ABSTRACT
Development of the plant aerial organs epidermis involves a complex interplay of cytoskeletal rearrangements, membrane trafficking-dependent cell surface expansion, and intra- and intercellular signaling, resulting in a pattern of perfectly interlocking pavement cells. While recent detailed in vivo observations convincingly identify microtubules rather than actin as key players at the early stages of development of pavement cell lobes in Arabidopsis, mutations affecting the actin-nucleating ARP2/3 complex are long known to reduce pavement cell lobing, suggesting a central role for actin. We have now shown that functional impairment of the Arabidopsis formin FH1 enhances both microtubule dynamics and pavement cell lobing. While formins are best known for their ability to nucleate actin, many members of this old gene family now emerge as direct or indirect regulators of the microtubule cytoskeleton, and our findings suggest that they might co-ordinate action of the two cytoskeletal systems during pavement cell morphogenesis.
KEYWORDS: Actin, cell growth, epidermal pavement cells, formins, FH2 proteins, microtubules
Abbreviations
- ABP1
auxin binding protein 1
- ARP2/3
actin-related proteins 2 and 3
- DAG
days after germination
- FH1
formin homolog 1
- FH2
formin homology domain 2
- FH17
formin homolog 17
- ICR1
interactor of constitutively active ROPs 1
- PIN
pin-formed
- PTEN
phosphatase and tensin homolog
- RIC
RHO-interacting CRIB
- RHO
RAS homolog
- ROP
RHO of plants
- SMIFH2
small molecule inhibitor of FH2
In the second edition of his influential monograph “On Growth and Form,” the great D´Arcy Thompson already included the notion of auxin-modulated local differences in plant cell wall extensibility as a pre-requisite of non-isotropic cell growth.1,p. 281 In the epidermis of above-ground plant organs, in particular cotyledons and leaves, cell wall expansion is precisely coordinated among neighboring cells, resulting in the development of a layer of intimately packed, interdigitating pavement cells whose shape often resembles jigsaw puzzle pieces (reviewed in refs. 2, 3). The concerted development of lobes and indentations (necks) in neighboring cells involves assembly of prominent microtubule bundles at the neck regions,4 and requires proper function of the actin cytoskeleton (or at least the ARP2/3 actin nucleation complex; summarized in ref. 5), myosins,6 the microtubule cytoskeleton including some microtubule-binding proteins,7,8 and the membrane trafficking apparatus.9,10
Pavement cell morphogenesis is also controlled or modulated by a number of signaling pathways, involving those depending on RHO (ROP) GTPases and their effectors including RIC family and ICR1 proteins,11-13 and (not surprisingly) also auxin.14-16 However, the cytokinin response pathway, as well as some genes previously described in the context of sugar signaling (see ref. 3), brassinosteroid response,17 and plant defense18 have been also implicated. Part of the support for auxin involvement comes, unfortunately, from the phenotype of the Arabidopsis abp1-5 mutant which carries a point mutation in the Auxin Binding Protein 1 gene and exhibits decreased pavement cell lobing.14,16 However, genuine null mutations in this gene have normal pavement cells,19 and the abp1-5 line carries numerous additional mutations outside the ABP1 locus, obviously contributing to its phenotype.20 Nevertheless, pavement cell lobing is also altered in auxin biosynthesis mutants (reviewed in ref. 14), and the ROP-dependent pathway, mediated by ICR1 and the exocyst complex subunit SEC3, acts at least in part through modulation of membrane trafficking,12 which important not only for cell expansion (see ref. 21) but also for the localization of PIN auxin transporters, also implicated in the regulation of pavement cell morphogenesis.14,15
Relationships between these molecular mechanisms and signaling pathways are still far from clear, including the relative contribution of the two main cytoskeletal systems at various stages of pavement cell morphogenesis. While microtubule bundles are generally acknowledged as restricting growth at the pavement cell indentations,3,4,13 a prominent role has been proposed for a fine actin meshwork assembling at expanding lobes, presumably driving lobe outgrowth in a manner reminiscent of tip growth or of leading edge advancement in metazoan cells.2,11 However, detailed in vivo time-lapse observations of fluorescent protein-labeled microtubules and microfilaments in Arabidopsis cotyledon epidermis, recently reported by Armour et al.,22 convincingly identify establishment of microtubule bands at incipient indentation sites as the first detectable event of pavement cell shaping, taking place long before actual lobe outgrowth and prior to any noticeable actin enrichment in future lobes. Although actin apparently accumulates in growing lobes at advanced stages of expansion,11 a recent study did not confirm the presumed lobe-specific localization of the ARP2/3 actin nucleation complex.23 This is not at odds with the previously reported reduced pavement cell lobing in mutants defective in the function of the ARP2/3 complex5 or in other microfilament-dependent processes,6,22 since these mutants might suffer by partial impairment of actin- and myosin-dependent intracellular transport, resulting in reduced cell expansion within an existing pattern of lobes and indentations rather than an alteration of this pattern.2 Even more likely, altered actin dynamics may affect microtubule organization,5 a phenomenon nowadays well documented.24
We recently reported that impairment of formin function in Arabidopsis (either by mutations in the FH1 locus or by pharmacological intervention) leads to increased complexity of cotyledon pavement cell shape, associated with stabilization and bundling of microfilaments, reduced density of fine cortical actin meshwork, and increased dynamics of cortical microtubules.25 Thus, formins, members of an evolutionarily ancient family of eukaryotic proteins possessing the FH2 domain whose dimer can nucleate actin, may play a key part in such actin-microtubule co-ordination. Indeed, formins often participate in microtubule organization in both metazoans and plants (reviewed in refs. 26, 27). Angiosperms have numerous formin paralogs that form clades termed Class I (usually with a transmembrane domain) and Class II (often with PTEN-like membrane binding domain). In Arabidopsis, FH1 is the most ubiquitously expressed Class I formin during vegetative development (see refs. 25, 27). This may explain the similarity of pavement cell shape changes caused by fh1 loss-of-function mutation and by general inhibition of formin-dependent actin polymerization by treatment with the SMIFH2 compound.28,29
In mature tobacco epidermal cells, heterologous FH1 specifically decorates plasmalemma regions free of cortical microtubules30 but its natural localization during early pavement cell development awaits characterization. Most other Class I formins are also inserted into membranes; additional plant formins either possess membrane-binding domains (Class II, see above), or at least may bind other membrane-associated proteins; this might perhaps include heterodimerization with membrane-bound formins via the conserved FH2 domain. Thus, formins are emerging as plausible candidates for co-ordinating cytoskeletal and membrane dynamics at the cell cortex.27 Intriguingly, the Arabidopsis abp1-5 line carries several mutations inactivating the FH17 gene, encoding a yet uncharacterized Class II formin.20 It remains to be seen if this locus contributes to the abp1-5 pavement cell lobing defect that was originally attributed to impairment of auxin signaling.
Regardless of the above-outlined open questions (and many others bound to emerge from future research), results of the recent studies can be summarized (Fig. 1), updating previously published models of pavement cell morphogenesis,3,11 and advancing thereby our understanding of what the classic has termed “the heterogenous chemistry of the cell”1,p. 400 - or, in today's´ language, the complex interplay of structural and regulatory components resulting in precise specification and coordination of the plant cell morphogenesis required for formation of functional tissues and organs.
Figure 1.
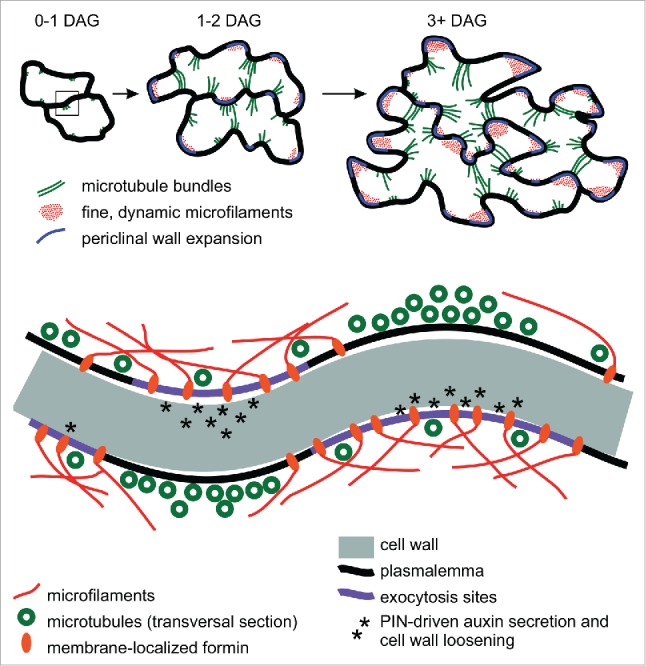
A model of the structural aspects of pavement cell shape specification. Top: temporal sequence of cytoskeletal rearrangements22 during early stages of pavement cell development.11 Bottom: close-up of a periclinal section of the interface of two neighboring cells at an early stage of pattern establishment (box in the top part of the figure). Membrane-anchored formins may contribute to the focusing of microtubule bundles to future indentations by restricting lateral mobility of cortical microtubules through nucleation of membrane-attached microfilaments, promoting assembly of cortically anchored actin cables.25 Unlike ARP2/3-nucleated actin arrays, these cables might form only a sparse meshwork, sufficient to prevent microtubule bundling but not interfering with exocytosis (compare ref. 21). At later stages, ARP2/3-mediated actin nucleation might take place on the existing formin-generated actin filaments, aiding actomyosin-driven vesicle delivery to expanding areas of the cell cortex. DAG - days after germination.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Amparo Rosero for the many inspiring ideas shared during her time in our laboratory, and Ministry of Education, Youth and Sports of the Czech Republic for support from the NPUI LO1417 project.
References
- 1.Thompson DW. On growth and form 2nd rev. ed. Cambridge: University Press, New York: The Macmillan Company, 1945, 1116 p; https://archive.org/details/ongrowthform00thom [Google Scholar]
- 2.Ivakov A, Persson S. Plant cell shape: modulators and measurements. Front Plant Sci 2013; 4:439; PMID:24312104; http://dx.doi.org/ 10.3389/fpls.2013.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacques E, Verbelen JP, Vissenberg K. Review on shape formation in epidermal pavement cells of the Arabidopsis leaf. Funct Plant Biol 2014; 41:914-621; PMID:26047974; http://dx.doi.org/ 10.1071/FP13338 [DOI] [PubMed] [Google Scholar]
- 4.Panteris E, Galatis B. The morphogenesis of lobed plant cells in the mesophyll and epidermis: organization and distinct roles of cortical microtubules and actin filaments. New Phytol 2005; 167:721-32; PMID:16101909; http://dx.doi.org/ 10.1111/j.1469-8137.2005.01464.x [DOI] [PubMed] [Google Scholar]
- 5.Mathur J. The ARP2/3 complex: giving plant cells a leading edge. Bioessays 2005; 27:377-87; PMID:15770684; http://dx.doi.org/ 10.1002/bies.20206 [DOI] [PubMed] [Google Scholar]
- 6.Ojangu EL, Tanner K, Pata P, Järve K, Holweg CL, Truve E, Paves H. Myosins XI-K, XI-1, and XI-2 are required for development of pavement cells, trichomes, and stigmatic papillae in Arabidopsis. BMC Plant Biol 2012; 12:81; PMID:22672737; http://dx.doi.org/ 10.1186/1471-2229-12-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Zhu L, Liu B, Wang C, Jin L, Zhao Q, Yuan M. Arabidopsis MICROTUBULE-ASSOCIATED PROTEIN18 functions in directional cell growth by destabilizing cortical microtubules. Plant Cell 2007; 19:877-89; PMID:17337629; http://dx.doi.org/ 10.1105/tpc.106.048579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrose JC, Shoji T, Kotzer AM, Pighin JA, Wasteneys GO. The Arabidopsis CLASP gene encodes a microtubule-associated protein involved in cell expansion and division. Plant Cell 2007; 19:2763-75; PMID:17873093; http://dx.doi.org/ 10.1105/tpc.107.053777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falbel TG, Koch LM, Nadeau JA, Segui-Simarro JM, Sack FD, Bednarek SY. SCD1 is required for cytokinesis and polarized cell expansion in Arabidopsis thaliana. Development 2003; 130:4011-24; PMID:12874123; http://dx.doi.org/ 10.1242/dev.00619 [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Zhang H, Liu P, Hao H, Jin JB, Lin J. Arabidopsis R-SNARE proteins VAMP721 and VAMP722 are required for cell plate formation. PLoS One 2011; 6:e26129; PMID:22022536; http://dx.doi.org/ 10.1371/journal.pone.0026129 Epub 2011 Oct 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 2005; 120:687-700; PMID:15766531; http://dx.doi.org/ 10.1016/j.cell.2004.12.026 [DOI] [PubMed] [Google Scholar]
- 12.Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S. A novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol 2007; 17:947-52; PMID:17493810; http://dx.doi.org/ 10.1016/j.cub.2007.04.038 [DOI] [PubMed] [Google Scholar]
- 13.Fu Y, Xu T, Wen M, Yang Z. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr Biol 2009; 19:1827-32; PMID:19818614; http://dx.doi.org/ 10.1016/j.cub.2009.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, Perrot-Rechenmann C, Friml J, Jones AM, Yang Z. Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 2010; 143:99-110; PMID:20887895; http://dx.doi.org/ 10.1016/j.cell.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagawa S, Xu T, Lin D, Dhonukshe P, Zhang X, Friml J, Scheres B, Fu Y, Yang Z. ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS Biol 2012; 10:e1001299; PMID:22509133; http://dx.doi.org/ 10.1371/journal.pbio.1001299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu T, Dai N, Chen J, Nagawa S, Cao M, Li H, Zhou Z, Chen X, De Rycke R, Rakusová H, et al.. Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 2014; 343:1025-8; PMID:24578577; http://dx.doi.org/ 10.1126/science.1245125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhiponova MK, Vanhoutte I, Boudolf V, Betti C, Dhondt S, Coppens F, Mylle E, Maes S, González-García MP, Caño-Delgado AI, et al.. Brassinosteroid production and signaling differentially control cell division and expansion in the leaf. New Phytol 2013; 197:490-502; PMID:23253334; http://dx.doi.org/ 10.1111/nph.12036 [DOI] [PubMed] [Google Scholar]
- 18.Han B, Chen L, Wang J, Wu Z, Yan L, Hou S. Constitutive Expresser of Pathogenesis Related Genes 1 is required for pavement cell morphogenesis in Arabidopsis. PloS One 2015; 10:e0133249; PMID:26193674; http://dx.doi.org/ 10.1371/journal.pone.0133249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Y, Zhang Y, Zhang D, Dai X, Estelle M, Zhao Y. Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc Natl Acad Sci USA 2015; 112:2275-80; PMID:25646447; http://dx.doi.org/ 10.1073/pnas.1500365112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enders TA, Oh S, Yang Z, Montgomery BL, Strader LC. Genome sequencing of Arabidopsis abp1-5 reveals second-site mutations that may affect phenotypes. Plant Cell 2015; 27:1820-186; PMID:26106149; http://dx.doi.org/ 10.1105/tpc.15.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Žárský V, Cvrčková F, Potocký M, Hála M. Exocytosis and cell polarity in plants - exocyst and recycling domains. New Phytol 2009; 183:255-72; PMID:19496948; http://dx.doi.org/ 10.1111/j.1469-8137.2009.02880.x [DOI] [PubMed] [Google Scholar]
- 22.Armour WJ, Barton DA, Law AML, Overall RL. Differential growth in periclinal and anticlinal walls during lobe formation in Arabidopsis cotyledon pavement cells. Plant Cell 2015; 27:2484-500; PMID:26296967; http://dx.doi.org/ 10.1105/tpc.114.126664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Mallery EL, Szymanski DB. ARP2/3 localization in Arabidopsis leaf pavement cells: a diversity of intracellular pools and cytoskeletal interactions. Front Plant Sci 2013; 4:238; PMID:23874346; http://dx.doi.org/ 10.3389/fpls.2013.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sampathkumar A, Lindeboom JJ, Debolt S, Gutierrez R, Ehrhardt DW, Ketelaar T, Persson S. Live cell imaging reveals structural associations between the actin and microtubule cytoskeleton in Arabidopsis. Plant Cell 2011; 23:2302-13; PMID:21693695; http://dx.doi.org/ 10.1105/tpc.111.087940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosero A, Oulehlová D, Stillerová L, Schiebertová P, Grunt M, Žárský V, Cvrčková F. Arabidopsis FH1 formin affects cotyledon pavement cell shape by modulating cytoskeleton dynamics. Plant Cell Physiol in press; PMID:26738547; http://dx.doi.org/ 10.1093/pcp/pcv209 [DOI] [PubMed] [Google Scholar]
- 26.Bartolini F, Gundersen GG. Formins and microtubules. Biochim Biophys Acta 2010; 1803:164-73; PMID:19631698; http://dx.doi.org/ 10.1016/j.bbamcr.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cvrčková F, Oulehlová D, Žárský V. Formins: linking cytoskeleton and endomembranes in plant cells. Int J Mol Sci 2015; 16:1-18; PMID:25546384; http://dx.doi.org/ 10.3390/ijms16010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizvi SA, Neidt EM, Cui J, Feiger Z, Skau CT, Gardel ML, Kozmin SA, Kovar DR. Identification and characterization of a small molecule inhibitor of formin-mediated actin assembly. Chem Biol 2009; 16:1158-68; PMID:19942139; http://dx.doi.org/ 10.1016/j.chembiol.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao L, Henty-Ridilla JL, Blanchoin L, Staiger CJ. Profilin-dependent nucleation and assembly of actin filaments controls cell elongation in Arabidopsis. Plant Physiol 2016; 170:220-323; PMID:2657459; http://dx.doi.org/ 10.1104/pp.15.01321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinière A, Gayral P, Hawes C, Runions J. Building bridges: formin1 of Arabidopsis forms a connection between the cell wall and the actin cytoskeleton. Plant J 2011; 66:354-65; PMID:21241388; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04497.x [DOI] [PubMed] [Google Scholar]


