Abstract
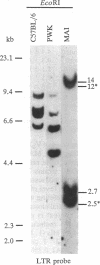
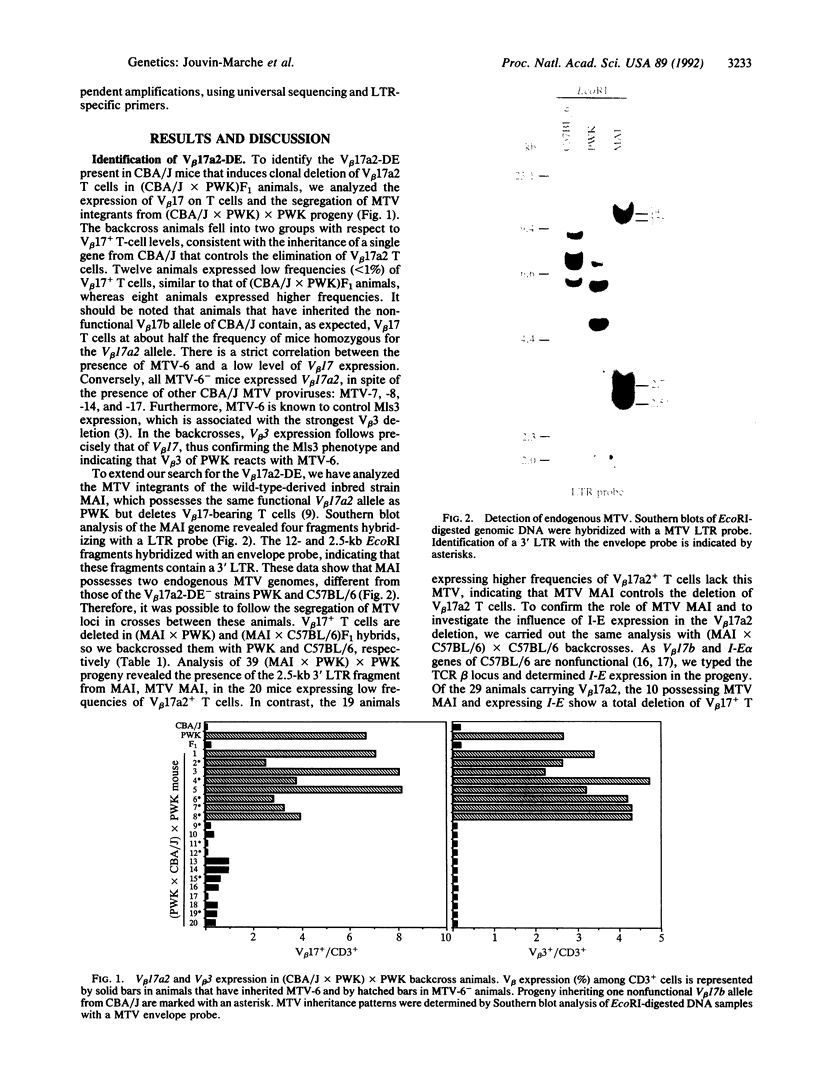
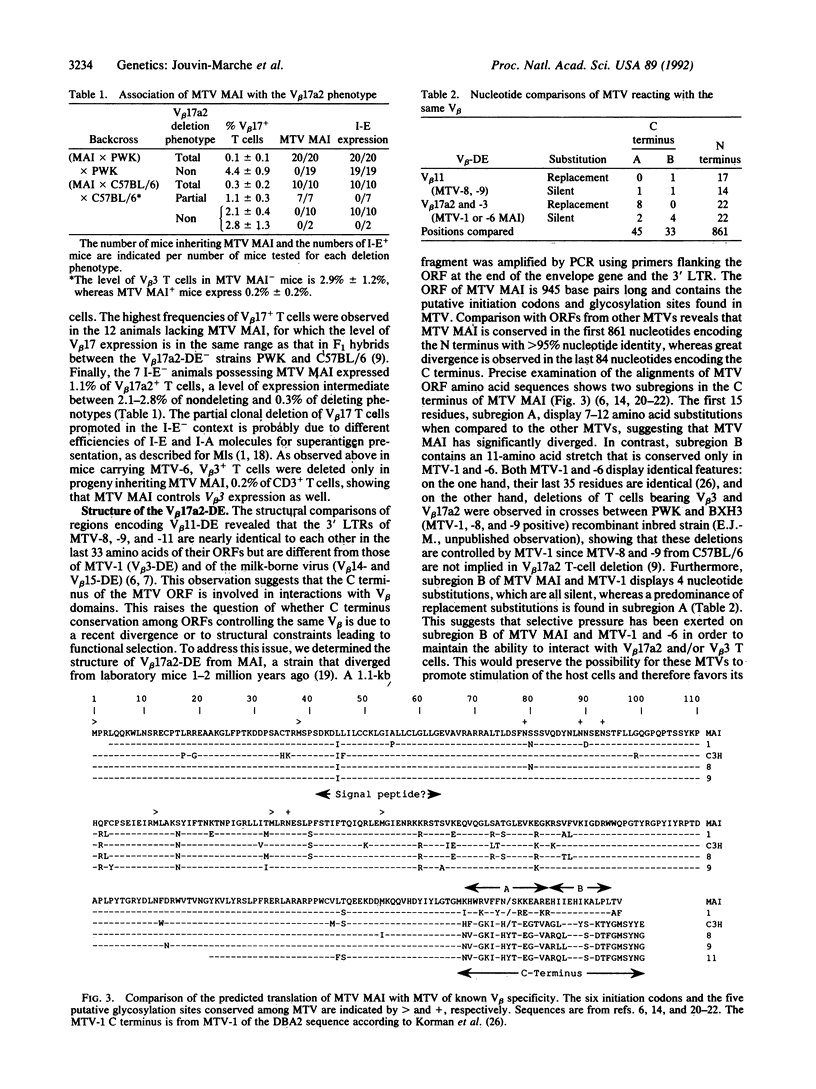
The wild-type-derived mouse strain PWK possesses a beta-chain variable region V beta 17a2 allele, which is expressed on mature T cells as part of the T-cell receptor of most mice expressing I-E, whereas V beta 17 T cells are deleted in all I-E+ laboratory mice bearing a V beta 17a1 allele. However, (PWK x CBA/J)F1 progeny and the wild-type-derived mouse strain MAI, which possesses the V beta 17a2 allele, display deletion of V beta 17 T cells. Analysis of (PWK x CBA/J) x PWK and of (PWK x MAI) x PWK backcrosses demonstrates that endogenous mouse mammary tumor virus MTV-6 from CBA/J and a MTV from strain MAI control the clonal deletion of V beta 17a2 as well as V beta 3 T cells. Furthermore, among I-E- progeny of a (MAI x C57BL/6) x C57BL/6 backcross, we observed that mice inheriting MTV of MAI have a reduced level of V beta 17 T cells, suggesting that the clonal deletion of V beta 17a2 T cells can be mediated in the absence of the I-E molecule. The 3' long terminal repeat of MTV MAI was cloned and translation of the open reading frame was compared to those of MTV known to encode superantigens. Comparisons indicate that MTV MAI has significantly diverged from the other MTVs. However, MTV MAI and MTV-6 share a stretch of 11 identical amino acids at the C terminus, which is divergent in MTV reacting with other V beta s. This suggests that this region is involved in determining the specificity toward V beta s and has been selectively conserved through evolution of the Mus species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe R., Hodes R. J. Properties of the Mls system: a revised formulation of Mls genetics and an analysis of T-cell recognition of Mls determinants. Immunol Rev. 1989 Feb;107:5–28. doi: 10.1111/j.1600-065x.1989.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Acha-Orbea H., Palmer E. Mls--a retrovirus exploits the immune system. Immunol Today. 1991 Oct;12(10):356–361. doi: 10.1016/0167-5699(91)90066-3. [DOI] [PubMed] [Google Scholar]
- Acha-Orbea H., Shakhov A. N., Scarpellino L., Kolb E., Müller V., Vessaz-Shaw A., Fuchs R., Blöchlinger K., Rollini P., Billotte J. Clonal deletion of V beta 14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991 Mar 21;350(6315):207–211. doi: 10.1038/350207a0. [DOI] [PubMed] [Google Scholar]
- Bonhomme F. Evolutionary relationships in the genus Mus. Curr Top Microbiol Immunol. 1986;127:19–34. doi: 10.1007/978-3-642-71304-0_3. [DOI] [PubMed] [Google Scholar]
- Callahan R., Drohan W., Gallahan D., D'Hoostelaere L., Potter M. Novel class of mouse mammary tumor virus-related DNA sequences found in all species of Mus, including mice lacking the virus proviral genome. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4113–4117. doi: 10.1073/pnas.79.13.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Gallahan D., D'Hoostelaere L. A., Potter M. Endogenous MMTV proviral genomes in feral Mus musculus domesticus. Curr Top Microbiol Immunol. 1986;127:362–370. doi: 10.1007/978-3-642-71304-0_44. [DOI] [PubMed] [Google Scholar]
- Cazenave P. A., Marche P. N., Jouvin-Marche E., Voegtlé D., Bonhomme F., Bandeira A., Coutinho A. V beta 17 gene polymorphism in wild-derived mouse strains: two amino acid substitutions in the V beta 17 region greatly alter T cell receptor specificity. Cell. 1990 Nov 16;63(4):717–728. doi: 10.1016/0092-8674(90)90138-5. [DOI] [PubMed] [Google Scholar]
- Choi Y., Kappler J. W., Marrack P. A superantigen encoded in the open reading frame of the 3' long terminal repeat of mouse mammary tumour virus. Nature. 1991 Mar 21;350(6315):203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- Crouse C. A., Pauley R. J. Molecular cloning and sequencing of the MTV-1 LTR: evidence for a LTR sequence alteration. Virus Res. 1989 Feb;12(2):123–137. doi: 10.1016/0168-1702(89)90059-2. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Singer P. A., Klein J. Eo: a history of a mutation. EMBO J. 1984 Jul;3(7):1647–1654. doi: 10.1002/j.1460-2075.1984.tb02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson P. J., Knight A. M., Fairchild S., Simpson E., Tomonari K. Genes encoding ligands for deletion of V beta 11 T cells cosegregate with mammary tumour virus genomes. Nature. 1991 Feb 7;349(6309):531–532. doi: 10.1038/349531a0. [DOI] [PubMed] [Google Scholar]
- Frankel W. N., Rudy C., Coffin J. M., Huber B. T. Linkage of Mls genes to endogenous mammary tumour viruses of inbred mice. Nature. 1991 Feb 7;349(6309):526–528. doi: 10.1038/349526a0. [DOI] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Jouvin-Marche E., Hue I., Marche P. N., Liebe-Gris C., Marolleau J. P., Malissen B., Cazenave P. A., Malissen M. Genomic organization of the mouse T cell receptor V alpha family. EMBO J. 1990 Jul;9(7):2141–2150. doi: 10.1002/j.1460-2075.1990.tb07383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvin-Marche E., Trede N. S., Bandeira A., Tomas A., Loh D. Y., Cazenave P. A. Different large deletions of T cell receptor V beta genes in natural populations of mice. Eur J Immunol. 1989 Oct;19(10):1921–1926. doi: 10.1002/eji.1830191024. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Wade T., White J., Kushnir E., Blackman M., Bill J., Roehm N., Marrack P. A T cell receptor V beta segment that imparts reactivity to a class II major histocompatibility complex product. Cell. 1987 Apr 24;49(2):263–271. doi: 10.1016/0092-8674(87)90567-8. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Knedlitschek G., Groner B., Hynes N. E., Herrlich P., Michalides R., van Ooyen A. J. Long terminal repeats of endogenous mouse mammary tumour virus contain a long open reading frame which extends into adjacent sequences. Nature. 1982 Feb 18;295(5850):622–624. doi: 10.1038/295622a0. [DOI] [PubMed] [Google Scholar]
- King L. B., Lund F. E., White D. A., Sharma S., Corley R. B. Molecular events in B lymphocyte differentiation. Inducible expression of the endogenous mouse mammary tumor proviral gene, Mtv-9. J Immunol. 1990 Apr 15;144(8):3218–3227. [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequencing of an apparent proviral copy of env mRNA defines determinants of expression of the mouse mammary tumor virus env gene. J Virol. 1983 Sep;47(3):495–504. doi: 10.1128/jvi.47.3.495-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kushnir E., Kappler J. A maternally inherited superantigen encoded by a mammary tumour virus. Nature. 1991 Feb 7;349(6309):524–526. doi: 10.1038/349524a0. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Wade T., Marrack P., Kappler J. W. Identification of the region of T cell receptor beta chain that interacts with the self-superantigen MIs-1a. Cell. 1990 Jun 29;61(7):1365–1374. doi: 10.1016/0092-8674(90)90700-o. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade T., Bill J., Marrack P. C., Palmer E., Kappler J. W. Molecular basis for the nonexpression of V beta 17 in some strains of mice. J Immunol. 1988 Sep 15;141(6):2165–2167. [PubMed] [Google Scholar]
- Woodland D. L., Happ M. P., Gollob K. J., Palmer E. An endogenous retrovirus mediating deletion of alpha beta T cells? Nature. 1991 Feb 7;349(6309):529–530. doi: 10.1038/349529a0. [DOI] [PubMed] [Google Scholar]