Abstract
On decaying wood or litter in forests, plasmodial slime molds (myxomycetes) represent a large fraction of eukaryotic protists that feed on bacteria. In his seminal book Experimental Physiology of Plants (1865), Julius Sachs referred to the multinucleate plasmodium of myxomycetes, which were considered at that time as primitive plants (or fungi). Today it is well established that myxomycetes are members of the Amoebozoa (Protista). In this study we compare the mobility of myxamoebae of 3 European species, Lycogala epidendrum (order Liceales), Tubulifera arachnoidea, and Trichia decipiens (order Trichiales). Using agar plates, on which 3 separate bacterial species were cultivated as prey organisms (Methylobacterium mesophilicum, Escherichia coli, Agrobacterium tumefaciens), we document large differences in cell motility between the myxomycetes investigated. In addition, we show that the 3 species of myxamoebae can be distinguished based on their average cell size. These data shed light on the mode of co-occurrence via differential substrate utilization in these members of the Amoebozoa.
Keywords: cell mobility, myxamoebae, plasmodial slime molds
Introduction
In October 1865, Wilhelm Hofmeister (1824–1877) finished the work on his first major book, entitled Die Lehre von der Pflanzenzelle (Plant Cell Biology) which appeared in print 2 y later. In the last chapter of his book, Hofmeister (1867),1 explained why botanists consider cells as the “elementary organs” of the plant body, and described the metabolically active content of these “units of life,” the protoplasm. With reference to a key publication of Anton de Bary (1831–1888) on the plasmodial slime molds (myxomycetes),2 Hofmeister argued that these microorganisms must be interpreted as descendants of plants (and not as animals or fungi). Accordingly, he described the properties of the “protoplasm of the plant cell” with reference to the plasmodium of myxomycetes, such as Physarum polycephalum and other well-studied species.3-5
In his Experimental-Physiologie der Pflanzen (Experimental Physiology of Plants), Sachs (1865),6 adopted de Bary's and Hofmeister's view, and described intracellular processes related to the properties of the protoplasm with reference to the myxomycetes.
Six years ago, 2 research groups provided unequivocal molecular evidence for the hypothesis that myxomycetes are neither plants, animals or fungi, but members of the amoebozoans,3,7 as originally suggested by de Bary.2 The life cycle of plasmodial slime molds has been reconstructed based on observations on numerous morphospecies (essentially defined by the structure of their fruiting bodies) that were cultivated in the laboratory. First, after spore germination in liquid medium, uni-nucleate flagellated swarm cells develop that are capable to transform into a myxamoeba. Second, after the fusion of 2 amoebae, a zygote is formed that develops, via nuclear divisions, into a multi-nucleate plasmodium. Finally, new spores are produced via the development of fruiting bodies (sporocarps). Both forms, the myxamoebae and the plasmodia, primarily feed on bacteria.3
We have observed that, under laboratory conditions, myxamoebae cultivated on agar plates move toward the source of prey organisms. However, to our knowledge, no quantitative comparative study has been published so far wherein different species of separate orders have been compared. In this report, we document that the myxamoebae of 3 morphospecies that co-occur in their natural habitat (decaying wood of European forests) display different cell motilities when exposed to the same bacterial prey organisms. In addition, we analyzed whether or not the 3 amoebae are distinguishable based on their average cell size.
The experiments were carried out with 3 well-defined morphospecies of plasmodial slime molds: Lycogala epidendrum, Tubulifera arachnoidea (order Liceales), and Trichia decipiens (order Trichiales). Swarm cells (myxamoebae) were obtained and cultivated on agar plates (Reasoner's 2A agar) as described by Hoppe and Kutschera.3,4 The swarming behavior of samples of myxamoebae (50 – 200 cells per experiment) was analyzed using an Inverse-light microscope (ID 03, Zeiss Germany) via series of photographs taken on the same samples from days zero to 10 after start of the swarming experiment (25°C, darkness)
As prey organisms, cultures of methylobacteria (Methylobacterium mesophilicum), agrobacteria (Agrobacterium tumefaciens) and a reference bacterial strain (Escherichia coli K12) were maintained in the lab. Diluted cell suspension (ca. 106 – 107 bacteria/ml) were added to the agar plates at day zero (Fig. 1A). Scanning electron microscopy was performed as described by Hoppe and Kutschera.3 Cell size (i.e., surface area)-measurements were carried out on single myxamoebae using light microscopy and a special computer program (AxioVision SE64 Rel. Four.9, Zeiss, Germany).
Figure 1.
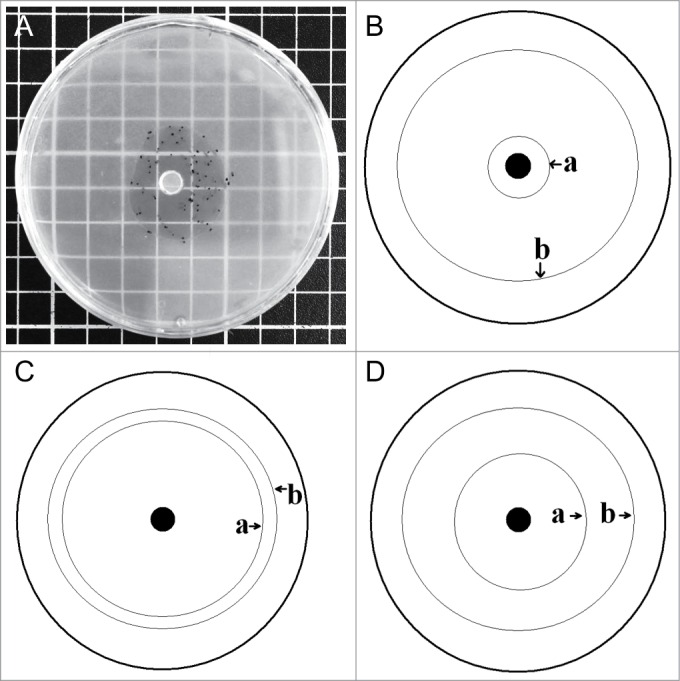
Agar plate (diameter: 9 cm) for comparative experimental analysis of cell motility in myxamoebae (A). In the center of the plate the amoebae were placed into a hole (diameter: 5 mm) at time zero. The movement of the myxamoebae, in the presence of methylobacteria, away from the center, was measured at daily intervals. Species-specific differences between Trichia decipiens (B), Lycogala epidendrum (C), and Tubulifera arachnoidea (D) with respect to the areas of highest population density (1) and the largest distance from the center (2) are shown schematically.
When a defined piece from a amoebal culture was placed into the center of an agar plate, on the surface of which bacterial prey organisms were present, the myxamoebae actively moved toward the periphery of their artificial habitat (Fig. 1 A). As indicated in Figures 1 B – D, the populations of myxamoebae of the taxa Trichia decipiens, Tubulifera arachnoidea, and Lycogala epidendrum behaved in a species-specific way with respect to the region of largest cell density, and the distance traveled away from the starting point wherein they were placed in at time zero, respectively.
Scanning electron micrographs revealed that the myxamoebae fed on the bacteria via phagocytosis (Fig. 2). Since the prey organisms by far outnumbered their eukaryotic predators, our experimental conditions do not reflect the real-world situation. However, for a quantitative comparison under the same (ideal) conditions, our approach is acceptable.
Figure 2.
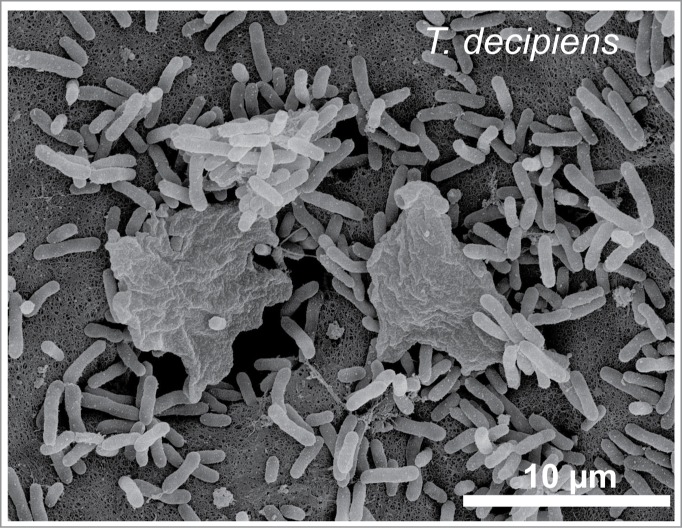
Morphology of the eukaryotic myxamoeba of Trichia decipiens and bacterial cells (M. mesophilicum) as revealed by scanning electron microscopy. Note that the 2 myxamoebae, equipped with pseudopodia, feed on the methylobacteria via phagocytosis.
In a series of quantitative experiments, populations of myxamoebae of the species studied here were exposed to 3 bacterial prey organisms: the plant-associated microbial taxa Methylobacterium mesophilicum and Agrobacterium tumefaciens, and the gut microbe Escherichia coli (reference strain K12). In the presence of M. mesophilicum (Figs. 2, 3), myxamoebae of T. decipiens displayed the highest rate of cell movements, followed by those of T. arachnoidea. Amoebae of L. epidendrum traveled at a very low rate toward the periphery of their artificial habitat. Similar results are obtained when the standard microbe E. coli K12 was offered as prey organism (Fig. 4). However, striking different movements were recorded in the presence of the soil bacterium A. tumefaciens (Fig. 5). Only amoebae of T. decipiens moved at a high rate, whereas those of L. epidendrum and T. arachnoidea displayed a moderate dispersal behavior (Fig. 5). These data document that the myxamoebae investigated here show species-specific patterns of cell motility, and apparently consume bacterial prey organisms differentially.
Figure 3.
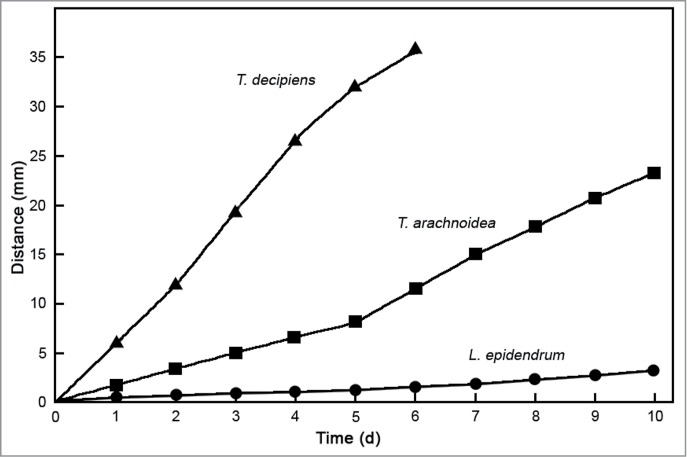
Comparative analysis of cell motility in populations of 3 species of myxamoebae in the presence of bacterial prey organisms (M. mesophilicum). The distance from the center of the agar plate was measured at daily intervals as shown in Figure 1A. Data points represent means (± s.e.m.) of 63 measurements each (standard errors of the means are smaller than the size of the symbols).
Figure 4.
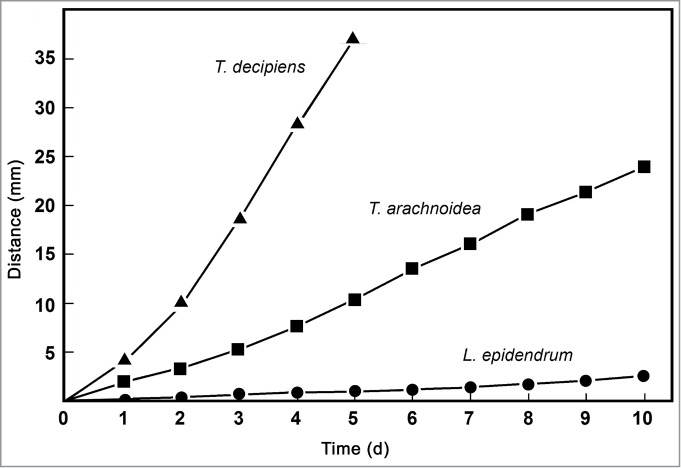
Comparative analysis of cell motility in populations of 3 species of myxamoebae in the presence of bacterial prey organisms (E. coli K12). The distance from the center of the agar plate was measured at daily intervals as shown in Figure 1A. Data points represent means (± s.e.m.) of 49 measurements each (standard errors of the means are smaller than the size of the symbols).
Figure 5.
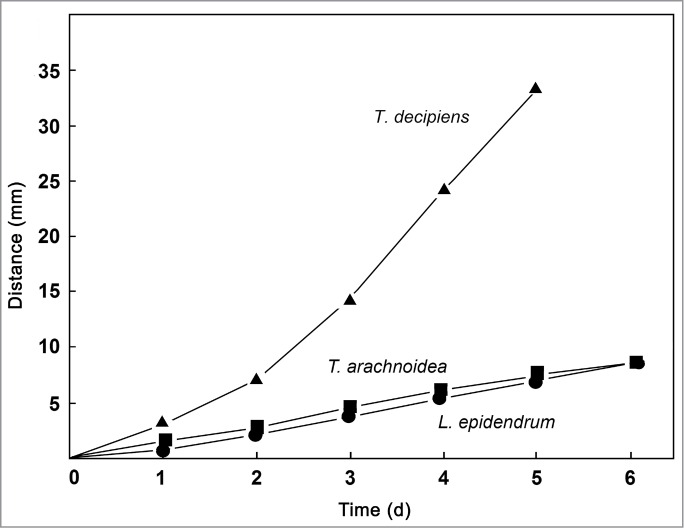
Comparative analysis of cell motility in populations of 3 species of myxamoebae in the presence of bacterial prey organisms (A. tumefaciens). The distance from the center of the agar plate was measured at daily intervals as shown in Figure 1A. Data points represent means (± s.e.m.) of 51 measurements each (standard errors of the means are smaller than the size of the symbols).
Finally, we addressed the question as to possible species-specific differences in cell size. The data summarized in Fig. 6 show that the cell surface areas of the 3 myxamoebae investigated here differ significantly. For the morphospecies T. decipiens, average values of 11.75 ± 3.2 µm (n = 74) were measured, which were similar to those of T. arachnoidea (11.31 ± 2.5 µm) (n = 197). Considerably larger cells were present in our lab-populations of L. epidendrum, with sizes of 16.77 ± 4.3 µm (n = 81). Hence, the 3 myxomycete species investigated are characterized by taxon-specific amoebae, at least with respect to their average cell size.
Figure 6.
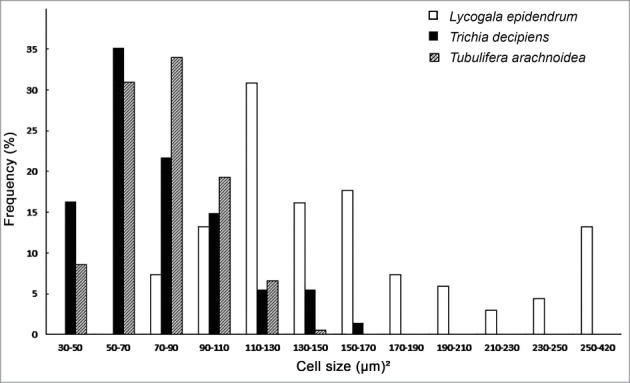
Species-specific distributions of cell size (surface area) of myxamoebae of 3 species of plasmodial slime molds. Note that T. arachnioides and T. decipiens are characterized by smaller cells compared to L. epidendrum (n = 197, 74 and 81 measurements for T. arachnioida, T. decipiens, L. epidendrum, respectively).
In his Experimental Physiology of Plants, Sachs6 referred to the earlier work of de Bary2 when he described intracellular movements in the plasmodia of myxomycetes. Since these “slime molds” were considered to be modified plants,1 Sachs' inclusion of these heterotrophic microorganisms, which are characterized by the ability to fruit, was justified at that time. Today we know that the myxomycetes are neither plants, nor fungi or animals – they are highly modified amoeboid protists and members of the Amoebozoa.3,7-10
A recent, comprehensive phylogenetic analysis for 109 eukaryotic protists (17 – 18 Amoebozoa) based on 60 – 188 genes revealed that this key phylum is monophyletic. The myxomycetes are, together with the related Dictyostelea, members of the sub-phylum Conosa, which, together with the sister group Lobosa, form the monophyletic taxon Amoebozoa.11
In this study, we addressed the question as to possible species-specific rates of cell movement in 3 different myxomycetes of the orders Liceales and Tricheales. Under defined laboratory conditions, these myxamoebae, which co-occur on decaying wood or litter in European forests, displayed taxon-specific patterns of cell movements (Figs. 1; 3–5). Moreover, when 3 defined bacterial prey organisms were offered in separate trials, the rates of myxamoeba-gliding over the agar surface, toward the periphery of their artificial habitat, were strikingly different. This results indicates that the myxamoebae of the co-occurring morphospecies T. decipiens, T. arachnioides and L. epidendrum exploit different prey organisms and hence occupy not the same ecological niche in their habitat. Our finding that the 3 species of myxamoebae investigated here differ with respect to their average cell size is in accordance with this conclusion. However, more work is required to further elucidate the way closely related Amoebozoa can co-exist in the same arboreal habitat as documented in recent publications.12,13
Finally, we want to note that the biophysical mode of “amoeboid migration” is still a matter of debate. Lämmermann and Sixt,14 summarized our knowledge concerning the mechanisms of cell gliding with a focus on the cellular slime mold Dictyostelium. However, myxomycetes are not addressed by these authors, so that it is likely that the biophysical principles of myxamoeba-movements are still unexplored.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Alexander von Humboldt Foundation, Bonn, Germany (AvH-Fellowships Stanford 2014/15 to U. K.).
References
- 1.Hofmeister W, Die Lehre von der Pflanzenzelle. Wilhelm Engelmann, Leipzig, 1867 [Google Scholar]
- 2.de Bary A, Die Mycetozoen. Ein Beitrag zur Kenntnis der niedersten Thiere. Z Wiss Zool 1859; 10:88-175 [Google Scholar]
- 3.Hoppe T, Kutschera U, In the shadow of Darwin: Anton de Bary's origin of myxomycetology and a molecular phylogeny of the plasmodial slime molds. Theory Biosci 2010; 129:15-23; PMID:19997788; http://dx.doi.org/ 10.1007/s12064-009-0079-7 [DOI] [PubMed] [Google Scholar]
- 4.Hoppe T, Müller H, Kutschera U, A new species of Physarum (Myxomycetes) from a boreal pine forest in Thuringia (Germany). Mycotaxon 2010; 114:7-14; http://dx.doi.org/ 10.5248/114.7 [DOI] [Google Scholar]
- 5.Kaplan DR, Cooke TJ, The genius of Wilhelm Hofmeister: the origin of causal-analytical research in plant development. Am J Bot 1996; 83:1647-60; http://dx.doi.org/ 10.2307/2445841 [DOI] [Google Scholar]
- 6.Sachs J, Handbuch der. Experimental-Physiologie der Pflanzen. Wilhelm Engelmann, Leipzig, 1865 [Google Scholar]
- 7.Fiore-Donno AM, Meyer M, Baldauf SL, Pawlowski J, Evolution of dark-spored Myxomycetes (slime-molds) Molecular versus morphology. Mol Phylogenet Evol 2008; 46:878-89; PMID:18221895; http://dx.doi.org/ 10.1016/j.ympev.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 8.Stephenson SL, From morphological to molecular: studies of myxomycetes since the publication of the Martin and Alexopoulos (1969) monograph. Fungal Diversity 2011; 50:21-34; http://dx.doi.org/ 10.1007/s13225-011-0113-1 [DOI] [Google Scholar]
- 9.Stephenson SL, Feest A, Ecology of soil eumycetozoans. Acta Protozool 2012; 51:201-8 [Google Scholar]
- 10.Stephenson SL, Fiore-Donno AM, Schnittler M, Myxomycetes in soil. Soil Biol Biochem 2011, 43: 2237-42; http://dx.doi.org/ 10.1016/j.soilbio.2011.07.007 [DOI] [Google Scholar]
- 11.Cavalier-Smith T, Fiore-Donno AM, Chao E, Kudryavtsev A, Berney C, Snell EA, Lewis R, Multigene phylogeny resolves deep branching of Amoebozoa. Mol Phylogenet Evol 2015; 83:293-304; PMID:25150787; http://dx.doi.org/ 10.1016/j.ympev.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 12.Hoppe T, Kutschera U, Chromosome numbers in representative myxomycetes: a cytogenetic study. Mycol Progr 2014; 13:189-92; http://dx.doi.org/ 10.1007/s11557-013-0934-2 [DOI] [Google Scholar]
- 13.Hoppe T, Schnittler M, Characterization of myxomycetes in two different soils by TRFLT-analysis of partial 18S rRNA gene sequences. Mycosphere 2015; 6:216-27 [Google Scholar]
- 14.Lämmermann T, Sixt M, Mechanical modes of ‘amoeboid’ cell migration. Curr Opin Cell Biol 2009; 21:636-44; http://dx.doi.org/ 10.1016/j.ceb.2009.05.003 [DOI] [PubMed] [Google Scholar]


