Abstract
Background
Choroidal thickness (CTh) and choroidal vessel diameter (VD) in the Haler’s layer were evaluated as markers of inflammatory insult in non-infectious uveitis (NIU). Spectral-domain optical coherence tomography (Spectralis®, Heidelberg Engineering Inc.) scans were acquired from 23 normal subjects (39 eyes – group 1), 7 subjects with high myopia (14 eyes – group 2), and 19 patients with NIU (23 eyes – group 3). In groups 1 and 2, CTh and VD were measured at 3 different points of the same horizontal OCT scan passing through the fovea and a mean calculated. Mean CTh and VD were calculated in 2 other locations, 2 mm superior and inferior from the chosen foveal horizontal scan. In group 3, three measurements of CTh and VD were obtained within 1 mm of a horizontal scan passing through a retinal lesion; mean CTh and VD were then computed. A ratio (R) between the VD and the corresponding CTh was calculated.
Results
Group 1, 2 and 3 mean age was 29.6, 29.1 and 45.9 years, respectively. Sixteen normal subjects, three myopic subjects and six NIU patients were male.. Group 1 mean CTh did not differ from group 2 (261.6±45.6 vs. 260.2±50.6 µm µm; p>0.05); mean VD was marginally higher in Group 2 (159.8±32.2 vs. 163.2±33.2 µm; p>0.05). Group 3 demonstrated thinner CTh (193.6±54.6 µm) than Groups 1 and 2 (p = 0.02 and <0.001). Group 3 mean VD (123.6±37.4 µm) was also less than that in Groups 1 and 2; the difference was statistically significant only when compared to group 2, p = 0.01. R did not differ across groups (p-values >0.05), indicating that variations in CTh and VD followed the same trend.
Conclusions
The study reports potential quantitative OCT-derived parameters that may be explored in future trials of non-infectious uveitis. Thinning of choroid and decrease of vessel diameter are observed in patients with chronic NIU compared to controls.
Electronic supplementary material
The online version of this article (doi:10.1186/s12348-014-0014-z) contains supplementary material, which is available to authorized users.
Keywords: Choroidal inflammatory disease, Choroidal thickness, Optical coherence tomography, Non-infectious uveitis and white dot syndrome
Background
Management of non-infectious uveitis (NIU) presents a unique challenge given the multitude of presentations, associated complications, and a lack of a definitive treatment thereof. Its pathophysiology and triggers are yet to be fully understood, consequently making it difficult to identify and observe biomarkers (anatomic and biochemical) that may help in early diagnosis and allow objective assessment of response to therapy.
Careful evaluation is required on part of the care providers to overcome the diagnostic challenge, which is often made by excluding a list of possible pathologies. Despite the great advances in diagnostic tests, a definitive diagnosis still remains elusive in more than 20% of uveitis cases [1]. In addition to infectious and autoimmune serologic assays, interdisciplinary evaluations, radiologic examination, ultrasonography, and fluorescein and indocyanine angiography, optical coherence tomography (OCT), a relatively recent imaging modality, has been used to accurately characterize structural damage to the retina and choroid in patients with NIU. It allows imaging of the retina and choroid with microscopic resolution, facilitating the differentiation and follow-up of subtle changes.
Various studies have been conducted to study the morphological changes seen in NIU employing OCT. Qualitative changes in the retinal and choroidal microstructures, such as choroidal, retinal pigment epithelium (RPE), and retinal intra-layer hyper reflectivity, have been described in many of the NIU diagnoses [2]-[4]. Despite the advances in the characterization of NIU provided by OCT, OCT-derived quantitative outcomes are yet to be evaluated as predictors of inflammation and functional outcomes.
Since inflammatory diseases of the choriocapillaris and stromal inflammatory vasculopathy play a role in the pathogenesis of many of the NIU conditions, changes in the normal blood circulation in the choroid are expected [5]-[9]. We hypothesized that inflammatory mediators produced in the course of these disorders may yield changes in choroidal thickness and vessel size and therefore could potentially be used as outcomes in future clinical trials. The purpose of our study was to quantify the role of vessel diameter and choroidal thickness as biomarkers of chronic inflammatory insults in non-infectious uveitis.
Methods
Cross-sectional clinical and imaging data were retrospectively collected from patients in the Retina Division of the Wilmer Eye Institute, Johns Hopkins Hospital. Only spectral domain OCT images acquired with Spectralis HRA+OCT (version 1.5.12.0, Heidelberg Engineering Inc., Carlsbad, CA, USA) were used in the study. The standards of the Helsinki Declaration were followed; written informed consent was obtained from all participants after the study approval by the Johns Hopkins Hospital Research Ethics Committee has been granted.
Demographic information was collected. Spectral domain optical coherence tomography (SD-OCT) images from participants were divided into three groups: group 1 (normal), group 2 (high myopia), and group 3 (NIU). Images from normal volunteers with no known ocular diseases and spherical equivalent refractive error >−6.00 D were assigned to group 1. As some patients in the NIU cohort had multifocal choroiditis (MFC) and punctate inner choroidopathy (PIC), which are commonly associated with high myopia, a cohort with highly myopic individuals was included in the study. Highly myopic individuals should have spherical equivalent refractive error ≤−6.00 D and fundus changes compatible with myopia and no other pathology associated. Subjects assigned to group 3 had their diagnoses confirmed after extensive evaluation by a retina and uveitis specialist (QDN). Among the ancillary studies employed to reach the diagnosis were ophthalmic examinations, infectious and autoimmune serologic assays, radiologic images, and interdisciplinary consultation when required, as well as SD-OCT and fluorescein angiography. Subjects <18 years old and with any other ocular pathology different from chronic NIU were excluded from the study.
OCT images with signal-to-noise ratio >15 were included in the study. Images used for analysis followed a standardized OCT scanning protocol. All images were acquired using 20° × 15° rectangle with horizontal raster scans, each comprised at least ≥16 frames per b-scan, encompassing the macula and optic nerve temporal border. Manual calipers were used to measure choroidal thickness (CTh) from the outer hyper-reflective border of the base of the retinal pigment epithelium (RPE) - RPE/BM complex - to the hypo-reflective inner border of the sclera (Figure 1) [10]. The choroidal layer was further subdivided into Haller's large vessel layer (HLVL) and Sattler's medium vessel layer (SMVL). Choroidal vessel diameter (VD) was measured in the HLVL of the choroid, which is defined as outer choroid large hypo-intense spaces, representing large vascular luminal spaces [8]. The imaging measurement algorithm is illustrated in Figure 1.
Figure 1.
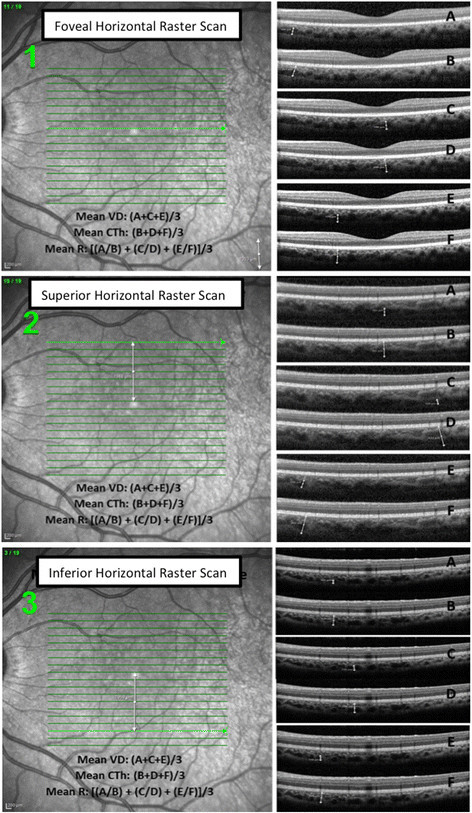
Measurement algorithm. Measurement algorithm in the OCT of a normal subject. A: VD measurement 1, B: CTh measurement 1, C: VD measurement 2, D: CTh measurement 2, E: VD measurement 3, F: CTh measurement 3
In normal and myopic subjects, CTh and VD were measured at three different points of the same OCT horizontal line and a mean was calculated. Each subject had the mean CTh and VD calculated for three horizontal lines: one sub-foveal, one 2 mm superior, and one 2 mm inferior from the chosen foveal horizontal scan. These three locations were used to compute a mean macular CTh and VD and served as a reference to analyze the CTh and VD of group 3.
In subjects with NIU, retinochoroidal lesions within any of the macular quadrants were identified, and the mean CTh and VD were obtained from three measurements of a given horizontal line passing through each lesion.
A ratio (R) between the VD measurement and the corresponding CTh measurement was calculated (R = VD/CTh) and used for comparison among normal, myopic, and NIU patients.
Statistical analysis
The SPSS (IBM® Inc., Chicago, IL, USA) release 19.0.0 was used for statistical analysis. Demographic characteristics of the patients were summarized using descriptive statistics and expressed as mean and 95% confidence intervals. Exploratory data analysis (EDA) was done to verify the data set characteristics, including covariates, and to orient the comparison analysis.
A multilevel analysis using a general model with random intercepts, which took into consideration age, spherical equivalent refractive error, measurement location, gender, and ethnicity, was used to compare the CTh, VD, and ratio among the three groups. In addition, as the mean CTh, VD, and R for each horizontal line for the same individual were analyzed individually, the model was adjusted to assume that there would be a correlation of retinal scans at two different levels: eyes and patients. Therefore, a random intercept for each of these two levels was also included. Kruskal-Wallis test was used to evaluate potential differences across different diagnoses of NIU. Similarly, Mann-Whitney test was conducted to compare the CTh, VD, and ratio between pairs of diagnosis. Mean and confidence interval were calculated to better visualize the possible overlapping distributions. A p value of <0.05 was considered statistically significant.
Results
Among the subjects enrolled, 23 had no known ocular pathology (39 eyes), 7 were highly myopic without any other retinal pathology (14 eyes), and 19 were patients with various types of NIU (23 eyes). Sixteen (51.6%) of the normal subjects, three (50%) of the myopic subjects, and six (35.2%) of the NUI patients were male. Mean age for normal, myopic, and NIU patients was 29.6, 29.1, and 45.9 years, respectively. The mean spherical refractive error in groups 1, 2, and 3 was −1.80 D (SD ±1.4), −7.30 D (SD ± 2.6), and −4.68 D (SD ±4), respectively. The best corrected visual acuity in ETDRS letters was 84 ± 3.6 in group 1, 75 ± 6.4 in group 2, and 69 ± 17 in group 3. In group 3, eight eyes were from patients diagnosed with multifocal choroiditis (MFC), four from patients with punctate inner choroidopathy (PIC), six from patients with birdshot chorioretinopathy (BSCR), three from patients with acute zonal occult outer retinopathy (AZOOR), while one eye was from a patient with serpiginous chorioretinitis (SC) and one with Vogt-Koyanagi-Harada (VKH) syndrome.
The NIU mean duration was 7 (±4.6 SD) years. All patients were in disease remission by the time of the study visit (for the analyses in this study). Four eyes (17.4%) were in remission for at least 2 years, ten eyes (43.5%) were in remission for at least 1 year, six eyes (26.1%) were in remission for at least 6 months, and three eyes (13%) were in remission for more than 1 month but less than 6 months. The mean time of controlled disease was 15.3 (±12.3 SD) months, 60.9% of the eyes were in remission for at least 1 year and 39.1% were in remission for less than 1 year. Twelve patients were on immunomodulatory therapy (IMT) alone, three patients were on both IMT and corticosteroids, three patients were taking less than 10 mg/day of prednisone as monotherapy, and one was not on any treatment. The 23 eyes were considered as having chronic disease in remission at the time of the study. Three patients had bilateral and 16 patients had unilateral ocular involvement. Demographic data and clinical characteristics of study subjects are summarized in Table 1.
Table 1.
Characteristics of study subjects
| Neyes | Age | Gender | Ethnicity | Refractive error | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Years | SD | Male | Female | C | AF | A | Spherical equivalent | SD | ||
| Group 1 | 39 | 29.6 | 7.4 | 18 | 18 | 18 | 4 | 17 | −1.8 | 1.40 |
| Group 2 | 14 | 29.1 | 8.7 | 8 | 6 | 4 | 2 | 8 | −7.3 | 2.60 |
| Group 3 | 23 | 45.9 | 14.4 | 7 | 16 | 19 | 3 | 1 | −4.68 | 4.00 |
| MFC | 8 | 44.4 | 8.5 | 2 | 6 | 5 | 2 | 1 | −5.54 | 3.50 |
| PIC | 4 | 33.6 | 3.3 | 2 | 2 | 4 | 0 | 0 | −8.86 | 2.30 |
| AZOOR | 3 | 56.0 | 20.7 | 0 | 3 | 3 | 0 | 0 | −1.16 | 0.28 |
| BSCR | 6 | 63.8 | 9.0 | 1 | 5 | 6 | 0 | 0 | −1.30 | 3.00 |
| VKH | 1 | 25 | 0 | 1 | 0 | 0 | 1 | 0 | −3.00 | 0.00 |
| SC | 1 | 38 | 0 | 1 | 0 | 1 | 0 | 0 | −1.00 | 0.00 |
Group 1: normal subjects; group 2: highly myopic subjects; group 3: subjects with non-infectious uveitis, multifocal choroiditis (MFC), punctate inner choroidopathy (PIC), acute zonal occult outer retinopathy (AZOOR), birdshot choroidoretinopathy (BSCR), Vogt-Koyanagi-Harada syndrome (VKH), serpiginous choroiditis (SC). C, Caucasian; AF, African; A, Asian.
The mean CTh was similar in normal subjects, 261.6 ± 45.6 μm, and myopic subjects, 260.2 ± 50.6 μm. Patients with NIU had thinner mean CTh, 193.6 ± 54.6 μm, as compared to the control groups. The mean VD was found to be larger in myopic subjects, 163.2 ± 33.2 μm, than in normal subjects and patients with NIU, 159.8 ± 32.2 μm and 123.6 ± 37.4 μm, respectively. Groups 1, 2, and 3 had approximately the same mean ratio, 0.61 ± 0.1, 0.61 ± 0.05, and 0.62 ± 0.01, respectively (p > 0.05) (Table 2).
Table 2.
Choroidal thickness, vessel diameter, and ratio characteristics
| Choroidal thickness (μm) | Vessel vertical diameter (μm) | Ratio | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | 95% CI | Mean | SE | 95% CI | Mean | SE | 95% CI | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| Group 1 | 261.6 | 4.5 | 253.1 | 271.3 | 159.8 | 3.2 | 154.0 | 166.2 | 0.61 | 0.01 | 0.59 | 0.65 |
| Group 2 | 260.2 | 7.8 | 244.5 | 275.5 | 163.2 | 5.1 | 152.9 | 174.1 | 0.62 | 0.00 | 0.61 | 0.64 |
| Group 3 | 193.6 | 8.5 | 176.9 | 213.3 | 123.6 | 5.8 | 111.7 | 135.5 | 0.61 | 0.01 | 0.60 | 0.67 |
| MFC | 187.7 | 10.4 | 165.3 | 210.0 | 123.1 | 8.8 | 104.2 | 142.0 | 0.65 | 0.02 | 0.6 | 0.69 |
| PIC | 198.4 | 16.9 | 159.3 | 237.5 | 127.6 | 11.5 | 101.0 | 154.3 | 0.64 | 0.02 | 0.58 | 0.70 |
| AZOOR | 318.8 | 18.2 | 240.2 | 397.3 | 177.3 | 13.5 | 119.1 | 235.5 | 0.55 | 0.04 | 0.36 | 0.74 |
| BSCR | 160.4 | 7.1 | 143.8 | 177.1 | 98.2 | 8.9 | 77.5 | 118.9 | 0.61 | 0.04 | 0.51 | 0.71 |
| VKH | 192.5 | 3.5 | 148.0 | 236.9 | 130.5 | 26.5 | 104.0 | 157.0 | 0.68 | 0.15 | 0.53 | 0.83 |
| SC | 183.2 | 66.2 | 117.0 | 249.5 | 137.5 | 45.5 | 92.0 | 183.1 | 0.75 | 0.02 | 0.73 | 0.79 |
Group 1: normal subjects; group 2: highly myopic subjects; group 3: subjects with non-infectious uveitis, multifocal choroiditis (MFC), punctate inner choroidopathy (PIC), acute zonal occult outer retinopathy (AZOOR), birdshot choroidoretinopathy (BSCR), Vogt-Koyanagi-Harada syndrome (VKH), serpiginous choroiditis (SC).
In normal subjects, the mean CTh has shown to be greater towards the arcades (mean foveal CTh = 242.8 ± 37.5 μm, mean supra-foveal CTh = 271.8 ± 50.4 μm, mean infra-foveal CTh = 275.3 ± 43 μm; p < 0.05). Mean VD was larger superiorly (173.9 ± 36.8 μm) than inferiorly (159.5 ± 26.6 μm, p > 0.06) and in the fovea (148.5 ± 28.1 μm, p < 0.001); the difference between mean VD in the fovea and inferiorly was not significant (p = 0.16). The variation of R across subfield was not statistically significant, p values >0.05. The CTh, VD, and R values have not shown statistically significant differences between macular locations in group 2, which may indicate a more homogeneous thickness across myopic retinal subfields (Tables 3 and 4).
Table 3.
Choroidal thickness, vessel diameter, and ratio characteristics according to location in the fovea
| Locations | Normal subjects | Highly myopic subjects | |||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| Mean CTh | Foveal | 39 | 242.8 | 37.5 | 14 | 248.1 | 42.0 |
| Supra-foveal | 32 | 271.8 | 50.4 | 14 | 273.1 | 58.6 | |
| Infra-foveal | 30 | 275.3 | 43.0 | 14 | 259.6 | 50.7 | |
| Mean VD | Foveal | 39 | 148.5 | 28.1 | 14 | 152.6 | 26.9 |
| Supra-foveal | 32 | 173.9 | 36.8 | 14 | 174.8 | 37.0 | |
| Infra-foveal | 30 | 159.5 | 26.6 | 14 | 162.4 | 33.5 | |
| Mean ratio | Foveal | 39 | 0.614 | 0.086 | 14 | 0.618 | 0.055 |
| Supra-foveal | 32 | 0.660 | 0.242 | 14 | 0.644 | 0.059 | |
| Infra-foveal | 30 | 0.582 | 0.150 | 14 | 0.628 | 0.065 | |
Supra-foveal is defined as 2 mm superiorly to the fovea; infra-foveal is defined as 2 mm inferiorly to the fovea.
Table 4.
Choroidal thickness, vessel diameter, and ratio characteristics according to comparison of locations in the fovea
| Comparison of locations | Normal subjects | Highly myopic subjects | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CI | pvalue | Mean difference | 95% CI | pvalue | ||||
| Lower bound | Upper bound | Lower bound | Upper bound | ||||||
| Mean CTh | Foveal × supra-foveal | −27.9 | −47.5 | −8.4 | 0.006 | −25.0 | −64.4 | 14.4 | 0.206 |
| Foveal × infra-foveal | −30.6 | −50.6 | −10.6 | 0.003 | −11.5 | −50.9 | 27.9 | 0.557 | |
| Supra-7foveal × infra-foveal | −2.7 | −23.5 | 18.2 | 0.800 | 13.5 | −25.9 | 52.9 | 0.492 | |
| Mean VD | Foveal × supra-foveal | −25.1 | −39.6 | −10.5 | 0.001 | −22.2 | −47.6 | 3.2 | 0.085 |
| Foveal × infra-foveal | −10.5 | −25.3 | 4.3 | 0.162 | −9.8 | −35.1 | 15.6 | 0.440 | |
| Supra-foveal × infra-foveal | 14.6 | −0.9 | 30.1 | 0.065 | 12.4 | −13.0 | 37.8 | 0.328 | |
| Mean ratio | Foveal × supra-foveal | −0.048 | −0.119 | 0.022 | 0.176 | 0.026 | −0.026 | −0.026 | 0.253 |
| Foveal × infra-foveal | 0.028 | −0.044 | 0.100 | 0.446 | 0.016 | −0.010 | −0.010 | 0.482 | |
| Supra-foveal × infra-foveal | 0.076 | 0.001 | 0.151 | 0.057 | −0.016 | −0.062 | 0.030 | 0.482 | |
Supra-foveal is defined as 2 mm superiorly to the fovea; infra-foveal is defined as 2 mm inferiorly to the fovea.
The relationship between age, refractive error, gender, ethnicity, and the study variables were analyzed using multiple regression analysis. An increase of 1 year has shown to decrease the CTh by 1.7 μm and the VD by 0.98 μm, p values <0.001. Conversely, the decrease of 1 diopter has shown to increase the CTh by 3.6 μm (Table 5). Ethnicity was found to have no association with CTh, VD, and R. In group 3, the association between remission duration and CTh did not show significant strength (r2 = 0.389, p value = 0.96); therefore, this variable was not included in the model. Likewise, the association between remission duration and vessel diameter was also not significant (r2 = 0.276, p value = 1.02).
Table 5.
Association of different co-factors with choroidal thickness and vessel diameter
| Choroidal thickness (μm) | Vessel vertical diameter (μm) | |||
|---|---|---|---|---|
| Effect | pvalue | Effect | pvalue | |
| Age (⬆ 1 year) | −1.7 | <0.001 | −0.98 | <0.001 |
| Refractive error (⬇ 1 D) | 3.6 | 0.01 | 0.85 | 0.390 |
| Gender (M) | 18.9 | 0.07 | 8.18 | 0.100 |
| Ethnicity | - | 0.22 | - | 0.666 |
D, diopter, M, male.
The comparisons among groups were adjusted for possible differences in age, refractive error, and gender, and for intra-eye and intra-patient correlation of repeated measurements. Mean CTh was slightly thicker in group 1 as compared to group 2; the difference, however, did not differ significantly (p value >0.05). Conversely, VD was higher in group 2, but not statistically significant (p = 0.92). Group 3 demonstrated thinner choroid when compared to groups 1 and 2. The difference was statistically significant with p values <0.05. The VD was also smaller in this group compared to groups 1 and 2; however, the difference was statistically significant only when compared to group 2, p = 0.008. The thin CTh of group 3 was not reflected by its group ratio when compared to groups 1 and 2, p value >0.05 (Table 6).
Table 6.
Group comparisons
| Choroidal thickness (μm) | Vessel vertical diameter (μm) | Ratio | ||||
|---|---|---|---|---|---|---|
| Difference | pvalue | Difference | pvalue | Difference | pvalue | |
| Group 1 vs. group 2a | 18.84 | 0.29 | −8.1 | 0.92 | 0.029 | 1.00 |
| Group 1 vs. group 3a | 31.3 | 0.02 | 17.96 | 0.07 | 0.006 | 1.00 |
| Group 2 vs. group 3a | 50.2 | <0.001 | 26.0 | 0.01 | −0.023 | 0.31 |
aMultivariate model using age and refractive error as covariates (age = 33.16 and Rx = −3.7). Bonferroni test was used to adjust for multiple comparisons.
Among patients with NIU, the mean CTh was found to be greatest in the AZOOR patients (318.8 μm) followed by patients with PIC (198.4 μm), VKH (192.5 μm), MFC (187.7 μm), SC (183.2 μm), and finally BSCR (160.4 μm). The CTh difference among the various entities was significant (p = 0.04). Mean VD was largest in AZOOR patients (177.3 μm), followed by SC (137.5 μm), VKH (130.5 μm), PIC (127.6 μm), MFC (123.1 μm), and BSCR (98.2 μm); however, the differences among subgroup were not significant (p = 0.06). R was higher in patients with SC (0.75) followed by VKH (0.68), MFC (0.65), PIC (0.64), BSCR (0.61), and then AZOOR (0.55), p = 0.23. The complete description of the CTh, VD, and ratio distribution across patients with different NIU diagnoses is assembled in Table 2. The CTh and VD intervals are illustrated in Figures 2 and 3. Figure 4 shows the measurements of the VD and CTh of a patient with BSCR.
Figure 2.
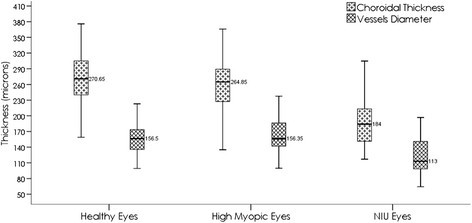
Group choroidal thickness and vessel diameter intervals. Choroidal thickness and vessel diameter intervals showing the difference between group 1 and group3 and between group 2 and group 3.
Figure 3.
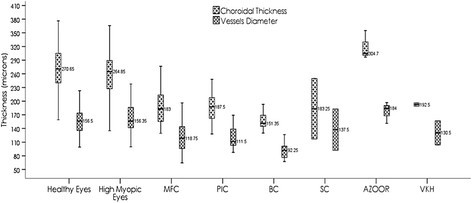
Non-infectious uveitis choroidal thickness and vessel diameter intervals. Choroidal thickness and vessel diameter intervals for different entities of non-infectious uveitis (NIU), showing the differences among AZOOR and other diagnosis.
Figure 4.
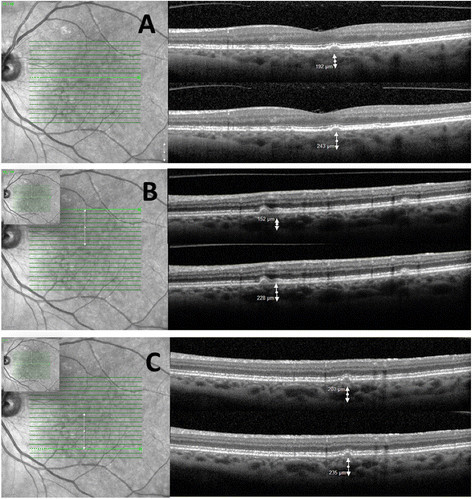
Patient with birdshot retinochoroidopathy. Spectral domain OCT of the left eye of a patient with birdshot retinochoroidopathy. The infrared SLO images (A to C) show the location of the horizontal b-scan. The correspondent b-scans show the vessel diameter and the choroidal thickness below the different lesions.
Mean differences in CTh and VD were found to be significant only across AZOOR patients and patients with MFC, PIC, and BSCR, p values <0.05. The R did not reflect any differences among subgroups (Table 7).
Table 7.
Comparisons between NIU diagnoses
| Choroidal thickness (μm) | Vessel vertical diameter (μm) | Ratio | ||||
|---|---|---|---|---|---|---|
| Among NIUa | pvalue = 0.04 | pvalue = 0.06 | pvalue = 0.23 | |||
| Pair-wise comparisonb | Z | pvalue | Z | pvalue | Z | pvalue |
| MFC vs. PIC | −0.48 | 0.63 | −0.28 | 0.80 | −0.05 | 0.97 |
| MFC vs. AZOOR | −2.66 | 0.01 | −2.23 | 0.02 | −1.67 | 0.10 |
| MFC vs. BSCR | −1.41 | 0.16 | −1.72 | 0.08 | −0.45 | 0.67 |
| PIC vs. AZOOR | −2.49 | 0.01 | −1.75 | 0.07 | −1.57 | 0.11 |
| PIC vs. BSCR | −1.76 | 0.07 | −2.16 | 0.03 | −0.30 | 0.75 |
| AZOOR vs. BSCR | −2.50 | 0.01 | −2.50 | 0.01 | −1.20 | 0.22 |
aKruskal-Wallis test; bMann-Whitney test. VKH and SC were not used for comparison due to the small number of subjects enrolled (1).
Discussion
In this cross-sectional study, OCT-derived parameters were employed to quantitatively evaluate choroidal changes in a variety of posterior NIUs. The NIU cohort has shown demographic features in accordance with those described in the literature for patients with different white dot syndromes (WDSs). The strong female predilection seen in MFC and PIC, as well as the high incidence in the third and fourth decades, was confirmed in the study cohort [11]. Similarly, patients with BSCR, SC, AZOOR, and VKH have revealed characteristics similar to those previously described for these pathologies [12],[13].
Variation of CTh and VD were found across different subfields of the macula. This difference may be explained by physiological and anatomical differences between the retina fields. Light-dark adaptation, exercise, and blue-flickering stimulus have been shown to induce choroidal blood flow responses and diversely influence the CTh and VD across various locations in the posterior pole [14]. It is not unusual to find the thickest portion of the choroid to be underneath the fovea, where the oxygen requirement is highest, thus requiring high blood flow [15]. In addition, peripheral, equatorial, and sub-macular choroid has been shown to differ on the density of capillaries and inter-capillary space, being more compacted and thicker in the macula.
Recent studies of macular CTh employing enhanced depth imaging (EDI)-OCT have made known that sub-foveal CTh decreases rapidly in the nasal direction and less impressively in the temporal direction [10],[16]. In our study, the mean foveal CTh was averaged from three measurements taken at any point of the foveal horizontal scan, including the nasal and temporal subfields. Therefore, the overall foveal CTh found in our cohort was thinner than the central foveal CTh reported by other authors [10],[16],[17]. However, by using such method, the averaged foveal CTh could then be used as reference to evaluate uveitis lesions occurring at any point of the foveal horizontal scan.
The mean CTh measured in normal OCTs was found to be within the normal range described by Ding et al. In this study of 420 eyes (mean CTh = 261.93 ± 88.42 μm), the thickness range was found to be very close to the one reported by Fujiwara and colleagues in a study with 145 eyes (mean CTh 265.5 ± 82.4 μm) [18],[19].
The cohort of patients with NIU showed thinner CTh and VD than the normal and myopic controls. These findings support the hypothesis that inflammatory and ischemic insults to the choroid may occur during posterior non-infectious uveitis or may lead to atrophic changes in the choroidal layers [5],[6],[20],[7]. The discrepancy in the CTh and VD measurements between groups 2 and 3 shows that co-existing myopia in NIU patient is not a confounding factor in final NIU thickness.
Not surprisingly, some variations on the intensity of CTh and VD atrophy occur across different diagnosis of NIU. Most likely, it reflects the distinct inflammatory profile involved in pathophysiology of each entity as well as the variable vascular involvement.
Recently, Aoyagi and colleagues have reported two cases of Japanese patients with multiple evanescent white dot syndrome (MEWDS) who had CTh decrease from the acute to convalescent phase [21]. Similarly, Karampelas and collaborators have described loss of hyporeflectivity in HLVL and thinning of both choroid and HLVL layer in patients with panuveitis [8]. Furthermore, Keane and colleagues evaluated qualitative and quantitative choroidal changes in patients with BSCR in comparison to normal controls using enhanced depth OCT scans. Their study revealed generalized choroidal thinning, absence of Sattler's layer, discrete hyper-reflective foci, focal depigmentation, and the presence of supra-choroidal hypo-reflective space [4]. The present study supports these previous findings; the choroid of patients with BSCR, SC, and MFC have shown more profound thinning than those with PIC and VKH, when compared to normal and highly myopic controls. Even though it is still controversial whether AZOOR should be among the so-called white dot syndromes, it is well known that the majority of the pathologic insults to the fundus structures occur at the level of RPE and photoreceptor layers, more commonly sparing the choroidal layers [22],[23]. Normal CTh and VD are expected findings for AZOOR, yet it was not seen in our cohort. Our patients with AZOOR have shown thicker CTh and VD than normal and highly myopic controls. There are two conceivable explanations: the small number of subjects with AZOOR, which may not be representative of the diseases as a whole, and subclinical acute inflammation leading to the thickening effect described by Aoyagi and colleagues. Either explanations warrant further evaluation.
The ratio of VD and CTh did not vary significantly across groups or even within different NIU diagnoses. Changes in VD followed the overall choroidal thickness changes. In addition, our cohort was characterized by patients under effective immunomodulation and therefore was composed of patients with inactive disease. As described by Aoyagi, vessel dilatation or increased CTh is more expected in acute inflammatory phase, which was not the case in the major part of patients enrolled in our study. The present study shows that the ratio could not accurately characterize NIU damage.
To our knowledge, our study is the first to evaluate CTh and VD in various forms of NIU and compared to controls such as myopic patients and normal subjects. CTh and VD thinning have shown to be altered in the remission phase of various NIU and should be further explored as a potential parameter to monitor disease regression or progression. Our next step is to validate a semi-automated method to measure CTh and vessel volume.
There are several study limitations, including the small number of patients in the subgroup of NIU and the use of standard SD-OCT images. As the study collected data retrospectively, the availability of OCTs using EDI was limited; therefore, it was not incorporated in the study design. However, this limitation was considered an intrinsic factor, common to all images, which does not affect the validity of the results [15],[10],[24].
Conclusions
The present study reports potential OCT-derived parameters in patients with non-infectious uveitis. We detected thinning of choroid and decrease of vessel diameter when compared to matched controls, which indicates that choroidal thickness and vessel size may serve as potential (follow-up) parameters to monitor patients with non-infectious uveitis in future studies. Further analysis with a larger sample size and semi-automated measurements is indicated to evaluate the role of choroidal thickness and vessel diameter in the management of active and inactive uveitic diseases.
Acknowledgements
The study was presented in part at the 2013 ARVO Annual Meeting, Seattle, WA, USA.
Abbreviations
- AZOOR
acute zonal occult outer retinopathy
- BSCR
birdshot choroidoretinopathy
- CTh
choroidal thickness
- EDI
enhanced depth imaging
- FA
fluorescein angiography
- HLVL
Haller's large vessel layer
- MFC
multifocal choroiditis
- OCT
optical coherence tomography
- PIC
punctate inner choroidopathy
- RPE
retinal pigment epithelium
- SC
serpiginous choroiditis
- SMVL
Sattler's medium vessel layer
- VD
vessel diameter
- VKH
Vogt-Koyanagi-Harada syndrome
- WDS
white dot syndrome
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Footnotes
Competing interests
The Johns Hopkins University and the University of Nebraska Medical Center have received research funding from Heidelberg Engineering, Inc., CA, USA. Dr. Quan Dong Nguyen and Dr. Diana Do have served on the Scientific Advisory Board for Heidelberg Engineering, Inc. The other authors have no competing interests.
Authors' contributions
MGB, QDN, and DVD designed and conducted the study. MGB, YJS, DAF, SK, MA, HN, MH, QDN, and DVD collected the data. MGB, YJS, QDN, and DVD managed the data. MGB analyzed the data. MGB, SK, DAF, YJS, QDN, and DVD interpreted the data. MGB, SK, MA, and HN prepared the manuscript. MGB, YJS, QDN, and DVD reviewed and approved the manuscript. All authors read and approved the final manuscript.
Contributor Information
Millena G Bittencourt, Email: mbitte2@jhmi.edu.
Saleema Kherani, Email: salimakherani@gmail.com.
Daniel A Ferraz, Email: danielferraz1@hotmail.com.
Mehreen Ansari, Email: mehreen.ansari91@hotmail.com.
Humzah Nasir, hhnasir@email.wm.edu.
Yasir J Sepah, Email: yasir.sepah@unmc.edu.
Mostafa Hanout, Email: mostafa.hanout@gmail.com.
Diana V Do, Email: diana.do@unmc.edu.
Quan Dong Nguyen, Email: quan.nguyen@unmc.edu.
References
- 1.Rodriguez A, Calonge M, Pedroza-Seres M, Akova YA, Messmer EM, D'Amico DJ, Foster CS. Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol. 1996;114(5):593–599. doi: 10.1001/archopht.1996.01100130585016. [DOI] [PubMed] [Google Scholar]
- 2.Channa R, Ibrahim M, Sepah Y, Turkcuoglu P, Lee JH, Khwaja A, Hatef E, Bittencourt M, Heo J, Do DV, Nguyen QD. Characterization of macular lesions in punctate inner choroidopathy with spectral domain optical coherence tomography. J Ophthalmic Inflamm Infect. 2012;2(3):113–120. doi: 10.1007/s12348-011-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vance SK, Khan S, Klancnik JM, Freund KB. Characteristic spectral-domain optical coherence tomography findings of multifocal choroiditis. Retina. 2011;31(4):717–723. doi: 10.1097/IAE.0b013e318203c1ef. [DOI] [PubMed] [Google Scholar]
- 4.Keane PA, Allie M, Turner SJ, Southworth HS, Sadda SR, Murray PI, Denniston AK. Characterization of birdshot chorioretinopathy using extramacular enhanced depth optical coherence tomography. JAMA Ophthalmol. 2013;131(3):341–350. doi: 10.1001/jamaophthalmol.2013.1724. [DOI] [PubMed] [Google Scholar]
- 5.Howe LJ, Stanford MR, Graham EM, Marshall J. Choroidal abnormalities in birdshot chorioretinopathy: an indocyanine green angiography study. Eye (Lond) 1997;11(Pt 4):554–559. doi: 10.1038/eye.1997.142. [DOI] [PubMed] [Google Scholar]
- 6.Vadala M, Lodato G, Cillino S. Multifocal choroiditis: indocyanine green angiographic features. Ophthalmologica. 2001;215(1):16–21. doi: 10.1159/000050820. [DOI] [PubMed] [Google Scholar]
- 7.Howe LJ, Tufail A. ICG angiography and uveitis. Ocul Immunol Inflamm. 2004;12(1):1–5. doi: 10.1076/ocii.12.1.1.28064. [DOI] [PubMed] [Google Scholar]
- 8.Karampelas M, Sim DA, Keane PA, Zarranz-Ventura J, Patel PJ, Tufail A, Westcott M, Lee R, Pavesio CE. Graefes Arch Clin Exp Ophthalmol. 2013. Choroidal assessment in idiopathic panuveitis using optical coherence tomography. [DOI] [PubMed] [Google Scholar]
- 9.Bouchenaki N, Cimino L, Auer C, Tao Tran V, Herbort CP. Assessment and classification of choroidal vasculitis in posterior uveitis using indocyanine green angiography. Klin Monbl Augenheilkd. 2002;219(4):243–249. doi: 10.1055/s-2002-30661. [DOI] [PubMed] [Google Scholar]
- 10.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147(5):811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Quillen DA, Davis JB, Gottlieb JL, Blodi BA, Callanan DG, Chang TS, Equi RA. The white dot syndromes. Am J Ophthalmol. 2004;137(3):538–550. doi: 10.1016/j.ajo.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 12.Kedhar SR, Thorne JE, Wittenberg S, Dunn JP, Jabs DA. Multifocal choroiditis with panuveitis and punctate inner choroidopathy: comparison of clinical characteristics at presentation. Retina. 2007;27(9):1174–1179. doi: 10.1097/IAE.0b013e318068de72. [DOI] [PubMed] [Google Scholar]
- 13.Francis PJ, Marinescu A, Fitzke FW, Bird AC, Holder GE. Acute zonal occult outer retinopathy: towards a set of diagnostic criteria. Br J Ophthalmol. 2005;89(1):70–73. doi: 10.1136/bjo.2004.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31(5):377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Yang ZK, Dong FT. Choroidal thickness in normal subjects measured by enhanced depth imaging optical coherence tomography. Zhonghua Yan Ke Za Zhi. 2012;48(9):819–823. [PubMed] [Google Scholar]
- 17.Hirata M, Tsujikawa A, Matsumoto A, Hangai M, Ooto S, Yamashiro K, Akiba M, Yoshimura N. Macular choroidal thickness and volume in normal subjects measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):4971–4978. doi: 10.1167/iovs.11-7729. [DOI] [PubMed] [Google Scholar]
- 18.Ding X, Li J, Zeng J, Ma W, Liu R, Li T, Yu S, Tang S. Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci. 2011;52(13):9555–9560. doi: 10.1167/iovs.11-8076. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148(3):445–450. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Atmaca LS, Sonmez PA. Fluorescein and indocyanine green angiography findings in Behcet's disease. Br J Ophthalmol. 2003;87(12):1466–1468. doi: 10.1136/bjo.87.12.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoyagi R, Hayashi T, Masai A, Mitooka K, Gekka T, Kozaki K, Tsuneoka H. Subfoveal choroidal thickness in multiple evanescent white dot syndrome. Clin Exp Optom. 2012;95(2):212–217. doi: 10.1111/j.1444-0938.2011.00668.x. [DOI] [PubMed] [Google Scholar]
- 22.Fine HF, Spaide RF, Ryan EH, Jr, Matsumoto Y, Yannuzzi LA. Acute zonal occult outer retinopathy in patients with multiple evanescent white dot syndrome. Arch Ophthalmol. 2009;127(1):66–70. doi: 10.1001/archophthalmol.2008.530. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara T, Imamura Y, Giovinazzo VJ, Spaide RF. Fundus autofluorescence and optical coherence tomographic findings in acute zonal occult outer retinopathy. Retina. 2010;30(8):1206–1216. doi: 10.1097/IAE.0b013e3181e097f0. [DOI] [PubMed] [Google Scholar]
- 24.Wong IY, Koizumi H, Lai WW. Enhanced depth imaging optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2011;42(Suppl):S75–S84. doi: 10.3928/15428877-20110627-07. [DOI] [PubMed] [Google Scholar]


