Abstract
Long-distance intercellular electrical signals, including variation potential (VP) in higher plants, are a potential mechanism of coordinate functional responses in different plant cells under action of stressors. VP, which is caused by damaging factors (e.g., heating, crushing), is transient depolarization with an irregular shape. It can include a long-term depolarization and fast impulse depolarization (‘AP-like’ spikes). Mechanisms of VP generation and propagation are still under investigation. It is probable that VP is a local electrical response induced by propagation of hydraulic wave and (or) chemical agent. Both hypotheses are based on numerous experimental results but they predict VP velocities which are not in a good accordance with speed of variation potential propagation. Thus combination of hydraulic and chemical signals is the probable mechanism of VP propagation. VP generation is traditionally connected with transient H+-ATPase inactivation, but AP-like spikes are also connected with passive ions fluxes. Ca2+ influx is a probable mechanism which triggers H+-ATPase inactivation and ions channels activation at VP.
Keywords: generation, higher plants, propagation, simulation, variation potential
Abbreviations
- AP
action potential
- ES
electrical signals
- VP
variation potential.
Introduction
Plants live under variable environmental conditions, but they are immovable organisms and cannot escape from stressors. As a result, adaptive physiological responses are very important for plant life. Development of these responses requires intracellular and intercellular signals that coordinate functional responses in different plant cells. In particular, long-distance intercellular signals are necessary under local action of stressors because they induce functional changes in nonstimulated organs and tissues, i.e., the systemic plant response.1,2
Chemical, hydraulic and electrical signals are considered to be potential long-distance intercellular signals in plants.2-10 Electrical signals (ESs) traditionally include action potential (AP), which is induced by non-damaging stimuli (e.g., cooling, touching), and variation potential (VP), which is caused by damaging factors (e.g., heating, crushing) (Fig. 1).8,11-14 Both signals are affected via transient depolarization, although the dynamics of membrane potential changes at AP and VP are different.7,11,12 Recently, an ES, termed ‘system potential’ (SP), has been shown in higher plants.15 In contrast to AP and VP, SP is transient hyperpolarization that can be induced by different stimuli and is likely connected with H+-ATPase activation.15
Figure 1.
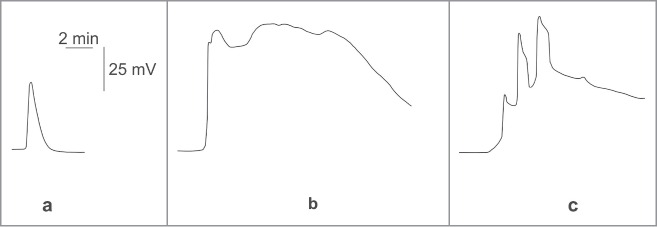
Electrical signals in hypocotyl of pumpkin seedling induced by different stimuli. (a) action potential induced by ice water;11 (b) variation potential without spikes induced by leaf burning;46 (c) variation potential with AP-like spikes induced by leaf burning.56 Electrical signals were registered in 7 cm from zone of stimulation. Redrawn from works.11,46,56
Propagation of AP and VP affects numerous physiological processes in plants. For example, ESs induce gene expression,16-18 phytohormone synthesis,17,19,20 phloem transport decrease,21 changes in root absorption,11 activation of respiration,22-26 and inactivation of photosynthesis.23,24,26-31 Retivin et al.32 hypothesized that the ultimate role of these physiological changes is the increase of plant resistance to stressors. Indeed, it has been shown that ESs can have a positive influence on resistance to stressors at the whole plant level,32 including increasing photosynthetic machinery resistance,30,33 which is strongly connected with inactivation of photosynthesis. The precise mechanisms of ESs' influence on physiological processes are under investigation. It has been hypothesized34 that changes in ion concentrations could be the initial mechanism that leads to a functional response. Investigation of ESs-induced photosynthetic response in Chara algae and higher plants showed that Ca2+27,28 and (or) H+26,29 influxes, which participate in development of electrical reactions, can be initiators of the functional response.
Thus ESs-induced physiological changes associated with AP and VP generation depend on ion mechanisms. AP generation is mainly based on passive Ca2+, Cl− and K+ fluxes,35 but transient H+-ATPase inactivation also participates in this process.36 VP generation is still under investigation7,11-13 and it has been difficult to determine its precise functional role. AP is subjected to the ‘all-or-none law’; i.e., propagation of this signal isn't directly related to its functional role. In contrast, VP propagation isn't subjected to the ‘all-or-none law’, and propagation parameters can directly influence plant physiological responses. As a result, a better understanding of VP generation and its propagation principles is important for future investigations of its functional role. The aim of our review is to provide an analysis of modern conceptions about mechanisms of VP generation and propagation.
General information about variation potential
In higher plants, local damage, including burning and other mechanical injuries7,11,13,15,37, induces a unique ES referred to as variation potential (VP), which is defined as a transient depolarization with an irregular shape. Burning is the most commonly used stressor for inducing VP in a wide variety of plants, including barley,37 faba bean,37 soybean,38 tobacco,39 sunflower,40 mimosa,11,41,42 tomato,6,43 Bidens pilosa,44,45 pumpkin,11,46 wheat,47 and pea.26,30,31 Mechanical injury also can induce VP. It was shown that pricking sunflower hypocotyls induced VP,7 and cutting stems and roots induced VP in sunflower,7 maize,48 and pea.49 However, similar mechanical injuries did not induce VP in tomato and wheat.47,50 Because the presence/absence of VP induction depends on the type of damage, burning has become the preferred inductor of VP.
VP has an irregular shape that can include 2 components. The first is long-term depolarization over the course of a minute to several minutes,5,7,12 and which is an absolutely necessary component of VP. It has wide-range variable amplitude (∼ tens mV) and low velocity propagation (∼ mm s−1).46,47 However, VP may also include fast impulse depolarization, a similarity it shares with AP.
These ‘AP-like’ spikes can take the lead over the long-term depolarization in mimosa, sunflower and bean,42,51,52 join the depolarization and form the first fast depolarization in pumpkin, geranium and pea,24,30,46 and be generated during the depolarization in mimosa, sunflower, tomato, pumpkin and cucumber.11,43,47,51,53 However, VP can occur without ‘AP-like’ spikes, as well. Only long-term depolarization was observed in pea epicotyls,49,53,54 wheat47 and spiderwort.55 It should be noted that presence and absence of ‘AP-like’ spikes may be observed in the same plants, i.e., its generation is determined by damage parameters and distance from the zone of stimulation (Fig. 1).46,56
Propagation of VP has a number of specific properties. It is known that VP amplitude is proportional to the intensity of the damaging stimulus.11 Amplitude and propagation velocity of VP decreases with increasing distance from the damage zone.46,47,56 This decrement was about 10% cm−1 in pumpkin46 and wheat.47 Our theoretical investigation56 showed that this decrement depends on initial damage intensity. Another interesting property of VP propagation is its transmission through inactive or dead plant parts.11
Thus, parameters of VP are essentially distinguished from AP in major 3 ways:7,11,13,15 (i) AP is induced by undamaging stimuli and VP is caused by damage; (ii) AP is a spike whereas VP has an irregular shape, including long-term depolarization and ‘AP-like’ spikes; and (iii) AP is subjected to the ‘all-or-none law’ and VP depends on stimulus type, distance from zone of damage, and plant species. As a result, generation and propagation mechanisms of VP are considered to be distinguishable from those for AP.
Mechanism of variation potential propagation
There are 3 basic hypotheses that describe potential mechanisms of VP propagation in plants. The first hypothesis55 supposes that VP propagation is a self-propagating ES, which is similar with AP transmission in principle. It was shown that VP propagation velocity is well described by a cable equation,55 i.e., its process may be based on active ES transmission. However, this hypothesis doesn't explain several important properties of VP transmission. In particular, VP can propagate through zones where its generation was suppressed by low and high temperatures,42,57,58 potassium cyanide,53 sodium azide,46 and EGTA.46 Moreover, it was shown that VP could propagate between cut stems, if plant parts were joined by solution.11 These results are contrary to a hypothesis regarding self-propagating VP and show that xylem is likely to be the main pathway of variation potential transmission. Data of Tzaplev and Zatzepina55 can be connected with measurement of propagation velocity of ‘AP-like’ spike, which may take the lead over long-term depolarization and is similar to AP.
According to the other 2 hypotheses, VP (or only long-term depolarization) is a local electrical response induced by propagation of a specific factor. This factor may be a hydraulic wave4,7 or chemical agent (‘wound substance’, ‘Ricca's factor’).3,11,13 A combination of hydraulic and chemical signals is also proposed in some studies.59
The hydraulic hypothesis maintains4,7 that damage increases hydraulic pressure in the stimulated zone that induces a hydraulic wave that is propagated through the plant body. A number of experimental data support this hypothesis. First, it was shown in several studies that local damage induced changes in stem or leaf thickness that reflected hydraulic wave propagation through the plant.4,47,53,60,61 Changes in thickness started before the electrical reaction was initiated4,40,47,51 thus supporting their key role in VP induction. Additionally, artificially increasing xylem hydraulic pressure in the positive direction7,49,53 induces electrical reactions that are similar to VP.
Thus, participation of a hydraulic wave in VP propagation is likely. However, the velocity of hydraulic wave propagation (up to the speed of sound in water) is essentially greater than the velocity of VP transmission (∼mm s−1). Stahlberg and Cosgrove53 supposed that low VP velocity was connected with a lag-phase prior to the start of VP generation. This lag-phase was extended as hydraulic wave amplitude decreased; in turn, the amplitude was reduced with distance from the damage zone.
The chemical hypothesis proposes that damage induces synthesis and (or) excretion of a wound substance that propagates through xylem vessels and induces a local electrical reaction.3,13 This wound substance can be contained in plant homogenates because homogenization breaks cells in a manner similar to a damage stimulus. It was shown that homogenates can induce an electrical reaction in mimosa,11 biophytum,62 and tomato.63-65 In contrast, the homogenate didn't change VP, induced by cut, in pea.54 The exact nature of the wound substance isn't known. This substance must: (i) be synthesized quickly in response to damage, (ii) induce electrical reactions, and (iii) propagate easily in plants. Oligosaccharides of broken cell walls,66 systemin,67 jasmonate,18,68 salycilic acid,69 ethylene19,70 and abscisic acid1,2 are considered to be potential wound substances. In recent studies,71 hydrogen peroxide (H2O2) has been shown to propagate from the damage zone and induce an electrical reaction. It is known that H2O2 can activate Ca2+ channels72-74 that can trigger VP development (see below), and hydrogen peroxide molecules can be quickly synthesized and propagated when a plant is under stress.75-78 These properties support the hypothesis of H2O2 participation in VP transmission.
The mechanism of wound substance propagation through plants is not well known. The first modification of the chemical hypothesis supposed that wound substances propagate through the plant body with water flow in the xylem.11 However, this supposition cannot explain basipetal VP transmission, which has been shown in a number of studies.12,26,30,31 Molecular diffusion in water solutions is a relatively slow process,79 and it is unlikely that this mechanism is the basis for wound substance propagation. Alternative possible mechanism of electrical signal propagation may be connected with diffusion of volatile wound substance (e.g., ethylene) in air spaces of plant. This mechanism has not been shown for VP; however, propagation of light-induced electrical signals through Coleus leaves on 12–20 mm is possible to be caused by CO2 diffusion in leaf air spaces.80 It shows potential possibility of this mechanism of VP propagation in plants.
It was also shown that water-soluble chemical agents (fluorescent dyes, radioactive isotopes) can propagate throughout the plant body with a velocity that was similar to the velocity of VP transmission.6,47,50,81 As a result, an alternative mechanism of wound substance transmission was suggested. According to Malone,59 local damage increases hydraulic pressure in the stimulated zone; in turn, this pressure growth induces acropetal and basipetal water flows in xylem, which transfers wound substances (‘hydraulic dispersion’). Another possible way of wound substance propagation was suggested previously.47,56 It is known that water flows in xylem are rather turbulent,82 and diffusion in turbulent flow is 3–4 orders of magnitude faster than molecular diffusion,79 and velocity of turbulent diffusion is similar to the speed of VP propagation.47 Therefore, we proposed that wound substance propagation is based on turbulent diffusion.47,56
Both mechanisms of wound substance propagation (hydraulic dispersion and turbulent diffusion) suppose that this propagation can be intensified by damage-induced changes in hydraulic pressure (hydraulic wave transmission), i.e., the chemical and hydraulic mechanisms cooperate during VP propagation.47,59
Mechanisms of variation potential generation
Both potential mechanisms of VP propagation rely on damage-induced signals that influence ion transporters in plant cell plasma membranes, which are ligand-dependent (chemical mechanism) or mechano-sensitive (hydraulic mechanism). Therefore, discerning the nature of these transporters is key to understanding VP generation mechanisms.
In contrast to AP, which is mainly based on passive ions fluxes, VP generation is traditionally connected with transient H+-ATPase inactivation.7,13,14 This hypothesis was initially supported by inhibitor analyses. It was shown that metabolic inhibitors (СN−, NaN3) decreased VP amplitude and velocity of depolarization, or suppressed generation of variation potential in pea, pumpkin, tomato and Bidens pilosa.43-46,54 Katicheva et al.83 showed that amplitude of VP and its depolarization and repolarization velocities were essentially reduced in wheat after treatment by sodium orthovanadate, a specific inhibitor of H+-ATPase. It should be noted that the repolarization phase of VP was more suppressed by inhibitor treatment than depolarization.44-47,54,83 Activation of H+-ATPase by fusicoccin induced the opposite effect and VP amplitude increased under the treatment.49
Participation of H+-ATPase inactivation in VP generation has also been shown by using different external pH conditions. Decrease in pH from 7 to 4 induced depolarization that was caused by lowering of H+-ATPase activity44,45. VP amplitude was low under these conditions. Application of the protonophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP), which increases permeability of the plasma membrane for H+, decreased VP amplitude.44,45
Changes in extracellular and intracellular pH, which were observed during VP development, also supported an important role of H+-ATPase inactivation in generating variation potentials. Using potentiometric and fluorescence methods, it was shown that VP generation was accompanied with apoplast alkalization26,29,37,46,49,84 and that its magnitude varied from about 0.223 to about 0.726,34 pH units. pH in cytoplasm was also affected by VP and decreased in the range of 0.3 to 0.6 pH units.23,26 Dynamics of pH changes were similar to the dynamics of the electrical response;23,46 VP amplitude was correlated with magnitude of pH change in the apoplast and cytoplasm.23
These results show that changes in H+-ATPase activity are an important mechanism of VP generation: depolarization caused its inactivation and repolarization was associated with its reactivation. However, the question ‘Is H+-ATPase inactivation the sole mechanism of variation potential?’ is still under investigation. Presence/absence of changes in conductivity of the plasma membrane is one potential argument that supports/rebuts the participation of ion channels in VP generation. According to Stahlberg et al.,54 VP development did not induce changes in plasma membrane conductivity in pea, and that was considered as an argument supporting absence of ion channel participation in VP generation. However, investigation of wheat leaves83 showed that VP generation was accompanied with increased conductivity of plasma membranes, and that observation supports activation of ions channels during VP development. These contradictory data can be explained by the complicated nature of total plasma membrane conductivity, which includes ion channel conductivities as well as H+-ATPase conductivity. As a result, total conductivity can be weakly changed with increase of ion channel conductivities (ions channel activation) and decrease of H+-ATPase conductivity (H+-ATPase inactivation) taken together. Therefore, participation of passive ion channels in VP generation requires more detailed analysis.
Participation of passive Ca2+ influx is the most thoroughly investigated VP generation mechanism. It was shown that inhibition of Ca2+ channels and (or) lowering of external concentrations of calcium ions essentially decreased VP amplitude or suppressed this electrical reaction in pumpkin, wheat, barley, tomato and Bidens pilosa.37,44,46,83 Removal of Ca2+ can also decrease number of AP-like spikes during VP development43. Moreover, VP generation was accompanied with decreased concentration of calcium ions in the apoplast34. These results show that Ca2+ influx is needed for development of VP. Also, it should be noted that strong local stressor can induce propagation of long-distance calcium signals in plant leaves, and parameters of these signals are similar with parameters of VP propagation.85
According to several studies,43,45,46,56,83 Ca2+ influx initiates VP generation, inducing H+-ATPase inactivation and, possibly, Cl− channel activation;56 i.e., Ca2+ plays a regulator role in VP development. However, other studies49 have shown that inhibition of Ca2+ channels only weakly influenced VP parameters in pea that could be connected with Ca2+ flux from internal sources during VP development.
Possible participation of anion and potassium channels on generation of VP has received significant attention. Several works44 showed that Cl− and K+ permeability of the plasma membrane was not changed during the repolarization phase of VP in Bidens pilosa. Moreover, inhibitor analysis did not show the influence of anion and potassium channels on slow wave parameters in pea and tomato.43,49 However, we have shown that VP generation was accompanied with a decrease in plasma membrane electrical resistance;83 and that this resistance change was affected by anion and potassium channel blockers. VP amplitude decrease or full suppression of these electrical signals under the action of ion channel blockers were also shown.46,86 Potentiometric analysis showed that VP generation was accompanied by an increase in external concentrations of Cl−.37,46 What seems apparently contradictory in these different studies may be explained by differences in ion mechanisms among different VP components. We hypothesized56 that activation of anion and potassium channels is the main mechanism of AP-like spikes and initial fast depolarization, and transient inactivation of H+-ATPase is the key mechanism for long-term depolarization. Ca2+ influx triggered both H+-ATPase inactivation and anion channel activation.
Figure 2 shows hypothetical mechanisms of VP generation and propagation. Accordingly, it shows propagation of a chemical or hydraulic signal or combination of these signals (hydraulic dispersion and turbulent diffusion) activated via ligand-dependent or mechano-sensitive Ca2+ channels. Influx of calcium ions inactivates H+-ATPase and induces development of long-term depolarization. Depolarization to the AP threshold activates potential-dependent Ca2+ channels. Additional Ca2+ influx and plasma membrane depolarization activates potential-dependent Cl− channels, and, later, K+-channels. As a result, an AP-like spike develops. Thus, according to the scheme, VP generation is connected with long-term H+-ATPase inactivation (mainly long-term depolarization) and with anion and potassium channel activation (mainly AP-like spikes); and Ca2+ influx is possible to trigger both mechanisms.
Figure 2.
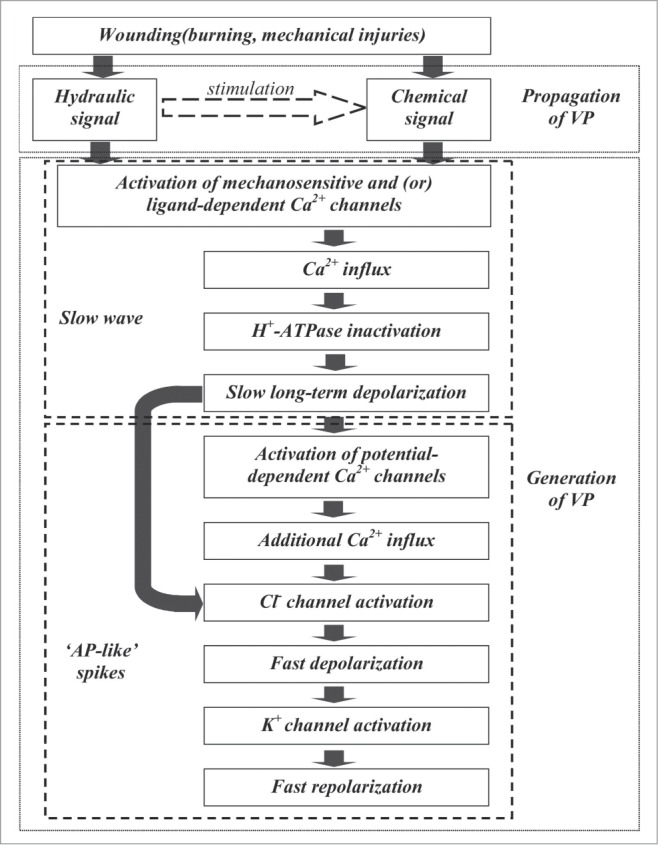
Hypothetical mechanism of VP generation and propagation. Propagation of chemical signal, hydraulic signal or combination of these signals (hydraulic dispersion and turbulent diffusion) activated ligand-dependent or mechano-sensitive Ca2+ channels. Ca2+ influx inactivates H+-ATPase and induces long-term depolarization. Depolarization to AP threshold activates potential-dependent Ca2+ channels. Additional Ca2+ influx and plasma membrane depolarization activate potential-dependent Cl− channels, and, later, K+-channels. As a result AP-like spike is formed.
Mechanisms of VP and AP generation are very similar. Depolarization of both electrical responses is connected with passive ion fluxes as well as with H+-ATPase inactivation. Differences of VP and AP properties are possibly caused by different initiation mechanisms for these signals.14 Activation of potential-dependent Ca2+ channels is the first stage of AP generation; whereas VP initiation is connected with activation of ligand-dependent (chemical mechanism) or mechano-sensitive (hydraulic mechanism) calcium channels. These differences allow for different participation of active and passive mechanisms in VP and AP generation.
Simulation of variation potential
VP simulation can be an effective method for the theoretical analysis of mechanisms of variation potential generation and propagation.47,56 There are several interconnected problems for simulation of VP: (i) modeling of VP propagation, (ii) modeling of VP generation, and (iii) description of irregular dynamics of the electrical potential, which can reflect VP propagation and VP generation.
One-dimensional systems can be suitable models for theoretical investigations of VP propagation,44 subject to significant excess of stem length compared with the transverse dimension. The chemical mechanism of VP propagation has been theoretically tested in our previous work47,56 using the diffusion equation. It was assumed that a wound substance was instantly generated at wounding, and then it diffused along xylem vessels and induced VP generation when its concentration reached the threshold mean. Under this set of assumptions, the diffusion equation described well VP propagation at the diffusion coefficient (D), equaling about 0.05 cm2 s−1 in wheat.44 This D magnitude was 2000 times larger than the coefficient of diffusion of small molecules in a water solution (molecular diffusion).79 This difference has been explained using turbulent diffusion,44 which can develop in turbulent flows (e.g., in xylem flow)82 and may be thousands of times larger than coefficients of molecular diffusion.79 Experimental analysis using a radioactive marker showed that D equaled 0.06 cm2 s−1 after wounding in wheat.44 Theoretical analysis of VP propagation in pumpkin showed that D was about 0.12 cm2 s−1.56 Thus simulation of VP propagation supports participation of turbulent diffusion in the transmission of chemical signals under wounding.
Modeling VP generation requires a detailed electrophysiological model of the plant cell. These models vary widely in range and scope; there are models that were based on Hodgkin-Huxley model,87,88 and models that were based on detailed descriptions of ion transport in plant plasma membranes.56,89-92 An electrophysiological model that was based on our previous simulation of VP generation56 takes into account K+, Cl− and Ca2+ channels, H+- and Ca2+-ATPase, 2H+/Cl−–symporter and H+/K+-antiporter, changes of ion concentrations in the cell and in the extracellular space, and buffers in the cytoplasm and apoplast. Analysis of the VP generation model56 supports the necessity of Ca2+ influx for VP generation, the key role of H+-ATPase inactivation in long-term depolarization, and participation of Cl−channel activation in the generation of AP-like spikes. Intensities of ions fluxes during VP generation and their dynamics are also calculated using the model. Thus, simulation of VP generation supports mechanisms of VP that have been shown in experimental investigations.
An additional important result of theoretical analyses of VP generation and propagation is the simulation of irregular dynamics of electrical potential during VP. Investigations using the VP model show56 that imitation of damage with different intensity (different extraction of wound substance) induces different electrical potential dynamics during VP. This result is in good accord with data on variability of VP shape7,46 and about dependence of VP parameters on wounding intensity. Also, the electrical potential dynamics depend on distance from the wounding zone; and that the distance dependence is in good accord with experimental data.46,56 The VP model has taken into account VP generation as well as VP propagation, and can describe the irregular shape of variation potential.
Conclusions
There are 2 main questions that are connected with generation and propagation of variation potential. First, is the wound-induced signal chemical, hydraulic, or a combination of the 2? Second, are the passive ion fluxes that participate in VP generation or H+-ATPase inactivation the sole mechanism of variation potential development? We propose that the solution to both questions involves a combination of mechanisms. There is good evidence to connect VP propagation with an interaction between chemical signals and a hydraulic wave. VP generation is a combined process, including long-term H+-ATPase inactivation (long-term depolarization) and short-term activation of anion and potassium channels (AP-like spikes). Testing these hypotheses should form the basis for future VP investigations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Russian Science Foundation (Project No. 14-26-00098).
References
- 1.Pena-Cortes H, Fisahn J, Willmitzer L. Signals involved in wound-induced proteinase-inhibitor-ii gene-expression in tomato and potato plants. Proc Natl Acad Sci U S A 1995; 92:4106-13; PMID:11607535; http://dx.doi.org/ 10.1073/pnas.92.10.4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leon J, Rojo E, Sanchez-Serrano JJ. Wound signalling in plants. J Exp Bot 2001; 52:1-9; PMID:11181708; http://dx.doi.org/ 10.1093/jexbot/52.354.1 [DOI] [PubMed] [Google Scholar]
- 3.Malone M. Rapid, long-distance signal transmission in higher plants In: Callow JA, ed. Advances in Botanical Research, 1996:163-228 [Google Scholar]
- 4.Mancuso S. Hydraulic and electrical transmission of wound-induced signals in Vitis vinifera. Aust J Plant Physiol 1999; 26:55-61; http://dx.doi.org/ 10.1071/PP98098 [DOI] [Google Scholar]
- 5.Fromm J, Lautner S. Characteristics and functions of phloem-transmitted electrical signals in higher plants. 2006 [Google Scholar]
- 6.Rhodes JD, Thain JF, Wildon DC. Signals and signalling pathways in plant wound responses. 2006 [Google Scholar]
- 7.Stahlberg R, Cleland RE, Van Volkenburgh E. Slow wave potentials – a propagating electrical signal unique to higher plants. 2006 [Google Scholar]
- 8.Harrison M. Cross-talk between phytohormone signaling pathways under both optimal and stressful environmental conditions In: Khan NA, Nazar R, Iqbal N, Anjum NA, eds. Phytohormones and Abiotic Stress Tolerance in Plants: Springer; Berlin Heidelberg, 2012:49-76 [Google Scholar]
- 9.Gallé A, Lautner S, Flexas J, Fromm J. Environmental stimuli and physiological responses: the current view on electrical signalling. Environ Exp Bot 2015; 114: 15-21; http://dx.doi.org/ 10.1016/j.envexpbot.2014.06.013 [DOI] [Google Scholar]
- 10.Notaguchi M, Okamoto S. Dynamics of long-distance signaling via plant vascular tissues. Front Plant Sci 2015; 6:161; PMID:25852714; http://dx.doi.org/ 10.3389/fpls.2015.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opritov VA, Pyatygin SS, Retivin VG. Bioelectrogenesis in higher plants. Moskow: Nauka, 1991 [Google Scholar]
- 12.Trebacz K, Dziubinska H, Krol E. Electrical signals in long-distance communication in plants. 2006 [Google Scholar]
- 13.Davies E. Electrical signals in plants: Facts and hypotheses. Plant Electrophysiol 2006:407-22 [Google Scholar]
- 14.van Bel AJE, Furch ACU, Hafke JB, Knoblauch M, Patrick JW. (Questions)(n) on phloem biology. 2. Mass flow, molecular hopping, distribution patterns and macromolecular signalling. Plant Sci 2011; 181:325-30; PMID:21889037; http://dx.doi.org/ 10.1016/j.plantsci.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann MR, Maischak H, Mithoefer A, Boland W, Felle HH. System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol 2009; 149:1593-600; PMID:19129416; http://dx.doi.org/ 10.1104/pp.108.133884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stankovic B, Davies E. Both action potentials and variation potentials induce proteinase inhibitor gene expression in tomato. Febs Lett 1996; 390:275-9; PMID:8706876; http://dx.doi.org/ 10.1016/0014-5793(96)00672-2 [DOI] [PubMed] [Google Scholar]
- 17.Fisahn J, Herde O, Willmitzer L, Pena-Cortes H. Analysis of the transient increase in cytosolic Ca2+ during the action potential of higher plants with high temporal resolution: Requirement of Ca2+ transients for induction of jasmonic acid biosynthesis and PINII gene expression. Plant Cell Physiol 2004; 45:456-9; PMID:15111720; http://dx.doi.org/ 10.1093/pcp/pch054 [DOI] [PubMed] [Google Scholar]
- 18.Mousavi SAR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 2013; 500:422-6; PMID:23969459; http://dx.doi.org/ 10.1038/nature12478 [DOI] [PubMed] [Google Scholar]
- 19.Dziubinska H, Filek M, Koscielniak J, Trebacz K. Variation and action potentials evoked by thermal stimuli accompany enhancement of ethylene emission in distant non-stimulated leaves of Vicia faba minor seedlings. J Plant Physiol 2003; 160:1203-10; PMID:14610889; http://dx.doi.org/ 10.1078/0176-1617-00914 [DOI] [PubMed] [Google Scholar]
- 20.Hlavackova V, Krchnak P, Naus J, Novak O, Spundova M, Strnad M. Electrical and chemical signals involved in short-term systemic photosynthetic responses of tobacco plants to local burning. Planta 2006; 225:235-44; PMID:16773374; http://dx.doi.org/ 10.1007/s00425-006-0325-x [DOI] [PubMed] [Google Scholar]
- 21.Fromm J, Bauer T. Action-potentials in maize sieve tubes change phloem translocation. J Exp Bot 1994; 45:463-9; http://dx.doi.org/ 10.1093/jxb/45.4.463 [DOI] [Google Scholar]
- 22.Filek M, Koscielniak J. The effect of wounding the roots by high temperature on the respiration rate of the shoot and propagation of electric signal in horse bean seedlings (Vicia faba L minor). Plant Sci 1997; 123:39-46; http://dx.doi.org/ 10.1016/S0168-9452(96)04567-0 [DOI] [Google Scholar]
- 23.Pavlovic A, Slovakova Lu, Pandolfi C, Mancuso S. On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis). J Exp Bot 2011; 62:1991-2000; PMID:21289078; http://dx.doi.org/ 10.1093/jxb/erq404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukhov V, Orlova L, Mysyagin S, Sinitsina J, Vodeneev V. Analysis of the photosynthetic response induced by variation potential in geranium. Planta 2012; 235:703-12; PMID:22020752; http://dx.doi.org/ 10.1007/s00425-011-1529-2 [DOI] [PubMed] [Google Scholar]
- 25.Lautner S, Stummer M, Matyssek R, Fromm J, Grams TEE. Involvement of respiratory processes in the transient knockout of net CO2 uptake in Mimosa pudica upon heat stimulation. Plant Cell Environ 2014; 37:254-60; http://dx.doi.org/ 10.1111/pce.12150 [DOI] [PubMed] [Google Scholar]
- 26.Sukhov V, Sherstneva O, Surova L, Katicheva L, Vodeneev V. Proton cellular influx as a probable mechanism of variation potential influence on photosynthesis in pea. Plant Cell Environ 2014; 37:2532-41; http://dx.doi.org/ 10.1111/pce.12321 [DOI] [PubMed] [Google Scholar]
- 27.Krupenina NA, Bulychev AA. Action potential in a plant cell lowers the non-photochemical energy-dependent quenching light requirement for of chlorophyll fluorescence. Biochim Biophys Acta Bioenerg 2007; 1767:781-8; http://dx.doi.org/ 10.1016/j.bbabio.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 28.Krupenina NA, Bulychev AA, Roelfsema MRG, Schreiber U. Action potential in Chara cells intensifies spatial patterns of photosynthetic electron flow and non-photochemical quenching in parallel with inhibition of pH banding. Photochem Photobiol Sci 2008; 7:681-8; PMID:18528552; http://dx.doi.org/ 10.1039/b802243g [DOI] [PubMed] [Google Scholar]
- 29.Grams TEE, Lautner S, Felle HH, Matyssek R, Fromm J. Heat-induced electrical signals affect cytoplasmic and apoplastic pH as well as photosynthesis during propagation through the maize leaf. Plant Cell Environ 2009; 32:319-26; http://dx.doi.org/ 10.1111/j.1365-3040.2008.01922.x [DOI] [PubMed] [Google Scholar]
- 30.Sukhov V, Surova L, Sherstneva O, Vodeneev V. Influence of variation potential on resistance of the photosynthetic machinery to heating in pea. Physiol Plant 2014; 152:773-83; PMID: 24730552; http://dx.doi.org/ 10.1111/ppl.12208 [DOI] [PubMed] [Google Scholar]
- 31.Sukhov V, Surova L, Sherstneva O, Katicheva L, Vodeneev V. Variation potential influence on photosynthetic cyclic electron flow in pea. Front Plant Sci 2015; 5:766; PMID:25610447; http://dx.doi.org/ 10.3389/fpls.2014.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Retivin VG, Opritov VA, Fedulina SB. Generation of action potential induces preadaptation of Cucurbita pepo L stem tissues to freezing injury. Russ J Plant Physiol 1997; 44:432-42 [Google Scholar]
- 33.Retivin VG, Opritov VA, Lobov SA, Tarakanov SA, Khudyakov VA. Changes in the resistance of photosynthesizing cotyledon cells of pumpkin seedlings to cooling and heating, as induced by the stimulation of the root system with CKl solution. Fiziol Rastenii (Moscow) 1999; 46:790-8 [Google Scholar]
- 34.Pyatygin SS, Opritov VA, Vodeneev VA. Signaling role of action potential in higher plants. Russ J Plant Physiol 2008; 55:285-91; http://dx.doi.org/ 10.1134/S1021443708020179 [DOI] [Google Scholar]
- 35.Felle HH, Zimmermann MR. Systemic signalling in barley through action potentials. Planta 2007; 226:203-14; PMID:17226028; http://dx.doi.org/ 10.1007/s00425-006-0458-y [DOI] [PubMed] [Google Scholar]
- 36.Vodeneev VA, Opritov VA, Pyatygin SS. Reversible changes of extracellular pH during action potential generation in a higher plant Cucurbita pepo. Russi J Plant Physiol 2006; 53:481-7; http://dx.doi.org/ 10.1134/S102144370604008X [DOI] [Google Scholar]
- 37.Zimmermann MR, Felle HH. Dissection of heat-induced systemic signals: superiority of ion fluxes to voltage changes in substomatal cavities. Planta 2009; 229:539-47; PMID:19011895; http://dx.doi.org/ 10.1007/s00425-008-0850-x [DOI] [PubMed] [Google Scholar]
- 38.Galle A, Lautner S, Flexas J, Ribas-Carbo M, Hanson D, Roesgen J, Fromm J. Photosynthetic responses of soybean (Glycine max L.) to heat-induced electrical signalling are predominantly governed by modifications of mesophyll conductance for CO2. Plant Cell Environ 2013; 36:542-52; PMID:22897236; http://dx.doi.org/ 10.1111/j.1365-3040.2012.02594.x [DOI] [PubMed] [Google Scholar]
- 39.Hlavackova V, Naus J. Chemical signal as a rapid long-distance information messenger after local wounding of a plant? Plant Signal Behav 2007; 2:103-5; PMID:19704749; http://dx.doi.org/ 10.4161/psb.2.2.3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stankovic B, Zawadzki T, Davies E. Characterization of the variation potential in sunflower. Plant Physiol 1997; 115:1083-8; PMID:12223859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sibaoka T. Some aspects of the slow conduction of stimuli in the leaf of Mimosa pudica. Sci Rep Tohoku Univ 1953; 20:72-88 [Google Scholar]
- 42.Roblin G. Analysis of the variation potential induced by wounding in plants. Plant Cell Physiol 1985; 26:455-61 [Google Scholar]
- 43.Rousset M, de Roo M, Le Guennec JY, Pichon O. Electrophysiological characterization of tomato hypocotyl putative action potentials induced by cotyledon heating. Physiol Plant 2002; 115:197-203; PMID:12060236; http://dx.doi.org/ 10.1034/j.1399-3054.2002.1150204.x [DOI] [PubMed] [Google Scholar]
- 44.Julien JL, Desbiez MO, Dejaegher G, Frachisse JM. Characteristics of the wave of depolarization induced by wounding in Bidens pilosa L. J Exp Bot 1991; 42:131-7; http://dx.doi.org/ 10.1093/jxb/42.1.131 [DOI] [Google Scholar]
- 45.Julien JL, Frachisse JM. Involvement of the proton pump and proton conductance change in the wave of depolarization induced by wounding in Bidens pilosa. Can J Bot-Rev 1992; 70:1451-8 [Google Scholar]
- 46.Vodeneev VA, Akinchits EK, Orlova LA, Sukhov VS. The Role of Ca2+, H+, and Cl− Ions in Generation of Variation Potential in Pumpkin Plants. Russ J Plant Physiol 2011; 58:974-81; http://dx.doi.org/ 10.1134/S1021443711050256 [DOI] [Google Scholar]
- 47.Vodeneev V, Orlova A, Morozova E, Orlova L, Akinchits E, Orlova O, Sukhov V. The mechanism of propagation of variation potentials in wheat leaves. J Plant Physiol 2012; 169:949-54; PMID:22533926; http://dx.doi.org/ 10.1016/j.jplph.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 48.Meyer AJ, Weisenseel MH. Wound-induced changes of membrane voltage, endogenous currents, and ion fluxes in primary roots of maize. Plant Physiol 1997; 114:989-98; PMID:12223755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stahlberg R, Cosgrove DJ. Induction and ionic basis of slow wave potentials in seedlings of Pisum sativum L. Planta 1996; 200:416-25; PMID:11541124; http://dx.doi.org/ 10.1007/BF00231397 [DOI] [PubMed] [Google Scholar]
- 50.Rhodes JD, Thain JF, Wildon DC. Evidence for physically distinct systemic signalling pathways in the wounded tomato plant. Ann Bot 1999; 84:109-16; http://dx.doi.org/ 10.1006/anbo.1999.0900 [DOI] [Google Scholar]
- 51.Stankovic B, Witters DL, Zawadzki T, Davies E. Action potentials and variation potentials in sunflower: An analysis of their relationships and distinguishing characteristics. Physiol Plant 1998; 103:51-8; http://dx.doi.org/ 10.1034/j.1399-3054.1998.1030107.x [DOI] [Google Scholar]
- 52.Dziubinska H, Trebacz K, Zawadzki T. Transmission route for action potentials and variation potentials in Helianthus annuus L. J Plant Physiol 2001; 158:1167-72; http://dx.doi.org/ 10.1078/S0176-1617(04)70143-1 [DOI] [Google Scholar]
- 53.Stahlberg R, Cosgrove DJ. The propagation of slow wave potentials in pea epicotyls. Plant Physiol 1997; 113:209-17; PMID:12223601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stahlberg R, Cosgrove DJ. Rapid alterations in growth rate and electrical potentials upon stem excision in pea seedlings. Planta 1992; 187:523-31; PMID:11538115; http://dx.doi.org/ 10.1007/BF00199972 [DOI] [PubMed] [Google Scholar]
- 55.Tsaplev YB, Zatsepina GN. Electric nature of variable potential propagation in tradescantia. Biofizika 1980; 25:708-12; PMID:7417550 [PubMed] [Google Scholar]
- 56.Sukhov V, Akinchits E, Katicheva L, Vodeneev V. Simulation of variation potential in higher plant cells. J Membr Biol 2013; 246:287-96; PMID:23417063; http://dx.doi.org/ 10.1007/s00232-013-9529-8 [DOI] [PubMed] [Google Scholar]
- 57.Roblin G, Bonnemain JL. Propagation in vicia faba stem of a potential variation induced by wounding. Plant Cell Physiol 1985; 26:1273-83 [Google Scholar]
- 58.Wildon DC, Thain JF, Minchin PEH, Gubb IR, Reilly AJ, Skipper YD, Doherty HM, O'Donnell PJ, Bowles DJ. Electrical signaling and systemic proteinase inhibitor induction in the wounded plant. Nature 1992; 360:62-5 [Google Scholar]
- 59.Malone M. Wound induced hydraulic signals and stimulus transmission in Mimosa pudica L. New Phytol 1994; 128:49-56; http://dx.doi.org/ 10.1111/j.1469-8137.1994.tb03985.x [DOI] [PubMed] [Google Scholar]
- 60.Malone M. Kinetics of wound-induced hydraulic signals and variation potentials in wheat seedlings. Planta 1992; 187:505-10; PMID:24178145; http://dx.doi.org/ 10.1007/BF00199969 [DOI] [PubMed] [Google Scholar]
- 61.Stankovic B, Davies E. Intercellular communication in plants: electrical stimulation of proteinase inhibitor gene expression in tomato. Planta 1997; 202:402-6; http://dx.doi.org/ 10.1007/s004250050143 [DOI] [Google Scholar]
- 62.Sibaoka T. Application of leaf extract causes repetitive action potentials in Biophytum sensitivum. J Plant Res 1997; 110:485-7; http://dx.doi.org/ 10.1007/BF02506809 [DOI] [Google Scholar]
- 63.K U. Der Erregungsvorgang In: W R , ed. Handbuch der Pflanzenphysiologie. Berlin Heidelberg New York: Springer, 1959:24-110 [Google Scholar]
- 64.Van Sambeek JW, Pickard BG, Ulbright CE. Mediation of rapid electrical metabolic transpirational and photosynthetic changes by factors released from wounds. Part 2: mediation of the variation potential by riccas factor. Can J Bot 1976; 54:2651-61; http://dx.doi.org/ 10.1139/b76-285 [DOI] [Google Scholar]
- 65.Cheeseman JM, Pickard BG. Electrical characteristics of cells from leaves of lycopersicon. Can J Bot-Rev 1977; 55:497-510 [Google Scholar]
- 66.Bishop PD, Makus DJ, Pearce G, Ryan CA. Proteinase inhibitor inducing factor activity in tomato leaves resides in oligosaccharides enzymically released from cell walls. Proc Natl Acad Sci U S A Biol Sci 1981; 78:3536-40; http://dx.doi.org/ 10.1073/pnas.78.6.3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pearce G, Strydom D, Johnson S, Ryan CA. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 1991; 253:895-8; PMID:17751827; http://dx.doi.org/ 10.1126/science.253.5022.895 [DOI] [PubMed] [Google Scholar]
- 68.Farmer EE, Ryan CA. Interplant communication – airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A 1990; 87:7713-6; PMID:11607107; http://dx.doi.org/ 10.1073/pnas.87.19.7713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dempsey DA, Shah J, Klessig DF. Salicylic acid and disease resistance in plants. Crit Rev Plant Sci 1999; 18:547-75; http://dx.doi.org/ 10.1080/07352689991309397 [DOI] [Google Scholar]
- 70.O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science 1996; 274:1914-7; PMID:8943205; http://dx.doi.org/ 10.1126/science.274.5294.1914 [DOI] [PubMed] [Google Scholar]
- 71.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trends Plant Sci 2011; 16:300-9; PMID:21482172; http://dx.doi.org/ 10.1016/j.tplants.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 72.Demidchik V, Nichols C, Oliynyk M, Dark A, Glover BJ, Davies JM. Is ATP a signaling agent in plants? Plant Physiol 2003; 133:456-61; PMID:14555773; http://dx.doi.org/ 10.1104/pp.103.024091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwak JM, Nguyen V, Schroeder JI. The role of reactive oxygen species in hormonal responses. Plant Physiol 2006; 141:323-9; PMID:16760482; http://dx.doi.org/ 10.1104/pp.106.079004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mazars C, Thuleau P, Lamotte O, Bourque S. Cross-Talk between ROS and calcium in regulation of nuclear activities. Mol Plant 2010; 3:706-18; PMID:20522524; http://dx.doi.org/ 10.1093/mp/ssq024 [DOI] [PubMed] [Google Scholar]
- 75.Neill S, Desikan R, Hancock J. Hydrogen peroxide signalling. Curr Opin Plant Biol 2002; 5:388-95; PMID:12183176; http://dx.doi.org/ 10.1016/S1369-5266(02)00282-0 [DOI] [PubMed] [Google Scholar]
- 76.Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann Rev Plant Biol 2004; 55:373-99; PMID:15377225; http://dx.doi.org/ 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- 77.Kreslavski VD, Los DA, Allakhverdiev SI, Kuznetsov VV. Signaling role of reactive oxygen species in plants under stress. Russ J Plant Physiol 2012; 59:141-54; http://dx.doi.org/ 10.1134/S1021443712020057 [DOI] [Google Scholar]
- 78.Petrov VD, Van Breusegem F. Hydrogen peroxide-a central hub for information flow in plant cells. Aob Plants 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levich VG. Physicochemical hydrodynamics. Englewood Cliffs, N.J.: Prentice-Hall, 1962 [Google Scholar]
- 80.Stahlberg R, Van Volkenburgh E, Cleland RE. Long-distance signaling within Coleus x hybridus leaves; mediated by changes in intra-leaf CO2? Planta 2001; 213:342-51; PMID:11506356; http://dx.doi.org/ 10.1007/s004250000514 [DOI] [PubMed] [Google Scholar]
- 81.Malone M, Alarcon JJ, Palumbo L. An hydraulic interpretation of rapid, long-distance wound signaling in the tomato. Planta 1994; 193:181-5; http://dx.doi.org/ 10.1007/BF00192528 [DOI] [Google Scholar]
- 82.Roth A. Water transport in xylem conduits with ring thickenings. Plant Cell Environ 1996; 19:622-9; http://dx.doi.org/ 10.1111/j.1365-3040.1996.tb00397.x [DOI] [Google Scholar]
- 83.Katicheva L, Sukhov V, Akinchits E, Vodeneev V. Ionic nature of burn-induced variation potential in wheat leaves. Plant Cell Physiol 2014; 55:1511-9; PMID:24928219; http://dx.doi.org/ 10.1093/pcp/pcu082 [DOI] [PubMed] [Google Scholar]
- 84.Vodeneev VA, Akinchits EK, Orlova LA, Sukhov VS, Balalaeva IV. An extracellular pH changes registration by confocal microscopy in higher plant at the excitation potentials generation. Tsitologiia 2010; 52:549-54; PMID:20799619 [PubMed] [Google Scholar]
- 85.Xiong TC, Ronzier E, Sanchez F, Corratge-Faillie C, Mazars C, Thibaud J-B. Imaging long distance propagating calcium signals in intact plant leaves with the BRET-based GFP-aequorin reporter. Front Plant Sci 2014; 5:43; PMID:24600459; http://dx.doi.org/ 10.3389/fpls.2014.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lautner S, Grams TEE, Matyssek R, Fromm J. Characteristics of electrical signals in poplar and responses in photosynthesis. Plant Physiol 2005; 138:2200-9; PMID:16040648; http://dx.doi.org/ 10.1104/pp.105.064196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beilby MJ. Cl− channels in chara. Philosophical Trans R Soc Lond B Biol Sci 1982; 299:435-45; http://dx.doi.org/ 10.1098/rstb.1982.0142 [DOI] [Google Scholar]
- 88.Beilby MJ. Action potential in charophytes. In: Jeon KW, ed. Int Rev Cytol Surv Cell Biol 2007; 257:43-82 [DOI] [PubMed] [Google Scholar]
- 89.Gradmann D. Impact of apoplast volume on ionic relations in plant cells. J Membr Biol 2001; 184:61-9; PMID:11687879; http://dx.doi.org/ 10.1007/s00232-001-0074-5 [DOI] [PubMed] [Google Scholar]
- 90.Gradmann D. Models for oscillations in plants. Aust J Plant Physiol 2001; 28:577-90 [Google Scholar]
- 91.Sukhov V, Vodeneev V. A mathematical model of action potential in cells of vascular plants. J Membr Biol 2009; 232:59-67; PMID:19921324; http://dx.doi.org/ 10.1007/s00232-009-9218-9 [DOI] [PubMed] [Google Scholar]
- 92.Sukhov V, Nerush V, Lova LO, Vodeneev V. Simulation of action potential propagation in plants. J Theor Biol 2011; 291:47-55; PMID:21959317; http://dx.doi.org/ 10.1016/j.jtbi.2011.09.019 [DOI] [PubMed] [Google Scholar]


