Abstract
REDOX RESPONSIVE TRANSCRIPTION FACTOR1 (RRTF1) regulates redox homeostasis under stress, however the mechanism is mainly unknown. In a recent publication, we analyzed rrtf1 knockout (ko) and RRTF1 overexpressor lines of Arabidopsis thaliana and showed that RRTF1 plays a crucial role in reactive oxygen species (ROS) production. Ko line produces less and overexpressor lines constitutively high levels of ROS under stress, and the amount of ROS increases with increase in stress and the RRTF1 level in the plant. The transcription factor also activates systemic ROS signaling under stress.1 In this report, we show that RRTF1 exerts different roles in young and old leaves. While RRTF1 enhances defense responses to high light (HL) stress in young leaves, it induces senescence and chlorosis in older leaves. These findings suggest that RRTF1 and/or RRTF1-mediated ROS signaling induce stress responses in an age-dependent manner, and the age-dependent alteration in the RRTF1 function might be important for plants' acclimation to the stress environment.
Keywords: REDOX RESPSONSIVE TRANSCRIPTION fACTOR1, senescence, systemic stress signaling
Introduction
RRTF1 was first reported as a transcription factor which is regulated by redox signals from the photosynthetic electron transport. Analyses of expression profiles demonstrated that RRTF1 belongs to a complex redox regulation network.2 The transcription factor is up-regulated directly and systemically by diverse stress stimuli and stress-related phytohormones,1,3-8 and a rrtf1 ko plant was more sensitive to HL.2 A survey of the literature uncovered that the transcription factor is also involved in various ROS-related stress responses induced by pathogens and pathogen-associated molecular patterns.1 RRTF1 expression itself is upregulated by ROS and ROS-generating stresses, and more than 800 genes for stress-, redox-, cell death- and senescence-related functions responded to elevated RRTF1 levels in young and mature leaves.1 Therefore, the transcription factor might be a general regulator of cellular redox homeostasis under various stress conditions and part of a regulatory circuit, in which it amplifies local and systemic ROS formation.1 The mechanisms of how RRTF1 regulates the cellular redox homeostasis and confers oxidative stress tolerance in plants is unknown. However, our microarray analyses uncovered that the RRTF1-responive gene pattern is different in young and older leaves and different organs (shoots/roots), suggesting that the transcription factor has distinct physiological roles depending on the age, tissue and organ of the analyzed material.
Purpose
To gain insight into the age-dependent regulation of RRTF1-mediated stress responses, we analyzed the effect of HL stress on young and old leaves of the RRTF1 overexpressor line oe18 1 and compared it with the responses of the wild-type (WT) and the rrtf1 ko line.
Result
Figure 1A shows the phenotypes of Arabidopsis thaliana WT (Col-0), rrtf1 ko and RRTF1 overexpressor (oe181) lines 48 hours after exposure to HL (700 µmol/m2s) stress. Since oe18 is sensitive to light stress,1 the oe18 shows stronger symptoms of chlorosis in the leaves than the rrtf1 and WT. The chlorotic phenotype appears mainly in the older leaves of oe18, and is not or less detectable in the younger leaves, suggesting that RRTF1 induces leaf-senescence preferentially in the old leaves (Fig. 1A). Most of the younger oe18 leaves continued to grow under the HL condition, implicating that they acquired HL stress tolerance rather than inducing senescence. The opposite was observed for older leaves.
Figure 1.
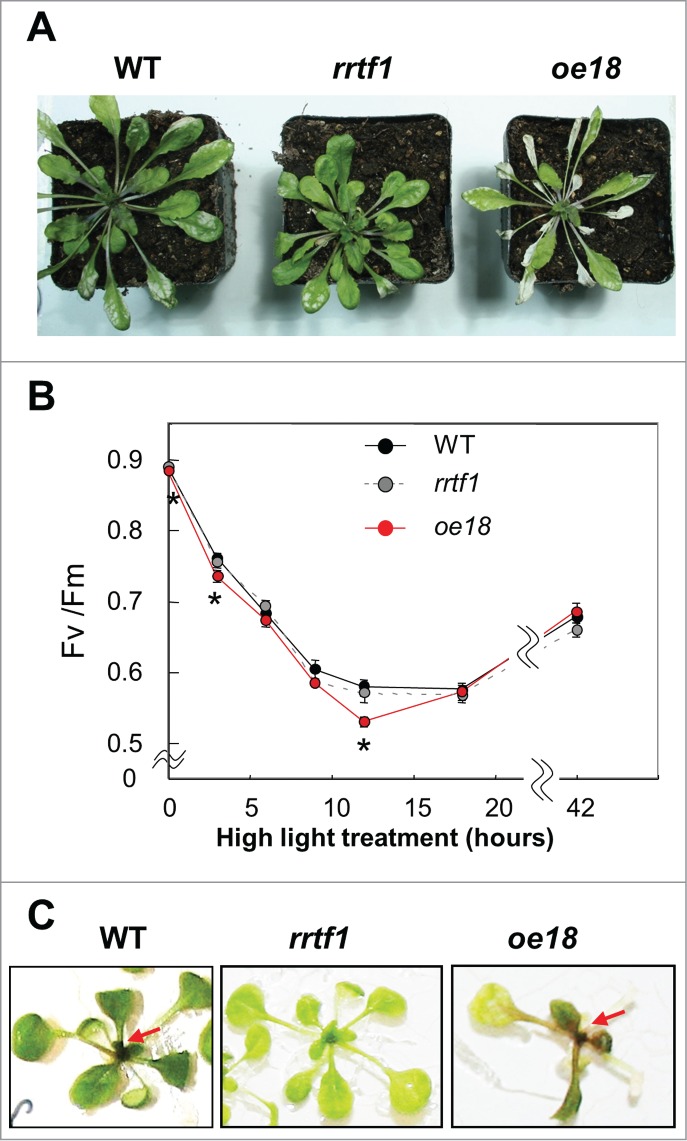
RRTF overexpressor (oe18) phenotype under high light condition. (A) Phenotype of WT (Col-0), rrtf1 (RRTF1 knockout line: SALK_150614) and oe18 after 48 hours high light (HL:700 μmol/m2s) illumination. (B) Time course experiment for the change of maximum quantum efficiency of PSII photochemistry (Fv/Fm) under HL condition. (C) Deltection of intensive anthocyanin accumulation in young leaves of seedlings after 48 hour HL treatment (200 μmol/m2s). 4–5 weeks old plants, grown under short day condition (80 μmol/m2s) (A,B), and 12 days old seedlings, growing on MS plate supplemented with 1.37% sucrose under continuous low light condition (20 μmol/m2s) (C) were transferred to HL condition. Whole plant's Fv/Fm vlaues were measured with a FluorCam 700F (Photon System Instruments, Czech Republic). Astertisk: data with significant differences (n ≥ 5, t-test p <0.05).Red arrows: anthocyaninsin WT and oe18.
A time course experiment for the photosynthetic parameter “maximum quantum efficiency of photosystem (PS)II photochemistry (Fv/Fm)” during HL illumination revealed that the Fv/Fm values of the oe18 decreased stronger than those of WT and ko plants during the early phase of the HL treatment (Fig. 1B). However, after 12 hours of HL, the Fv/Fm values of the oe18 plants recovered faster and reached the levels of the WT and ko plants. The faster recovery could have 2 reasons. One explanation could be that impaired oe18 leaves died due to chlorosis, while healthier and less impaired leaves with higher Fv/Fm values recover during later phases of the HL treatment. This results in a faster increase in the Fv/Fm values during later phases of the measurement (Fig. 1B). Another explanation could be that the surviving oe18 leaves develop a stronger defense capacity against oxidative HL stress and ROS accumulation which is further amplified by RRTF1.1
We have also shown that anthocyanins, photoprotective compounds, accumulate to higher levels in HL-exposed RRTF1 overexpressor lines compared to WT and rrtf1 plants,(1 supporting Figure S4). The oe18 seedlings mainly accumulated anthocyanins in young leaves surrounding the meristem (Fig. 1C), and not in older and more developed leaves. This supports the idea that the photoprotective capacity is higher in young leaves.
Conclusion
RRTF1 and RRTF1-mediated ROS signaling cause different responses dependent on the leaf age. In younger leaves, RRTF1 and RRTF1-mediated ROS signaling activate predominantly stress defense responses. In older leaves, RRTF1 accelerates senescence, which becomes visible by the chlorotic leaf phenotype. It is reasonable to assume that stress-induced senescence in the older leaves produces catabolites which can be transported to the young leaves to support their fitness, development and growth. The combination of these 2 distinct responses may be crucial for the plant to acclimate to the stress environment. RRTF1 is a core component of a stress-related transcriptional network, regulating stress responses not only at cellular and organ level but also at the plant level. Therefore, the transcription factor might connect these 2 stress strategies via systemic signaling. This might allow the plant to regulate the turnover of their leaves during adaptation to stress. Elucidation of the role of RRTF1 in this regulatory network will give essential clues to understand the stress-induced communication within the plant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Matsuo M, Johnson JM, Hieno A, Tokizawa M, Nomoto M, Tada Y, Godfrey R, Obokata J, Sherameti I, Yamamoto YY, et al.. High REDOX RESPONSIVE TRANSCRIPTION FACTOR1 levels result in accumulation of reactive oxygen species in Arabidopsis thaliana shoots and roots. Mol Plant (2015); 8;1253-73. doi: 10.1016/j.molp.2015.03.011. http://dx.doi.org/ 10.1016/j.molp.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 2.Khandelwal A, Elvitigala T, Ghosh B, Quatrano RS. Arabidopsis transcriptome reveals control circuits regulating redox homeostasis and the role of an AP2 transcription factor1. Plant Physiol (2008); 148:2050-8; PMID:18829981; http://dx.doi.org/ 10.1104/pp.108.128488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toufighi K, Brady SM, Austin R, Ly E, Provart NJ. The Botany Array Resource: e-Northerns, Expression Angling, and promoter analyses. Plant J (2005); 43:153-63; PMID:15960624; http://dx.doi.org/ 10.1111/j.1365-313X.2005.02437.x [DOI] [PubMed] [Google Scholar]
- 4.Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inzé D, Mittler R, Van Breusegem F. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol (2006); 141:436-45; PMID:16603662; http://dx.doi.org/ 10.1104/pp.106.078717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossel JB, Wilson PB, Hussain D, Woo NS, Gordon MJ, Mewett OP, Howell KA, Whelan J, Kazan K, Pogson BJ. Systemic and intracellular responses to photooxidative stress in Arabidopsis. Plant Cell (2007); 19:4091-110; PMID:18156220; http://dx.doi.org/ 10.1105/tpc.106.045898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsui A, Ishida J, Morosawa T, Mochizuki Y, Kaminuma E, Endo TA, Okamoto M, Nambara E, Nakajima M, Kawashima M, et al.. Arabidopsis transcriptome analysis under drought, cold, high-salinity and ABA treatment conditions using a tiling array. Plant Cell Physiol (2008); 49:1135-49; PMID:18625610; http://dx.doi.org/ 10.1093/pcp/pcn101 [DOI] [PubMed] [Google Scholar]
- 7.Wang P, Song CP. Guard-cell signaling for hydrogen peroxide and abscisic acid. New Phytol (2008); 178:703-18; PMID:18373649; http://dx.doi.org/ 10.1111/j.1469-8137.2008.02431.x [DOI] [PubMed] [Google Scholar]
- 8.Vogel MO, Moore M, König K, Pecher P, Alsharafa K, Lee J, Dietz KJ. Fast retrograde signaling in response to high light involves metabolite export, MITOGEN-ACTIVATED PROTEIN KINASE6, and AP2/ERF transcription factors in Arabidopsis. Plant Cell (2014); 26:1151-65; PMID:24668746; http://dx.doi.org/ 10.1105/tpc.113.121061 [DOI] [PMC free article] [PubMed] [Google Scholar]


