Abstract
Consolidation of motor memories associated with skilled practice can occur both online, concurrent with practice, and offline, after practice has ended. The current study investigated the role of dorsal premotor cortex (PMd) in early offline motor memory consolidation of implicit sequence specific learning. Thirty-three participants were assigned to one of three groups of repetitive TMS over left PMd (5 Hz, 1 Hz or control) immediately following practice of a novel continuous tracking task. There was no additional practice following repetitive TMS. This procedure was repeated for 4 days. The continuous tracking task contained a repeated sequence that could be learned implicitly and random sequences that could not. On a separate fifth day, a retention test was performed to assess implicit sequence-specific motor learning of the task. Tracking error was decreased for the group who received 1 Hz repetitive TMS over the PMd during the early consolidation period immediately following practice compared to control or 5 Hz repetitive TMS. Enhanced sequence specific learning with 1 Hz repetitive TMS following practice was due to greater offline consolidation, not differences in online learning between the groups within practice days. A follow-up experiment revealed that stimulation of PMd following practice did not differentially change motor cortical excitability, suggesting that changes in offline consolidation can be largely attributed to stimulation induced changes in PMd. These findings support a differential role for the PMd in support of online and offline sequence specific learning of a visuomotor task and offer converging evidence for competing memory systems.
Keywords: Offline Motor Learning, Online Motor Learning, Implicit Motor Learning, Repetitive Transcranial Magnetic Stimulation, Dorsal Premotor Cortex
Introduction
Skilled motor practice facilitates the formation of an internal model of movement, which may then be used to anticipate task specific requirements at a later time (Shadmehr and Holcomb, 1997). Internal models are most susceptible to interference during and immediately following practice and become less susceptible to interference over time through persistent neural activity, a process called consolidation (Brashers-Krug et al., 1996; Robertson et al., 2004).
Motor memory consolidation can take place both explicitly, with conscious awareness, or implicitly, without conscious awareness of the skill being performed (Robertson et al., 2004). The neural processes of consolidation can take two forms: 1) online improvements that occur concurrent with practice or 2) offline improvements that develop following the termination of practice (Brashers-Krug et al., 1996; Robertson et al., 2004). Importantly, these two processes are not completely independent or mutually exclusive.
Given its known role in the selection of movements (Kalaska and Crammond, 1995; Rushworth et al., 2003) and implicit motor learning (Meehan et al., 2011; Ohbayashi et al., 2003), the dorsal premotor cortex (PMd) is a logical candidate for involvement in motor memory consolidation. Our group reported improved implicit sequence specific motor learning when 5 Hz repetitive TMS was delivered over the PMd prior to skilled motor practice of a continuous tracking task (Boyd and Linsdell, 2009). However, it is not clear whether improvements noted when PMd stimulation precedes motor practice result from only online or a combination of online and offline consolidation of sequence specific elements.
The current work sought to directly assess the involvement of PMd in offline consolidation of skilled motor practice. In contrast to our previous work (Boyd and Linsdell, 2009), three groups received either 1 Hz, 5 Hz or control repetitive TMS immediately following practice of a continuous visuomotor tracking task (Experiment 1). Based upon our previous work, it was hypothesized that 5 Hz repetitive TMS immediately following practice would enhance while 1 Hz repetitive TMS would suppress motor learning compared to control stimulation. However, the effects of TMS are known to be “state dependent” (Silvanto et al., 2008). State-dependence has been demonstrated during both perceptual and cognitive tasks where prior or concurrent neural activity (Arai et al., 2011; Silvanto et al., 2007b) and/or task specific elements (Bestmann et al., 2008; Cohen and Robertson, 2011) influence expected outcomes. An alternate hypothesis is that 1 Hz rTMS, typically associated with inhibition, over PMd immediately following practice may enhance implicit sequence specific motor learning through state-dependent mechanisms.
To confirm changes in offline consolidation could be attributed to PMd rather than altered primary motor cortex (M1) excitability, via PMd projections to M1 (Cavada and Goldman-Rakic, 1989; Hanakawa, 2011) motor evoked potentials were elicited before and after practice preceded by either 1 Hz, 5 Hz or control repetitive TMS (Experiment 2). We hypothesized that repetitive TMS over the PMd immediately following practice would not alter M1 excitability and that any change in offline consolidation noted in Experiment 1 could be attributed to the PMd.
Methods
Experiment 1
Participants
Thirty-three healthy, right-handed participants (20 males and 13 females, age range 20 to 48 years) were enrolled in the study (Table 1a). All participants provided informed consent; the University of British Columbia Clinical Research Ethics Board approved the protocol. Participants were excluded from the study if they showed any sign of neurological impairment or disease, or if they had any colour blindness that might impair response ability.
Table 1.
Participant characteristics for Experiments 1 and 2. Age is reported in years (± SD).
| Experiment 1 | Experiment 2 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 5 Hz | 1 Hz | Control | 5 Hz | 1 Hz | Control | |
| Age | 24±2.9 | 28±5.6 | 23±2.3 | 27±4.8 | 27±4.5 | 27±4.5 |
| Gender | 6M, 5F | 6M, 5F | 8M, 3F | 4M, 6F | 4M, 6F | 4M, 6F |
Experimental Design
The experiment took place over 5 testing sessions, on separate days, completed within two weeks. Prior to the start of the experiment participants were randomly assigned to one of three groups. The protocol was the same for each group, with the exception of the type of repetitive TMS that followed practice of the continuous tracking (CT) task. One group received 1 Hz repetitive TMS over the left PMd, the second group received 5 Hz repetitive TMS over the left PMd, while the third group received sham stimulation over the left PMd as a control condition.
Each group completed four CT practice sessions; practice was immediately followed by repetitive TMS according to group (days 1–4) (Figure 1). To evaluate motor learning, a retention test was conducted on a separate day (day 5). In each practice session participants performed 3 blocks (30 trials) of the CT task. Practice sessions were scheduled to accommodate the participant however no more than 48 hours elapsed between any of the sessions. On day 5, the retention test consisted of 1 block (10 trials) of continuous tracking without application of repetitive TMS. The retention test was used to disentangle performance effects from more permanent changes in behaviour associated with motor learning (Salmoni et al., 1984).
Figure 1.
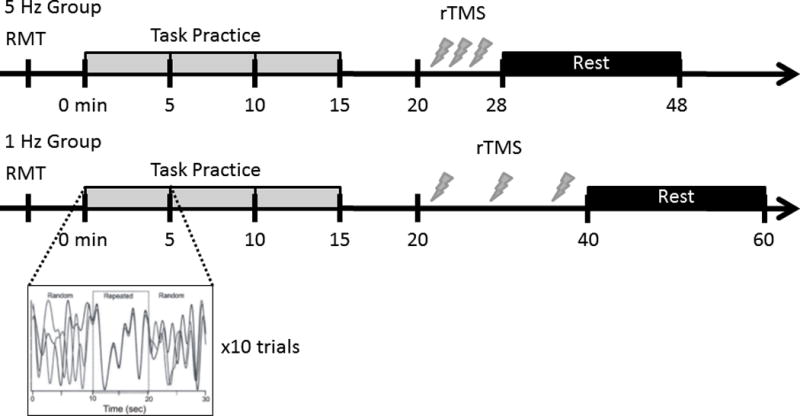
Experimental protocol for each group. Note that the Control group followed either the protocol for the 5 Hz or 1 Hz group. Protocol for the control group was randomized across participant. Inset represents one 30 second trial within a 5 minute block of task practice. This 30 second trial was repeated 10 times within a 5 minute block.
Continuous Tracking (CT) Task
The CT task used in the current study was similar to that previously reported (Boyd and Linsdell, 2009). During the CT task participants were seated in front of a computer monitor. Holding a joystick in their right hand, participants tracked a target as it moved in a sine-cosine waveform. The target appeared as an open white circle and participant movements were shown as a red dot (Boyd and Linsdell, 2009). Joystick position sampling and all stimuli were presented at 40 Hz using custom software developed on the LabView platform (v. 8.6; National Instruments Co.).
The pattern of the target movement was predefined according to a method modified from Wulf and Schmidt (1997). A unique 32-second trial was constructed from one 2-second baseline and three 10-second sine-cosine segments. One block consisted of ten 32-second trials. The middle third of each tracking pattern was repeated and identical across practice and retention (Boyd and Linsdell, 2009). The pattern was unknown to the participants and was constructed from the polynomial equation described by Wulf and Schmidt (1997) with the following general form:
The middle (repeated) segment was constructed by using the same coefficients for every trial (b0=2.0, a1=−4.0, b1=3.0, b2=−3.6, a3=3.9, b3=4.5, a4=0.0, b4=1.0, a5=−3.8, b5=−0.5, a6=1.0 and b6=2.5). The first and third segments of the tracking pattern were generated randomly using coefficients ranging from −5.0 to 5.0. A different random sequence was used for both the first and third segments of every trial. There were ten separate reversals in the direction in each third of the tracking pattern. The random and repeated patterns were equated for difficulty by ensuring that the overall excursion of each random sequence fell within a range of that required by the repeated sequence. Neither the trajectories of the target or the participants’ movements left a visible train on the screen and thus, participants could not visualize the entire pattern. The same sets of trials were practiced by all of the participants to ensure uniformity so that the random segments were the same for each participant.
Participants were not informed of the repeating sequence but were instructed daily to track the target as accurately as possible by controlling the position of the cursor with the joystick.
Transcranial Magnetic Stimulation (TMS)
TMS was delivered with a Magstim Super Rapid2 stimulator using a 70 mm figure-8 air-cooled coil (Magstim Company, Ltd., Wales, UK). Participants were seated in a semi-reclined dental chair with their arms bent and supported by armrests. The TMS coil was oriented tangentially to the scalp with the handle at 45 degrees to the midline in a posterior lateral orientation. Prior to the experiment, high-resolution anatomical magnetic resonance images (MRI) were acquired for each participant (TR=12.4ms, TE=5.4ms, flip angle θ=35°, FOV=256mm, 170 slices, 1mm thickness) at the UBC MRI Research Centre on a Philips Achieva 3.0 T whole body MRI scanner (Phillips Healthcare, Andover, MD) using a sensitivity encoding head coil (SENSE). These images were then imported into the BrainSight™ TMS neuronavigation software (BrainSight 2.0, Rogue Research Inc., Montreal, QC) to allow for stereotaxic registration of the TMS coil with the participants’ anatomy for online control of coil positioning during each session and across days.
Surface electromyography (EMG) over the participants right flexor carpi radialis (FCR) was monitored using the evoked potential unit of the Super Rapid2 control unit (Magstim Super Rapid2, Magstim Company, Ltd) (Boyd and Linsdell, 2009). Initially, the FCR representation was marked on the participants anatomical MRI as the medial edge of the left “hand knob”. This point acted as a starting point for determination of the motor “hot spot” for FCR. Motor evoked potentials (MEP) were then used to determine the coil position that evoked the maximal response in the right FCR. The location and trajectory of the coil over left primary motor cortex (M1) were marked using the BrainSight™ stereotaxic software to minimize variability within and across days. Resting motor threshold (RMT) was determined for each participant as the percentage of stimulator output that elicited an MEP of ≥ 50 μV peak to peak on 5 out of 10 trials.
The site of stimulation for the left PMd was marked in Brainsight™ by moving one gyrus forward from the FCR “hot spot” (Boyd and Linsdell, 2009). The PMd location was confirmed as the posterior aspect of the middle frontal gyrus (Fridman et al., 2004; Munchau et al., 2002). Isolation of this area from M1 was verified using single-pulses to ensure that: 1) there was no EMG record of muscle activity recorded over the FCR, and 2) there were no visually apparent muscle twitches in the forearm or hand. Once confirmed, the location and trajectory of the coil were recorded using BrainSight™ to ensure the consistent stimulation of the PMd across days (Boyd and Linsdell, 2009).
5 Hz stimulation consisted of 1200 pulses delivered in 10-second trains with an inter-train interval of 10s. Intensity was set to 110% of RMT. 1 Hz stimulation consisted of 1200 pulses delivered in 10 seconds trains with an inter-train interval of 1s and an intensity of 110% RMT. Control stimulation was delivered using a custom sham coil that looks and sounds similar to the repetitive TMS coil but does not induce any current in the underlying cortex (Magstim Company Ltd.). The parameters of the control stimulation were counterbalanced across participants such that six participants received control stimulation that mimicked 5 Hz stimulation and five participants received control stimulation that mimicked 1 Hz stimulation.
The repetitive TMS parameters employed have been shown to induce an after-effect of approximately 15-minutes (Chen et al., 2003). To ensure that there was no interference with the effects of the repetitive TMS protocols upon consolidation of motor practice, participants were required to remain quietly seated for 15 minutes following the end of stimulation.
Explicit Awareness of the Repeated Sequence
Following the retention test on day 5, participants were shown 10, 30-second trials of continuous target movements and asked to decide if they recognized any pattern that they performed during the practice sessions. Out of the 10 trials, 3 contained the “true” middle sequence i.e., the same as the repeated practice sequence and 7 were foils. Individuals were considered to have explicit awareness of the repeated sequence if they could both correctly recognize 2 of the 3 repeated sequences and properly label 5 of 7 of the foils as having not been seen before (Boyd and Linsdell, 2009).
Behavioural Outcome Measures
Motor performance was evaluated across practice and retention in two ways. Our primary analysis consisted of the overall root mean square error (RMSE). Overall RMSE reflects the average of the difference waveform derived by subtracting the instantaneous position of the target from the participant’s location. This score was calculated separately for random and repeated sequences and averaged for all trials within a block (Boyd and Winstein, 2004b; Vidoni and Boyd, 2009; Wulf and Schmidt, 1997). The difference between overall RMSE during random and repeated sequence tracking reflects implicit learning and was used to evaluate reductions in tracking errors across practice and at retention. Random tracking performance was assessed using the second random sequence (Boyd and Winstein, 2004b; Boyd and Linsdell, 2009).
As overall RMSE reflects both spatial accuracy and temporal lag, improvement on each of these components of movement was also assessed (Boyd and Winstein, 2004a). Time lag of tracking is the time (in milliseconds) corresponding to the maximal cross-correlation co-efficient and represents the temporal distance from the target. Spatial error is the residual RMSE score that remains following adjustment of the participant’s cursor position to account for the time lag of tracking. Time lag scores in larger negative numbers indicate greater time lag of tracking, while a zero value represents no tracking time lag between participant and target. Lower RMSE scores indicate less overall error and show improved motor performance.
Statistical Analysis
Statistical analyses were performed in three steps. First, improvement in performance during the acquisition phase (days 1–4) was assessed for overall RMSE, spatial error and time lag using separate 3 (Group: 1 Hz, 5 Hz, Control repetitive TMS) × 12 (Block: 1–12) mixed measures ANOVAs for the random and repeated sequences. Group was treated as a between subjects factor and Block was treated as a repeated measures factor. In all cases the dependent variables (overall RMSE, spatial error and time lag) were log transformed as Maulchy’s sphericity test revealed that raw scores across blocks violated of the sphericity assumption for each dependent variable and both sequences.
Second, implicit sequence specific learning at retention was examined for overall RMSE, spatial error and time lag using three separate 3 (Group: 1 Hz, 5 Hz, Control repetitive TMS) × 2 (Sequence: Random, Repeated) mixed measures ANOVAs. Group was treated as a between subjects factor and Sequence was treated as a repeated measures factor. As implicit sequence specific learning is defined as lower error/less lag during repeated compared to random sequence tracking, significant Group × Sequence interactions were investigated using contrasts comparing repeated versus random sequence tracking performance within each group to determine if implicit sequence specific learning was evident in each group. Bonferroni correction was applied with the corrected threshold of p = 0.033 to correct for multiple comparisons.
Finally, we assessed the impact of the rTMS post-practice upon the retention of implicit learning from day to day. Offline sequence-specific motor learning was defined as the change in sequence specific learning (random performance – repeated performance) from the previous day to the first block of the subsequent day (Robertson et al., 2004; Robertson and Cohen, 2006). Separate 3 (Group: 1 Hz, 5 Hz, Control rTMS) × 4 (Consolidation Period: Day 1, Day 2, Day 3 and Day 4) mixed measures ANOVAs were run to assess offline sequence specific motor learning for RMSE, spatial error and time lag. Group was treated as a between subjects factor and Consolidation Period was treated as a repeated measures factor.
To ensure that differences in offline learning could not be attributed to differences across the groups in online consolidation we also ran three separate 3 (Group: 1 Hz, 5 Hz, Control rTMS) × 4 (Day: Day 1, Day 2, Day 3 and Day 4) mixed measures ANOVAs to assess difference in online sequence specific learning for RMSE, spatial error and time lag. Group was treated as a between subjects factor and Consolidation Period was treated as a repeated measure. Online sequence specific learning was defined as the change in sequence-specific performance from Block 1 to Block 3 within each day. Statistical analyses were performed in SPSS v.20. For all analyses Group was treated as a between-subjects factor. All other variables were treated as a repeated measures factor. Greenhouse-Geisser epsilon corrections and Bonferonni corrections were applied where appropriate.
Experiment 2
The aim of experiment 2 was to determine whether motor practice followed by stimulation over the left PMd had an effect of the excitability of M1.
Participants
Thirty healthy, right-handed participants (12 males and 18 females, age range 20 to 33 years) were enrolled in the study (Table 1b). All participants provided informed consent; the University of British Columbia Clinical Research Ethics Board approved the protocol. Participants were excluded from the study if they showed any sign of neurological impairment or disease, or if they had any colour blindness that might impair response ability.
Procedure
The experiment consisted of a single session. Prior to the start of the experiment participants were randomly assigned to one of three groups. For each group, RMT and M1 excitability (indexed by the amplitude of MEPs) were assessed before and after each participant completed three blocks of continuous tracking practice paired with repetitive TMS. The testing protocol was the same for each group; only the type of repetitive TMS that followed task practice differed. As in Experiment 1, one group received 1 Hz repetitive TMS over the left PMd, the second received 5 Hz repetitive TMS over the left PMd, while the third group received sham stimulation over the left PMd.
Continuous Tracking
The CT task was the same as that described for Experiment 1. Only one practice session containing three blocks of CT task practice was completed.
Transcranial Magnetic Stimulation
The procedures for delivering repetitive TMS were the same as those outlined in Experiment 1. However, to gauge the effect of repetitive TMS over the PMd on M1 two additional measures were collected: 1) RMT was established before and after CT task practice plus repetitive TMS, and 2) MEPs were elicited by 10 single pulses at 110% of RMT before and after practice plus repetitive TMS.
Analyses
The effect of stimulation over the PMd on RMT and MEP amplitude was assessed using separate 3 (Group: 1 Hz, 5 Hz, Control repetitive TMS) × 2 (Time: Pre, Post) mixed measures ANOVAs. Group was treated as a between subjects factor. Time was treated as a repeated measures factor. Linear contrasts corrected for multiple comparisons using the Bonferonni correction were applied where appropriate.
Results
Experiment 1
Practice
The Group by Block mixed measures ANOVA considering practice performance with RMSE as the dependent measure revealed a main effect of Block for both the random [F(11, 330) = 19.66, p<0.001] and repeated [F(11, 330) = 14.70, p<0.001] sequences. The main effect of Block can be attributed to a decrease in RMSE across blocks with practice for both repeated and random sequences (Figure 2A & B). Group by Block mixed measures ANOVAs upon spatial error and lag revealed that the improvement in RMSE across practice block can be attributed to both reduced spatial error [Random: F(11, 330) = 13.33, p<0.001 ; Repeated: F(11, 330) = 9.41, p<0.001] (Figure 2C & D) and time lag [Random: F(11, 330) = 19.66, p<0.001; Repeated: F(11, 330) = 12.17, p<0.001] (Figure 2E & F).
Figure 2.
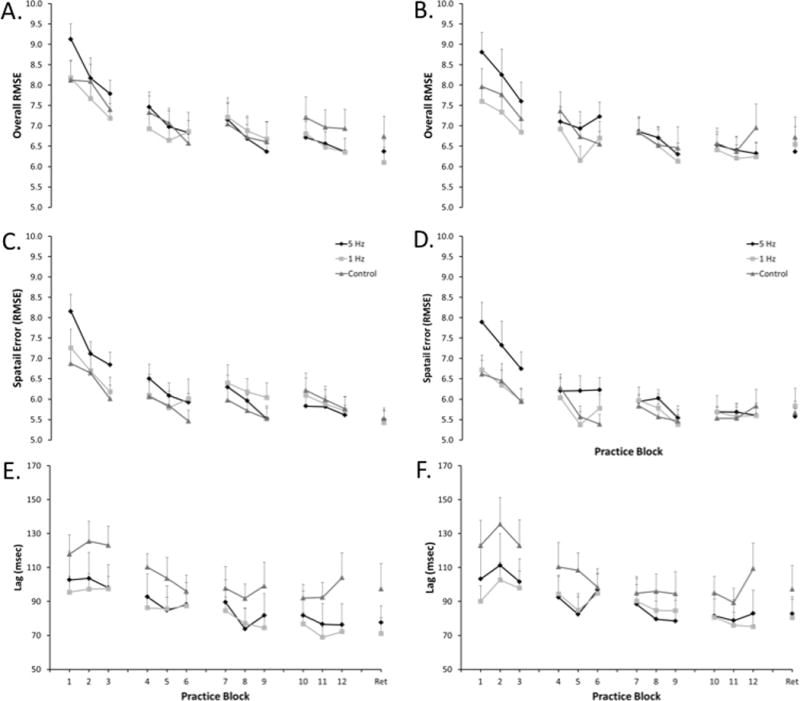
Tracking performance across the 4 days of practice (3 blocks per day) and retention testing (1 block labeled “Ret”) for A) Overall tracking error for the repeated sequence, B) Overall tracking error for random sequences, C) Spatial tracking error for the repeated sequence, D) Spatial tracking error for random sequences, E) Time lag of tracking for the repeated sequence, and F) Time lag of tracking for the random sequences. For overall and spatial tracking error lower scores indicate improved performance. For time lag of tracking scores closer to zero represent improved performance. Data are mean; error bars are SEM.
Implicit Sequence Specific Motor Learning
The mixed measures Group by Sequence ANOVA on Overall RMSE at retention (Day 5) revealed a significant interaction [F(2,30) = 3.81; p=0.033], as well as a trend for a main effect of Sequence [F(2,30) = 3.27, p=0.081]. Inspection of the data (Figure 3A) shows that the interaction can be attributed to lower Overall RMSE (i.e. improved performance) during repeated compared to random sequence tracking at retention in individuals who received 1 Hz repetitive TMS during the consolidation period immediately following practice (contrast, p = 0.007). Reduced error during repeated compared to during random sequence tracking is indicative of implicit sequence specific learning in this group. In contrast, overall RMSE during repeated compared to random sequence tracking at the retention test was not different for the groups that received 5 Hz repetitive TMS or control stimulation (p = 0.96, p = 0.89).
Figure 3.
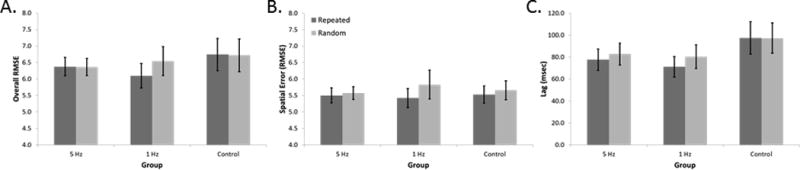
Change in RMSE for random and repeated sequences from Early Practice to Retention for each group for A) Overall tracking error and B) Spatial tracking error. Sequence specific learning is reflected by greater change in repeated sequence tracking performance from Early Practice to Retention relative to random sequence tracking performance. Data are mean; error bars are SEM.
The corresponding Group by Sequence ANOVA using spatial RMSE as the dependent measure revealed a main effect of Sequence [F(1,30) = 3.84, p=0.06]. Post-hoc t-tests comparing repeated versus random sequence spatial RMSE suggest that the trend for a main effect can be attributed to reduced spatial RMSE during repeated compared to random sequence tracking at Retention (p = 0.014; Figure 3B) in the 1 Hz group. There were no differences in spatial RMSE for individuals who received 5 Hz repetitive TMS or control stimulation.
The Group by Sequence ANOVA for time lag of tracking failed to reveal any significant effects.
Off-Line Learning
Mixed measures Group by Sequence ANOVA on change in RMSE reflecting implicit motor learning from the previous day to the first block of the subsequent day revealed main effects of Group [F(2,30) = 6.60, p = 0.004] and Consolidation Period [F(3, 90) = 4.23, p = 0.017]. Scheffe’s post-hoc tests revealed that the main effect of Group can be attributed to significantly greater sequence-specific offline learning in the 1 Hz group compared to the Control and 5 Hz rTMS groups (p = 0.030 and p = 0.003, respectively) (Figure 4A – dark grey bars). The main effect of Sequence can be attributed to greater consolidation of implicit motor learning from Day 4 to the retention test compared to consolidation between Day 2 to Day 3 and Day 3 to Day 4 (p < 0.001 and p = 0.024, respectively) (Figure 4B – dark grey bars). The Group by Sequence ANOVA on spatial error revealed main effects of Group [F(2,30) = 5.10, p < 0.012] and Consolidation Period [F(3,90) = 4.09, p < 0.014]. The main effects of Group (Figure 4A – light grey bars) and Consolidation Period (Figure 4B – light grey bars) reveal that the changes in RMSE can be attributed to consolidation of spatial accuracy.
Figure 4.
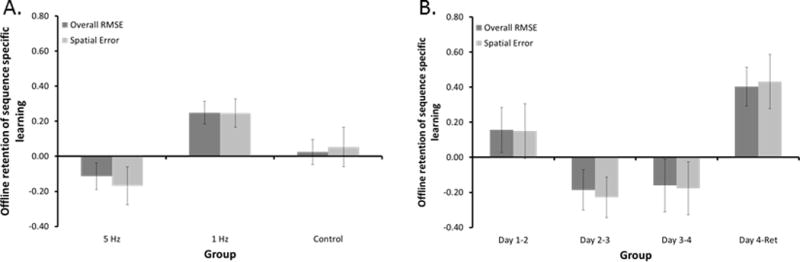
Main effects for offline learning sequence specific learning. A) Main effect of Group – Mean change in offline retention of sequence specific learning for each group, collapsed across retention period. Means are shown for both overall RMSE (dark grey) and spatial error (light grey). B) Main effect of Day – Mean change in offline retention of sequence specific learning for each retention period, collapsed across group. Means are shown for overall RMSE (dark grey) and spatial error (light grey). “Ret” = Retention Day. Bars represent SEM.
The mixed measures Group by Sequence ANOVA with time lag as the dependent measure failed to reveal any effects.
Online Learning
None of the analyses on RMSE, spatial accuracy or lag revealed any effects associated with change in implicit performance from Block 1 to Block 3 on each day of practice. Online learning within each practice day was consistent for all groups.
Explicit Awareness of the Repeating Sequence
Three of the 11 individuals in the 5 Hz repetitive TMS group acquired sufficient explicit awareness of the repeating sequence to be able to recognize it at the recognition test. This was also the case for 2 individuals in the 1 Hz repetitive TMS group and 1 individual in the Control group.
Experiment 2
The mixed measures Group by Time ANOVAs performed upon RMT and MEP amplitude failed to reveal any significant effects of the varied forms of repetitive TMS following continuous tracking on excitability in M1.
Discussion
The present study is the first to demonstrate the cumulative impact of repetitive TMS over PMd immediately following practice upon consolidation of implicit sequence-specific motor learning. While all three experimental groups (1 Hz repetitive TMS, 5 Hz repetitive TMS and sham stimulation) demonstrated improvement in performance over time only the group receiving 1 Hz repetitive TMS over the PMd immediately following task practice enhanced offline learning of an implicit motor skill (Experiment 1). Enhanced implicit sequence specific learning with 1 Hz repetitive TMS following practice was largely explained by improved spatial rather than temporal accuracy of movements (Experiment 1). Further, enhanced motor learning associated with 1 Hz repetitive TMS over the PMd during early consolidation does not appear to be attributable to spread of stimulation to M1 or to PMd to M1 connections, as M1 excitability was not changed by repetitive TMS over PMd (Experiment 2).
The enhancement of motor learning following application of 1 Hz repetitive TMS over PMd immediately after practice of the continuous visuomotor tracking task differs from our previous results (Boyd and Linsdell, 2009). In our past work 5 Hz repetitive TMS delivered prior to practice of the same visuomotor task as was employed here enhanced implicit sequence specific motor learning across multiple days of practice. The difference between our current and our previous studies suggests state-dependency in the form of a task-dependent role for PMd during online performance and offline consolidation of implicit sequence specific learning of a visuomotor task.
Given its anatomical location and functional connectivity, the PMd is a likely convergence point for cognition and motor control. PMd is generally associated with explicit declarative aspects of motor learning. While the PMd has been implicated in facilitating the transition between implicitly learned movements that constitute a sequence (Mushiake et al., 1991), activity in the PMd is reduced when explicit awareness of the implicit motor sequences is gained (Hazeltine et al., 1997; Honda et al., 1998). During online learning it is likely that the PMd serves to enhance implicit sequence specific learning by linking specific movements which are dependent upon sensory cues (Nowak et al., 2009; Taubert et al., 2010). This role may be particularly important during interleaved practice and may explain why anodal transcranial direct current stimulation over the PMd during constant repetitive practice does not result in improved consolidation of performance gains (Kantak et al., 2012; Nitsche et al., 2003). In contrast, early offline consolidation of information relating to sequencing of action selection may interfere with early consolidation of more procedural elements relating to individual movements, which are most likely represented in M1 (Muellbacher et al., 2002; Wilkinson et al., 2010). This may result from early offline consolidation of information being more reliant on a declarative memory and thus more explicit. This assumption is consistent with observations of differential rates of consolidation for explicit declarative memories relative to procedural memory (Brown and Robertson, 2007a; Galea et al., 2010; Ghilardi et al., 2009) and competition between procedural and declarative memory systems (Brown and Robertson, 2007a; Brown and Robertson, 2007b; Galea et al., 2010). Interference may occur even when explicit instruction is not given and participants have not autogenously acquired declarative knowledge of a sequence through practice (Vidoni and Boyd, 2007). Therefore, reducing the cortical excitability of PMd through 1 Hz repetitive TMS during early offline consolidation may relieve suppression of procedural representations in M1 during this critical period and facilitate an early boost in procedural learning not seen at later stages of offline consolidation (Hotermans et al., 2008).
Another interesting result was the lack of dissociation between implicit motor sequence learning for the 5 Hz and sham stimulation groups. Relative to the sham control group, one might expect the 5 Hz group to show the opposite effect to that induced by 1 Hz repetitive TMS. The fact that 5 Hz repetitive TMS immediately following practice had no impact upon offline consolidation of implicit sequence specific learning suggests that increasing the cortical excitability of the PMd during early off-line learning was not sufficient to enhance declarative consolidation beyond that already occurring during the period of early offline consolidation probed here (~5–30 minutes post-practice). The lack of sequence specific learning despite the same amount of practice as the 1 Hz group suggests a state-dependent element where current activity in PMd, the activity producing the interference effect, is not enhanced by stimulating PMd. The net result is that offline consolidation and implicit sequence specific motor learning similar to that seen in the control group in the absence of stimulation, where any learning is associated with gains in sensorimotor efficiency rather than sequence specific elements. This further supports a competitive model of declarative/procedural consolidation where competition is biased towards the developing declarative memories.
Interestingly, the enhancement associated with cumulative 1 Hz repetitive TMS over the PMd appeared to reflect retained improvement in spatial accuracy rather than a reduction in response lag. While these two variables are not completely independent of each other our results suggest that consolidation of spatial aspects of a motor sequence may be mediated by PMd and M1 networks but that procedural elements of these representations are stored in M1(Muellbacher et al., 2002). The relative insensitivity of temporal aspects to 1 Hz repetitive TMS during early offline consolidation highlights the importance of other cortical areas for implicit sequence specific learning, such as the supplementary motor area (SMA) (Mushiake et al., 1991) and cerebellum (Boyd and Winstein, 2004a). In particular, the changes in spatial tracking error may relate to the role of the PMd in preparing aspects of spatial working memory during externally guided movements (Mushiake et al., 1991).
Traditionally, 1 Hz repetitive TMS has been associated with inhibitory effects that persist beyond cessation of stimulation (Chen et al., 2003; Vidoni et al., 2010; Wassermann et al., 1996). Our interpretation of our results is based upon this assumption however, an alternate explanation may be that enhanced implicit sequence specific learning observed following 1 Hz repetitive TMS post-practice is linked to state dependent effects present during application of the 1 Hz repetitive TMS. Silvanto et al. (2007a; 2007b; Silvanto and Pascual-Leone, 2008) demonstrated similar state dependent effects in the visual cortex using adaptation paradigms. Therefore, it cannot be ruled out that resonant activity within the PMd, tied to online learning that persisted into the early period of offline consolidation may have caused 1 Hz repetitive TMS to enhance the PMd contributions to early offline consolidation. Under this scenario, 1 Hz repetitive TMS may serve to reduce neural noise of weak non-sequence related neural activity within the PMd to a greater extent than the elevated residual sequence related neural activity tied to online practice. The net result would be akin to the phenomena of surround inhibition reported in the motor cortex that enhances motor ability (Beck and Hallett, 2010; Hallett, 2004), the visual cortex that enhances visual perception (Angelucci et al., 2002) and the somatosensory cortex that enhances tactile acuity(Drevets et al., 1995). State dependency would also explain the lack of effect elicited by 5 Hz repetitive TMS where both the sequence related and non-sequence related neural activity would be facilitated. However, given the already elevated excitability in the neurons involved with the repeated sequence representation, the effects of the repetitive TMS would be more pronounced in the less active neural pathways representing the random sequence compared to the already excited neural pathways representing the repeated sequence (Bienenstock et al., 1982; Kuo et al., 2008). The net result would be a reduction in the difference between the signal (repeated sequence neural activity) and the noise (random sequence neural activity). One limitation to the current work is that we are unable to directly assess changes in cortical excitability of the PMd itself. Future work is needed to determine whether repetitive TMS following practice of interleaved random and repeated sequences can elicit state dependency during the period of early offline consolidation.
Conclusions
Our data highlights the potential differential roles for the PMd in implicit motor learning and early offline motor memory consolidation of a novel motor task. The results confirm past work demonstrating that with practice participants can implicitly learn a repeated sequence (Brashers-Krug et al., 1996; Meehan et al., 2011; Shadmehr and Holcomb, 1997) and that sequence specific learning can be altered via repetitive TMS (Boyd and Linsdell, 2009). Importantly, we found that 1 Hz repetitive TMS over the PMd during early consolidation improved sequence-specific implicit motor learning likely by reducing competition between consolidation of motor parameters and action selection following interleaved practice. Applying repetitive TMS during early consolidation may be an adjunctive mechanism to enhance gains associated with practice through consolidation of specific elements of motor memory.
Table 2.
Mean change score (pre- to post-stimulation) for peak-to-peak MEP amplitude and resting motor threshold across groups (Experiment 2). Mean ± Standard Error of the Mean
| 5 Hz | 1 Hz | Control | |
|---|---|---|---|
|
|
|||
| MEP Amplitude (μV) | 79 ± 46 | 27 ± 28 | 61 ± 106 |
| RMT (% Stimulator Output) | −0.3 ± 0.5 | −0.6 ± 0.4 | 0.1 ± 0.2 |
Acknowledgments
Support was provided to SKM by the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research and to LAB by the Canada Research Chairs and the Michael Smith Foundation for Health Research. This work was also supported by awards from the Natural Sciences and Engineering Research Council of Canada (Award #401890) and the Vancouver Coastal Health Research Institute to LAB.
References
- Angelucci A, Levitt JB, Lund JS. Anatomical origins of the classical receptive field and modulatory surround field of single neurons in macaque visual cortical area V1. Prog Brain Res. 2002;136:373–388. doi: 10.1016/s0079-6123(02)36031-x. [DOI] [PubMed] [Google Scholar]
- Arai N, Muller-Dahlhaus F, Murakami T, Bliem B, Lu MK, Ugawa Y, Ziemann U. State-dependent and timing-dependent bidirectional associative plasticity in the human SMA-M1 network. J Neurosci. 2011;31:15376–15383. doi: 10.1523/JNEUROSCI.2271-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Hallett M. Surround inhibition is modulated by task difficulty. Clin Neurophysiol. 2010;121:98–103. doi: 10.1016/j.clinph.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Haggard P, Weiskopf N, Josephs O, Driver J, Rothwell JC, Ward NS. Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cereb Cortex. 2008;18:1281–1291. doi: 10.1093/cercor/bhm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Linsdell MA. Excitatory repetitive transcranial magnetic stimulation to left dorsal premotor cortex enhances motor consolidation of new skills. BMC Neurosci. 2009;10:72. doi: 10.1186/1471-2202-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ. Cerebellar stroke impairs temporal but not spatial accuracy during implicit motor learning. Neurorehabil Neural Repair. 2004a;18:134–143. doi: 10.1177/0888439004269072. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ. Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learn Mem. 2004b;11:388–396. doi: 10.1101/lm.80104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Off-line processing: Reciprocal interactions between declarative and procedural memories. J Neurosci. 2007a;27:10468–10475. doi: 10.1523/JNEUROSCI.2799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Inducing motor skill improvements with a declarative task. Nat Neurosci. 2007b;10:148–149. doi: 10.1038/nn1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Chen WH, Mima T, Siebner HR, Oga T, Hara H, Satow T, Begum T, Nagamine T, Shibasaki H. Low-frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clin Neurophysiol. 2003;114:1628–1637. doi: 10.1016/s1388-2457(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Cohen DA, Robertson EM. Preventing interference between different memory tasks. Nat Neurosci. 2011;14:953–955. doi: 10.1038/nn.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Burton H, Videen TO, Snyder AZ, Simpson JR, Jr, Raichle ME. Blood flow changes in human somatosensory cortex during anticipated stimulation. Nature. 1995;373:249–252. doi: 10.1038/373249a0. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Galea JM, Albert NB, Ditye T, Miall RC. Disruption of the dorsolateral prefrontal cortex facilitates the consolidation of procedural skills. J Cogn Neurosci. 2010;22:1158–1164. doi: 10.1162/jocn.2009.21259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi MF, Moisello C, Silvestri G, Ghez C, Krakauer JW. Learning of a sequential motor skill comprises explicit and implicit components that consolidate differently. J Neurophysiol. 2009;101:2218–2229. doi: 10.1152/jn.01138.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Dystonia: Abnormal movements result from loss of inhibition. Adv Neurol. 2004;94:1–9. [PubMed] [Google Scholar]
- Hanakawa T. Rostral premotor cortex as a gateway between motor and cognitive networks. Neurosci Res. 2011;70:144–154. doi: 10.1016/j.neures.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry R. Attention and stimulus characteristics determine the locus of motor-sequence encoding. A PET study Brain. 1997;120(Pt 1):123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Honda M, Deiber MP, Ibanez V, Pascual-Leone A, Zhuang P, Hallett M. Dynamic cortical involvement in implicit and explicit motor sequence learning. A PET study Brain. 1998;121(Pt 11):2159–2173. doi: 10.1093/brain/121.11.2159. [DOI] [PubMed] [Google Scholar]
- Hotermans C, Peigneux P, de Noordhout AM, Moonen G, Maquet P. Repetitive transcranial magnetic stimulation over the primary motor cortex disrupts early boost but not delayed gains in performance in motor sequence learning. Eur J Neurosci. 2008;28:1216–1221. doi: 10.1111/j.1460-9568.2008.06421.x. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ. Deciding not to GO: Neuronal correlates of response selection in a GO/NOGO task in primate premotor and parietal cortex. Cereb Cortex. 1995;5:410–428. doi: 10.1093/cercor/5.5.410. [DOI] [PubMed] [Google Scholar]
- Kantak SS, Mummidisetty CK, Stinear JW. Primary motor and premotor cortex in implicit sequence learning – evidence for competition between implicit and explicit human motor memory systems. Eur J Neurosci. 2012;36:2710–2715. doi: 10.1111/j.1460-9568.2012.08175.x. [DOI] [PubMed] [Google Scholar]
- Kuo MF, Unger M, Liebetanz D, Lang N, Tergau F, Paulus W, Nitsche MA. Limited impact of homeostatic plasticity on motor learning in humans. Neuropsychologia. 2008;46:2122–2128. doi: 10.1016/j.neuropsychologia.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Meehan SK, Randhawa B, Wessel B, Boyd LA. Implicit sequence-specific motor learning after subcortical stroke is associated with increased prefrontal brain activations: An fMRI study. Hum Brain Mapp. 2011;32:290–303. doi: 10.1002/hbm.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Munchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci. 2002;22:554–561. doi: 10.1523/JNEUROSCI.22-02-00554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Berner J, Herrnberger B, Kammer T, Gron G, Schonfeldt-Lecuona C. Continuous theta-burst stimulation over the dorsal premotor cortex interferes with associative learning during object lifting. Cortex. 2009;45:473–482. doi: 10.1016/j.cortex.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Ohbayashi M, Ohki K, Miyashita Y. Conversion of working memory to motor sequence in the monkey premotor cortex. Science. 2003;301:233–236. doi: 10.1126/science.1084884. [DOI] [PubMed] [Google Scholar]
- Robertson EM. The serial reaction time task: Implicit motor skill learning? J Neurosci. 2007;27:10073–10075. doi: 10.1523/JNEUROSCI.2747-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Cohen DA. Understanding consolidation through the architecture of memories. Neuroscientist. 2006;12:261–271. doi: 10.1177/1073858406287935. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Miall RC. Current concepts in procedural consolidation. Nat Rev Neurosci. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: Motor attention and selection. Neuroimage. 2003;20(Suppl 1):S89–100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Salmoni AW, Schmidt RA, Walter CB. Knowledge of results and motor learning: A review and critical reappraisal. Psychol Bull. 1984;95:355–386. [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277:821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci. 2008;12:447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG, Cowey A, Walsh V. Neural activation state determines behavioral susceptibility to modified theta burst transcranial magnetic stimulation. Eur J Neurosci. 2007a;26:523–528. doi: 10.1111/j.1460-9568.2007.05682.x. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG, Cowey A, Walsh V. Neural adaptation reveals state-dependent effects of transcranial magnetic stimulation. Eur J Neurosci. 2007b;25:1874–1881. doi: 10.1111/j.1460-9568.2007.05440.x. [DOI] [PubMed] [Google Scholar]
- Taubert M, Dafotakis M, Sparing R, Eickhoff S, Leuchte S, Fink GR, Nowak DA. Inhibition of the anterior intraparietal area and the dorsal premotor cortex interfere with arbitrary visuo-motor mapping. Clin Neurophysiol. 2010;121:408–413. doi: 10.1016/j.clinph.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Acerra NE, Dao E, Meehan SK, Boyd LA. Role of the primary somatosensory cortex in motor learning: An rTMS study. Neurobiol Learn Mem. 2010;93:532–539. doi: 10.1016/j.nlm.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Boyd LA. Preserved motor learning after stroke is related to the degree of proprioceptive deficit. Behav Brain Funct. 2009;5:36. doi: 10.1186/1744-9081-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, Boyd LA. Achieving enlightenment: What do we know about the implicit learning system and its interaction with explicit knowledge? J Neurol Phys Ther. 2007;31:145–154. doi: 10.1097/NPT.0b013e31814b148e. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Wang B, Zeffiro TA, Sadato N, Pascual-Leone A, Toro C, Hallett M. Locating the motor cortex on the MRI with transcranial magnetic stimulation and PET. Neuroimage. 1996;3:1–9. doi: 10.1006/nimg.1996.0001. [DOI] [PubMed] [Google Scholar]
- Wilkinson L, Teo JT, Obeso I, Rothwell JC, Jahanshahi M. The contribution of primary motor cortex is essential for probabilistic implicit sequence learning: Evidence from theta burst magnetic stimulation. J Cogn Neurosci. 2010;22:427–436. doi: 10.1162/jocn.2009.21208. [DOI] [PubMed] [Google Scholar]
- Wulf G, Schmidt RA. Variability of practice and implicit motor learning. Journal of Experimental Psychology-Learning Memory and Cognition. 1997;23:987–1006. [Google Scholar]


