Abstract
Autophagy is a pathway in which a cell degrades part of its cytoplasm in vacuoles or lysosomes. To identify the physiological functions of autophagy in plants, we disrupted ATG5, an autophagy-related gene, in Physcomitrella, and confirmed that atg5 mutants are deficient in the process of autophagy. On carbon or nitrogen starvation medium, atg5 colonies turned yellow earlier than the wild-type (WT) colonies, showing that Physcomitrella atg5 mutants, like yeast and Arabidopsis, are sensitive to nutrient starvation. In the dark, even under nutrient-sufficient conditions, colonies turned yellow and the net degradation of chlorophyll and Rubisco protein occurred together with the upregulation of several senescence-associated genes. Yellowing reactions were inhibited by the protein synthesis inhibitor cycloheximide, suggesting that protonemal colonies undergo dark-induced senescence like the green leaves of higher plants. Such senescence responses in the dark occurred earlier in atg5 colonies than WT colonies. The sugar content was almost the same between WT and atg5 colonies, indicating that the early-senescence phenotype of atg5 is not explained by sugar deficiency. However, the levels of 7 amino acids showed significantly different alteration between atg5 and WT in the dark: 6 amino acids, particularly arginine and alanine, were much more deficient in the atg5 mutants, irrespective of the early degradation of Rubisco protein. On nutrient-sufficient medium supplemented with casamino acids, the early-senescence phenotype was slightly moderated. We propose that the early-senescence phenotype in atg5 mutants is partly explained by amino acid imbalance because of the lack of cytoplasmic degradation by autophagy in Physcomitrella.
Keywords: amino acid, autophagy, bryophyte, Physcomitrella, senescence, sugar
Abbreviations
- ATG gene
autophagy-related gene
- CHX
cycloheximide
- Rubisco
ribulose 1,5-bisphosphate carboxylase/oxygenase
- SAG
senescence-associated gene
- WT
wild-type.
Introduction
Autophagy is a pathway responsible for the degradation of cytoplasm in the lytic organelles lysosomes and vacuoles. Previous studies have identified several autophagy pathways including macroautophagy, microautophagy and chaperone-mediated autophagy.1-4 Of these, macroautophagy has been studied the most. In macroautophagy, cytoplasmic components including cytosolic proteins and organelles are sequestered in double-membrane-enclosed autophagosomes, and then transported into vacuoles and/or lysosomes for degradation.5 This process is performed by 15 core Atg proteins plus other Atg proteins, all of which are encoded by autophagy-related genes (ATG genes).
Atg5 protein, one of Atg proteins, is involved in the formation of the precursor for the autophagosome.6-8 The precursor formation requires the conjugation of Atg5 with a ubiquitin-like Atg protein, Atg12. The resulting Atg5-Atg12 protein complex functions as a ligase that catalyzes the transfer of another ubiquitin-like protein, Atg8 from a conjugating enzyme, Atg3 to phosphatidylethanolamine on autophagosome membrane.9 Indeed, in the atg5 mutants of yeast and Arabidopsis, macroautophagy does not proceed.10,11
Given that macroautophagy (hereafter, autophagy) is induced in nutrient-starved and stressful environments, its physiological roles have been thought to be the supply of degradation products as nutrients and the removal of denatured proteins by degradation, although it is also believed to be involved in protein turnover.12 Recent studies of autophagy in plants using Arabidopsis and rice atg mutants have revealed several physiological roles of autophagy in plants. They include the recycling of amino acids under nutrient starvation and stress conditions and removing damaged proteins under oxidative and osmotic stress 13-15 and the degradation of chloroplasts, peroxisomes and starch particles.16-21 They also include involvement in hypersensitive cell death and senescence.6,22,23
Senescence in plants is a process characterized by cessation of photosynthesis, disintegration of organelle structure, net degradation of chlorophyll and chloroplast proteins, and upregulation of senescence-associated genes (SAGs).24 Senescence needs the expression of genes, including some SAGs, for its proceeding. Thus it is inhibited by cycloheximide (CHX), an inhibitor of protein synthesis on cytoplasmic ribosomes. It has been reported that CHX delays the degradation of chlorophyll and proteins accompanied by senescence of barley leaves placed in darkness 25 and inhibits senescence in the acd2 (accelerated cell death 2) mutant of Arabidopsis.26 Senescence is affected and often induced by environmental changes, although it occurs as a developmental process. It has been known that when green leaves are transferred into the dark, senescence is induced, which is called dark-induced senescence.
Studies in Arabidopsis and rice have shown that atg mutants show early-senescence phenotypes, including the yellowing of leaves and the net degradation of chlorophyll and chloroplast proteins 6,18,19 as well as the upregulation of SAGs.13,27 However, the details of autophagy's function in the early-senescence phenotype are unknown.
In this study, to elucidate the physiological significance of autophagy in plant cells, we constructed ATG5 knockout (atg5) mutants of Physcomitrella. On carbon or nitrogen starvation medium, atg5 colonies turned yellow earlier than wild-type (WT) colonies, showing that atg5 colonies are sensitive to nutrient starvation like higher plants. Also, atg5 mutant colonies showed the early-senescence phenotype: the mutant colonies turned yellow earlier than WT colonies in the dark. Taking advantage of the simple cellular structure of Physcomitrella colonies, we further analyzed changes in sugar and amino acid levels during dark treatment. From the results, we propose the function of autophagy in the acceleration of dark-induced senescence.
Results
Preparation of ATG5 knockout lines
Arabidopsis thaliana has a single gene (AtATG5: AT5G17290) encoding the Atg5 protein. We performed a BLAST search using the NCBI database and found that Pyscomitrella also has a single gene (PpATG5) encoding a protein highly homologous to the AtAtg5 protein (score, 300; E-value, 6e-99). We planned to construct PpATG5 knockout mutants as illustrated in Figure 1A. To validate the mutants, several candidates were analyzed for the absence of PpATG5 transcript by reverse transcription PCR, and 3 lines were chosen and named atg5–1, atg5–2, and atg5–3 (Fig. 1B, C). RNA was extracted from these lines and cDNA was synthesized. A section of the PpATG5 transcript was amplified using the obtained cDNA as a template (see Materials and Methods). No bands were amplified in any of the 3 mutants, whereas a band of the expected size (1,016 bp) was amplified in the WT plants (Fig. 1B). Furthermore, the genomic DNAs were extracted and checked for the expected homologous recombination of the endogenous ATG5 gene with the exogenous drug-resistant gene NPTII or APH4 in atg mutants. For this analysis, primers were designed for the first exon of ATG5 and for the promoter of the exogenous drug-resistance gene cassette (Fig. 1A). The WT plants yielded no PCR product, but atg5 mutants showed a band of the expected size (Fig. 1C).
Figure 1.
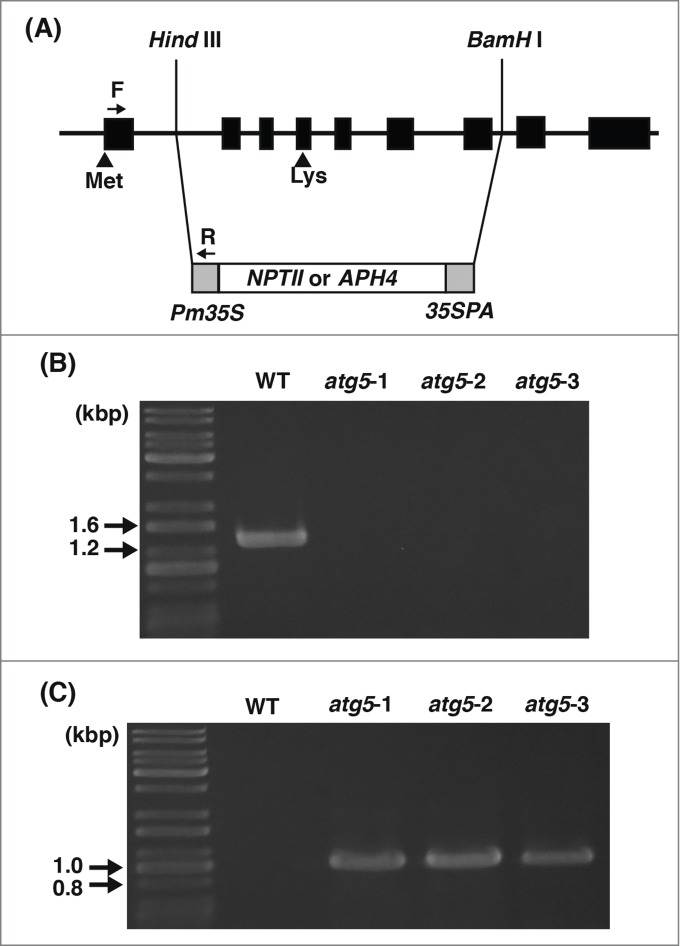
Disruption of the ATG5 gene in Physcomitrella. (A) A white box represents either the NPTII or APH4 gene cassette with its promoter (Pm35S) and terminator (35SPA) shown in gray. Black boxes represent 9 exons of the ATG5 gene in the Physcomitrella genome (solid line). Met indicates the coding region for the first methionine of Atg5 protein and Lys for the lysine that is to conjugate with Atg12 protein. The region from Hind III to BamH I sites was replaced with the NPTII cassette in the atg5–1 and atg5–2 mutants, and with the APH4 cassette in the atg5–3 mutant. (B) RNA was extracted from 7-day-old colonies of wild-type (WT), atg5–1, atg5–2, or atg5–3, and analyzed for the presence of PpATG5 transcript by reverse-transcription PCR. Size markers are shown on the left. (C) DNA was extracted from the 7-day-old colonies of WT, atg5–1, atg5–2, or atg5–3, and analyzed for recombination of the genome by PCR using the primers (F and R) shown in (A). DNA size markers are shown on the left.
Growth of atg5 mutants on nutrient-sufficient medium
The protonemal colonies of WT and atg5 mutants that were grown on nutrient-sufficient medium for 7 d were further cultured on fresh nutrient-sufficient medium for 14 d (Fig. 2). On day 0, WT and atg5 colonies were green overall and similar in size; thus were indistinguishable by their appearance (0 d). All of the colonies expanded in a similar manner on the medium. In the 3 atg5 mutants, however, the central part of each colony gradually turned yellow with the surrounding part remaining green (Figs. 2, 7D), and the yellow area extended during 14 d of culture. In contrast, WT colonies largely stayed green overall for the first 7 d., and only a small area in their center turned brown after 14 d. (Fig. 2, 14D)
Figure 2.
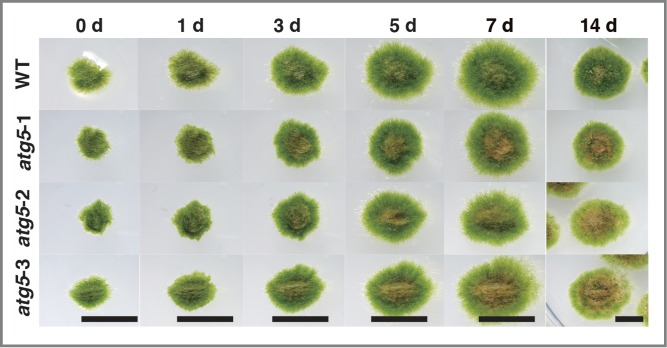
Growth and morphological changes of the atg5 mutants of Physcomitrella on nutrient-sufficient medium. Seven-day-old colonies of wild-type (WT) and atg5 mutants (atg5–1, atg5–2 and atg5–3) of Physcomitrella were further cultured on fresh nutrient-sufficient medium for 14 d. The same colonies were photographed from 0 to 14 d. Bar, 5 mm.
The results show that natural senescent reaction occurs according to their developmental ages earlier in atg5 mutants than in WT plants, or alternatively, yellowing occurred as a starvation response, described in the following section, because the central parts of colonies are likely to be deficient in nutrients.
Growth of atg5 mutants on nutrient-deficient medium
Seven-day-old protonemal colonies of atg5 mutants and WT plants were transferred onto glucose-deficient medium and cultured in the dark for 7 d This carbon starvation treatment arrested the growth of colonies and induced yellowing of colonies in both atg5 and WT plants. However, yellowing occurred earlier in atg5 mutants than in WT plants (Fig. 3A). On nitrogen-deficient medium, 7-day-old colonies of WT and atg5 mutants grew to the same extent for 5 d.; however, the atg5 mutants lost the green color faster than the WT after 5 d of nitrogen deficiency (Fig. 3B)
Figure 3.
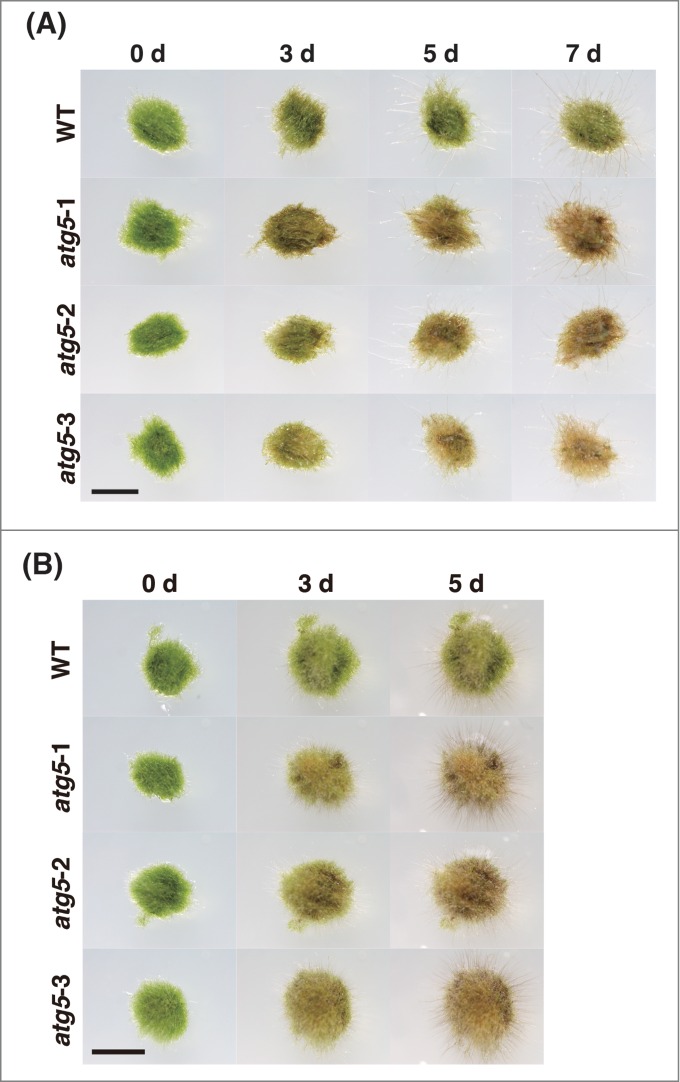
Growth and morphological changes of the atg5 mutants of Physcomitrella on nutrient-deficient medium. (A) Seven-day-old colonies of wild-type (WT) and atg5 mutants of Physcomitrella were cultured in the dark on glucose-depleted medium for 7 d Bar, 3 mm. (B) Seven-day-old colonies of WT and atg5 mutants of Physcomitrella were cultured on nitrogen starvation medium for 5 d Bar, 5 mm.
These results show that atg5 mutants of Physcomitrella were more sensitive to nutrient starvation than WT plants.
Autophagic activities were not detected in atg5 mutants
In tobacco BY-2 cells and barley and Arabidopsis root cells, autophagy can be blocked by protease inhibitors such as E-64c and E-64d, and the resulting accumulation of degradation intermediates can be detected by light microscopy equipped with Nomarski optics.28 In the present study, using E-64d, we examined the atg5 mutants for the absence of autophagy. Because autophagy is generally supposed to be induced under nutrient starvation conditions, we transferred the colonies that had been cultured on nutrient-sufficient medium for 7 d to glucose-depleted liquid medium containing E-64d, and further cultured them in the dark for 1 d. In the protonemal cells of atg5 mutants, the accumulation of aggregates, which are most likely the degradation intermediates of a part of the cytoplasm, was not observed in the presence of E-64d, whereas aggregates were found in WT cells (Fig. 4, arrow). It is notable that in the protonemal cells of Physcomitrella, the aggregates seemed to accumulate near the nucleus as in tobacco BY-2 cells.
Figure 4.
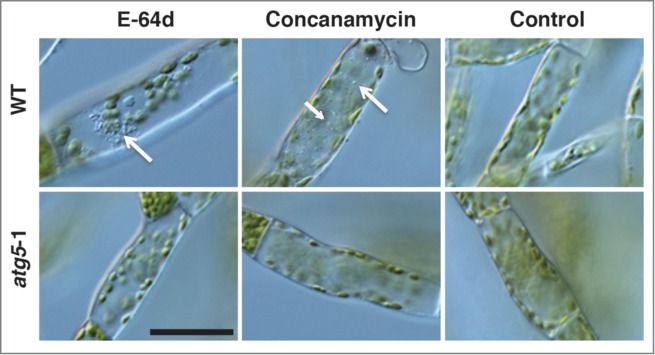
Detection of autophagy in the protonemal cells of Physcomitrella using E-64d and concanamycin A. The protonemal cells from 7-day-old colonies of wild-type (WT) and atg5–1 plants were incubated in glucose-depleted liquid medium including 100 μM E-64d, 0.1 μM concanamycin, or 1% (v/v) methanol (Control) for 1 d. in the dark. Arrows point to aggregates. Bar, 50 μm.
The vacuolar H+-ATPase inhibitor concanamycin A also blocks the macroautophagic pathway in a different manner from E-64c and E-64d; thus, autophagic bodies accumulate in the vacuoles of Arabidopsis roots.29 The colonies of WT and atg5 mutants were cultured in glucose-depleted liquid medium containing concanamycin A in the dark and observed after 1 d. of culture. Although many autophagic bodies were found in the vacuole of WT cells (arrow), such structures were not observed in atg5 mutant cells (Fig. 4)
These results strongly suggest that macroautophagy does not proceed in atg5 mutants.
Dark induces chlorophyll degradation
To investigate whether atg5 mutants undergo senescence in the dark earlier and more rapidly than WT plants, 7-day-old protonemal colonies of WT and atg5 mutants were transferred onto a fresh nutrient-sufficient medium, which contained glucose, and placed in the dark, and their morphological changes were observed. atg mutant colonies turned slightly yellow after 3 d and mostly yellow after 5–7 d., whereas WT colonies stayed slightly yellow even after 7 d (Fig. 5A). In general, changes of color in the colonies proceeded in a manner similar to that on glucose-depleted medium in the dark. This result shows that glucose in the medium does not affect the progress of senescence under these experimental conditions.
Figure 5.
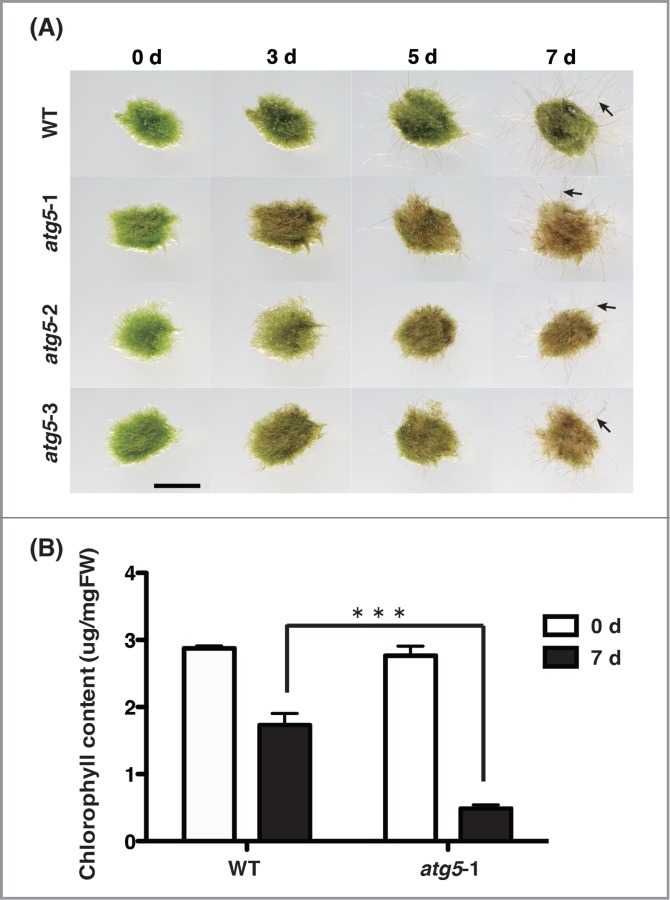
Growth and morphological changes of wild-type (WT) and atg5 mutants of Physcomitrella in the dark. (A) Seven-day-old colonies of WT and atg5 mutants of Physcomitrella were cultured on nutrient-sufficient medium in the dark. Arrows point to diaphanous cells. Bar, 3 mm. (B) Chlorophyll was extracted from the colonies of WT and atg5 mutants on the days 0 and 7, and measured. Values are shown as means ± SE. *** indicates that the difference in chlorophyll contents between WT and atg5–1 on day 7 is significant (p < 0.001).
The chlorophyll contents in WT and atg5–1 mutant plants before dark treatment were almost the same (Fig. 5B). After 7 d., however, the content decreased sharply in atg5–1 mutant, whereas it decreased slightly in WT colonies (Fig. 5B). The results show that like green leaves of higher plants, the protonemal colonies of Physcomitrella undergo the net degradation of chlorophyll in the dark, suggesting that autophagy decelerates this degradation. Both WT and atg5 colonies extended diaphanous cells, which appeared different from normal green protonemal cells, under dark conditions (Fig. 5A, arrow). Few such cells were observed under light conditions (Fig. 2)
Dark induces net degradation of ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco)
The total protein content in colonies of WT plants and atg5 mutants did not change much during 7 d of dark treatment (Fig. 6). However, the amount of the large subunit of Rubisco (52.6 kD), which is the most abundant polypeptide in the protonemata of Physcomitrella,30 decreased rapidly and drastically so that the net degradation for 7 d amounted to more than 70% of the initial amount in atg5 mutants, whereas it decreased to a small extent in WT plants (Fig. 6)
Figure 6.
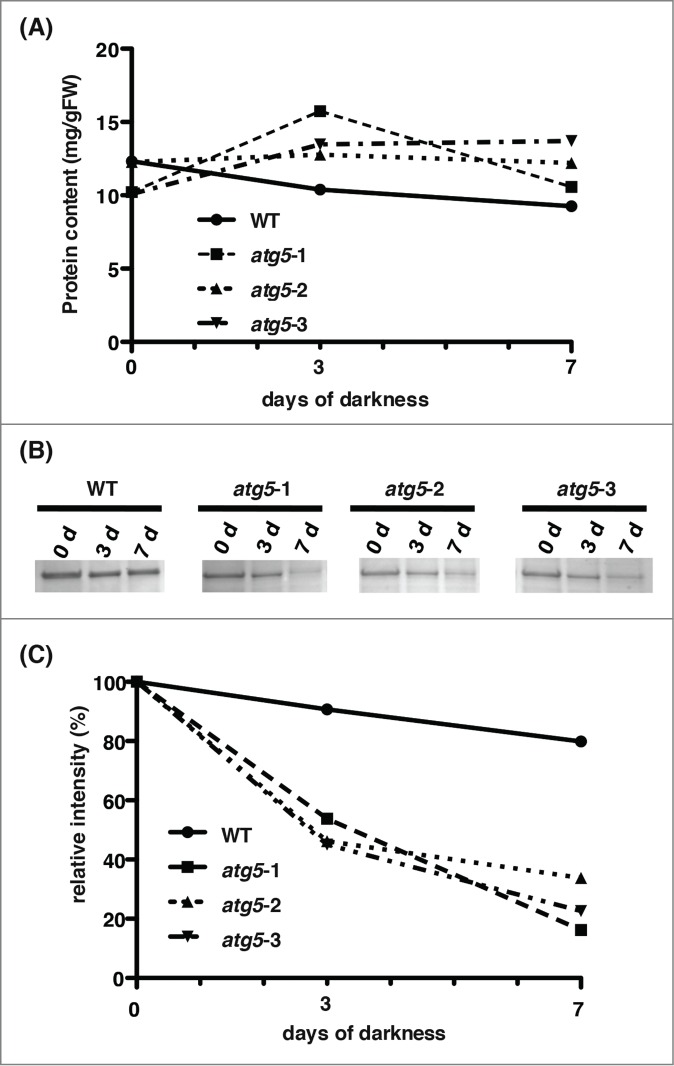
Change in total protein and Rubisco large subunit levels in the dark. Proteins were extracted from colonies of wild-type (WT) and atg5 mutants on days 0, 3, and 7. (A) Total proteins were measured. (B) They were separated on SDS-PAGE and stained with Coomassie brilliant blue. The same amount of protein was loaded into each lane. Bands of Rubisco large subunit, which is the most abundant polypeptide, are shown. (C) The amount of Rubisco large subunit was estimated by densitometry using ChemiDoc (Bio-Rad).
Expression of senescence-associated genes in the dark
We analyzed changes in the expression of several SAGs in WT and atg5 mutants subjected to darkness over 7 d These included SEN1 (encoding a protein strongly induced by phosphate starvation), SAG12 (encoding a cysteine protease induced by cytokinin, auxin, and sugars), SAG13 (encoding a short-chain alcohol dehydrogenase), and SAG18 (encoding a plant-specific protein of unknown function). The expressions of PpSEN1, PpSAG13 and PpSAG18 were kept at low levels during 7 d of darkness in WT plants. In contrast, the expression of all 3 genes was upregulated by dark treatment in atg5–1 mutant. SAG12 showed a slightly different response from that of the other 3 genes. Its expression increased after 5 d even in WT plants, although in atg5–1 mutant plants, the increase occurred earlier than in WT plants upon dark treatment. The expression of PpSEN1 and PpSAG12 was also slightly higher than that before dark treatment in the atg5–1 mutant than in WT plants (Fig. 7), suggesting that the mutants entered the senescent stage of development at the end of their previous culture on nutrient-sufficient medium under light conditions.
Figure 7.
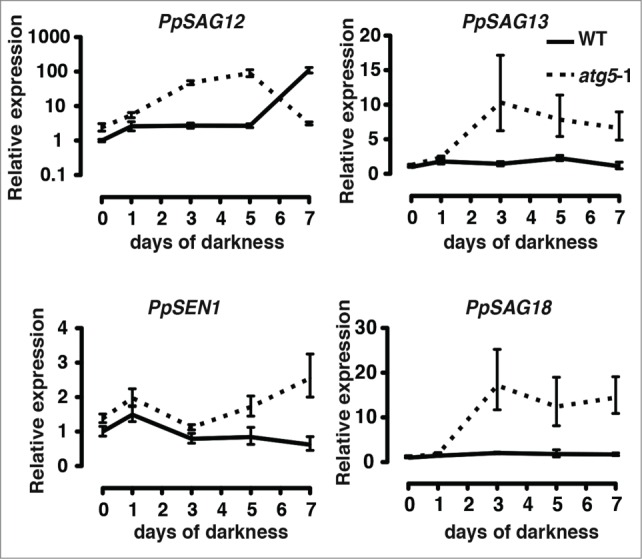
Expression of senescence-associated genes (SAGs) in the protonemal colonies of wild-type (WT) and atg5 mutants in Physcomitrella placed in the dark. Seven-day-old colonies of the WT and atg5–1 mutant of Physcomitrella were placed in the dark. RNA was extracted from 1, 3, 5 and 7 d after dark treatment. Expressions of PpSAG12, PpSAG13, PpSAG18 and PpSEN1 were analyzed by real-time PCR using specific primers for each gene (shown in Table S1).
CHX inhibits senescence
We investigated whether the senescence of Physcomitrella induced by darkness was also inhibited by CHX like the senescence of green leaves from higher plants. In the atg5–1 mutant, yellowing of the colony was clearly inhibited by the addition of CHX to the culture medium (Fig. S1A). However, the effect of CHX on senescence in WT colonies was difficult to evaluate because they did not show a clear senescence phenotype after 5 d of darkness. Longer treatment with CHX made colonies more yellow and we obtained no clear results showing the retardation of senescence by CHX in WT plants (Fig. S1B).
Autophagy is activated in the dark
The results obtained thus far showed that senescence is induced by dark treatment in the protonemal cells of Physcomitrella and suggested that autophagy has a decelerating effect on the process of senescence. Accordingly, we investigated the expression of ATG genes in protonemal cells placed in the dark. The transcripts of PpATG5, PpATG7 and PpATG8a were detected from 3 d after darkness, and seemed to slightly increase during darkness (Fig. 8), although PpATG8a was upregulated earlier than the others after 1 d. of darkness. This result suggests that the flux of the macroautophagy pathway increases under darkness.
Figure 8.
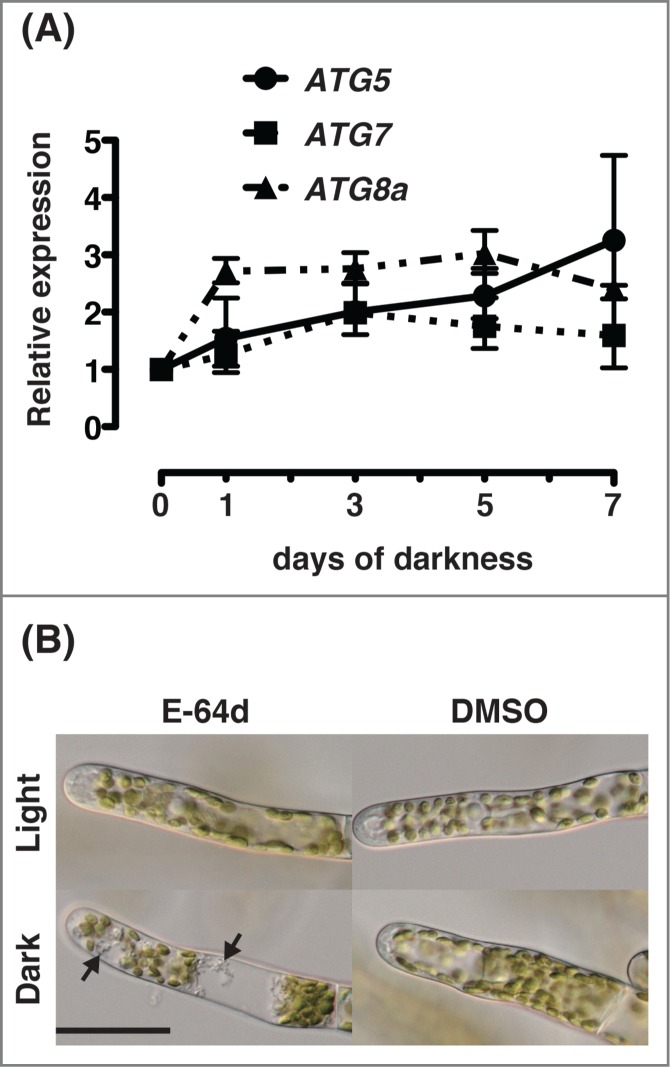
Activation of ATG gene expression and autophagy in protonemal cells of Physcomitrella cultured in the dark. (A) Seven-day-old colonies of wild-type (WT) Physcomitrella were cultured on nutrient-sufficient medium in the dark. RNA was isolated and the expressions of PpATG5, PpATG7 and PpATG8a genes were analyzed by real-time PCR using specific primers for each gene (shown in Table S1). All values are shown as means ± SE. (B) Protonemal cells from 7-day-old colonies of wild-type (WT) plants were incubated in nutrient-sufficient liquid culture medium including 100 μM E-64d or 1% (v/v) DMSO for 1 d. in the dark or in light. Arrows point to aggregates that appeared in response to the inhibition of protein degradation by E-64d. Bar, 40 μm.
We investigated whether autophagy is truly activated in the dark. WT colonies that had been cultured on nutrient-sufficient medium for 7 d were transferred to nutrient-sufficient liquid culture medium containing E-64d, and cultured in the dark or in light for 1 d. In protonemal cells cultured in the dark, accumulation of aggregates was observed (Fig. 8B, arrow), whereas aggregates were rarely observed in cells cultured in light (Fig. 8B). No such accumulation occurred in cells cultured in the absence of E-64d. The observations strongly suggest that in protonemal cells of Physcomitrella, autophagy is activated in the dark.
Change in sugar content during darkness
It is conceivable that earlier dark-induced senescence in atg5 mutants was due to poor nutrient conditions in cells resulting from autophagy-deficiency. In the dark, sugars should be insufficiently supplied to cells of Physcomitrella, particularly in atg5 mutants. Thus, total sugar contents were estimated using anthrone staining. In WT and atg5 mutants, total sugar content before dark culture was almost the same: autophagy did not affect the total sugar content of the colonies that had been cultured on nutrient-sufficient medium. During culture in the dark, the total sugar content in both WT and atg5 mutants increased less than 2-fold after 3 d., and decreased slightly from this level after 7 d (Fig. 9A). However, there was no significant difference in total sugar content between WT plants and atg5 mutants.
Figure 9.
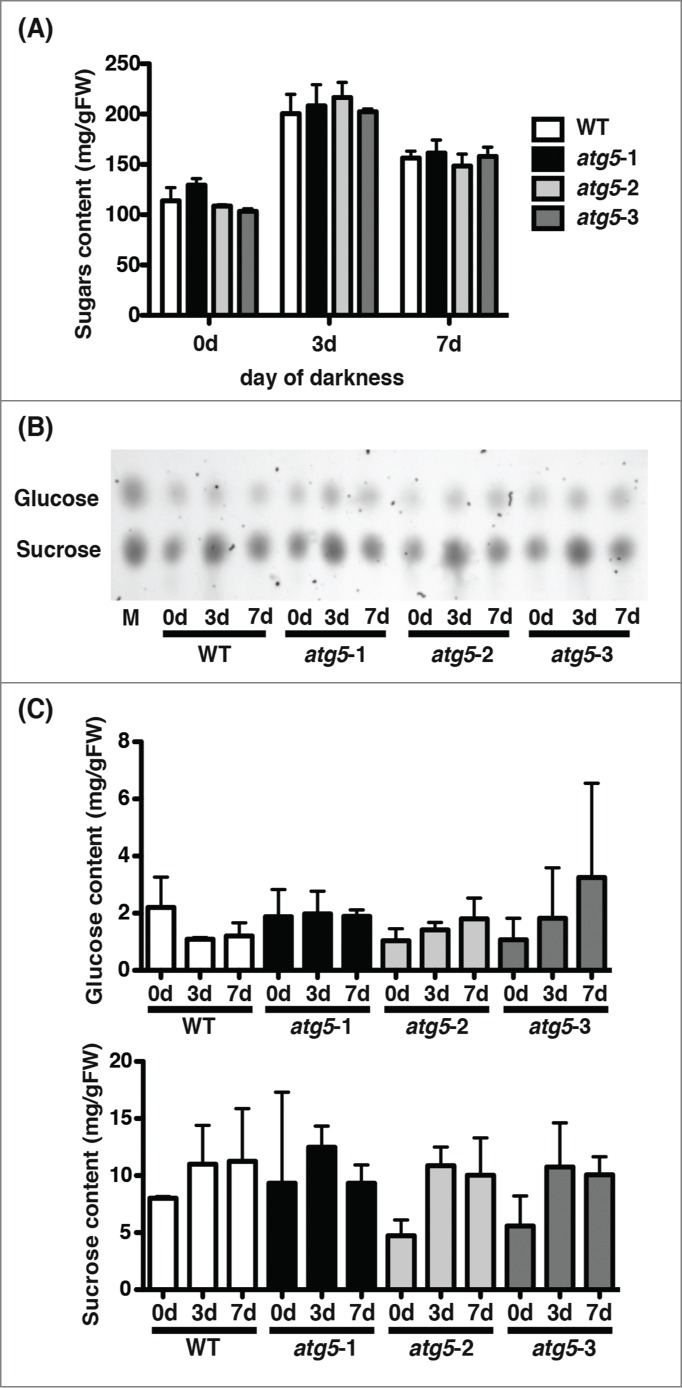
Changes in soluble sugar levels in colonies of the wild-type (WT) and atg5 mutants of Physcomitrella cultured in the dark. Seven-day-old colonies of WT and atg5 mutants of Physcomitrella were cultured for 7 d in the dark. Soluble sugars were extracted from the colonies on days 0, 3, and 7. (A) Total amounts of sugars were measured by the anthrone method using glucose as standard. (B) Extracted sugars were separated by thin-layer chromatography and stained with sulfuric acid. In the lane M, authentic glucose and sucrose were separated. (C) Levels of glucose and sucrose in the colonies of WT and atg5 mutants were estimated by densitometry using ChemiDoc (Bio-Rad).
Thin-layer chromatography showed that the major sugar components were sucrose and glucose in both WT and atg5 mutants (Fig. 9B). In both plants, glucose did not change but sucrose increased during culture in the dark. However, no significant difference in glucose or sucrose was found between WT and atg5 plants (Fig. 9B, C). These results show that a difference in sugar content is not responsible for early-senescence in atg mutants.
Changes in amino acid contents during darkness
The intracellular amino acid pool is substantially reduced in the atg mutants of yeast.31 It was conceivable that the reduction of the amino acid pool caused early-senescence in atg5 mutants in Physcomitrella. Thus intracellular amino acids were measured. We could separate and detect 26 substances including 17 protein amino acids, ammonia, and 8 non-protein amino acids including γ-aminobutyric acid. The three amino acids missing from all the protein amino acids are tryptophan, cysteine, and proline: proline was not detected, whereas tryptophan or cysteine could not be measured correctly by the method used in this study. First, the total concentration of these 17 amino acids were not significantly different between atg5 and WT colonies, and these values changed little during the 7 d of dark treatment (Fig. 10). Of the 17 protein amino acids, the concentrations of 9 behaved like the total concentration: their concentrations were almost the same between the cells of the atg5 and WT plants at the start of dark treatment, and changed negligibly during the 7 d of dark culture (data not shown). In contrast, the concentrations of 8 amino acids changed differentially between atg5 and WT plants. Of the 8, 6 amino acids, Arg, Lys, Ala, Val, Leu, and Tyr, were nearly at the same levels between atg5 and WT plants at the start of dark treatment but changed upon the transfer into the dark and were kept lower in atg5 mutants than in WT plants during the dark culture: Arg, Lys, Val, Leu, and Tyr increased upon dark treatment in both WT and atg5 plants but the increment was lower in atg5 mutants than in WT plants. Further, Ala decreased upon dark treatment but the decrease was higher in atg5 mutants than in WT plants. His behaved in the opposite manner to the other basic amino acids such as Arg and Lys, although its concentration was much less than that of Arg. Unlike the other 16 amino acids measured in this study, the concentration of Glu in atg5 mutants was significantly higher than that in WT plants before dark treatment. Upon dark treatment, however, it decreased to the same level as that in WT plants, and there was no difference between atg5 mutants and WT plants under dark conditions. The concentration of ammonia changed in a manner similar to that of His and was significantly higher in atg5 mutants than in WT plants. In addition, the level of γ-aminobutyric acid was significantly higher in WT than in atg5 mutants before and during the 7 d of dark treatment.
Figure 10.
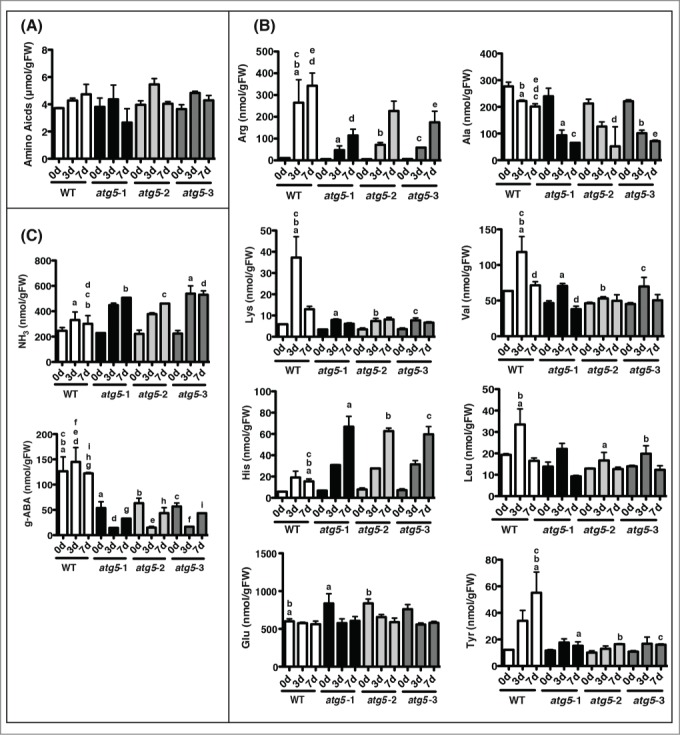
Changes in amino acid levels in colonies of the wild-type (WT) and atg5 mutants of Physcomitrella. Seven-day-old colonies of WT and atg5 mutants of Physcomitrella were cultured for 7 d in the dark. Amino acids were extracted from the colonies on days 0, 3, and 7. (A) Changes in the total amounts of 17 protein-amino acids accurately measured in this analysis are shown. Proline was not detected, and tryptophan and cysteine could not be measured correctly. (B) Changes in the amount of arginine, lysine, histidine, alanine, valine, leucine, tyrosine, and glutamic acid, all of which showed significant differences between WT and atg5 mutant colonies, are shown. (C) Changes in the amounts of ammonium and γ-aminobutyric acid, which showed significant difference between WT and atg5 mutant colonies, are shown. The letters (a, b, c, d, e, f, g, h, and i) in each graph indicate significant differences (P < 0.05) determined by Tukey's Multiple Comparison Test.
The addition of casamino acids relieves the early-senescence phenotype in atg5 mutants
If amino acid imbalance is one of the causes of early senescence of atg5 mutants, supplementing the culture medium with amino acids would diminish the early-senescence phenotype in the dark. Thus, we investigated whether addition of various amino acid supplements would affect the progress of senescence in the dark. We tested the addition of 5 mM arginine, 1 mM arginine, 1 mM arginine and 0.1 mM tyrosine, 0.1% (w/v) yeast extract, or 0.5% (w/v) casamino acids (Fig. S2). On the medium containing yeast extract, WT and atg5 colonies turned dark green and dark brown, respectively; both of these colors had not been observed in the de-greening process of senescence on the usual nutrient-sufficient medium. Addition of supplements other than casamino acids also slightly darkened the color of WT colonies, whereas it did not appear to delay the de-greening process in atg5 mutants. Only on the medium containing casamino acids, atg5 colonies remained green longer, until day 7, than on the medium without any supplement (Fig. 11). These results suggest that addition of casamino acids reduced the time lag in senescence between atg5 mutants and WT plants and supports the notion that the early-senescence phenotype of atg5 mutants is partly due to amino acid imbalance caused by the impairment of amino acid production on account of autophagy.
Figure 11.
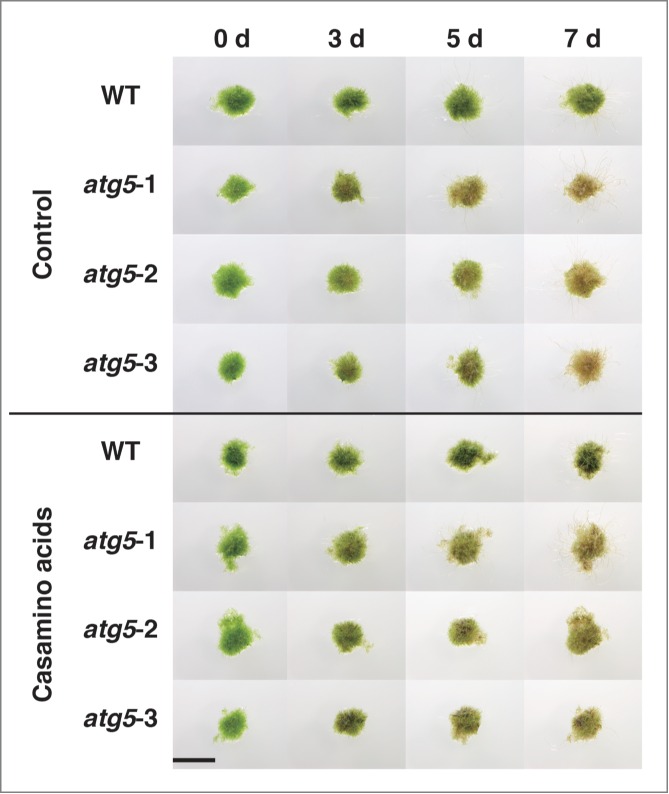
Effects of casamino acid addition to culture medium on dark-induced senescence in protonemal colonies of Physcomitrella. Seven-day-old colonies of WT and atg5 mutants (atg5–1, atg5–2 and atg5–3) of Physcomitrella were cultured on nutrient-sufficient medium supplemented with 0.5% (w/v) casamino acids for 7 d As a control, senescence on nutrient-sufficient medium with no supplement is shown (Control). Bar, 4 mm.
Discussion
We prepared ATG5 gene-knockout mutants in the moss Physcomitrella and confirmed that the mutants atg5–1, atg5–2, and atg5–3 did not perform autophagy (Fig. 4). atg5 colonies that had been cultured on the nutrient-sufficient medium for 7 d were indistinguishable in appearance from one another and from WT colonies cultured under the same conditions.(Fig. 2)
When such colonies were subjected to carbon or nitrogen starvation treatment, yellowing occurred earlier and more severely in atg5 mutants than in WT plants (Fig. 3). Yeast and Arabidopsis atg mutants are sensitive to nutrient starvation. In yeast, the survival rate decreased in nitrogen-deficient medium earlier in atg cells than in WT cells.32 In Arabidopsis, yellowing of cotyledon and rosette leaves was induced earlier in atg mutants under nutrient starvation than in WT plants.6,13 Thus, we interpret the results as showing that the atg mutants of Physcomitrella also are more sensitive to nutrient starvation and confirming that autophagy generally contributes to nutrient supply within cells when that from the outside is cut off.
Colonies of Physcomitrella senesced in the dark (Fig. 5). When Physcomitrella colonies were placed in the dark, they yellowed, net degradation of Rubisco protein and chlorophyll was induced, and SAGs genes were expressed. Yellowing of the colonies was inhibited by the protein synthesis inhibitor CHX (Fig. S1), as had been shown in green leaves of higher plants.33 These results show that protonemal colonies of Physcomitrella display senescence syndrome like green leaves of higher plants.
In Physcomitrella, atg5 mutants underwent earlier senescence than the WT in the dark, manifested as yellowing of colonies, net degradation of chlorophyll and Rubisco protein, and expression of SAGs genes (Fig. 5, 6 and 7). The early-senescence phenotype has been shown in the leaves of Arabidopsis and rice.13,19 These results suggest that accelerated senescence in the dark is a general phenotype in plant autophagy-deficient mutants.
This study has shown that autophagy is not a primary contributor to the degradation of chlorophyll and Rubisco protein that occurs in the dark. In Arabidopsis, autophagy contributes to the degradation of chloroplasts during senescence processes,18 and under moderate stress conditions, yellowing reaction occurs faster in WT leaves than in atg5 leaves.34 Although we do not know whether chloroplast autophagy that occurs in Arabidopsis and rice also occurs in Physcomitrella, the result shows that the degradation of chlorophyll and Rubisco protein is performed mainly by chloroplast degradation machinery, not by autophagy, under dark conditions.
In the present study, we addressed the question of what functions of autophagy accelerate the degradation of chlorophyll and Rubisco protein in chloroplasts. Colonies of Physomitrella consist of 2 types of protonemal cells that resemble each other, and we found that these cells appeared to undergo senescence processes in a similar manner in the dark. We expected that this simple colony structure would facilitate analysis of the involvement of autophagy in dark-induced senescence at the cellular level. We hypothesized that some nutrients are deficient in atg mutants and that accordingly senescence proceeds faster than in WT colonies given that a primary physiological function of autophagy is the degradation of proteins for amino acid recycling and energy supply. Therefore, we compared WT and atg5 mutants with respect to intracellular sugars and amino acids during darkness.
The contents of glucose and sucrose, the 2 major sugars in the protonemal cells of Physcomitrella, were almost the same in atg5 mutant colonies as in the WT at the start of dark treatment (Fig. 9). They increased slightly in the dark in a similar manner possibly owing to protein degradation, and no difference between WT and atg5 mutant colonies was found (Fig. 9). Thus, sugar concentrations seemed to be strictly regulated even under dark conditions. The finding that dark-induced senescence is not accelerated by the depletion of glucose from culture medium supports this notion (compare Figs. 3A and 5A). It is unlikely that changes in sugar content explain the acceleration of dark-induced senescence in atg5 mutants.
Amino acid analysis showed that the levels of total amino acid and almost all individual protein amino acids except glutamic acid did not differ significantly among atg5 and WT colonies before dark treatment. The level of glutamic acid was higher in atg5 than in WT colonies before dark culture, but decreased to the same level as that of WT colonies upon the start of dark culture, and remained at the same level during the 7 d of dark treatment. Six amino acids including 2 dominant amino acids in Physcomitrella colonies, arginine and alanine, changed upon dark treatment differentially between atg5 and WT colonies and remained lower in atg5 mutants than in WT plants for 7 d (Fig. 10). Only histidine remained higher in atg5 mutants than in WT plants, but its levels were low compared to another basic amino acid, arginine. Overall, the levels of several amino acids are lower in atg5 mutants in the middle of senescence, when more degradation Rubisco by chloroplast degradation of machinery would produce more amino acids.
Taking these findings together, we propose that the early-senescence phenotype of atg5 mutants is due to amino acid imbalance caused by loss of amino acid production by autophagy. Amino acid imbalance in atg5 cells is likely to accelerate the degradation of chloroplast components including chlorophyll and Rubisco protein by the chloroplast degradation machinery, although the signaling mechanism is unknown. In cells of WT colonies transferred into the dark, autophagy is induced (Fig. 8) and release amino acids and sugars that compensate for cessation of photosynthetic production of energy and metabolites. In the cells of atg5 colonies, however, autophagy does not operate, and the intracellular concentrations of amino acids are unbalanced, with a deficiency of several amino acids (Fig. 10). The decrease in several amino acids leads in some way to the acceleration of chloroplast degeneration including the net degradation of chlorophyll and Rubisco protein by the chloroplast degradation machinery, compensating for the absence of production of amino acids by autophagy.
We aware that amino acid imbalance in atg5 mutants does not alone explain the early-senescence phenotype of atg5 mutants placed under dark conditions, but that there is another contributor to the early-senescence phenotype in atg5 mutants. Although supplementation with casamino acids moderated the early-senescence phenotype of atg5 mutants, its effect was not large enough to compensate for the full time lag in senescence between WT and atg5 plants (Fig. 11). Furthermore, SAGs were induced by dark treatment in atg5 mutants of Physcomitrella (Fig. 7). The PpSAG12 and PpSEN1 genes were upregulated in atg5 mutants from a time preceding dark treatment. Similarly, the concentration of glutamic acid was higher in atg mutants from the outset than in WT colonies. These results suggest that the deficiency of autophagy somehow leads to changes in cellular metabolism so that the atg mutants readily undergo senescence reactions once colonies are transferred into the dark.
Materials and Methods
Plant material
Physcomitrella cultures were grown on nutrient-sufficient medium, BCD medium 35 containing 5 mM ammonium tartrate, 0.5% (w/v) glucose, and 0.8% (w/v) agar, at 25°C under continuous light from fluorescent lamps (15 w/m2). Cultures were maintained by the transfer of a portion of protonemal cells excised from a colony using forceps to fresh culture medium. Colonies that had been grown for 7 d. were generally used for experiments. For carbon starvation, BCD medium containing ammonium tartrate and 0.8% (w/v) agar without glucose was used. To make culture medium for nitrogen starvation, KNO3 was omitted from the complete agar medium and ammonium tartrate was replaced with potassium tartrate. To evaluate the effects of amino acid addition to the medium on the progress of senescence, nutrient-sufficient medium containing either 5 mM arginine, 1 mM agrinine, 1 mM arginine and 0.1 mM tyrosine, 0.1% (w/v) yeast extract (Bacto Yeast Extract, BD, Sparks, USA), or 0.5% (w/v) casamino acids (Bacto Vitamin Assay Casamino Acids, BD) were prepared.
Construction of Ppatg5 knockout vectors
Genomic DNA was extracted from 7-day-old colonies of Physcomitrella, and a region of the PpATG5 gene that spans from the second to seventh exons and harbors native Hind III and BamH I recognition sites was amplified by PCR using the primers, 5′- GCGTTTGAGTTCATTCTTACATTTAGA-3′ and 5′-AAGACAGCAGAATACACATGAAAGC-3′ (Table S1). The amplified PCR product of 2,268 bp was cloned into pGEM-T Easy Vector (Promega). In contrast, the drug-resistance gene cassettes Pm35S-NPTII-35SPA or Pm35S-APH4–35SPA were excised with Hind III and BamH I from pTN82 or pTN86, respectively (http://moss.nibb.ac.jp/). They were swapped with the Hind III-BamH I region present in the PpATG5 fragment cloned in the pGEM-T Easy Vector. The resulting knockout vectors were digested with EcoR I and Not I, and the resulting DNA fragment, composed of the drug resistance gene cassette with approximately 1,000bp PpATG5 homologous regions at both ends, was introduced into the protoplast of Physcomitrella.
Polyethyleneglycol mediated transformation
Protoplasts were prepared from 4-day-old protonemal colonies. The colonies were treated with 1% (w/v) Driselase (Kyowa Hakko, Japan) in 8% (w/v) mannitol for 30 min and the suspension was filtered through a 50 μm nylon mesh. The protoplasts that passed the nylon mesh were washed 3 times with 8% (w/v) mannitol. Transformation of the protoplasts with DNA was performed as described by Nishiyama et al.35 For the selection of drug resistant lines, 20 μg/ml geneticin or 30 μg/ml hygromycin were used.35-37 The drug resistant lines were screened for expression of PpATG5 transcript and the disruption of the PpATG5 genomic locus.
Detection of autophagy by microscopy
To detect autophagy under carbon starvation, 7-day-old colonies of WT and atg5–1 mutant were incubated with rotation in liquid BCD medium containing 5 mM ammonium tartrate but not glucose at 23°C in the dark in the presence of 100 μM E-64d or 0.1 μM concanamycin A. To confirm the occurrence of autophagy in the dark, 7-day-old colonies of the WT were incubated with rotation in liquid BCD medium containing 5 mM ammonium tartrate and 0.5% (w/v) glucose in the dark or in light in the presence of 100 μM E-64d. After 1 d. of each incubation, protonemal cells were observed by light microscopy with Nomarski optics (FV1000-D, Olympus).
Chlorophyll measurement
Chlorophyll was extracted from colonies with 80% (v/v) acetone. To remove cell debris the extracts were centrifuged for 10 min at 1,000xg. A645 and A663 of the supernatant were measured and total chlorophyll amounts were calculated according to Arnon.38
SDS-PAGE
After the colonies were weighed, they were homogenized with a solution consisting of 0.1 M HEPES-Na (pH 7.5), 0.1 mM EDTA, 10 μM leupeptin, 0.1 mM phenylmethylsulfonyl fluoride, and 2-mercaptoethanol in a mortar and pestle on ice. The homogenate was centrifuged at 15,000xg for 10 min at 4°C and the protein content was measured according to the method of Lowry et al.,39 modified by Bensadoun and Weinstein.40 Bovine serum albumin was used as a standard. The resultant supernatant was mixed with 2×SDS sample buffer (Cosmo Bio Corporation, Tokyo, Japan) and boiled at 100°C for 2 min. These proteins were separated on SDS-polyacrylamide (10%) gels and stained with Coomassie brilliant blue. The band intensities of Rubisco large subunits were quantified by densitometry (ChemiDoc system, Bio-Rad).
Quantitative real-time PCR
Total RNA was extracted using RNAiso Plus (Takara) and the NucleoSpin′ RNA II (MACHEREY-NAGEL, Germany) and reverse transcribed using Transcriptor First Strand cDNA Synthesis Kit (Roche, Japan). Real-time PCR was performed using a StepOnePlus™ Real Time PCR System (Applied Biosystems, Japan) with SYBR® Green Realtime PCR Master Mix (TOYOBO, Japan). Amplifications were performed using 1×SYBR Green PCR mix (TaKaRa) and 0.4 pmol of each primer. PpRPL22 (Pp1s352_17) was used as an internal standard for data normalization and quantification. The cycling conditions were as follows: 1 min activation at 95°C, 50 cycles of 10 s at 95°C, 30 s at 55°C, and 30 s at 72°C and melting curves of 95°C for 15 s, 60°C 1 min, and 95°C for 15 s. The accompanying software was used for quantitative PCR data normalization and quantification. The full list of primers is described in Table S1.
Measurement of sugars
Protonemal colonies were homogenized at 4°C in 80% ethanol. The homogenate was centrifuged at 12,000rpm for 30 min at 4°C, and the supernatant was recovered. After evaporation of ethanol, the samples of soluble sugars were dissolved in distilled water and centrifuged at 15,000rpm for 30 min at 4°C to remove any debris. Total soluble sugars in the supernatant were quantified by the anthrone method using glucose as standard.41 Also, these sugar samples were subjected to thin-layer chromatography. The samples were developed by 75% acetonitrile in water. The mobilized sugar spots were visualized by spraying 50% sulfuric acid in ethanol supplemented with 0.1% (v/w) 1-naphtrol followed by baking at 110°C.
Amino acid analysis
Amino acids were extracted from protonemal colonies and prepared in the same way as the ample of soluble sugars. The contents of amino acids were determined using Hitachi L-8900 amino acid analyzer.
Accession numbers
Nucleotide sequence data for real-time and reverse transcription PCR from this article can be found in the Physcomitrella Cosmoss database (https://www.cosmoss.org/physcome_project/wiki/Downloads) under the following accession numbers: PpATG5 (Pp1s227_54), PpATG7 (Pp1s73_159), PpATG8a (Pp1s209_114), PpRPL22 (Pp1s352_17), PpSEN1 (Pp1s81_135), PpSAG12 (Pp1s19_362), PpSAG13 (Pp1s457_13), PpSAG18 (Pp1s74_138). The GenBank accession number of PpATG5 genome is NW_001865483.
Supplementary Material
Funding
This work was supported by the Japan Society for the Promotion of Science [Research Fellowship for Young Scientists (6733 to K.M.)]; and KAKENHI [grant number 23120504, 25120704 to Y.M.].
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Reference
- 1.Mortimore GE, Poso AR. Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu Rev Nutr 1987; 7:539-68; PMID:3300746; http://dx.doi.org/ 10.1146/annurev.nu.07.070187.002543 [DOI] [PubMed] [Google Scholar]
- 2.Dunn WA, Jr. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol 1994; 4:139-43; PMID:14731737; http://dx.doi.org/ 10.1016/0962-8924(94)90069-8 [DOI] [PubMed] [Google Scholar]
- 3.Ahlberg J, Marzella L, Glaumann H. Uptake and degradation of proteins by isolated rat liver lysosomes. Suggestion of a microautophagic pathway of proteolysis. Lab Invest 1982; 47:523-32; PMID:6755063 [PubMed] [Google Scholar]
- 4.Dice JF. Chaperone-mediated autophagy. Autophagy 2007; 3:295; PMID:17404494; http://dx.doi.org/ 10.4161/auto.4144 [DOI] [PubMed] [Google Scholar]
- 5.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000; 290:1717-21; PMID:11099404; http://dx.doi.org/ 10.1126/science.290.5497.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanaoka H, Noda T, Shirano Y, Kato T, Hayashi H, Shibata D, Tabata S, Ohsumi Y. Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol 2002; 129:1181-93; PMID:12114572; http://dx.doi.org/ 10.1104/pp.011024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kametaka S, Matsuura A, Wada Y, Ohsumi Y. Structural and functional analyses of APG5 a gene involved in autophagy in yeast. Gene 1996; 178:139-43; PMID:8921905; http://dx.doi.org/ 10.1016/0378-1119(96)00354-X [DOI] [PubMed] [Google Scholar]
- 8.Mizushima N, Sugita H, Yoshimori T, Ohsumi Y. A new protein conjugation system in human The counterpart of the yeast Apg12p conjugation system essential for autophagy. J Biol Chem 1998; 273:33889-92; PMID:9852036; http://dx.doi.org/ 10.1074/jbc.273.51.33889 [DOI] [PubMed] [Google Scholar]
- 9.Geng J, Klionsky DJ. The Atg8 and Atg12 ubiquitin‐like conjugation systems in macroautophagy. EMBO Rep 2008; 9:859-64; PMID:18704115; http://dx.doi.org/ 10.1038/embor.2008.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung T, Phillips AR, Vierstra RD. ATG8 lipidation and ATG8‐mediated autophagy in Arabidopsis require ATG12 expressed from the differentially controlled ATG12A AND ATG12B loci. Plant J 2010; 62:483-93; PMID:20136727; http://dx.doi.org/ 10.1111/j.1365-313X.2010.04166.x [DOI] [PubMed] [Google Scholar]
- 11.Romanov J, Walczak M, Ibiricu I, Schüchner S, Ogris E, Kraft C, Martens S. Mechanism and functions of membrane binding by the Atg5–Atg12/Atg16 complex during autophagosome formation. EMBO J 2012; 31:4304-17; PMID:23064152; http://dx.doi.org/ 10.1038/emboj.2012.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W-P, Klionsky DJ. Autophagy in yeast: a review of the molecular machinery. Cell Structure Function 2002; 27:409-20; PMID:12576634; http://dx.doi.org/ 10.1247/csf.27.409 [DOI] [PubMed] [Google Scholar]
- 13.Doelling JH, Walker JM, Friedman EM, Thompson AR, Vierstra RD. The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J Biol Chem 2002; 277:33105-14; PMID:12070171; http://dx.doi.org/ 10.1074/jbc.M204630200 [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Xiong Y, Bassham DC. Autophagy is required for tolerance of drought and salt stress in plants. Autophagy 2009; 5:954-63; PMID:19587533; http://dx.doi.org/ 10.4161/auto.5.7.9290 [DOI] [PubMed] [Google Scholar]
- 15.Xiong Y, Contento AL, Nguyen PQ, Bassham DC. Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol 2007; 143:291-9; PMID:17098847; http://dx.doi.org/ 10.1104/pp.106.092106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata M, Oikawa K, Yoshimoto K, Kondo M, Mano S, Yamada K, Hayashi M, Sakamoto W, Ohsumi Y, Nishimura M. Highly oxidized peroxisomes are selectively degraded via autophagy in Arabidopsis. Plant Cell 2013; 25:4967-83; PMID:24368788; http://dx.doi.org/ 10.1105/tpc.113.116947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Yu B, Zhao J, Guo J, Li Y, Han S, Huang L, Du Y, Hong Y, Tang D, et al.. Autophagy contributes to leaf starch degradation. Plant Cell Online 2013; 25:1383-99; http://dx.doi.org/ 10.1105/tpc.112.108993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wada S, Ishida H, Izumi M, Yoshimoto K, Ohsumi Y, Mae T, Makino F. Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiol 2009; 149:885-93; PMID:19074627; http://dx.doi.org/ 10.1104/pp.108.130013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izumi M, Hidema J, Wada S, Kondo E, Kurusu T, Kuchitsu K, Ishida H. Establishment of monitoring methods for autophagy in rice reveals autophagic recycling of chloroplasts and root plastids during energy limitation. Plant Physiol 2015; 167:1307-20; PMID:25717038; http://dx.doi.org/ 10.1104/pp.114.254078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida H, Yoshimoto K, Izumi M, Reisen D, Yano Y, Makino A, Makino A, Ishida H. Mobilization of rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiol 2008; 148:142-55; PMID:18614709; http://dx.doi.org/ 10.1104/pp.108.122770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshimoto K, Shibata M, Kondo M, Oikawa K, Sato M, Toyooka K, Shirasu K, Nishimura M, Ohsumi Y. Organ-specific quality control of plant peroxisomes is mediated by autophagy. J Cell Sci 2014; 127:1161-8; PMID:24463818; http://dx.doi.org/ 10.1242/jcs.139709 [DOI] [PubMed] [Google Scholar]
- 22.Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, Ohsumi Y, Shirasu K. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell Online 2009; 21:2914-27; http://dx.doi.org/ 10.1105/tpc.109.068635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Schiff M, Czymmek K, Tallóczy Z, Levine B, Dinesh-Kumar S. Autophagy regulates programmed cell death during the plant innate immune response. Cell 2005; 121:567-77; PMID:15907470; http://dx.doi.org/ 10.1016/j.cell.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 24.Buchanan-Wollaston V. The molecular biology of leaf senescence. J Exp Botany 1997; 48:181-99; http://dx.doi.org/ 10.1093/jxb/48.2.181 [DOI] [Google Scholar]
- 25.Martin C, Thimann KV. The role of protein synthesis in the senescence of leaves I. The formation of protease. Plant Physiol 1972; 49:64-71; PMID:16657898; http://dx.doi.org/ 10.1104/pp.49.1.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mach JM, Castillo AR, Hoogstraten R, Greenberg JT. The Arabidopsis-accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc Natl Acad Sci 2001; 98:771-6; http://dx.doi.org/ 10.1073/pnas.98.2.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Y, Contento AL, Bassham DC. AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. Plant J 2005; 42:535-46; PMID:15860012; http://dx.doi.org/ 10.1111/j.1365-313X.2005.02397.x [DOI] [PubMed] [Google Scholar]
- 28.Moriyasu Y, Inoue Y. Chapter Thirty‐Two Use of Protease Inhibitors for Detecting Autophagy in Plants. Methods Enzymol 2008; 451:557-80; PMID:19185740; http://dx.doi.org/ 10.1016/S0076-6879(08)03232-1 [DOI] [PubMed] [Google Scholar]
- 29.Yoshimoto K, Hanaoka H, Sato S, Kato T, Tabata S, Noda T, Ohsumi Y. Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell 2004; 16:2967-83; http://dx.doi.org/ 10.1105/tpc.104.025395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skripnikov AY, Polyakov N, Tolcheva E, Velikodvorskaya V, Dolgov S, Demina I, Rogova MA, Govorun VM. Proteome analysis of the moss Physcomitrella patens (Hedw.) BSG. Biochemistry (Mosc) 2009; 74:480-90; http://dx.doi.org/ 10.1134/S0006297909050022 [DOI] [PubMed] [Google Scholar]
- 31.Onodera J, Ohsumi Y. Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. J Biol Chem 2005; 280:31582-6; PMID:16027116; http://dx.doi.org/ 10.1074/jbc.M506736200 [DOI] [PubMed] [Google Scholar]
- 32.Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Letters 1993; 333:169-74; PMID:8224160; http://dx.doi.org/ 10.1016/0014-5793(93)80398-E [DOI] [PubMed] [Google Scholar]
- 33.Thimann KV, Satler S. Relation between senescence and stomatal opening: Senescence in darkness Proc Natl Acad Sci 1979; 76:2770-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakuraba Y, Lee S-H, Kim Y-S, Park OK, Hörtensteiner S, Paek NC. Delayed degradation of chlorophylls and photosynthetic proteins in Arabidopsis autophagy mutants during stress-induced leaf yellowing. J Exp Botany 2014; 65(14):3915-25, eru008; PMID:24510943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishiyama T, Hiwatashi Y, Sakakibara K, Kato M, Hasebe M. Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res 2000; 7:9-17; PMID:10718194; http://dx.doi.org/ 10.1093/dnares/7.1.9 [DOI] [PubMed] [Google Scholar]
- 36.Schaefer DG. A newmoss genetics: Targeted mutagenesis in Physcomitrella patens. Annu Rev Plant Biol 2002; 53:477-501; PMID:12221986; http://dx.doi.org/ 10.1146/annurev.arplant.53.100301.135202 [DOI] [PubMed] [Google Scholar]
- 37.Schaefer D, Zryd J-P, Knight C, Cove D. Stable transformation of the moss Physcomitrella patens. Mol Gen Genet 1991; 226:418-24; PMID:2038304; http://dx.doi.org/ 10.1007/BF00260654 [DOI] [PubMed] [Google Scholar]
- 38.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 1949; 24:1; PMID:16654194; http://dx.doi.org/ 10.1104/pp.24.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193:265-75; PMID:14907713 [PubMed] [Google Scholar]
- 40.Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Analytical Biochem 1976; 70:241-50; PMID:1259145; http://dx.doi.org/ 10.1016/S0003-2697(76)80064-4 [DOI] [PubMed] [Google Scholar]
- 41.Yemm E, Willis A. The estimation of carbohydrates in plant extracts by anthrone. Biochem J 1954; 57:508; PMID:13181867; http://dx.doi.org/ 10.1042/bj0570508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


