ABSTRACT
The holoparasitic angiosperm Cuscuta develops haustoria that enable it to feed on other plants. Recent findings corroborate the long-standing theory that cell wall modifications are required in order for the parasite to successfully infect a host, and further suggest that changes to xyloglucan through the activity of xyloglucan endotransglucosylases/hydrolases (XTHs) are essential. On the other hand, XTH expression was also detected in resistant tomato upon an attack by Cuscuta, which suggests that both host and parasite use these enzymes in their “arms race.” Here, we summarize existing data on the cell wall-modifying activities of XTHs during parasitization and present a model suggesting how XTHs might function to make the host's resources accessible to Cuscuta.
KEYWORDS: Cell wall; Cuscuta, host infection, parasitic plant, xyloglucan endotransglucosylase/hydrolase (XTH)
Abbreviations
- XEH
xyloglucan endohydrolysis
- XET
xyloglucan endotransglucosylation
- XTH
xyloglucan endotransglucosylase/hydrolase
- XyG
xyloglucan
Parasitic plants have steered away from the self-sufficient, photoautotrophic lifestyle of most plants and, rather than searching for light and producing photosynthates, instead search for other plants and consume what they produce.1 This way of life was made possible by the evolution of specialized infection/feeding organs called haustoria. However, before the parasite can establish direct contact with the vascular bundles of its host, the haustorium has to first penetrate the epidermis of the host plant and grow several millimeters through the host cortex. This invasion is impeded firstly by the cuticle and secondly by the polysaccharide-rich wall of the host plant cells. Correspondingly, the relevance of cell wall changes during host infection by the parasitic plant genus Cuscuta has long been postulated, and the current understanding is that the host-invasive growth of the haustorium is mediated by both mechanical pressure and modifications to host cell walls.1,2 However, although Cuscuta haustorium development has been studied in great morphological detail,3-5 the specific mechanisms underlying the formation of the haustorium and the progress of host infection have not been elucidated. In reviewing the relevant literature, we here propose that cell wall modifications through the activity of xyloglucan endotransglucosylases/hydrolases (XTHs) play a significant role in the Cuscuta parasitization strategy.
Cuscuta XTHs facilitate host plant infection
Xyloglucan (XyG) is a hemicellulose that associates with cellulose through hydrogen bonds. The suggested function of XyG is to cross-link the linear cellulose microfibrils and thus establish a cellulose/xyloglucan network that plays a significant role in wall architecture. Consequently, the strength and extensibility of the cell wall can be regulated by agents that act on XyG. XTHs are a family of enzymes that cleave the backbone of XyG employing 2 different activities: i) xyloglucan endotransglucosylation (XET) by which the reducing end of the cleaved XyG is grafted onto an acceptor XyG chain or ii) xyloglucan endohydrolysis (XEH) in which water acts as the acceptor.6 Modification of the cellulose/xyloglucan network resulting from XTH activities can promote turgor-driven cell expansion by loosening the wall.6,7 Thus, the activity of these enzymes is generally associated with growing plant tissues. However, it has also been reported that the activity of XTHs can inhibit plant cell growth,8 possibly by wall strengthening through increasing the number of XyGs that cross-link cellulose microfibrils.9 Whereas XTHs carrying out XET can both break and join cross-linking microfibrils, and thus loosen and strengthen the cell wall, respectively (Fig. 1A and B), the XEH activity of these enzymes can by itself only contribute to wall loosening (Fig. 1C).
Figure 1.
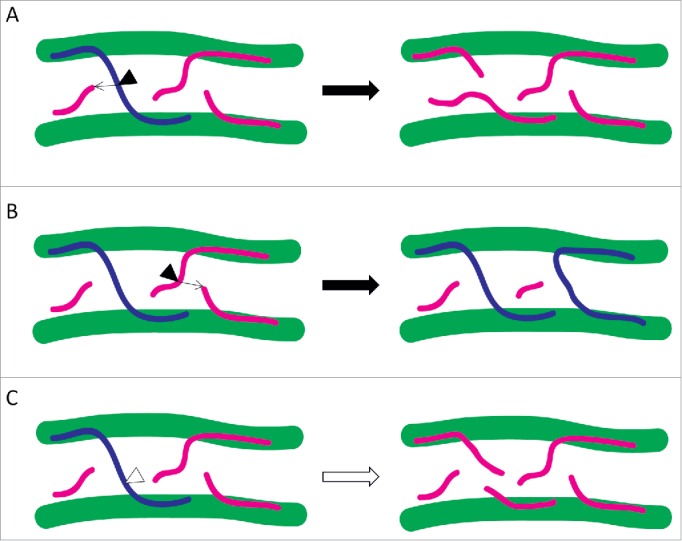
Cell wall loosening and strengthening activities of XTHs through the respective breaking or joining of XyGs that cross-link (blue) or do not cross-link (magenta) 2 cellulose microfibrils (green). XTHs carrying out XET (black triangles) are illustrated together with arrows indicating the respective acceptor XyGs. While XET activity can either (A) loosen or (B) strengthen cell walls, (C) XTHs performing XEH (white triangle) only contribute to wall loosening.
Genes encoding XTHs have been reported to be expressed more highly in the young haustorium than in the stem of Cuscuta pentagona.10 In a recent study,11 we identified 2 Cuscuta reflexa XTHs that were highly expressed at the onset of haustorium development. The closest homologues in another Cuscuta species also displayed high expression levels in young haustoria. Corroborating the gene expression data, immunolocalization and XET action assays indicated that XTHs were carrying out XET in the walls of elongating cells during the early stages of host infection.11 Taken together, these data suggest that the XET activity of Cuscuta XTHs loosen the wall of cells on the side of the parasite facing the host plant and thereby promote the swelling of the parasite stem toward the host surface (Fig. 2A). In the same study, the XET activity was shown to persist throughout host infection on the side of the parasite facing the host and in the endophytic part of the haustorium, indicating that Cuscuta XTHs could also play a role in the host-invasive growth of the infection organ. Increased levels of XyG degradation were reported in the haustorium of C. reflexa and in the infected host plant.12 This is in agreement with the reduced levels of XyG found in the phloic hyphae of the mature haustorium and in the adjoining host phloem cells.5 It is, therefore, a logical assumption that Cuscuta XTHs may not only promote growth within the parasites own borders, but may in addition be secreted from the haustorium into the adjacent host tissue, loosening host cell walls and consequently enabling the infection organ to expedite its growth into the host plant (Fig. 2B). When the haustorium reaches the vascular tissue of its host, searching hyphae emerge and differentiate into the necessary cell types responsible for the uptake of water, minerals and sugars.5 XTHs have previously been suggested to play a role in the formation of tracheary elements and sieve tubes,13 and could therefore be directly responsible for the formation of a vascular connection between host and parasite. It was also proposed that apoplastic transfer of sugars from host to parasite was enabled by a loosening of the cell wall through XyG degradation,5 which could be realized through the activity of Cuscuta XTHs (Fig. 2C). However, while the growth of haustoria and its hyphae requires a high turgor pressure in the respective cells, the redirection of nutrients into the parasite would be hampered by this. Thus, in order to prevent that excessive loosening of cell walls in conjunction with a drop in turgor pressure results in a structural collapse, Cuscuta XTHs could re-strengthen walls within the mature haustorium (Fig. 2C).
Figure 2.
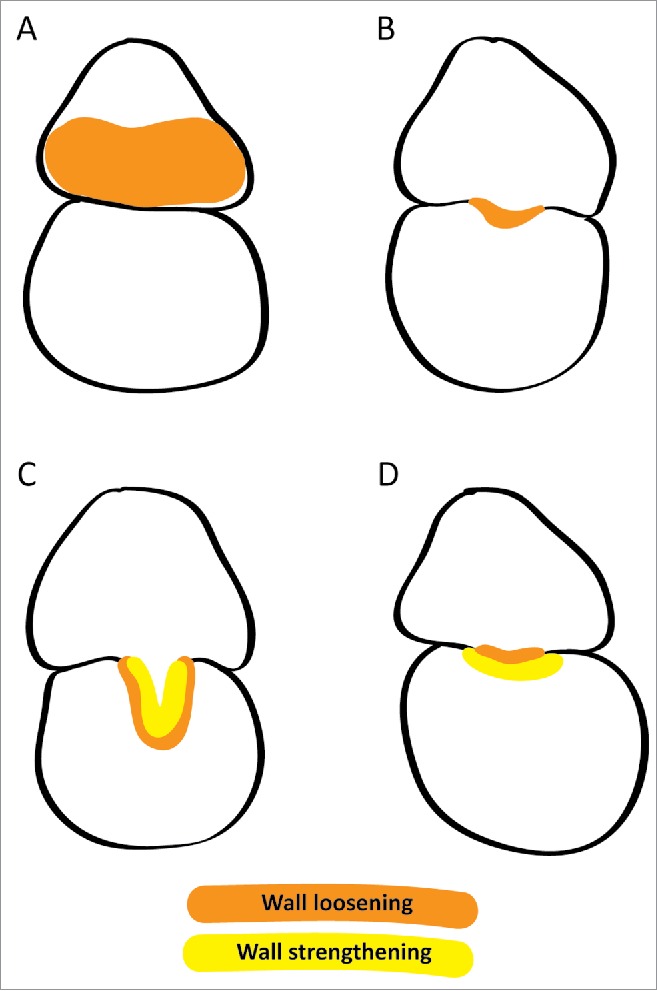
Hypothetical functions of XTHs in the parasitization strategy employed by Cuscuta and in the resistance mechanism of cultivated tomato. Illustrated are cross-sections of infection sites when Cuscuta (upper) infects a potential host plant (lower). Orange and yellow signify wall loosening and strengthening activities of XTHs, respectively. (A) At the onset of haustorium development, the swelling of the parasite stem facing the host plant is facilitated by Cuscuta XTHs that promote expansive cell growth through wall loosening. (B) As the haustorium begins its host-invasive growth, XTHs secreted from the infection organ aid tissue penetration by loosening host cell walls. (C) Upon reaching the vascular bundles of its host, the cell wall loosening activity of Cuscuta XTHs at the host-parasite interface enables parasite feeding through apoplastic sugar transfer and/or by promoting vascular tissue differentiation. To prevent exaggerated cell wall loosening under low turgor pressure, Cuscuta XTHs must also strengthen its own walls. (D) When Cuscuta attempts to invade cultivated tomato by deploying wall loosening XTHs at the interface, the counteractive wall strengthening activity of host-encoded XTHs (yellow area) prevents the haustorium from entering the host plant.
Role of an XTH in the resistance mechanism of cultivated tomato
The resistance mechanism of cultivated tomato (Solanum lycopersicum) toward C. reflexa has been thoroughly reviewed elsewhere.2 Interestingly, one of the defense responses of S. lycopersicum is the increased expression of LeXTH1, a gene encoding an XTH.14 The function of LeXTH1 could be to promote the elongation of tomato epidermal cells that takes place at the site of contact with the parasite.2 However, another possibility is that LeXTH1 is deployed to reinforce the tomato cell walls, presumably as a remedy against the cell wall loosening activity of Cuscuta XTHs at the interface (Fig. 2D). If XET action in tissue prints of Cuscuta infection sites can be analyzed at a higher resolution than recently demonstrated,11 this issue may be resolved.
Initiation of haustorium development
The formation of Cuscuta haustoria can be induced by the cooperative effects of far-red light and tactile stimuli,15 indicating that the signals required to initiate the infection of a host plant is of an abiotic nature. This also agrees with the observation that C. reflexa will try to infect rods of metal or plastic as earnestly as it will a suitable host plant. Moreover, cell wall-associated C. reflexa genes displayed similar expression patterns during the early stages of host-free induced haustorium development and host infection.11 Far-red light and touch (representing a tactile stimulus) have both been reported to increase the expression of several Arabidopsis XTHs.16,17 Hence, a direct pathway could exist from one or both of these stimuli to the expression of Cuscuta XTHs and the initiation of haustorium development. If corresponding regulatory elements are identified in Cuscuta, other genes engaged at the onset of haustorium development could be found.
Concluding remarks
As the primary cell wall of all tested land plants contains XyG,18 a parasitization strategy employing enzymes that modify this hemicellulose would prove most effective in enabling a plant parasite to accommodate a broad host range. In fact, many species of Cuscuta are generalists, being able to successfully parasitisize a number of different dicotyledonous plant species. The hypothesis that XyG modification is essential to host infection by Cuscuta is further substantiated by the fact that grasses, which contain far lower concentrations of XyG than other vascular plants,18 are with a few exceptions immune to Cuscuta.1,2 The model of the putative function of XTHs in the parasitization strategy of Cuscuta that is presented in this paper is intended to provide a basis for designing experiments to prove or reject this possibility.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
Work on Cuscuta by KK and SO was generously funded by Tromsø Forskningsstiftelse. We thank Dr. Frantisek Baluska for the invitation to write this article.
References
- 1.Dawson JH, Musselman LJ, Wolswinkel P, Dorr I. Biology and control of Cuscuta. Rev Weed Sci 1994; 6:265-317. [Google Scholar]
- 2.Kaiser B, Vogg G, Furst UB, Albert M. Parasitic plants of the genus Cuscuta and their interaction with susceptible and resistant host plants. Front Plant Sci 2015; 6:45; PMID:25699071; http://dx.doi.org/ 10.3389/fpls.2015.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughn KC. Dodder hyphae invade the host: a structural and immunocytochemical characterization. Protoplasma 2003; 220:189-200; PMID:12664283; http://dx.doi.org/ 10.1007/s00709-002-0038-3 [DOI] [PubMed] [Google Scholar]
- 4.Vaughn KC. Attachment of the parasitic weed dodder to the host. Protoplasma 2002; 219:227-37; PMID:12099223; http://dx.doi.org/ 10.1007/s007090200024 [DOI] [PubMed] [Google Scholar]
- 5.Vaughn KC. Conversion of the searching hyphae of dodder into xylic and phloic hyphae: a cytochemical and immunocytochemical investigation. Int J Plant Sci 2006; 167:1099-114; http://dx.doi.org/ 10.1086/507872 [DOI] [Google Scholar]
- 6.Rose JKC, Braam J, Fry SC, Nishitani K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol 2002; 43:1421-35; PMID:12514239; http://dx.doi.org/ 10.1093/pcp/pcf171 [DOI] [PubMed] [Google Scholar]
- 7.Van Sandt VST, Suslov D, Verbelen JP, Vissenberg K. Xyloglucan endotransglucosylase activity loosens a plant cell wall. Ann Bot 2007; 100:1467-73; PMID:17916584; http://dx.doi.org/ 10.1093/aob/mcm248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maris A, Suslov D, Fry SC, Verbelen JP, Vissenberg K. Enzymic characterization of two recombinant xyloglucan endotransglucosylase/hydrolase (XTH) proteins of Arabidopsis and their effect on root growth and cell wall extension. J Exp Bot 2009; 60:3959-72; PMID:19635745; http://dx.doi.org/ 10.1093/jxb/erp229 [DOI] [PubMed] [Google Scholar]
- 9.Takeda T, Furuta Y, Awano T, Mizuno K, Mitsuishi Y, Hayashi T. Suppression and acceleration of cell elongation by integration of xyloglucans in pea stem segments. Proc Natl Acad Sci USA 2002; 99:9055-60; PMID:12084943; http://dx.doi.org/ 10.1073/pnas.132080299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranjan A, Ichihashi Y, Farhi M, Zumstein K, Townsley B, David-Schwartz R, Sinha NR. De novo assembly and characterization of the transcriptome of the parasitic weed Cuscuta pentagona identifies genes associated with plant parasitism. Plant Physiol 2014; 166:1186-99; PMID:24399359; http://dx.doi.org/ 10.1104/pp.113.234864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen S, Striberny B, Hollmann J, Schwacke R, Popper Z, Krause K. Getting ready for host invasion: elevated expression and action of xyloglucan endotransglucosylases/hydrolases in developing haustoria of the holoparasitic angiosperm Cuscuta. J Exp Bot 2015; In press; PMID:26561437; http://dx.doi.org/10.1093/jxb/erv482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnsen HR, Striberny B, Olsen S, Vidal-Melgosa S, Fangel JU, Willats WG, Rose JK, Krause K. Cell wall composition profiling of parasitic giant dodder (Cuscuta reflexa) and its hosts: a priori differences and induced changes. New Phytol 2015; 207:805-16; PMID:25808919; http://dx.doi.org/ 10.1111/nph.13378 [DOI] [PubMed] [Google Scholar]
- 13.Antosiewicz DM, Purugganan MM, Polisensky DH, Braam J. Cellular localization of Arabidopsis xyloglucan endotransglycosylase-related proteins during development and after wind stimulation. Plant Physiol 1997; 115:1319-28; PMID:9414546; http://dx.doi.org/ 10.1104/pp.115.4.1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert M, Werner M, Proksch P, Fry SC, Kaldenhoff R. The cell wall-modifying xyloglucan endotransglycosylase/hydrolase LeXTH1 is expressed during the defence reaction of tomato against the plant parasite Cuscuta reflexa. Plant Biol (Stuttg) 2004; 6:402-7; PMID:15248122; http://dx.doi.org/ 10.1055/s-2004-817959 [DOI] [PubMed] [Google Scholar]
- 15.Tada Y, Sugai M, Furuhashi K. Haustoria of Cuscuta japonica, a holoparasitic flowering plant, are induced by the cooperative effects of far-red light and tactile stimuli. Plant Cell Physiol 1996; 37:1049-53; http://dx.doi.org/ 10.1093/oxfordjournals.pcp.a029052 [DOI] [Google Scholar]
- 16.Lee D, Polisensky DH, Braam J. Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: a focus on calmodulin-like and XTH genes. New Phytol 2005; 165:429-44; PMID:15720654; http://dx.doi.org/ 10.1111/j.1469-8137.2004.01238.x [DOI] [PubMed] [Google Scholar]
- 17.Sasidharan R, Chinnappa CC, Staal M, Elzenga JT, Yokoyama R, Nishitani K, Voesenek LA, Pierik R. Light quality-mediated petiole elongation in Arabidopsis during shade avoidance involves cell wall modification by xyloglucan endotransglucosylase/hydrolases. Plant Physiol 2010; 154:978-90; PMID:20688978; http://dx.doi.org/ 10.1104/pp.110.162057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popper ZA, Fry SC. Primary cell wall composition of bryophytes and charophytes. Ann Bot 2003; 91:1-12; PMID:12495914; http://dx.doi.org/ 10.1093/aob/mcg013 [DOI] [PMC free article] [PubMed] [Google Scholar]


