ABSTRACT
Local burning is known to generate and propagate variation potential (VP) in plants. VP affects different physiological processes, including reducing heat-related damage to photosystem I (PSI). We investigated mechanisms of the process. Photosynthesis parameters were measured with Dual-PAM-100 and GFS-3000. VP was induced by burning the first mature leaf and then waiting 5, 10, 15, or 20 min to initiate heating of the second mature leaf. Photosystems activities in the second leaf were investigated at 15 and 135 min after heating. In the absence of VP induction, when incubation in hot water (5 min) was used for heating the intact second leaf, PSI and PSII activities decreased after incubation at both exposure temperatures (45°C and 50°C). When local burning of the first leaf induced VP propagation into the second leaf, reduced photosynthesis (PSI) was observed. Arrival of VP in the second leaf prior to hot water incubation at 50°C decreased heating-induced suppression of PSI activity when measured 15 and 135 min later. Dependence of PSI activity on the time interval (5, 10, 15, or 20 min) between VP induction and heating of the second leaf was dissimilar at 15 and 135 min. Heat-induced suppression of PSII activity in the second leaf was stimulated after VP induction. In contrast, the effect of VP on PSI and PSII damage was weak when leaf 2 was heated at 45°C. VP-induced decrease of PSI activity suppression at 15 min after heating was correlated with stimulation of PSII activity suppression, but increase of PSI activity at 135 min after heating was not related to PSII activity. Thus, our results suggest the possibility of 2 different pathways of VP-induced decrease of heat-related PSI damage.
KEYWORDS: Heat damage, heat resistance, pea, photosystem I, photosystem II, variation potential
Abbreviations
- ACO2
CO2 assimilation rate
- E
surface potential
- PSI
photosystem I
- PSII
hotosystem II
- VP
variation potential
- фPSI
photosystem I effective quantum yield
- фPSII
photosystem II effective quantum yield
- фPSI0
photosystem I quantum yield after 10-s illumination by far-red light
- фPSII0
photosystem II maximum quantum yield.
Introduction
Variation potential (VP) in plants is a variable, long-term electrical signal that is induced by damage and propagated into undamaged areas.1-3 VP generation is mainly connected with transitory H+-ATPase inactivation1-4 although ion channels also participate in the processes.3 VP is propagated by the transmission of hydraulic1,5 or chemical6,7 signals.
VP influences many physiological processes,2 including gene expression, protein synthesis, phytohormone biosynthesis, respiration, and photosynthesis. In particular, VP can change resistance of photosynthetic machinery to heating.8,9 It has been shown that VP can increase photosystem I (PSI) resistance to high temperature;8 simultaneously, VP contributes to heat-related photosystem II (PSII) damage.9 These changes in photosynthetic machinery resistance to high temperature may increase whole plant resistance to temperature stress.9
Our previous analysis showed that increased heat tolerance of PSI was caused by an electrical signal-induced photosynthetic response8 that was mainly related to photosynthesis dark stage inactivation.8,10-13 VP-induced response is caused by changes in intra- and extracellular pH that is concomitant with VP generation.14-16 pH-dependent decrease of HCO3:CO2 ratio,16 changes in aquaporin permeability,12 and (or) changes in carbonic anhydrase activity14 are potential mechanisms by which VP influences dark stage photosynthesis.
It is unknown how VP enables the photosynthetic machinery to become more resistant to heat treatment. VP-induced increase in resistance can be potentially connected with changes in ATP:ADP ratio,17 modifications of reactive oxygen species production,18 and activation of cyclic electron flow.8,13 There are several questions that, when answered, can clarify mechanisms of VP influence on photosynthetic machinery resistance: (i) what characteristics of VP influence PSI and PSII damage that occur after fast, short-time heating; (ii) what characteristics of VP influence PSI and PSII damage that occur several hours after heating; and (iii) how are PSI damage and PSII damage related. Our previous studies8,9 did not address these questions because we used gradual, long-term heating (30 min) and did not investigate photosystems parameters beyond 20 min after application of heating. Thus, the objective of this study was to investigate the influence of VP on PSI and PSII damage 15 min after fast, short-time heating and again, about 2 hours later.
Results and discussion
Fig. 1 shows that local burning of the first mature leaf induced VP that propagated into the second leaf, resulting in inactivated photosynthesis. Maximums of changes in photosynthetic parameters were observed 5–6 min after burning; slow relaxation of ACO2, фPSI and NPQ changes were developed subsequently. A weak second increase in NPQ occurred at 15–20 min after the VP induction stimulus. The decrease in фPSII was not relaxed during this experiment. Our results are in a good accord with our earlier data8,9,13,15 that showed that VP inactivates photosynthesis in peas.
Figure 1.
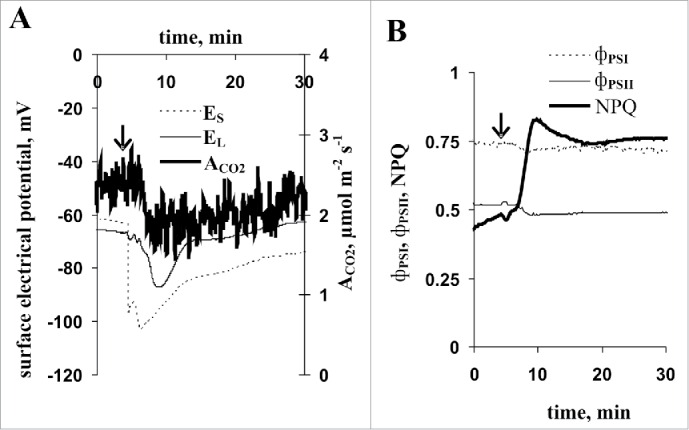
Local burning-induced changes in surface electrical potential and photosynthetic parameters of the second mature leaf of pea seedlings (n = 8). A. Changes in stem (ES) and leaf (EL) surface potential and CO2 assimilation rate (ACO2). B. Changes in quantum yields of PSI (фPSI) and PSII (фPSII) and non-photochemical quenching in PSII (NPQ). Local burning of the first mature leaf is indicated by the black arrow.
Earlier, it was shown that electrical signals changed damage to PSI and PSII after heating.8,9,19 The change was connected with photosynthetic response,8 but its properties and mechanisms were not clear. We used fast, short-time heating in hot water (5 min) and measurements of PSI and PSII activities (1-фPSI0 and фPSII0, respectively) shortly after heating (15 min) and about 2 hours later (135 min) for analysis of the problem.
Fig. 2 shows that heating to 40°C did not damage either photosystem; whereas, heating to 45°C and 50°C decreased activities of PSI and PSII. Therefore, we used 45°C and 50°C for further investigation. It should be noted that incubation of the second leaf in hot water (40°C, 45°C, or 50°C) did not induce VP in the pea plant (data not shown); i.e., only burning-induced VP from the first mature leaf was observed in these experiments.
Figure 2.
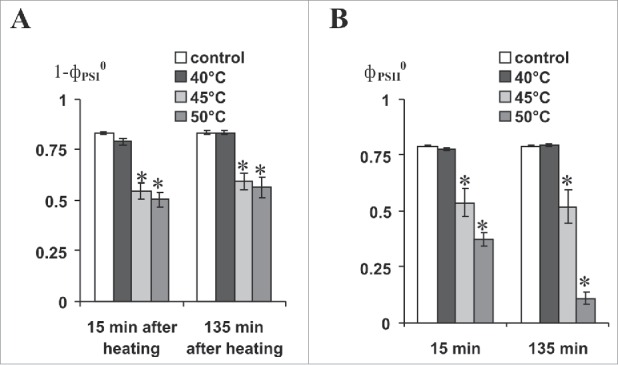
Activities of PSI (1-фPSI0, A) and PSII (фPSII0, B) at 15 min and 135 min after heating the second mature leaf in hot water (n = 6). фPSI0, PSI quantum yield after 10-s illumination by far red light; фPSII0, PSII maximum quantum yield. Second mature leaf incubation in hot water (5 min, at different temperatures) was used for heating. Leaf was dried for 5 min after incubation. Dark adaptation before фPSI0 and фPSII0 registration was 10 min. *, p < 0.05 compared with controls.
Fig. 3 shows that burning-induced VP did not influence the magnitudes of the decreases in PSI and PSII activities after the second leaf was heated to 45°C. The result was in a good accord with our earlier work.8 Fig. 4A shows that burning-induced VP increased PSI activity after the second leaf was heated to 50°C. The increase was expressed differently depending upon the time interval between VP induction and the start of heating. At the 5 min interval, PSI activity at 15 min after heating was more than activity in the non-heated control and PSI tended to increase following the 20-min time interval. When measured again after 135 min, PSI activity was greater than the control at 5- and 10-min time intervals between VP induction and start of heating. There was also a tendency toward increase at the 15-min time interval, whereas differences at the 20-min time interval were absent. VP induced decrease of PSII activity when measured 15 min after heating (Fig. 4B) at the 5- and 20-min time intervals between VP induction and start of heating.
Figure 3.
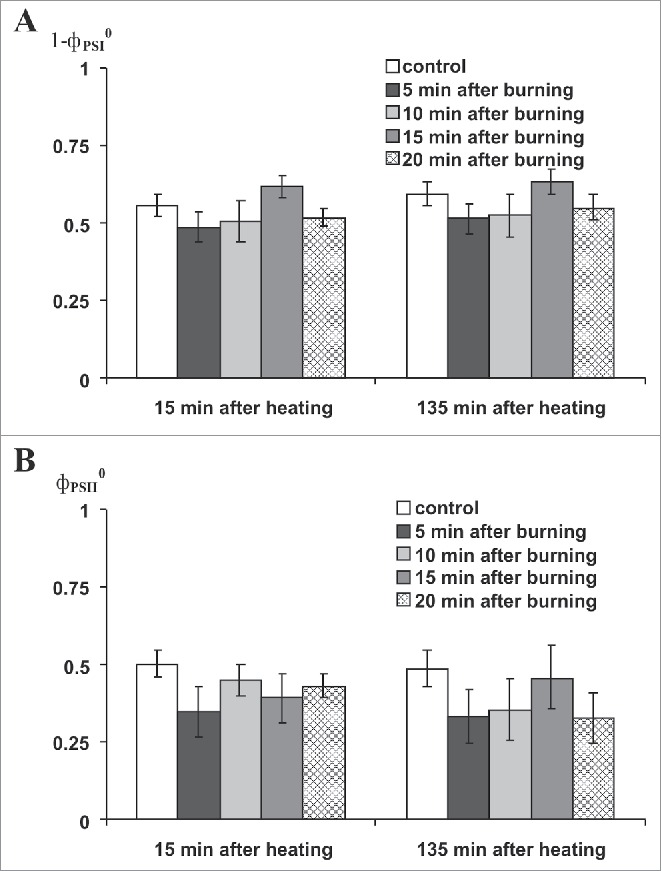
Influence of local burning on activities of PSI (1-фPSI0, A) and PSII (фPSII0, B) at 15 min and 135 min after heating to 45°C (n = 6-12). фPSI0, PSI quantum yield after 10-s illumination by far red light; фPSII0, PSII maximum quantum yield. First mature leaf was burned. Second mature leaf was incubated in hot water (heating, 5 min, 45°C). Leaf was dried for 5 min after incubation. Dark adaptation before фPSI0 and фPSII0 registration was 10 min. *, p < 0.05 compared with controls.
Figure 4.
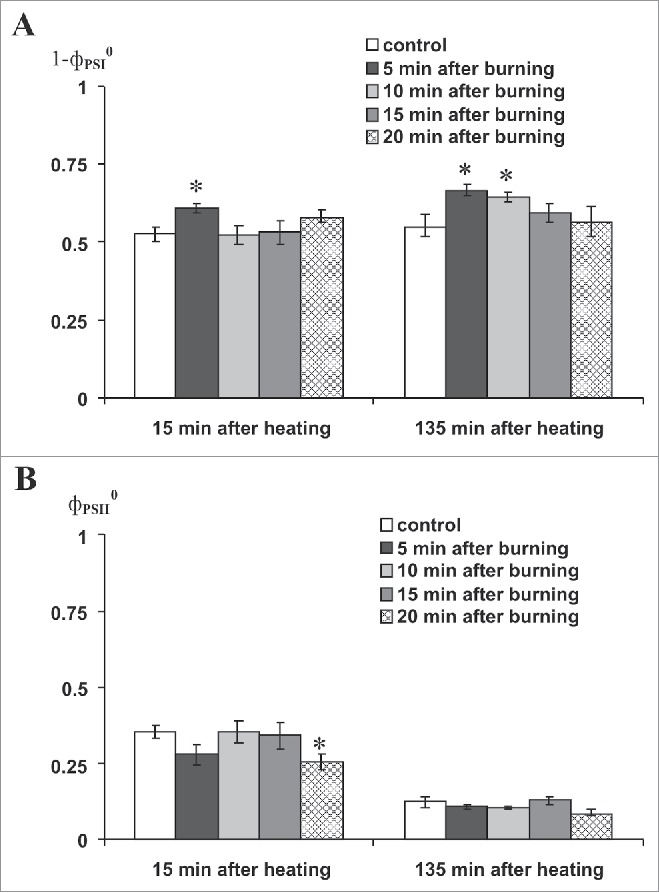
Influence of local burning on activities of PSI (1-фPSI0, A) and PSII (фPSII0, B) at 15 min and 135 min after heating to 50°C (n = 6–11). фPSI0, PSI quantum yield after 10-s illumination by far red light; фPSII0, PSII maximum quantum yield. First mature leaf was burned. Second mature leaf was incubated in hot water (heating, 5 min, 50°C). Leaf was dried for 5 min after incubation. Dark adaptation before фPSI0 and фPSII0 registration was 10 min. *, p < 0.05 compared with controls.
It was interesting that the maximum influence of VP on PSI activity after heating was observed at the 5-min time interval between VP induction and the start of heating, because VP-induced photosynthesis inactivation under control conditions (without heating) was highest at about 5 min after stimulation. The result is evidence for a key role of VP-induced photosynthetic response related to heat-induced PSI damage. This observation is in accord with our earlier findings.8
Burning-induced VP increased PSI activity and decreased PSII activity when measured at 15 and 135 min after heating. However, the observed changes were differently dependent upon the time interval between VP induction and the start of heating when measured at 15 and 135 min after incubation in hot water. The result showed that VP influence on photosystems damage during fast, short-term heating and VP influence on photosystems damage 2 hours after heating were likely induced by different mechanisms.
According to the hypothesis of Aro and co-workers,20,21 limitation of electron flow from PSII protects PSI against oxidative damage; therefore, an increase in PSII damage can decrease PSI damage. Taking into account efficient repair of PSII after damage and very weak repair of PSI damage, this mechanism could protect photosynthetic machinery when viewed in its entirety.20 Our earlier data8,9 and present results showed that VP stimulated heat-related PSII damage heating, therefore we propose that VP-related PSII damage provided protection against heat-related PSI damage.
Fig. 5 shows the correlation of PSI activities with PSII activities for all repetitions of the experiment. There were positive correlations between PSI and PSII activities after heating at 45°C (Fig. 5A and B). The result showed that the level of PSII damage did not have a positive influence on PSI activity after heating at 45°C. The positive correlation likely reflects that individual resistances among the different plants were very similar for PSI and PSII. A positive influence of VP was also absent after heating to 45°C. However, there was a strongly negative correlation between PSI and PSII activities at 15 min after heating to 50°C (Fig. 5C). The result was in a good accord with the hypothesis of Aro and coworkers.20,21 Moreover, VP increased PSI activity after heating under these conditions. Thus, the result supports our hypothesis that VP-stimulated PSII damage under heating protected PSI at the higher temperature. The hypothesis also helps explain our earlier data9 that showed that VP increased PSII damage, but protected PSI under gradual heating.
Figure 5.
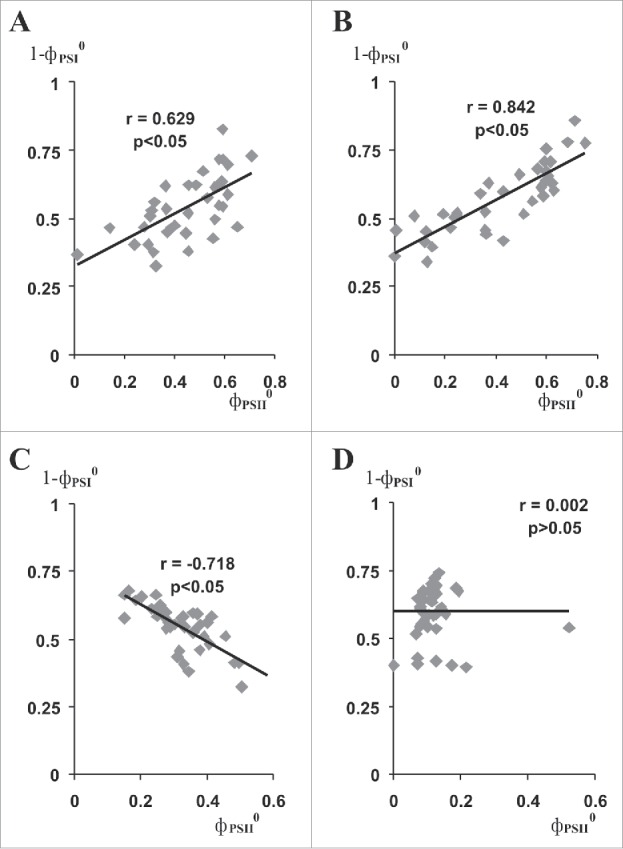
Correlations of PSI activity (1-фPSI0) with PSII activity (фPSII0) after heating (n = 37–42). A. Correlation of 1-фPSI0 with фPSII0 at 15 min after heating to 45°C. B. Correlation of 1-фPSI0 with фPSII0 at 135 min after heating to 45°C. C. Correlation of 1-фPSI0 with фPSII0 at 15 min after heating to 50°C. D. Correlation of 1-фPSI0 with фPSII0 at 135 min after heating to 50°C. All control and experimental repetitions from Figs. 3 and 4 were used for analysis. Each point represents one observation. r is Pearson's correlation coefficient.
It is likely that there were different mechanisms of VP-stimulated PSII damage under heating. Earlier, we showed that PSII damage was related to VP-induced transpiration decrease and intensification of leaf heating9; however, PSII damage after VP tended to increase under leaf heating to constant temperature8 and under heating, which was not regulated by transpiration (as seen in the current results with heating by hot water). It is known22 that strong inactivation of the Calvin cycle can be one of mechanisms causing PSII damage when plants are under stress. As a result, it was possible that VP-induced photosynthesis dark stage inactivation8,11-13 stimulated PSII damage under heating.
There was no correlation between PSI and PSII activities at 135 min after heating (Fig. 5D). Therefore, it is likely that PSII inactivation is not involved in the effect of VP on PSI activity at this timing. It is known that PSI damage is practically irreversible,20 therefore PSI repair was also an unlikely mechanism of VP-induced increase of PSI activity relative to the control at 135 min after heating. Investigation of ratio Pm(in 15 min after heating) : Pm(in 135 min after heating), where Pm reflected the quantity of active PSI,8,9 showed that the ratio was 0.5–0.7 and did not significantly depend on VP (data not shown). This result suggests that PSI damage continued after heating was finished and PSI repair was absent.
Taking into account the absence of PSI repair, the increase of PSI activity could be a reflection of increased light flow to this photosystem. The increase can be caused by ‘state-transition’, which is a redistribution of light energy from PSII to PSI.20,21 We did not observe state-transition after VP was propagated under control conditions,13 but its development after heating is possible.
Finally, our results show that at least 2 pathways are possibly responsible for the effects of VP on PSI damage after heating. The positive influence of VP on PSI activity during heating and soon after is probably connected with VP-induced stimulation of PSII damage after heating. The following chain of events could be occurring: local burning → VP propagation → photosynthesis inactivation, including dark stage suppression → increase of PSII damage under heating → decrease of PSI damage under heating. The positive influence of VP on PSI activity at 135 min after heating likely has another mechanism that is possibly related to an increase of light flow to PSI.
Conclusion
We conclude that local burning-induced VP had a positive influence on PSI activity in the second mature leaf after it was heated to 50°C in hot water, but the effect was absent at 45°C. The positive influence of VP on PSI activity at 15 min and 135 min after heating is probably facilitated by 2 different mechanisms or pathways. VP-induced increase of PSI activity at 15 min after heating is likely related to VP-induced stimulation of PSII damage after heating. VP-induced increase of PSI activity at 135 min after heating may be related to increase of light flow to PSI.
Materials and methods
Experimental plants were 14–21-d-old pea (Pisum sativum L.) seedlings that were grown hydroponically in a Binder KBW 240 plant growth chamber (Binder GmbH, Tuttlingen, Germany) at 24°C under a 16/8-h light/dark photoperiod.
Electrical signals were induced by burning ˜1 cm2 of the tip of the first mature leaf (a stimulated leaf) over a flame for 3–4 s, a standard damaging stimulus,8,9 after about 1.5 h of preconditioning. Measurements of surface electrical potential were described in earlier studies.8,9 Ag/AgCl electrodes (RUE Gomel Measuring instrument, Gomel Plant of Measuring Equipment, Gomel, Belarus), a high-impedance amplifier IPL-113 (Semico, Novosibirsk, Russia), and a PC were used. Measurement electrodes contacted the stem (ES) and center of a non-stimulated leaf (EL), and the reference electrode placed in standard solution (1 mM KCl, 0.5 mM CaCl2, and 0.1 mM NaCl) surrounding the roots (Fig. 6A).
Figure 6.
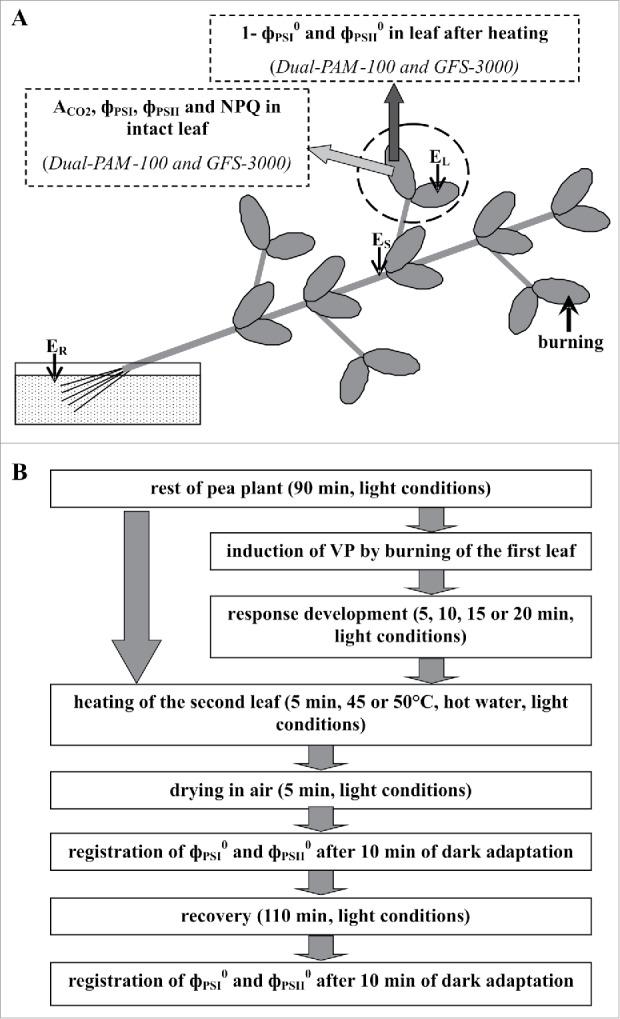
Positions of stimulation, electrical potential, and photosynthesis parameter measurements, and heating in pea plants (A), and procedure for investigating photosystem activities after heating (B). EL is the electrode connected to the leaf, ES is the electrode connected to the stem, and ER is the reference electrode. The distance between EL and ES was 3–4 cm. Burning of the tip of the first mature the leaf (with a flame for 3–4 s over an area of about 1 cm2) was used as the external stimulus inducing VP.8,9 Both leaflets of the second leaf (area marked by the dotted line) were heated in hot water at different times after VP induction. PSI and PSII activities after heating have been indicated using 1-фPSI0 and фPSII0.
Photosynthetic parameters were measured in the second mature leaf (non-stimulated leaf) using a system for photosynthetic analysis (Heinz Walz GmbH, Effeltrich, Germany) including a gas exchange measuring system (GFS-3000), a measuring system for simultaneous assessment of P700 oxidation and chlorophyll fluorescence (Dual-PAM-100), and a measuring head (Cuvette 3010-Dual). Saturation pulses of light (10000 μmol m−2 s−1, red light, 630 nm) were used for registration of photosystem parameters. Initial parameters of PSII fluorescence – dark fluorescence yield (F0) and maximal fluorescence yield (Fm) – were measured after dark adaptation. The maximal change of the P700 signal (Pm), reflecting maximal P700 oxidation, and P700 signal after far red light (PFR) were measured after preliminary illumination by far red light for 10 s. Steady-state fluorescence yield in light (F), maximal fluorescence yield in light (Fm′), steady-state P700 signal (P), and maximal change in the P700 signal in light (Pm′) were measured using saturation pulses generated every 10 s. Effective quantum yields of PSI ****23 and PSII ****24 and non-photochemical quenching of fluorescence ****,24,25 were calculated after every saturation pulse. The CO2 assimilation rate (ACO2, μmol CO2 m−2 s−1) was measured using the GFS-3000 and its associated software. Parameters were calculated in accordance with von Caemmerer and Farquahar.26 Initial external CO2 concentration was 360 ppm, photosynthetically active radiation at about 108 μmol m−2 s−1 (blue light, 460 nm), and relative air humidity approximately 65%.
According to several published reports,19,27 leaf heating in hot water can be used for fast damage of photosystems. We used this method in our experiments, the schema of which is shown in Fig. 6B. Incubation of the second leaf in hot water (5 min, at different temperatures) under white light of about 100 μmol m−2 s−1 was used for heating. PSI quantum yield after 10-s illumination by far red light ****23 and PSII maximum quantum yield ****24 were measured at 15 min and 135 min after heating. 1- фPSI0 reflected PSI activity because it showed relative velocity of photosystem I oxidizing under light; фPSII0 reflected PSII activity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Russian Science Foundation (Project No. 14-26-00098).
References
- 1.Stahlberg R, Cleland RE, Van Volkenburgh E. Slow wave potentials - a propagating electrical signal unique to higher plants In: Communication in Plants. Baluška F., Mancuso S. and Volkmann D. (eds.) Springer; Berlin Heidelberg: 2006; 291-308. [Google Scholar]
- 2.Fromm J, Lautner S. Electrical signals and their physiological significance in plants. Plant Cell Environ 2007; 30: 249-57; PMID:17263772; http://dx.doi.org/ 10.1111/j.1365-3040.2006.01614.x [DOI] [PubMed] [Google Scholar]
- 3.Vodeneev V, Akinchits E, Sukhov V. Variation potential in higher plants: Mechanisms of generation and propagation. Plant Signaling & Behavior 2015; 10:e1057365; PMID:26313506; http://dx.doi.org/ 10.1080/15592324.2015.1057365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies E. Electrical signals in plants: Facts and hypotheses In: Plant Electrophysiology: Theory and Methods. Volkov A. (ed.) Springer; Berlin Heidelberg: 2006; 407-22. [Google Scholar]
- 5.Mancuso S. Hydraulic and electrical transmission of wound-induced signals in Vitis vinifera. Aust J Plant Physiol 1999; 26: 55-61; http://dx.doi.org/ 10.1071/PP98098 [DOI] [Google Scholar]
- 6.Malone M. Wound induced hydraulic signals and stimulus transmission in Mimosa pudica L. New Phytol 1994; 128:49-56; http://dx.doi.org/ 10.1111/j.1469-8137.1994.tb03985.x [DOI] [PubMed] [Google Scholar]
- 7.Vodeneev V, Orlova A, Morozova E, Orlova L, Akinchits E, Orlova O, Sukhov V. The mechanism of propagation of variation potentials in wheat leaves. J Plant Physiol 2012; 169:949-54; PMID:22533926; http://dx.doi.org/ 10.1016/j.jplph.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 8.Sukhov V, Surova L, Sherstneva O, Vodeneev V. Influence of variation potential on resistance of the photosynthetic machinery to heating in pea. Physiol Plantarum 2014; 152: 773-83; PMID:24730552; http://dx.doi.org/ 10.1111/ppl.12208 [DOI] [PubMed] [Google Scholar]
- 9.Sukhov V, Surova L, Sherstneva O, Bushueva A. Vodeneev V.. Variation potential induces decreased PSI damage and increased PSII damage under high external temperatures in pea. Funct Plant Biol 2015; 42:727-36; http://dx.doi.org/ 10.1071/FP15052 [DOI] [PubMed] [Google Scholar]
- 10.Pavlovic A, Slovakova Lu, Pandolfi C, Mancuso S. On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis). J Exp Bot 2011; 62: 1991-2000; PMID:21289078; http://dx.doi.org/ 10.1093/jxb/erq404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukhov V, Orlova L, Mysyagin S, Sinitsina J, Vodeneev V. Analysis of the photosynthetic response induced by variation potential in geranium. Planta 2012; 235: 703-12; PMID:22020752; http://dx.doi.org/ 10.1007/s00425-011-1529-2 [DOI] [PubMed] [Google Scholar]
- 12.Galle A, Lautner S, Flexas J, Ribas-Carbo M, Hanson D, Roesgen J, et al.. Photosynthetic responses of soybean (Glycine max L.) to heat-induced electrical signalling are predominantly governed by modifications of mesophyll conductance for CO2. Plant Cell Environ 2013; 36: 542-52; PMID:22897236; http://dx.doi.org/ 10.1111/j.1365-3040.2012.02594.x [DOI] [PubMed] [Google Scholar]
- 13.Sukhov V, Surova L, Sherstneva O, Katicheva L, Vodeneev V. Variation potential influence on photosynthetic cyclic electron flow in pea. Front Plant Sci 2015; 5:766; PMID:25610447; http://dx.doi.org/ 10.3389/fpls.2014.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grams TEE, Lautner S, Felle HH, Matyssek R, Fromm J. Heat-induced electrical signals affect cytoplasmic and apoplastic pH as well as photosynthesis during propagation through the maize leaf. Plant Cell Environ 2009; 32:319-26; PMID:19054346; http://dx.doi.org/ 10.1111/j.1365-3040.2008.01922.x [DOI] [PubMed] [Google Scholar]
- 15.Sukhov V, Sherstneva O, Surova L, Katicheva L, Vodeneev V. Proton cellular influx as a probable mechanism of variation potential influence on photosynthesis in pea. Plant Cell Environ 2014; 37:2532-541; PMID:24635649; http://dx.doi.org/ 10.1111/pce.12321 [DOI] [PubMed] [Google Scholar]
- 16.Sherstneva ON, Vodeneev VA, Katicheva LA, Surova LM, Sukhov VS. Participation of intracellular and extracellular pH changes in photosynthetic response development induced by variation potential in pumpkin seedlings. Biochem (Moscow) 2015; 80:776-84; PMID:26531023; http://dx.doi.org/ 10.1134/S0006297915060139 [DOI] [PubMed] [Google Scholar]
- 17.Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P. Heat stress: an overview of molecular responses in photosynthesis. Photosynthesis Res 2008; 98: 541-50; PMID:18649006; http://dx.doi.org/ 10.1007/s11120-008-9331-0 [DOI] [PubMed] [Google Scholar]
- 18.Fischer BB, Hideg É, Krieger-Liszkay A. Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxidants & Redox Signaling 2013; 18:2145-62; PMID:23320833; http://dx.doi.org/ 10.1089/ars.2012.5124 [DOI] [PubMed] [Google Scholar]
- 19.Retivin VG, Opritov VA, Lobov SA, Tarakanov SA, Khudyakov VA. Changes in the resistance of photosynthesizing cotyledon cells of pumpkin seedlings to cooling and heating, as induced by the stimulation of the root system with KCl solution. Russian J Plant Physiol 1999; 46:689-96. [Google Scholar]
- 20.Tikkanen M, Mekala NR, Aro EM. Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochimica et Biophysica Acta 2014; 1837:210-15; PMID:24161359; http://dx.doi.org/ 10.1016/j.bbabio.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 21.Tikkanen M, Aro EM. Integrative regulatory network of plant thylakoid energy transduction. Trends in Plant Sciences 2014; 19:10-7; PMID:24120261; http://dx.doi.org/ 10.1016/j.tplants.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 22.Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. Photoinhibition of photosystem II under environmental stress. Biochimica et Biophysica Acta 2007; 1767:414-421; PMID:17207454; http://dx.doi.org/ 10.1016/j.bbabio.2006.11.019 [DOI] [PubMed] [Google Scholar]
- 23.Klughammer C, Schreiber U. Saturation pulse method for assessment of energy conversion in PS I. PAM Application Notes 2008; 1:11-4. [Google Scholar]
- 24.Maxwell K, Johnson GN. Chlorophyll fluorescence – a practical guide. J Exp Bot 2000; 51:659-68; PMID:10938857; http://dx.doi.org/ 10.1093/jexbot/51.345.659 [DOI] [PubMed] [Google Scholar]
- 25.Müller P, Li X-P, Niyogi KK. Non-photochemical quenching. A response to excess light energy. Plant Physiol 2001; 125:1558-66; PMID:11299337; http://dx.doi.org/ 10.1104/pp.125.4.1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981; 153:376-87; PMID:24276943; http://dx.doi.org/ 10.1007/BF00384257 [DOI] [PubMed] [Google Scholar]
- 27.Oukarroum A, Goltsev V, Strasser RJ. Temperature effects on pea plants probed by simultaneous measurements of the kinetics of prompt fluorescence, delayed fluorescence and modulated 820 nm reflection. PLoS One 2013; 8:e59433; PMID:23527194; http://dx.doi.org/ 10.1371/journal.pone.0059433 [DOI] [PMC free article] [PubMed] [Google Scholar]


