Abstract
Light signaling plays a pivotal role in controlling plant morphogenesis, metabolism, growth and development. The central process of light signaling pathway is to build the link between light signals and the expression of genes involved. Although studies focused on light signaling toward metabolism have been documented well in the past several decades, most regulation networks of light signaling in a specific metabolic production largely remained unknown. Anthocyanin accumulation in plant tissues depends on the availability of light signals, but only little is known about the potential regulation network underlying light signal controls anthocyanin biosynthesis. Here, we briefly review the recent progress on the light-triggered anthocyanin biosynthesis via ANGUSTIFOLIA3 (AN3) and CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) network in Arabidopsis.
Keywords: AN3, anthocyanin biosynthesis, COP1, light signaling, photomorphogenesis
Light signaling plays a pivotal role in controlling plant morphogenesis, metabolism, growth and development. Light signaling is able to activate various physiological processes by different photoreceptors such as the red- and far-red light-sensing phytochromes, the blue/ultraviolet (UV)-A-perceiving cryptochromes and phototropins, and the UV-B-sensing photoreceptor UVR8.1-4 With light activation photoreceptors can induce developmental and growth responses, for example, seedling de-etiolation, phototropism and the induction of flowering, as well as alternations in metabolism. Like anthocyanin, a natural pigment, its accumulation in plant tissues typically depends on the availability of light signals, but limited is known about the potential regulation network underlain light signal controls anthocyanin biosynthesis.
Anthocyanins play critical roles in diverse physiological processes, such as producers of photoprotective screens in vegetative tissue, visual attractors in pollination and seed dispersal, and antimicrobial agents and feeding deterrents in the defense response.5 Although the processes of anthocyanin biosynthesis are complicated, studies have revealed that the biosynthetic pathway of anthocyanin usually is activated by diverse Anthocyanin Biosynthesis Genes (ABGs), including the EBGs (early biosynthesis genes, involved in the initial stage of anthocyanin biosynthesis) such as chalcone synthase, chalcone isomerase (CHI), flavanone 3-hydroxylase and flavonoid 3′-hydroxylase (F3′H), and the LBGs (late biosynthesis genes, involved in the late stage of anthocyanin biosynthesis) such as dihydroflavonol 4-reductase, leucoanthocyanidin oxygenase, anthocyanidin reductase, and flavonoid 3-O-glucosyltransferase.6 Moreover, the anthocyanin biosynthesis is largely influenced by various plant hormones such as auxin,7 ABA,8 gibberellins,9 cytokinin10 and ethylene.11 In particular, the anthocyanin biosynthesis is greatly regulated by light signaling in that the anthocyanin biosynthesis is usually suppressed without light signals.
In the anthocyanin biosynthesis, the COP1 (CONSTITUTIVE PHOTOMORPHOGENIC1) is usually thought to be a central regulator in the light signaling pathway, where COP1 acts as a repressor to promote the ubiquitination and degradation of the positive regulators and itself is regulated by multiple photoreceptors.12 Specifically, COP1 interacts with the MYB transcription factors PRODUCTION OF ANTHOCYANIN PIGMENT1 (PAP1) and PAP2, 2 members of a small protein family that is required for anthocyanin accumulation and for the expression of structural genes in the anthocyanin biosynthesis pathway.12 The cop1 mutants are able to produce anthocyanins in the dark.13 Besides, it was found that SUPPRESSOR OF PHYA (SPA) genes were required for down-regulating PAP1 and PAP2 transcript levels in dark-grown seedlings, implying that SPA genes were necessary for the transcriptional control of PAP1 and PAP2 via light.14,15 In particular, the complex COP1/SPA ubiquitin ligase consisting of 2 COP1 and 2 SPA proteins was thought to be the central regulator for activating or repressing anthocyanins biosynthesis in light or in darkness, as cop1 and spa mutants produce anthocyanins also in the dark.12-14 Further, a few targets of the COP1/SPA ubiquitin ligase have been identified, including the transcription factors LONG HYPOCOTYL 5 (HY5) and its homolog HYH, plus HFR1, LAF1, and STH and its homologs.17 These studies concluded that COP1 formed a complex COP1/SPA ubiquitin ligase, which directly degraded the critical regulators (such as PAP1 and PAP2) resulting in the expressional suppression of structural genes in the anthocyanin biosynthesis pathway, leading to the suppression of the anthocyanin biosynthesis in darkness. In addition, the photoreceptors phytochrome A and B and cryptochrome 2 are subject to COP1-/SPA-mediated degradation, participating in mediating anthocyanin biosynthesis.17-20
Very recently, Meng's study21 found a novel regulator AN3 (ANGUSTIFOLIA3) for negatively regulating upstream the expression of the COP1 in the anthocyanin biosynthesis. The AN3 was found to be a growth regulating factor coactivator (GIF1), tightly associated with the Growth regulating factor1 (GRF1), involved in mediating leaf morphogenesis in Arabidopsis.22,23 In the expression profiles of the an3–4 mutant, many anthocyanin biosynthesis-related genes in seedlings were lower expressed compared to the wild types.24 Particularly, several flavonoid biosynthetic and regulatory genes (e.g., encoding CHI, F3'H, flavonol synthase (FLS), TRANSPARENT TESTA19, UDP-GLUCOSYL TRANSFERASE 79B1, and UGT89C1) in the an3–4 mutant were significantly down-expressed, strongly implying the potential function of AN3 might be involved in mediating flavonoid metabolism in Arabidopsis seedlings. But the connection of the AN3 function and flavonoid metabolism remains unknown. In Meng's study,22 the loss-of-function mutants of AN3 displayed a significantly lower anthocyanin accumulation compared to their wild types, meaning that the AN3 may be participated in mediating the anthocyanin accumulation. While investigating whether the AN3 directly regulate the main flavonoid biosynthetic and regulatory genes such as CHI, F3′H, and FLS by binding the cis-elements in the promoter regions of these genes, results showed that AN3 was not directly bound with those promoter regions (see Fig. 1). AN3 trans-repressed COP1 expression and it directly regulated the expression of COP1, leading to the expressional changes of the anthocyanin biosynthetic and regulatory genes as a consequence. A network of anthocyanin biosynthesis was proposed, as is showed in Figure 2.
Figure 1.
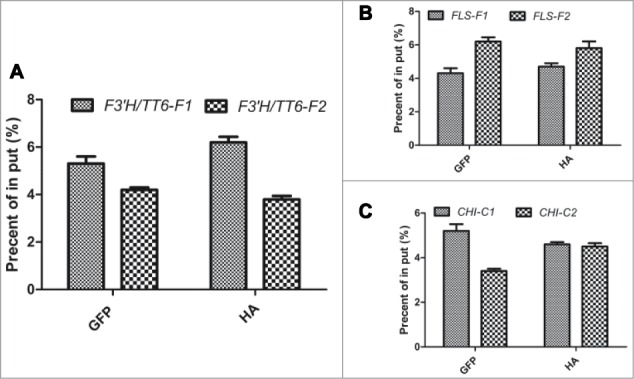
AN3 is not associated to promoters of TT6, FLS and CHI. (A–C). A chromatin immunoprecipitation (ChIP) analysis. Enrichment of particular chromatin regions with anti-HA antibody (as a control) or anti-GFP antibody in 35S:AN3-GFP transgenic plants as detected by real-time PCR analysis. Quantifications were normalized to the expression of UBQ5. Error bars represent SD (n = 3). In put is set as 100%.
Figure 2.
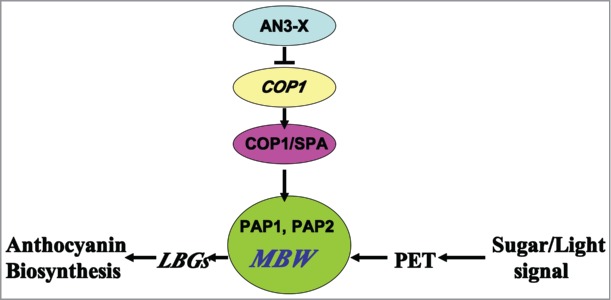
A working model is proposed for AN3 function in Arabidopsis anthocyanin accumulation. AN3 is recruited to the promoters of COP1 by protein X and regulates the expression of key genes such as COP1 that are required for anthocyanin accumulation. Light signal generated from PET activates the MYB/bHLH/TTG1 (MBW) regulatory complex(20). COP1 interacts with the MYB transcription factors PAP1 and PAP2 (belonging to MBW) (12).
This study not only demonstrated the first direct evidence for AN3 mediated anthocyanin biosynthesis by negatively regulating the expression of COP1, enriched the regulation network of anthocyanin biosynthesis, but also provided a novel insight in understanding the mechanism of anthocyanin biosynthesis regulated by light signaling. However, how light induces the activity of AN3 and what relationships between photoreceptors and AN3 remain unknown. It is possible that AN3 has a similar function to photoreceptors and its function is independent of photoreceptors. Thus, further research is necessary to discover the mystery of AN3 in mediating anthocyanin biosynthesis with light signaling.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was financially supported by NSFC (31401421 to TZ).
References
- 1.Demarsy E, Fankhauser C. Higher plants use LOV to perceive blue light. Curr Opin Plant Biol 2009; 12:69-74; PMID:18930433; http://dx.doi.org/ 10.1016/j.pbi.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 2.Nagatani A. Phytochrome: structural basis for its functions. Curr Opin Plant Biol 2010; 13:565-70; PMID:20801708; http://dx.doi.org/ 10.1016/j.pbi.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 3.Chaves I, Pokorny R, Byrdin M, Hoang N, Ritz T, Brettel K, Essen LO, van der Horst GT, Batschauer A, Ahmad M. The cryptochromes: blue light photoreceptors in plants and animals. Annu Rev Plant Biol 2011; 62:335-64; PMID:21526969; http://dx.doi.org/ 10.1146/annurev-arplant-042110-103759 [DOI] [PubMed] [Google Scholar]
- 4.Heijde M, Ulm R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci 2012; 17:230-7; PMID:22326562; http://dx.doi.org/ 10.1016/j.tplants.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 5.Winkel-Shirley B. It takes a garden. How work on diverse plant species has contributed to an understanding of flavonoid metabolism. Plant Physiol 2001; 127:1399-404; PMID:11743081; http://dx.doi.org/ 10.1104/pp.010675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelletier MK, Murrell JR, Shirley BW. Characterization of flavonol synthase and leucoanthocyanidin dioxygenase genes in Arabidopsis: further evidence for differential regulation of “early” and “late” genes. Plant Physiol 1997; 113:1437-45; PMID:9112784; http://dx.doi.org/ 10.1104/pp.113.4.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong ST, Goto-Yamamot N, Kobayashi S, Esaka M. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci 2004; 167:247-52; http://dx.doi.org/ 10.1016/j.plantsci.2004.03.021 [DOI] [Google Scholar]
- 8.Weiss D, Van Der Luit A, Knegt E, Vermeer E, Mol JNM, Kooter JM. Identification of endogenous gibberellins in petunia flowers (induction of anthocyanin biosynthetic gene expression and the antagonistic effect of abscisic acid). Plant Physiol 1995; 107:695-702; PMID:12228393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss D, van Blokland R, Kooter JM, Mol JN, van Tunen AJ. Gibberellic acid regulates chalcone synthase gene transcription in the corolla of Petunia hybrida. Plant Physiol 1992; 98:191-7; PMID:16668613; http://dx.doi.org/ 10.1104/pp.98.1.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deikman J, Hammer PE. Induction of anthocyanin accumulation by cytokinins in Arabidopsis thaliana. Plant Physiol 1995; 108:47-57; PMID:12228453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan PW, Drew MC. Ethylene and plant responses to stress. Physiol Plant 1997; 100:620-630; http://dx.doi.org/ 10.1111/j.1399-3054.1997.tb03068.x [DOI] [Google Scholar]
- 12.Maier A, Schrader A, Kokkelink L, Falke C, Welter B, Iniesto E, Rubio V, Uhrig JF, Hülskamp M, Hoecker U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J 2013; 74:638-51; PMID:23425305; http://dx.doi.org/ 10.1111/tpj.12153 [DOI] [PubMed] [Google Scholar]
- 13.McNellis TW, von Arnim AG, Araki T, Komeda Y, Miser S, Deng XW. Genetic and molecular analysis of an allelic series of cop1 mutants suggest functional roles for the multiple protein domains. Plant Cell 1994; 6:487-500; PMID:8205001; http://dx.doi.org/ 10.1105/tpc.6.4.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balcerowicz M, Fittinghoff K, Wirthmueller L, Maier A, Fackendahl P, Fiene G, Koncz C, Hoecker U. Light exposure of Arabidopsis seedlings causes rapid de-stabilization as well as selective post-translational inactivation of the repressor of photomorphogenesis SPA2. Plant J 201; 65:712-23; http://dx.doi.org/ 10.1111/j.1365-313X.2010.04456.x [DOI] [PubMed] [Google Scholar]
- 15.Hoecker U. Regulated proteolysis in light signaling. Curr Opin Plant Biol 2005; 8:469-76; PMID:16039154; http://dx.doi.org/ 10.1016/j.pbi.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 16.Yi C, Deng XW. COP1 - from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol 2005; 15:618-25; PMID:16198569; http://dx.doi.org/ 10.1016/j.tcb.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 17.Shalitin D, Yang HY, Mockler TC, Maymon M, Guo HW, Whitelam GC, Lin CT. Regulation of Arabidopsis cryptochrome 2 by blue-light-dependent phosphorylation. Nature 2002; 417:763-67; PMID:12066190; http://dx.doi.org/ 10.1038/nature00815 [DOI] [PubMed] [Google Scholar]
- 18.Seo HS, Watanabe E, Tokutomi S, Nagatani A, Chua NH. Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev 2004; 18:617-22; PMID:15031264; http://dx.doi.org/ 10.1101/gad.1187804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong SW, Das PK, Jeoung SC, Song JY, Lee HK, Kim YK, Kim WJ, Park YI, Yoo SD, Choi SB, et al.. Ethylene suppression of sugar-induced anthocyanin pigmentation in Arabidopsis. Plant Physiol 2010; 154:1514-31; PMID:20876338; http://dx.doi.org/ 10.1104/pp.110.161869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weidler G, Zur Oven-Krockhaus S, Heunemann M, Orth C, Schleifenbaum F, Harter K, Hoecker U, Batschauer A. Degradation of Arabidopsis CRY2 is regulated by SPA proteins and phytochrome A. Plant Cell 2012; 24:2610-23; PMID:22739826; http://dx.doi.org/ 10.1105/tpc.112.098210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng LS. Transcription Coactivator Arabidopsis ANGUSTIFOLIA3 modulates anthocyanin accumulation and light-induced root elongation through transrepression of Constitutive Photomorphogenic 1. Plant Cell Environ 2015; 38:838-851; PMID:25256341; http://dx.doi. 10.1111/pce.12456 [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Kende H. A transcriptional coactivator, AtGIF1, is involved in regulating leaf growth and morphology in Arabidopsis. Proc Natl Acad Sci USA 2004; 101:13374-9; PMID:15326298; http://dx.doi.org/ 10.1073/pnas.0405450101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horiguchi G, Kim GT, Tsukaya H. The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J 2005; 43:68-78; PMID:15960617; http://dx.doi.org/ 10.1111/j.1365-313X.2005.02429.x [DOI] [PubMed] [Google Scholar]
- 24.Horiguchi G, Nakayama H, Ishikawa N, Kubo M, Demura T, Fukuda H, Tsukaya H. ANGUSTIFOLIA3 plays roles in adaxial/abaxial patterning and growth in leaf morphogenesis. Plant Cell Physiol 2011; 52:112-24; PMID:21097896; http://dx.doi.org/ 10.1093/pcp/pcq178 [DOI] [PubMed] [Google Scholar]


