assays. Our data clearly show that the PP2Ac abundance is not the reason for ABA induced reduction of PP2A activity and other posttranslational events such as protein phosphorylation or de-phosphorylation might be the reason for the changed PP2A activity after ABA treatment.
Figure 2.
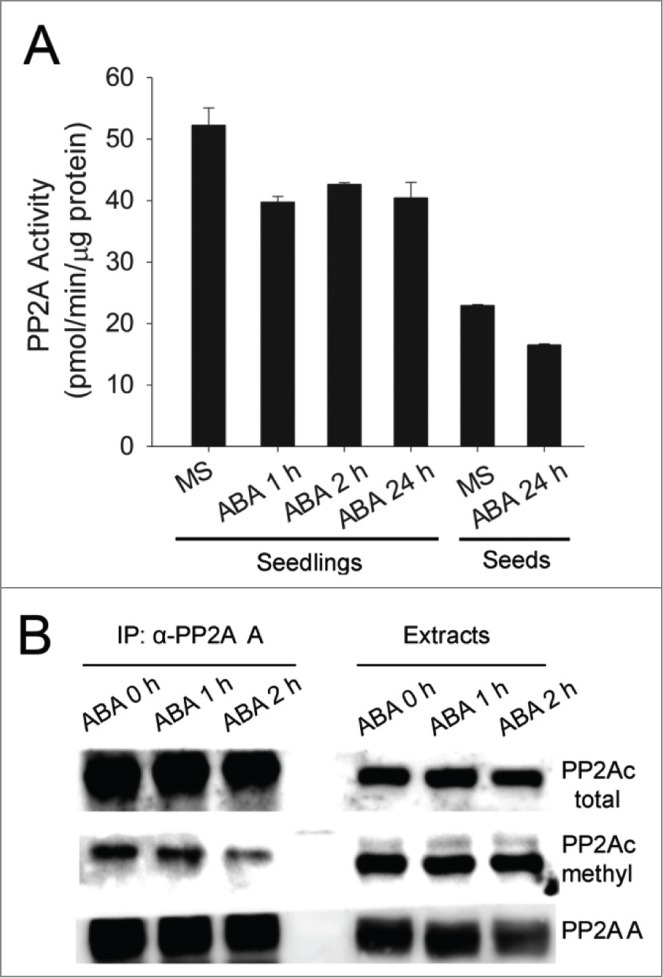
Effects of ABA on PP2A activity and formation of PP2A holoenzyme assembly. (A) ABA treatment and cold reduce PP2A activity in plant seedlings and seeds. MS, Murashige and Skoog medium; ABA 1 h, ABA 2 h, and ABA 24 h, plant seedlings were treated with ABA for 1, 2, and 24 hours, respectively. Bar indicates standard deviation. (B) Immunoprecipitation with PP2A-A antibodies brings down less PP2A-C subunits after ABA treatment. Proteins recognized by different antibodies are labeled on the right.
We then analyzed the AC dimer status after ABA treatment. Again, we used PP2A-A antibodies to immuno-precipitate PP2A from cellular extracts of GFP-AtPTPA overexpressing plants before and after ABA treatment. The result showed that total PP2Ac and methylated PP2Ac bound to A subunits were both reduced after plants were treated with ABA (left column, Fig. 2B). These data indicate that ABA might reduce PP2A holoenzyme formation by preventing C subunits from binding to A subunits, as less PP2Ac, especially methylated PP2Ac, were pulled down with PP2A-A antibodies, therefore leading to reduced PP2A activity after ABA treatment.
As one of the most important regulators in eukaryotes, PP2A is involved in diverse aspects of cellular signaling and metabolisms in plants.1-4,7-13 AtPTPA, the critical regulator for PP2A holoenzyme assembly, is essential for a number of PP2A mediated cellular processes like ABA signaling and abiotic stress responses.1,3 Here we provide evidence that the effect of ABA on PP2A activity is to prevent C subunits from binding to A subunits, thereby preventing the formation of active PP2A holoenzyme. To understand how PP2A is involved in ABA signaling in plants, it will be necessary to elucidate the molecular mechanism of how ABA affects the AC dimer formation during PP2A assembly, which will be the next challenge in studying how PP2A is regulated by plant hormone ABA.
Funding
This research was supported by Department of Biological Sciences of Texas Tech University, and partially supported by grants from the International Cooperation, Innovation and Upgrading Programs of Zhejiang Academy of Agricultural Sciences (2014CX005), and the National Natural Science Foundation of China (31170793 and 31402140).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Farkas I, Dombradi V, Miskei M, Szabados L, Koncz C. Arabidopsis PPP family of serine/threonine phosphatases. Trends Plant Sci 2007; 12:169-76; PMID:17368080; http://dx.doi.org/ 10.1016/j.tplants.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 2.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 2001; 353:417-39; PMID:11171037; http://dx.doi.org/ 10.1042/0264-6021:3530417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Hu R, Zhu Y, Shen G, Zhang H. Arabidopsis PHOSPHOTYROSYL PHOSPHATASE ACTIVATOR is essential for PROTEIN PHOSPHATASE 2A holoenzyme assembly and plays important roles in hormone signaling, salt stress response, and plant development. Plant Physiol 2014; 166:1519-34; PMID:25281708; http://dx.doi.org/ 10.1104/pp.114.250563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pernas M, Garcia-Casado G, Rojo E, Solano R, Sanchez-Serrano JJ. A protein phosphatase 2A catalytic subunit is a negative regulator of abscisic acid signalling. Plant J 2007; 51:763-78; PMID:17617176; http://dx.doi.org/ 10.1111/j.1365-313X.2007.03179.x [DOI] [PubMed] [Google Scholar]
- 5.Fellner T, Lackner DH, Hombauer H, Piribauer P, Mudrak I, Zaragoza K, Juno C, Ogris E. A novel and essential mechanism determining specificity and activity of protein phosphatase 2A (PP2A) in vivo. Genes Dev 2003; 17:2138-50; PMID:12952889; http://dx.doi.org/ 10.1101/gad.259903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang L, Stanevich V, Satyshur KA, Kong M, Watkins GR, Wadzinski BE, Sengupta R, Xing Y. Structural basis of protein phosphatase 2A stable latency. Nat Commun 2013; 4:1699; PMID:23591866; http://dx.doi.org/ 10.1038/ncomms2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu R, Zhu Y, Shen G, Zhang H. TAP46 plays a positive role in the ABSCISIC ACID INSENSITIVE5-regulated gene expression in Arabidopsis. Plant Physiol 2014;164:721-34; PMID:24357600; http://dx.doi.org/ 10.1104/pp.113.233684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garbers C, DeLong A, Deruere J, Bernasconi P, Soll D. A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J 1996; 15:2115-24; PMID:8641277 [PMC free article] [PubMed] [Google Scholar]
- 9.Kwak JM, Moon JH, Murata Y, Kuchitsu K, Leonhardt N, DeLong A, Schroeder JI. Disruption of a guard cell-expressed protein phosphatase 2A regulatory subunit, RCN1, confers abscisic acid insensitivity in Arabidopsis. Plant Cell 2002; 14:2849-61; PMID:12417706; http://dx.doi.org/ 10.1105/tpc.003335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen PB, Chang C. The Arabidopsis eer1 mutant has enhanced ethylene responses in the hypocotyl and stem. Plant Physiol 2001; 125:1061-73; PMID:11161061; http://dx.doi.org/ 10.1104/pp.125.2.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, et al.. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 2007; 130:1044-56; PMID:17889649; http://dx.doi.org/ 10.1016/j.cell.2007.07.033 [DOI] [PubMed] [Google Scholar]
- 12.Muday GK, Brady SR, Argueso C, Deruere J, Kieber JJ, DeLong A. RCN1-regulated phosphatase activity and EIN2 modulate hypocotyl gravitropism by a mechanism that does not require ethylene signaling. Plant Physiol 2006; 141:1617-29; PMID:16798939; http://dx.doi.org/ 10.1104/pp.106.083212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skottke KR, Yoon GM, Kieber JJ, DeLong A. Protein phosphatase 2A controls ethylene biosynthesis by differentially regulating the turnover of ACC synthase isoforms. PLoS Genet 2011; 7:e1001370; PMID:21533019; http://dx.doi.org/ 10.1371/journal.pgen.1001370 [DOI] [PMC free article] [PubMed] [Google Scholar]


