Abstract
The process of host plant penetration by parasitic dodder (genus Cuscuta) is accompanied by molecular and structural changes at the host/parasite interface. Recently, changes in pectin methyl esterification levels in the host cell walls abutting parasitic cells in established infection sites were reported. In addition to that, we show here that the composition of cell wall glycoproteins in Cuscuta-infected Pelargonium zonale undergoes substantial changes. While several arabinogalactan protein epitopes exhibit decreased abundances in the vicinity of the Cuscuta reflexa haustorium, extensins tend to increase in the infected areas.
Keywords: arabinogalactan proteins, Cuscuta reflexa, extensins, haustoria, parasitic plants, Pelargonium zonale
Abbreviations
- CoMPP
comprehensive microarray polymer profiling
- mAbs
monoclonal antibodies
- AGP
arabinogalactan protein.
Parasitic plants are specialized in the relocation of nutrients from autotrophic plants to their own shoots or roots, potentially harming their hosts in this way.1,2 One parasitic plant genus that has been classified as noxious weed in many countries is Cuscuta (colloquial name: dodder). With special feeding organs, termed haustoria in reminiscence of the infection organs of plant-pathogenic fungi, Cuscuta invades the shoots of their hosts and taps their water- and assimilate-conducting vessels.3 Unlike their fungal counterparts, however, Cuscuta haustoria are complex multicellular organs that, in essence, replace the absent roots.4
The cell wall compartment plays an important role in the protection of plants against pathogenic attacks of all sorts.5 Its main structural building blocks – cellulose, hemicellulose and pectins – form a physical barrier and are therefore targets for an enzymatic degradation by parasitic plants.6 Glycosylated proteins embedded in the cell wall or located on the plasma membrane like arabinogalactan proteins (AGPs), on the other hand, have been identified as key molecules in signaling and defense regulation7 and an elevated expression of one AGP was observed in tomato upon contact with C. reflexa prehaustoria.8 But also in rhinanthoid Orobanchaceae that are root parasites, Pielach et al.9 found that the hyaline body of the haustorium was enriched for arabinogalactan protein (AGP) epitopes and that this coincided with a depletion of de-esterified pectins. A high-throughput technique for comprehensive microarray-based carbohydrate polymer profiling10 applied to a shoot parasitic Cuscuta species revealed also a high amount of some AGP epitopes in the haustoria but did not support a coinciding depletion of de-esterified pectins.11
To map the occurence of AGPs and other glycoproteins more thoroughly, we extended the CoMPP-analysis described by Johnsen et al.11 with immunohistological analyses of mature C. reflexa/P. zonale infection sites. Binding of a set of monoclonal antibodies (mAbs) directed against AGP and extensin epitopes was assayed in vibratome sections encompassing the host/parasite interface and distal tissues of both interaction partners using the AlexaFluor 488 fluorophore-coupled secondary antibody (Invitrogen, Thermo Fischer Scientific Corp.) for detection. Controls without the mAbs showed only weak staining in sclerenchymatized tissue and no staining of cortex cells (data not shown). As expected, the labeling intensity of the mAbs was not uniform across tissue types and often also differed between tissue at the host/parasite interface and tissue distant to the interface. To facilitate meaningful comparisons between the specific staining patterns of each mAb, we therefore concentrated on seven specific areas (Fig. 1A) that represent different tissues at the infection interface (areas I, II and V) and tissue distant to the infection site of the parasite (areas III and IV) and of the host (areas VI and VII), respectively. Staining intensity within each of these areas was scored using an arbitrary scale as described for Fig. 1B. The compiled data in Fig. 1B represent average staining intensities of at least four stained intact cross sections.
Figure 1.
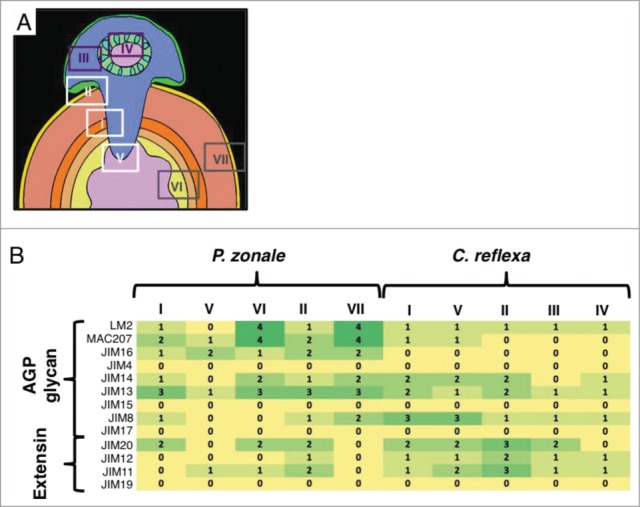
Immunohistolabeling survey of AGP glycan and extensin epitopes in stems of Pelargonium zonale infected with the parasitic plant Cuscuta reflexa. (A) Schematic picture of a cross section through an infection site. Areas, which were investigated in detail are indicated with numbered boxes: White boxes indicate interface areas, dark purple boxes represent non-infective parasite tissue and gray boxes represent host tissue distant from the infection site. (B) Heatmap of the relative intensities of the fluorescent signals in the C. reflexa/P. zonale cross section areas depicted in (A), based on a arbitrary scale from 0 (no fluorescence detected; yellow) to 4 (strong fluorescence; dark green). The nine mABs for AGP glycans and 4 mABs for extensins that were assayed (see Johnsen et al.11 for a description) are shown on the left. Sectioning and immunostaining of vibratome sections were performed as described.11
Overall, AGPs appeared to be more abundant in the host than in the parasite while the opposite was, by and large, observed for extensin-specific staining. The two mAbs that labeled the host most strongly were LM2 and MAC207, followed by JIM13 and JIM16. Only one AGP-specific mAb (JIM8) was observed to label parasitic tissue more strongly. JIM 14 reacted approximately equally well with both interaction partners while JIM 4, JIM15 and JIM17 did not show any specific immunolabeling. The infective tissues (parasitic parts of areas I, II and V) on average labeled equally strong or stronger for AGPs than the non-infective parts of the parasite (areas III and IV). Remarkably, the high abundance of the AGP epit-opes detected by LM2, MAC207 and Jim14 in the cortex and pith of the host (areas VI and VII) was strongly reduced in the vicinity of the penetrating haustorium of C. reflexa (areas I, II and V) (Fig. 1B and Fig. 2A-C) .
Figure 2.
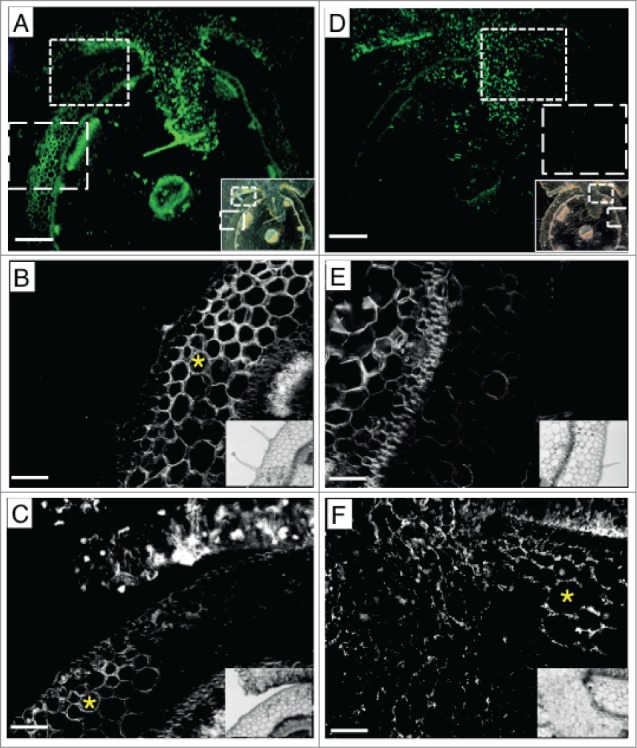
Distribution of AGP glycan and extensin epitopes at the interface between C. reflexa and P. zonale. (A-C) Fluorographs of mAb LM2 detecting an AGP glycan epitope with AlexaFluor 488 (green fluorescence). (D-F) Fluorographs of mAb JIM20 detecting an extensin epitope with AlexaFluor 488 (green fluorescence). Green pictures (in A and D) were taken with a color camera and a StereoLumar stereo microscope, black and white pictures were taken with a monochromal camera and an AxioVert 200M microscope. Scalebars represent 500 μm in A and D and 100 μm in B, C, E and F. The small insets show darkfield or brightfield pictures of the same sections. (B) and (E) show details from area VII, (C) and (F) from area II. Yellow asterisks mark cortex cells that show mAb labeling: labeling with mAb LM2 (AGP) occurred mainly in cell walls located at some distance from the parasite, while labeling with JIM20 (extensin) occurred mainly in the cortex immediately next to the haustorium.
Three of the extensin-specific mABs showed equal or stronger binding in the parasite (Fig. 1B) while one (JIM19) produced no signal. Interestingly, the uninfected cortex of P. zonale was not stained by any of the extensin epitope-binding antibodies. Area II representing infected cortex below the attachment site and adjacent to the penetrating haustorium, on the other hand, exhibited strong staining in the cell walls with mAB JIM20 (Fig. 2D-F) and, to a lesser extent with JIM11 and JIM12 (Fig. 1B).
The enrichment in AGP epitopes in the infectious organs of Cuscuta and the relative depletion of extensin epitopes are consistent with the results of the immunolabeling study with the hyaline body in the haustorium of the root parasitic Rhinanthus minor9 and tentatively support the notion that some common mechanisms underlying the parasitic lifestyle in shoot parasites (such as Cuscuta) and root parasites (e.g. Rhinanthus) do exist. The depletion of AGPs in the P. zonale tissue around the Cuscuta haustorium, on the other hand, has not been described before and contrasts with the observed induction of a tomato AGP upon attack by Cuscuta. With tomato being a resistant host while P. zonale is susceptible, this observation opens for speculations that AGPs may be involved in conferring resistance to incompatible hosts. The accumulation of extensins in the very same areas where AGP depletion was observed is also novel and intriguing and calls for more in-depth analyses on their interplay in the future.
The penetration of host tissue by the parasitic plant haustorium is an example for the intrusive growth of foreign plant cells in other individuals or species.12 An even better studied example is the growth of the pollen tube through stigma and style during angiosperm cross-fertilization. Indeed, pollen tip growth is also accompanied by a deposition of AGPs into the tips,13,14 and can be inhibited by treatment with an AGP binding reagent, β-Yariv reagent.15 The lack of two pollen-specific AGPs in Arabidopsis was, moreover, shown to invoke changes in the pollen tube gene expression profile.16 Calcium- and signaling-related genes that play a role in pollen tube growth but also in the interaction between Cuscuta and its hosts17,18 were among the genes found to be altered in AGP-lacking pollen.16 Comparative investigations of the different types of compatible cellular interactions between individual plants may in the future be able to reveal the full extent of such congruencies and allow deeper insight into the evolution of the parasitic plant haustorium.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Prof. W. Willats (University of Copenhagen, Denmark) for providing the mAbs.
Funding
This work was funded by the Tromsø Research Foundation.
References
- 1. Dawson JH, Musselman LJ, Wolswinkel P, Dörr I. Biology and control of Cuscuta. Rev Weed Sci 1994:265-317. [Google Scholar]
- 2. Parker C, Riches CR. Parasitic weeds of the world: biology and control. CAB International, Wallingford, UK: 1993. [Google Scholar]
- 3. Birschwilks M, Haupt S, Hofius D, Neumann S. Transfer of phloem-mobile substances from the host plants to the holoparasite Cuscuta sp. J Exp Bot 2006; 57:911-21; PMID:16467411; http://dx.doi.org/ 10.1093/jxb/erj076 [DOI] [PubMed] [Google Scholar]
- 4. Mayer AM. Pathogenesis by fungi and by parasitic plants: similarities and differences. Phytoparasitica 2006; 34:3-16; http://dx.doi.org/ 10.1007/BF02981333 [DOI] [Google Scholar]
- 5. Malinovsky FG, Fangel JU, Willats WG. The role of the cell wall in plant immunity. Frontiers Plant Sci 2014; 5:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnsen HR, Krause K. Cellulase activity screening using pure carboxymethyl cellulose: Application to soluble cellulolytic samples and to plant tissue prints. Int J Mol Sci 2014; 15:830-8; PMID:24413752; http://dx.doi.org/ 10.3390/ijms15010830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellis M, Egelund J, Schultz CJ, Bacic A. Arabinogalactan-proteins: key regulators at the cell surface? Plant Physiol 2010; 153:403-19; PMID:20388666; http://dx.doi.org/ 10.1104/pp.110.156000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albert M, Belastegui-Macadam X, Kaldenhoff R. An attack of the plant parasite Cuscuta reflexa induces the expression of attAGP, an attachment protein of the host tomato. Plant J 2006; 48:548-56; http://dx.doi.org/ 10.1111/j.1365-313X.2006.02897.x [DOI] [PubMed] [Google Scholar]
- 9. Pielach A, Leroux O, Domozych DS, Knox JP, Popper ZA. Arabinogalactan protein-rich cell walls, paramural deposits and ergastic globules define the hyaline bodies of rhinanthoid Orobanchaceae haustoria. Ann Bot 2014; 114:1359-73; PMID:25024256; http://dx.doi.org/ 10.1093/aob/mcu121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moller I, Sørensen I, Bernal AJ, Blaukopf C, Lee K, Øbro J, Pettolino F, Roberts A, Mikkelsen JD, Knox JP, et al. High-throughput mapping of cell wall polymers within and between plants using novel microarrays. Plant J 2007; 50:1118-28; PMID:17565618; http://dx.doi.org/ 10.1111/j.1365-313X.2007.03114.x [DOI] [PubMed] [Google Scholar]
- 11. Johnsen HR, Striberny B, Olsen S, Vidal-Melgosa S, Fangel JU, Willats WG, Rose JK, Krause K. Cell wall composition profiling of parasitic giant dodder (Cuscuta reflexa) and its hosts: a priori differences and induced changes. New Phytol 2015; 207:805-16; PMID:25808919; http://dx.doi.org/ 10.1111/nph.13378 [DOI] [PubMed] [Google Scholar]
- 12. Lev-Yadun S. Intrusive growth - the plant analog of dendrite and axon growth in animals. New Phytol 2001; 150:508-12; http://dx.doi.org/ 10.1046/j.1469-8137.2001.00143.x [DOI] [Google Scholar]
- 13. Pereira LG, Coimbra S, Oliveira H, Monteiro L, Sottomayor M. Expression of arabinogalactan protein genes in pollen tubes of Arabidopsis thaliana. Planta 2006; 223:374-80; PMID:16228244; http://dx.doi.org/ 10.1007/s00425-005-0137-4 [DOI] [PubMed] [Google Scholar]
- 14. Dardelle F, Lehner A, Ramdani Y, Bardor M, Lerouge P, Driouich A, Mollet JC. Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant Physiol 2010; 153:1563-76; PMID:20547702; http://dx.doi.org/ 10.1104/pp.110.158881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mollet JC, Kim S, Jauh GY, Lord EM. Arabinogalactan proteins, pollen tube growth, and the reversible effects of Yariv phenylglycoside. Protoplasma 2002; 219:89-98; PMID:11926071; http://dx.doi.org/ 10.1007/s007090200009 [DOI] [PubMed] [Google Scholar]
- 16. Costa M, Nobre MS, Becker JD, Masiero S, Amorim MI, Pereira LG, Coimbra S. Expression-based and co-localization detection of arabinogalactan protein 6 and arabinogalactan protein 11 interactors in Arabidopsis pollen and pollen tubes. BMC Plant Biol 2013; 13:7; PMID:23297674; http://dx.doi.org/ 10.1186/1471-2229-13-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albert M, Kaiser B, van der Krol S, Kaldenhoff R. Calcium signaling during the plant-plant interaction of parasitic Cuscuta reflexa with its hosts. Plant Signal Behav 2010; 5:1144-6; PMID:20818172; http://dx.doi.org/ 10.4161/psb.5.9.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Albert M, van der Krol S, Kaldenhoff R. Cuscuta reflexa invasion induces Ca release in its host. Plant Biol 2010; 12:554-7; PMID:20522193; http://dx.doi.org/ 10.1111/j.1438-8677.2010.00322.x [DOI] [PubMed] [Google Scholar]


