Abstract
Constitutive activation of the KRAS oncogene in human malignancies is associated with aggressive tumor growth and poor prognosis. Similar to other oncogenes, KRAS acts in a cell-intrinsic manner to affect tumor growth or survival. However, we describe here a different, cell-extrinsic, mechanism through which mutant KRAS contributes to tumor development. Tumor cells carrying mutated KRAS induced highly suppressive T cells, and silencing KRAS reversed this effect. Overexpression of the mutant KRASG12V gene in wild-type KRAS tumor cells led to Treg induction. We also demonstrate that mutant KRAS induces the secretion of interleukin-10 and transforming growth factor-β1 (both required for Treg induction) by tumor cells through the activation of the MEK-ERK-AP1 pathway. Finally, we report that inhibition of KRAS reduces the infiltration of Tregs in KRAS-driven lung tumorigenesis even before tumor formation. This cell-extrinsic mechanism allows tumor cells harboring a mutant KRAS oncogene to escape immune recognition. Thus, an oncogene can promote tumor progression independent of its transforming activity by increasing the number and function of Tregs. This has a significant clinical potential, in which targeting KRAS and its downstream signaling pathways could be used as powerful immune modulators in cancer immunotherapy.
Keywords: KRAS, Treg, induction, Oncogene, suppressive
INTRODUCTION
Oncogenes act through cell-intrinsic mechanisms to promote tumor cell growth and survival. RAS proteins control signaling pathways important for cell survival and are the most common oncogenes in human cancers (1). Single amino acid mutations place RAS in a constitutively active state promoting tumor growth, angiogenesis, and metastasis (2). Mutations in KRAS are found in various human cancers and are associated with poor prognosis (3, 4). Although peptides derived from mutated KRAS are presented on the surface of tumor cells in the context of MHC and recognizable as tumor-associated antigens, tumors carrying a KRAS mutation fail to be eliminated by the immune system (5, 6). This could be attributed to the immunosuppressive tumor microenvironment, in particular, the suppressive regulatory T cells (Tregs), that play a role in promoting tumor progression (7–9).
Cancer cells overexpress immunosuppressive factors such as interleukin-10 (IL10) and transforming growth factor beta-1 (TGFβ1), both of which inhibit effector T-cell activity and stimulate Treg development (10–12). It has been suggested that Tregs are required for KRAS-mediated lung tumorigenesis (13). However, whether KRAS is involved in the induction of Treg has not been determined.
We investigated whether oncogenic KRAS could enhance the induction of Tregs. We found that, in comparison to tumor cells with wild-type KRAS, tumor cells carrying mutated KRAS induce suppressive Tregs by enhancing the secretion of IL10 and TGFβ1. Conversely, the inhibition of KRAS reduced the infiltration of Tregs into sites of KRAS-driven tumorigenesis.
Here, we identify a cell-extrinsic mechanism by which tumors carrying a KRAS mutation induce Tregs. This negative regulation of adaptive immunity through the induction of functional Tregs, combined with the well known cell-intrinsic effects of mutant KRAS, leads to the promotion of tumorigenesis.
MATERIALS AND METHODS
Cell lines, culture conditions, and inhibitors
Human cell lines established from primary tumors were purchased from American Type Culture Collection (ATCC). SW620 and SW480 are mutated KRAS colon cancer cell lines harboring a G12V mutation. Colo320 and WiDr are wild-type KRAS colon cancer cell lines. Cells were cultured in RPMI-1640 with 10% FCS, 100IU/ml penicillin, 100µg/ml streptomycin and 2mmol/l L-glutamine. Cell lines were routinely tested and confirmed Mycoplasma negative (Hoechst stain, PCR, and standard culture tests). Cells were used within six months of purchase (between 2011 and 2012).
PD98059 and Curcumin (Sigma-Aldrich) were dissolved in DMSO at 10mM and used at 20µM.
kR4A4 (Synthetic Biologics and Drug Discovery Facility, NCI-Frederick) is a potent KRAS inhibitor; a lipopeptide that mimics the C-terminal alpha-helix of KRAS and binds directly to KRAS. It inhibits cancer cells with GI50 in nanomolar ranges.
In vitro simulation culture assay (IVA) of tumor microenvironment (TME)
Peripheral blood mononuclear cells (PBMC) from normal donors were processed for Treg generation as described (14). Briefly, PBMC were isolated by centrifugation over Ficoll-Hypaque gradients (GE Healthcare Bioscience) and separated into monocytes and lymphocytes via plastic adherence. Monocytes were differentiated into immature dendritic cells (iDC) by culturing in AIM-V with granulocyte macrophage colony-stimulating factor (GM-CSF; 1000IU/ml) and IL4 (4ng/ml) for 7 days. CD4+CD25− cells were isolated from the lymphocyte fraction using regulatory T cell Isolation Kit (Miltenyi). T cells (1 × 106) were co-incubated with iDC (1 × 105) and irradiated tumor cells (1 × 105) for 10 days in AIM-V medium. A cytokine cocktail optimized for Treg growth (IL2 (10 IU/ml), IL10 (20 IU/ml) and IL15 (20 IU/ml)) was added on days 0, 3 and 6. On day 9, culture medium was replaced by fresh medium containing mAb OKT-3 (1µg/ml) and Brefeldin-A (1µg/ml). On day 10, lymphocytes and cell supernatant were harvested for phenotypic, functional, and cytokine analyses. To some cocultures, neutralizing IL10 mAb (clone 25209 at1µg/ml) or neutralizing TGFβ mAb (clone 9016 at 1µg/ml; R&D Systems) were added on day 0, 3, and 6.
To rule out artefactual observations due to mixed-lymphocyte reactions resulting from HLA mismatches, experiments were repeated and results were consistent across multiple lymphocyte donors.
To assess whether cell-to-cell contact was necessary for tumor cells to mediate Treg induction, polycarbonate 24 well Transwell inserts (0.4µm; Corning Costar Corp) were used in the assay system.
Flow Cytometry
Cells were stained for flow cytometry as described (14). Briefly, cells were stained for surface markers (30 min, 4°C, in the dark), fixed, permeabilized, stained for intracellular markers (30 min, 4°C, in the dark), washed, resuspended in a flow solution and analyzed (EPICS® XL-MCL cytometer with Expo32 software (Beckman Coulter). Anti-human mAb used: anti-FOXP3 conjugated to fluorescein isothiocyanate (FITC, clone PCH101) from eBiosciences); anti–CTLA-4–phycoerythrin (PE), anti-IL10-PE (clone 127107), and anti-TGFβ1-PE (clone 9016) from R&D Systems; and anti-CD3–phycoerythrin-cyanine 5 (PC5, clone UCHT1), anti-CD4− phycoerythrin-Texas Red conjugate (Energy Coupled Dye) (ECD, clone SFCI12T4D11), anti-CD4-PC5 (clone SFCI12T4D11), anti-CD25-PE (clone B1.49.9), and isotype controls from Beckman Coulter.
Suppression Assay
CD4+CD25−T cells were stained with 1.5 µM CFSE (Life technologies/Invitrogen) and cocultured with Tregs as described (14). Briefly, CD4+CD25−T cells were stimulated with plate-bound CD3 mAb (2 µg/ml) and soluble CD28 mAb (2 µg/ml: Miltenyi Biotec) in complete AIM-V medium containing IL2 (150 IU/ml) in 96-well plates (1 × 105). Regulatory T cells obtained from the in vitro TME cultures were harvested, phenotyped (for expression of FOXP3, CTLA-4, CD122, IL10, and TGFβ1), counted, and added to CD4+CD25−T cells at 1:2 or 1:1. T-cell numbers ensured the proper CD4+CD25−:Treg. Cocultures were incubated for 5 days at 37°C. CFSE dilution was analysed using ModFit-LT software (Verity Software House), to assess T-cell proliferation and calculate the percent proliferation inhibition relative to proliferation of responder cells alone.
siRNA knockdown of endogenous KRAS, IL10, or TGFβ1
KRAS, IL10, or TGFβ1 was silenced using a pool of four siRNAs (SMARTpool siRNA; Dharmacon) containing targeting sequences against genes of interest.
KRAS smartpool sequences: GGAGGGCUUUCUUUGUGUA, UCAAAGACAAAGUGUGUAA, GAAGUUAUGGAAUUCCUUU, GAGAUAACACGAUGCGUAU.
IL10 smartpool sequences: UUAAUAAGCUCCAAGAGAA, UGGAGGACUUUAAGGGUUA, UGUCUGAGAUGAUCCAGUU, CAACCUGCCUAACAUGCUU.
TGFβ1 smartpool sequences: AUUGAGGGCUUUCGCCUUA, GCAGAGUACACACAGCAUA, CCGAGAAGCGGUACCUGAA, GGACUAUCCACCUGCAAGA.
Tumor cells were transfected with 100nM of KRAS-siRNA, IL10-siRNA, TGFβ1-siRNA, or control siRNA (siCONTROL nontargeting siRNA; Dharmacon) with Dharmafect 4 (Dharmacon) according to manufacturer’s instructions.
Real Time RT-PCR
Total RNA was extracted from cells using RNeasy Mini Spin Kit (Qiagen). Quantitative RT–PCR was performed using Express One-Step SYBR GreenER system (Invitrogen), and was carried out on a 7500 FAST Real-Time PCR System (Applied Biosystems). Relative quantification of target gene mRNA expression was calculated with comparative Ct method. Expression levels of target genes were normalized to an endogenous control (Gapdh housekeeping gene). Gene-specific PCR primers used: human KRAS; TCCTGACCTCAAGTGATTCACCCA (forward) and ACTGGCATCTGGTAGGCACTCAAT (reverse), human IL10; GGCGCTGTCATCGATTTCTT (forward) and TGGAGCTTATTAAAG GCATTCTTCA (reverse), human TGFβ1; ACAATTCCTGGCGATACCTCAG (forward) and TGCAGTGTGTTATCCCTGCTGTCA (reverse), human GAPDH; ACCCACTCCTCCACCTTTGA (forward) and GTCCACCACCCTGTTGCTGTA (reverse).
Establishment of Colo320 cells stably expressing KRASG12V mutation
Colo320 cells were stably transfected with plasmids encoding empty vector (pBabe-puro, Addgene) or KRASG12V (pBabe-puro-KRAS 12V, Addgene) using Amaxa system (Lonza). Cells were screened with puromycin (1µg/ml; Merck) for 7 days. Survival clones were pooled and cultured in RPMI containing 1µg/ml puromycin.
TAM67 transient transfection
TAM67 was a gift from Dr. MJ. Birrer. SW620 cells were transiently transfected using Lipofectamine 2000 (Invitrogen) according to manufacturer’s recommendations.
Western-blot immunoassay
mAb used: mouse anti-KRAS (1:1,000; Novus Biologicals), rabbit anti-cJun (1:1,000; Santa Cruz), rabbit anti-phospho ERK (1:1,000), rabbit anti-ERK (1:2,000), rabbit anti-GAPDH (1:5,000), rabbit anti-β-tubulin (1:5,000), and Horseradish peroxidase-linked secondary antibodies (1:10,000) (Cell Signaling). Bands were visualized by chemiluminescence using X-ray film.
ELISA
Tumor cells (106) were seeded in 6 well plates with 2 ml complete RPMI medium. Two days later, the culture medium was collected and dead cells removed by centrifugation. IL10 and TGFβ1 concentrations in the supernatant were assayed with sandwich ELISA Kit (R&D Systems) according to manufacturer’s instructions.
LUMINEX
IL10 concentrations in in vitro culture supernatants were analyzed by LUMINEX, using a human cytokine 10-plex Ab bead kit (Biosource/Invitrogen) according to manufacturer’s instructions.
Mouse Treatment
A/J mice (Jackson Laboratories, Bar Harbor, ME) were housed according to the guidelines of the Animal Care and Use Committee of the National Institutes of Health. Mice (6 weeks old) were treated with three weekly doses of the tobacco-specific carcinogen 4-methylnitrosamino-1-(3-pyridyl)-1-butanone NNK (EaglePicher Pharmaceuticals Lenexa, KS). NNK was prepared in 0.9% NaCl solution and delivered by i.p. injection at 100mg/kg. KRAS inhibitor kR4A4 was injected intravenously (twice a week for three weeks) or intraperitoneally (five times a week for three weeks) at 12.5mg/kg. At 9 weeks of age, and prior to tumor formation, mice were euthanized by cervical dislocation and lungs collected.
Detection of lung infiltrated Treg cells
Murine lung tissues were collected one week after the last NNK injection and dissociated using mechanical and enzymatic (Collagenase Type 1, Invitrogen) methods, as described (15). Lung-infiltrating CD4+Foxp3+ cells were analyzed using flow cytometry and the following anti-mouse mAbs (eBiosciences): anti-CD3-PE-Cy7 (clone 145-2C11), anti-CD4-FITC (clone RM 4-5), and anti-Foxp3-Alexa700 (clone FJK-16s).
Statistical analysis
Statistical parameters were calculated using GraphPad Prism and Microsoft Excel. Statistical significance was determined by paired t tests (P < 0.05 was considered statistically significant). Data are presented as means ± s.d. from three independent experiments.
RESULTS
Mutant KRAS tumor cells convert CD4+CD25− T cells into Tregs
Although mutant KRAS produces recognizable tumor antigens, these cells escape immune surveillance. This could be attributed to a suppressive microenvironment, to which Treg cells are major contributors. We therefore tested whether mutant KRAS can induce functional Tregs.
We used an in vitro culture assay (IVA) that simulated the human tumor microenvironment (TME) (14). Briefly, CD4+CD25− T cells were cocultured with autologous iDC and irradiated tumor cells in the presence of IL2, IL10, and IL15. Four colon cancer cell lines were assessed that expressed either wild type (WT) (Colo320, Widr) or mutant (SW620, SW480) KRAS. Lymphocytes were phenotyped and functionally evaluated after 10 days. We found that, in the presence of mutant KRAS tumor cells, a high percentage of CD4+CD25−T cells were converted to Tregs as characterized by expression of FOXP3, CTLA-4, and CD122 (Fig. 1A and B). In contrast, cocultures established with WT KRAS tumor cells contained significantly fewer Tregs (Fig. 1A and B).
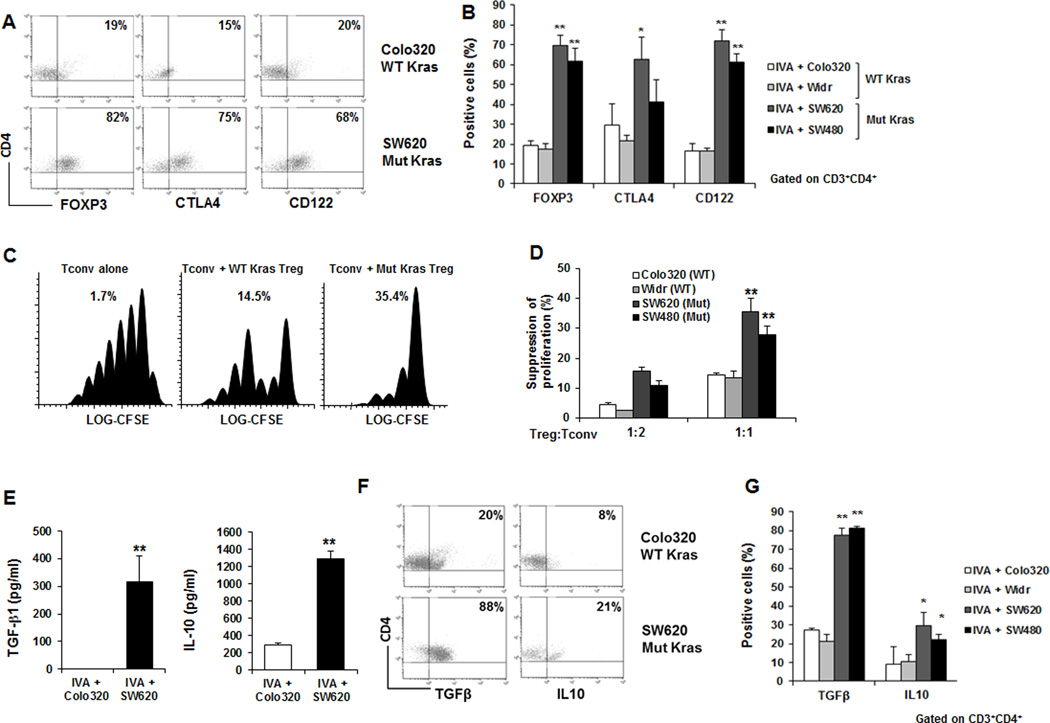
Figure 1. Tumors with mutated KRAS could induce Tregs.
(A and B) T lymphocytes were cocultured with tumor cells carrying WT (Colo320) or mutated (SW620) KRAS. (A) Representative dot plots (B) The percentage of CD4+FOXP3+, CD4+CTLA-4+ and CD4+CD122+ T cells cocultured with mutated KRAS (SW620, SW480) was significantly higher than WT KRAS (Colo320, Widr). (C and D) Suppressor activity of Tregs generated by in vitro assay (IVA) culture simulating the tumor microenvironment co-incubated at 1:2 or 1:1 with CFSE-labeled CD4+CD25−T cells (C) Representative example (Percentages represent percent inhibition of proliferation relative to proliferation of responder cells alone) (D) Cells generated in cultures with mutated KRAS tumor cells possessed a significantly higher suppressive ability than WT KRAS. (E) T cells generated from cocultures with mutant KRAS tumor cells secreted significantly higher amounts of IL10 and TGFβ1 than WT KRAS (Protein concentrations in supernatants). (F and G) (F) Representative dot plots (G) The percentage of IL10- and TGFβ1-positive cells was significantly higher in lymphocytes cocultured with mutant KRAS tumor cells than with WT KRAS (Intracellular expression). Data are means ± s.d. from three independent experiments, *P < 0.05, ** P < 0.005 (Student’s t-test).).
To determine whether Tregs generated in the presence of mutant KRAS were functional, we evaluated their ability to suppress proliferation of activated T cells. T cells from the in vitro TME culture were co-incubated with CFSE-labeled autologous CD4+CD25−responder cells stimulated with CD3 and CD28 mAbs. Cocultures were set up at the Treg: CD4+CD25− ratio of 1:2 or 1:1.Regulatory T cells generated in cocultures with mutated KRAS mediated stronger suppression than those generated with WT KRAS (Fig. 1C and D). As Tregs may exert their suppressive function through secretion of IL10 and TGFβ1 (16, 17), we examined the secretion of these cytokines in the supernatants. We found that T cells isolated from cocultures containing mutant KRAS secreted significantly more IL10 and TGFβ1 than those from culture containing WT KRAS (Figure 1E). This was further confirmed by intracellular staining (Fig. 1F and G).
Taken together, these data show that tumor cells with KRAS mutations can significantly enhance the induction and function of Tregs when compared to tumor cells with WT KRAS.
Mutated KRAS in tumors directly responsible for Treg induction
We have shown that tumor cells carrying mutated KRAS could convert a higher percentage of CD4+CD25−T cells to Tregs when compared to WT KRAS. To investigate whether constitutive activation of KRAS was the direct cause of conversion to Treg, we used siRNA to knockdown KRAS.
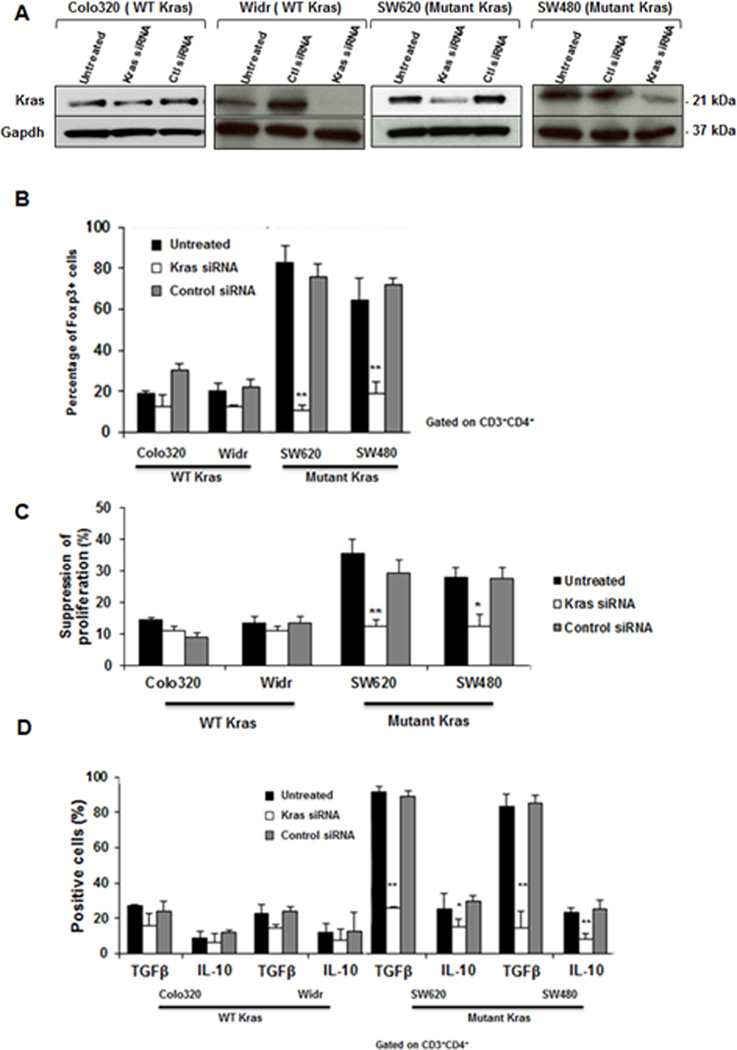
Endogenous KRAS protein levels were efficiently and specifically reduced with KRAS-specific siRNA pool (Fig. 2A). We found that disruption of KRAS abrogates the ability to convert CD4+CD25−T cells into Tregs. Indeed, knockdown of KRAS resulted in a significantly decreased number of FOXP3+, CTLA-4+ and CD122+ T cells (Fig. 2B). Whereas silencing KRAS in WT KRAS tumor cells had no effect on T-cell conversion (Fig. 2B). We also observed that T cells generated in cocultures containing silenced KRAS mediated significantly lower suppression, similar to those generated in the presence of WT KRAS (Fig. 2C). To further analyze the effect of silencing KRAS on Treg function, intracellular staining for the suppressive cytokines IL10 and TGFβ1 was performed. As expected, silencing KRAS in WT KRAS tumor cells did not affect the percentage of IL10- and TGFβ1-expressing cells (Fig. 2D). However, silencing KRAS in tumor cells with mutant KRAS, significantly reduced the number of Treg cells that express IL10 and TGFβ1 (Fig. 2D).
Figure 2. Silencing KRAS in mutated KRAS tumor cells prevented Treg induction.
Wild-type (Colo320, Widr) or mutated KRAS (SW620, SW480) tumor cells were treated with control or KRAS siRNA. (A) KRAS levels were evaluated three days after transfection by Western-blot. Silencing KRAS in SW620 and SW480 resulted in a significant reduction in (B) FOXP3+ T-cell generation, (C) the suppressive ability, and (D) the percentage of cells expressing intracellular IL10 and TGFβ1. Data are means ± s.d. from three independent experiments, *P < 0.05, ** P < 0.005 (compared to untransfected cells; Student’s t-test).).
These findings confirm that constitutive activation of KRAS in cancer cells was responsible for the induction of suppressive Treg.
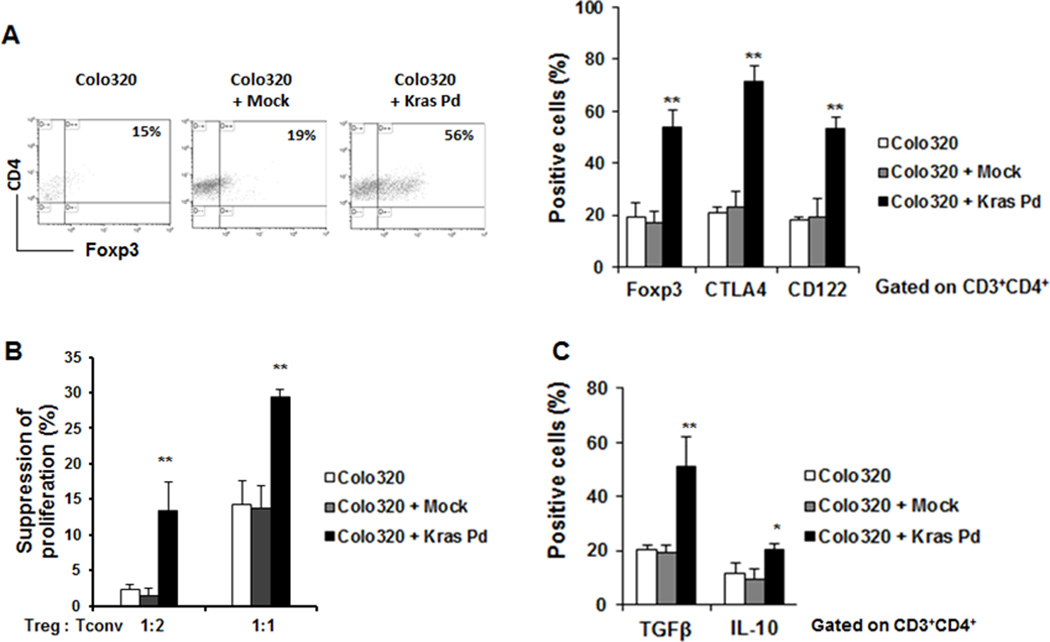
To further verify the role of KRAS in mediating Treg conversion, WT KRAS tumor cells were stably transfected with cDNA encoding the KRASG12V mutation or with an empty vector (pBABE-puro) (Supplemental Fig. S1A). T cells cocultured with tumor cells transfected with KRASG12V contained significantly more FOXP3+, CTLA-4+, and CD122+ cells (Fig. 3A and Supplemental Fig. S1B). Moreover, functional analysis of T lymphocytes cocultured with KRASG12V-transfected Colo320 cells had more suppressor activity (Fig. 3B). This correlated with an increase in intracellular IL10 and TGFβ1 (Fig. 3C and Supplemental Fig,S1B).
Figure 3. Overexpression of mutant KRASG12V in wild-type KRAS tumor cells induced Treg conversion.
(A) The percentage of FOXP3+ T lymphocytes, (B) the suppressive ability, and (C) the percentage of cells expressing intracellular IL10 and TGFβ1 in T cells generated with WT KRAS tumor cells overexpressing mutant KRASG12V plasmid (KRAS Pd) was significantly higher than WT cells. Data are means ± s.d. from three independent experiments, *P < 0.05, ** P < 0.005 (compared to untransfected Colo320 cells; Student’s t-test).
Treg induction by mutant KRAS mediated through IL10 and TGFβ1
We have shown that tumor cells with mutant KRAS induce a higher percentage of highly functional Treg in comparison to WT KRAS.
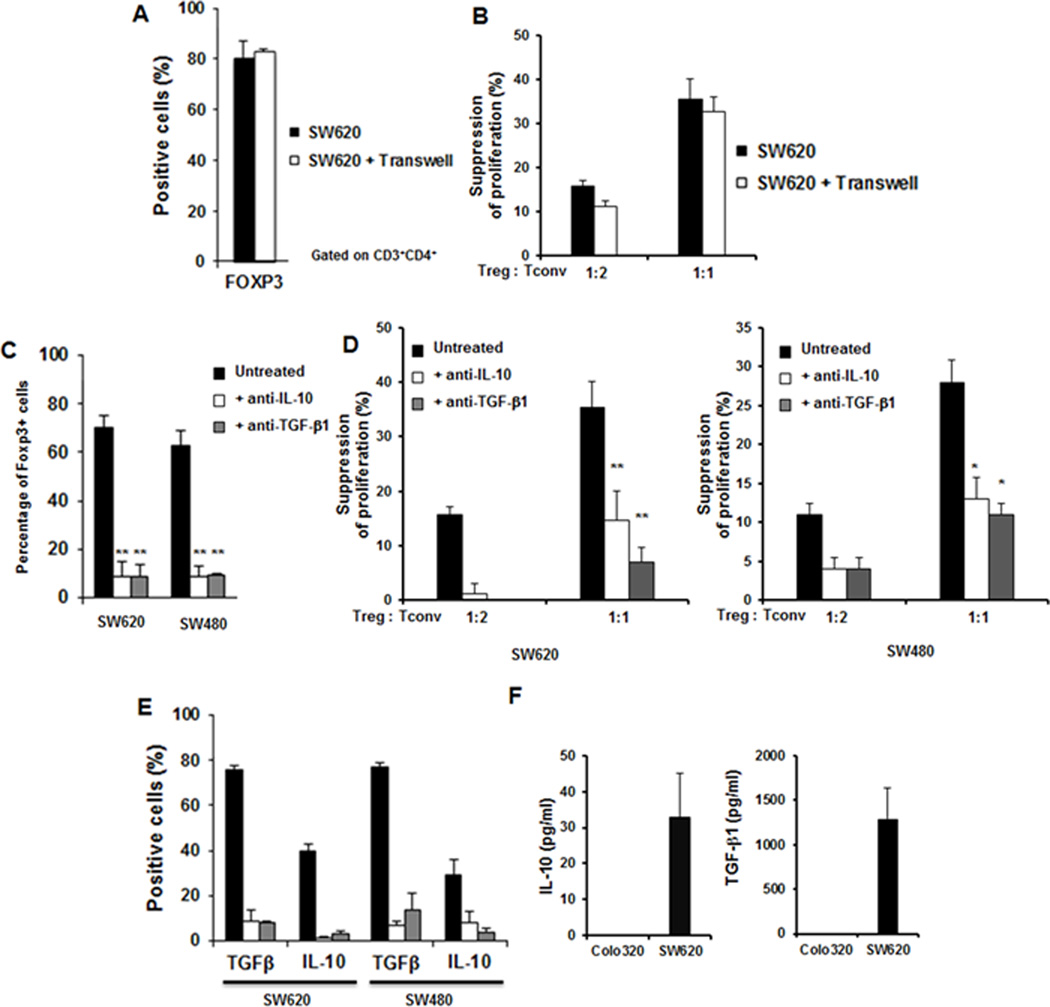
To delineate the exact mechanism by which constitutive activation of KRAS induces highly functional Treg, we investigated whether the induction requires direct cell-cell interaction. Transwell inserts separating SW620 cells from CD4+CD25−T cells were used. We observed that the percentage of FOXP3+ cells (Fig. 4A), as well as the suppressive activity and cytokine levels (Fig. 4B) were unaffected by the presence of transwell inserts. This indicated that direct contact between tumor cells and CD4+CD25−T cells was not required for Treg generation. These findings also suggested that mutated KRAS tumor cells may negatively modulate immune responses by secreting factors that promote Treg induction.
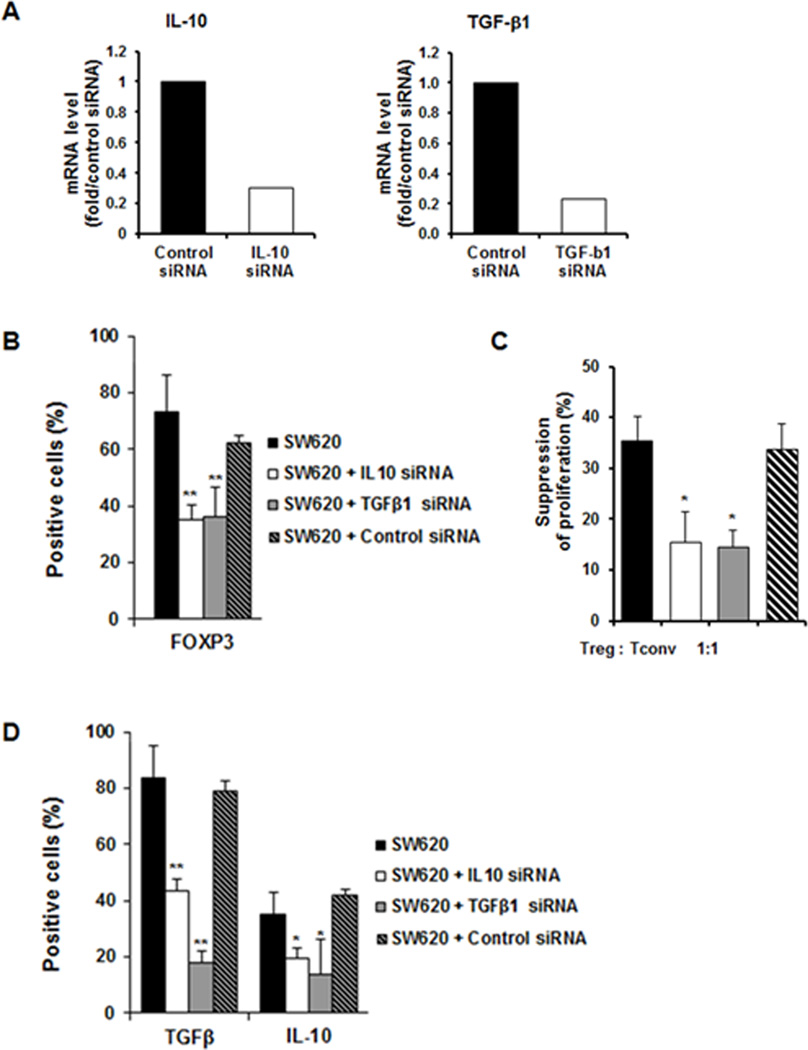
Figure 4. Treg conversion by mutated KRAS did not require direct cell-cell contact, and neutralization of IL10 and TGFβ1 prevened Treg induction.
(A and B) T cells generated from cocultures with mutated KRAS (SW620) in the presence or absence of transwell inserts did not show any differences in (A) the percentage of FOXP3+ T-lymphocytes and (B) the suppressive activity. (C–E) T cells generated from cocultures with mutated KRAS tumor cells in the presence of neutralizing mAbs to IL10 or -TGFβ1 had a significantly lower (C) percentage of FOXP3+ T lymphocytes, (D) suppressive activity, and (E) intracellular expression of IL10 and TGFβ1. (F) Secretion of IL10 and TGFβ1 in the supernatant of mutated KRAS tumor cells (SW620) was significantly higher than wild-type (Colo320).
Data are means ± s.d. from three independent experiments, *P < 0.05, ** P < 0.005 (compared to untreated cells; Student’s t-test).).
Interleukin-10 and TGFβ1 are secreted by many tumors and play a role in Treg induction (10, 18). We therefore investigated their effects by testing whether their neutralization in the in vitro TME culture could inhibit the ability to induce Treg. Neutralization of IL10 or TGFβ1 resulted in a significant reduction in FOXP3+ T cells (Fig. 4C) and mediated significantly less suppression (Fig. 4D). Not surprisingly, intracellular expression of IL10 and TGFβ1 was significantly reduced in the presence of neutralizing antibodies (Fig. 4E).
We next investigated whether IL10 and TGFβ1 are secreted by tumor cells carrying mutated KRAS. We analyzed supernatants of WT or mutated KRAS tumor cells and found that cells expressing mutated KRAS secreted more IL10 and TGFβ1 (Fig. 4F).
To further test whether the secretion of IL10 and TGFβ1 was directly responsible for Treg induction, we performed the in vitro TME culture with mutated KRAS tumor cells treated with IL10, TGFβ1, or control siRNA. We observed that silencing endogenous IL10 or TGFβ1 (Fig. 5A) resulted in a significant decrease in the conversion of CD4+CD25−T cells to Treg (Fig. 5B) and resulted in generating T cells with reduced suppressive ability (Fig. 5C) and lower number of IL10+ and TGFβ1+ T cells (Fig. 5D).
Figure 5. Silencing IL10 and TGFβ1 in mutated KRAS tumor cells prevented Treg induction.
SW620 cells were treated with control, IL10 or TGFβ1 siRNA. (A) IL10 or TGFβ1 levels were evaluated three days after transfection (Real-time PCR) (representative example of three independent experiments). When endogenous IL10 or TGFβ1 were silenced there was a reduction in (B) the percentage of FOXP3+, CTLA-4+, and CD122+ T lymphocytes, (C) suppressive activity, and (D) the percentage of T cells expressing intracellular IL10 and TGFβ1.
Data are means ± s.d. from three independent experiments, *P < 0.05, ** P < 0.005 (compared to untransfected SW620 cells; Student’s t-test).).
Mutant KRAS led to induction of IL10 and TGFβ1
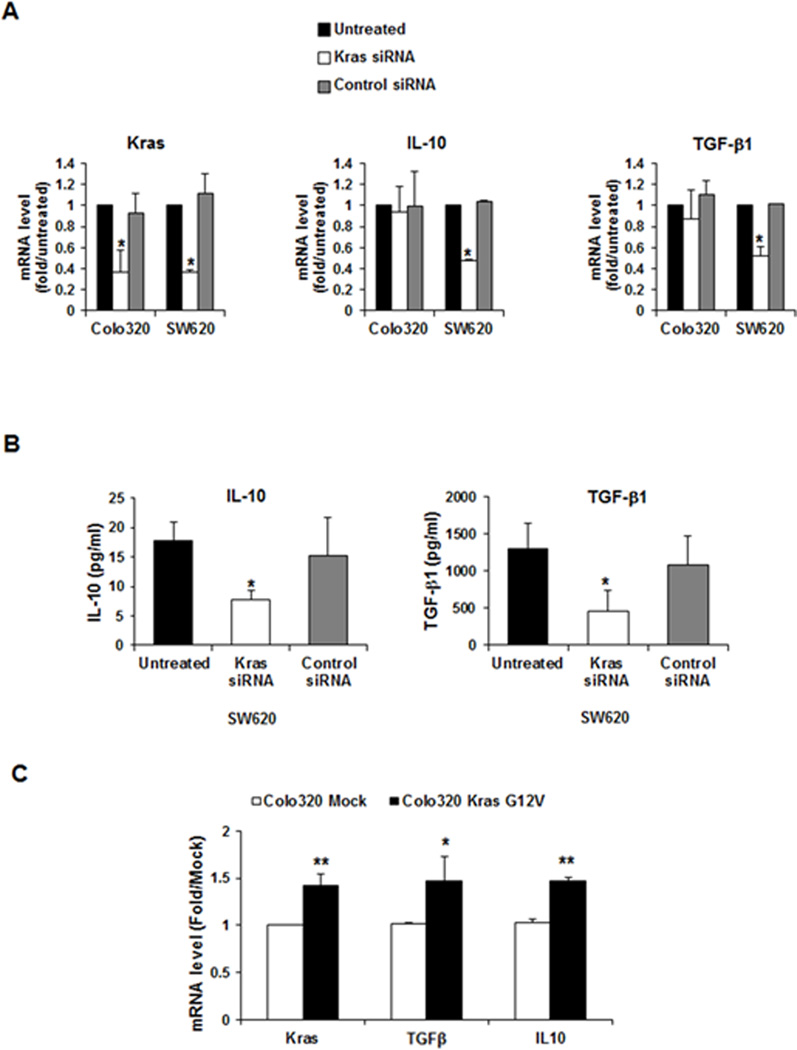
To examine whether mutated KRAS directly causes the increase of IL10 and TGFβ1 secretion, we disrupted KRAS signaling (KRAS siRNA) in tumor cells carrying mutated or WT KRAS and evaluated the effects on IL10 and TGFβ1. The mRNA expression these cytokines was attenuated by 50% in SW620, whereas knockdown of KRAS in Colo320 had no significant effect (Fig. 6A). These findings were confirmed at the protein level (Fig. 6B).
Figure 6. Mutated KRAS led to the secretion of IL10 and TGFβ1.
Silencing KRAS resulted in a significant reduction of (A) IL10 and TGFβ1 mRNA expression and (B) IL10 and TGFβ1 secretion from mutated KRAS tumor cells (SW620). (C) Overexpression of mutant KRAS in wild-type KRAS (Colo320) resulted in a significant increase in IL10 and TGFβ1 mRNA. Data are means ± s.d. from three independent experiments, *P < 0.05, ** P < 0.005.
We also found that overexpression of mutant KRAS in tumor cells harboring WT KRAS resulted in an increase of IL10 and TGFβ1 mRNA (Fig. 6C).
Taken together, these data indicated that constitutive KRAS activation drove the secretion of IL10 and TGFβ1.
MEK-ERK-AP1 pathway mediated IL10 and TGFβ1 increase
We have shown that tumors with mutated KRAS induced the development of highly suppressive Treg and that this induction was mediated through the production of IL10 and TGFβ1.
We next addressed the mechanisms through which mutant KRAS enhances the expression of these cytokines. Oncogenic KRAS leads to the activation of MEK-ERK pathway; we therefore confirmed that this pathway was activated in mutated KRAS cells. Indeed, silencing KRAS resulted in decreased activation of ERK as determined by less phospho-ERK (Fig. 7A).
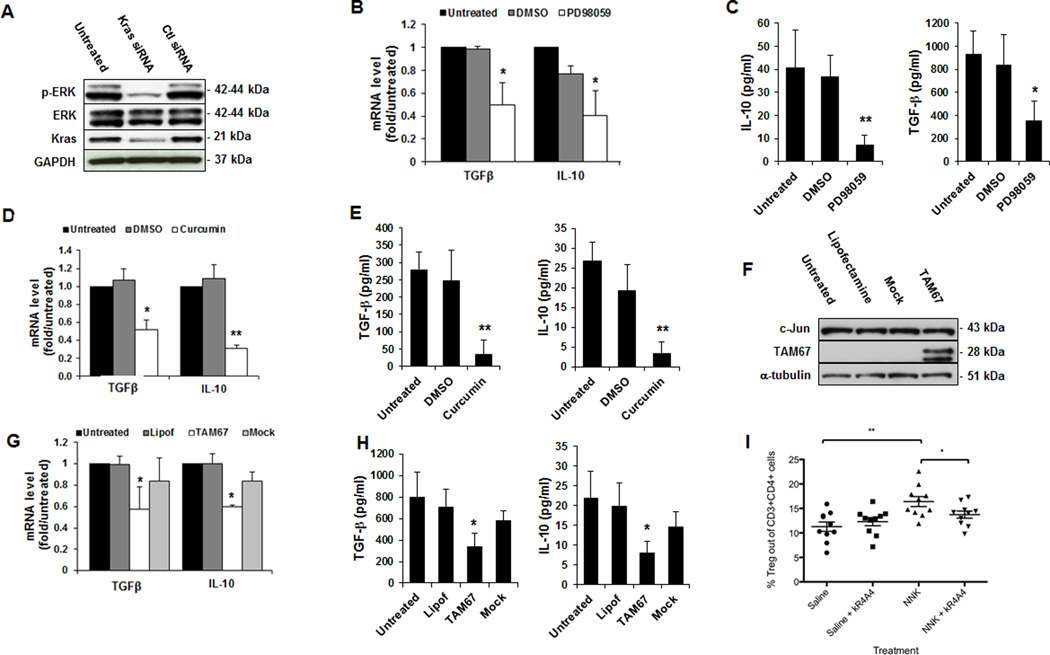
Figure 7. Mutated KRAS tumor cells upregulate IL10 and TGFβ1 expression through the activation of the MEK-ERK-AP-1 pathway.
(A) Silencing KRAS in SW620 resulted in a significant reduction in the phosphorylation of ERK. (B and C) MEK inhibitor (PD98059) significantly reduced IL10 and TGFβ1 (B) mRNA and (C) secreted protein. (D and E) AP-1 inhibitor (curcumin) significantly reduces IL10 and TGFβ1 (D) mRNA and (E) secreted protein. (F) Expression of TAM67 in SW620 after transfection with Lipofectamine 2000. (G and H) TAM67 significantly reduced IL10 and TGFβ1 (G) mRNA and (H) secreted protein. (I) Inhibition of KRAS prevented Treg infiltration in a tobacco carcinogen-driven lung tumorigenesis model. Three weekly doses of NNK were administered into AJ mice, and Treg lung infiltration was assessed one week after completion of NNK treatment (prior to tumor development). NNK markedly increased the number of Treg in lung tissues. Treatment with the KRAS inhibitor kR4A4 partially reversed the induction of Treg by NNK. Data are means ± s.d. from three independent experiments, *P < 0.05, ** P < 0.005.
We next examined the effect of MEK inhibitor, PD98059, on KRAS-induced IL10 and TGFβ1 production. Incubation of SW620 with PD98059 was associated with a significant decrease in IL10 and TGFβ1 mRNA and secreted protein (Fig. 7B and C), suggesting that MEK was critical for their expression.
Given that ERK can modulate gene expression by activating several transcription factors, such as AP-1, and that the human promoters of IL10 and TGFβ1 contain several binding sites for AP-1 (19–21), we hypothesized that AP-1 could be involved in KRAS-induced transcription of IL10 and TGFβ1. To explore this possibility, we incubated SW620 cells with curcumin, an AP-1 inhibitor (22). The addition of curcumin significantly reduced IL10 and TGFβ1 production (Fig. 7D and E), suggesting that AP-1 may play a role in their upregulation. Additional confirmation was obtained using a dominant negative mutant of c-Jun (component of AP-1), TAM67. This mutant specifically inhibits AP-1 activity (23). Expression of TAM67 protein was determined 24h after transient transfection of SW620 cells (Fig. 7F). Expression of IL10 and TGFβ1 was downregulated by TAM67 (Fig. 7G and H).
Together, these data demonstrate that oncogenic KRAS induced the expression of IL10 and TGFβ1 through activation of the MEK-ERK-AP-1 pathway.
KRAS inhibition prevented Treg infiltration in lung tumorigenesis model
Activating mutations in KRAS have been identified in approximately 25% of human lung adenocarcinomas primarily associated with smoking (24). In preclinical models, KRAS mutations are present in over 90% of lung tumors induced by the tobacco-specific carcinogen 4-methylnitrosamino-1-(3-pyridyl)-1-butanone (NNK) (25, 26). Exposure of A/J mice to NNK increases the number of lung-associated Tregs before tumors develop (13).
To assess whether NNK-induced lung Treg infiltration directly correlated with NNK-induced KRAS mutation, A/J mice were treated with NNK, with or without treatment with the KRAS inhibitor kR4A4. One week after the last NNK dose, mice were euthanized and their lungs analyzed to assess lung-infiltrating Tregs. The analysis was carried out before tumors formed, so that tumor size did not affect the number of detected Tregs. This specific model was used to avoid the disadvantage of other tumor models, in which the number of tumor-infiltrating cells could be misinterpreted based on variability of tumor size. This model also avoids conflating Treg homing to the tumor and intratumoral conversion, because Treg assessment is made prior to tumor formation, hence, no Treg homing can be detected at this stage.
Administration of NNK markedly increased the number of Treg in lung tissues (Fig. 7I). Treatment of NNK-treated mice with kR4A4 partially reversed the induction of lung associated Treg (Fig. 7I). Thus, we have demonstrated tumor KRAS–dependent modulation of Treg infiltration in lung cancer precursor lesions.
DISCUSSION
Constitutive activation of KRAS in human malignancies is associated with aggressive tumor growth and poor prognosis (3, 4). Similar to other oncogenes, KRAS acts in a cell-intrinsic manner to affect tumor growth by affecting apoptosis, angiogenesis and tumor invasiveness (2). Tumor cells carrying mutated KRAS upregulate cell cycle regulatory and anti-apoptotic genes (27, 28) and mutated KRAS proteins stimulate vascular endothelial growth factor and matrix metalloproteases production, promoting angiogenesis and metastasis (29, 30). However, a cell-extrinsic role of mutated KRAS in modulating the tumor microenvironment, in particular the immune response, has yet not been identified.
Despite the fact that mutated KRAS is a tumor-associated antigen (5), tumor cells carrying the mutated KRAS evade immune recognition. Indeed, although immunization of advanced cancer patients with mutant KRAS peptide vaccines generates specific immune responses, seldom have any clinical responses to these vaccines been demonstrated (6, 31, 32). Silencing mutated KRAS in colorectal cancer cells reduces the formation of subcutaneous tumors in immune-competent mice, but not in immune-deficient mice (33). Although these findings suggest that mutated KRAS may contribute to initiation and maintenance of tumor growth by evasion from the immune system, the role of mutated KRAS in this process has not been defined.
A growing body of evidence suggests the presence of interplay between oncognenic mutations and antitumor immunity. Oncogenic BRAF(V600E) promotes immune evasion by promoting internalization of MHC class I from melanoma cell surface (34), suppressing expression of melanocyte differentiation antigens (35), suppressing dendritic cell function (36), and enhancing the production of immune suppressive cytokines (37, 38). Inhibition of BRAF(V600E) reverses all these effects, rendering melanoma cells more recognizable by T cells (35, 36, 38).
In this work, we investigated whether the presence of mutated KRAS in tumors suppresses the immune system as a mechanism to escape immune recognition. We found that tumor cells expressing mutated KRAS generated suppressive Tregs and that silencing KRAS significantly reduced this ability. We also found that, although tumor cells with WT KRAS induced an insignificant number of Tregs, mutant KRASG12V gene transfection into these cells significantly enhanced their ability to induce suppressive Tregs.
We also show that mutant KRAS drove the secretion of IL10 and TGFβ1 by tumor cells, which are responsible—at least in part—for Treg induction (10, 18). When the secretion of these immunosuppressive cytokines was inhibited, Treg generation was significantly reduced. Silencing KRAS resulted in a significant reduction in the production of these cytokines. On the other hand, the introduction of mutant KRASG12V gene into tumor cells with WT KRAS significantly enhanced their ability to produce IL10 and TGFβ1, thus confirming the role of KRAS in the production of these cytokines.
Our data also demonstrate an important role for the MEK-ERK-AP-1 signaling pathway in mutant KRAS-driven secretion of IL10 and TGFβ1. Inhibition of MEK and AP-1 in tumor cells with mutated KRAS resulted in a significant reduction in the production of IL10 and TGFβ1. Our findings align with a recent report that showed that MEK inhibition mitigates TGFβ production in tumor cells, hence reducing their ability to induce Tregs (39).
The role of KRAS in the induction of Tregs can be utilized to mitigate tumor infiltrating Tregs. Here, we report that KRAS inhibition reduced the number of Tregs induced by tobacco carcinogen NNK in lung tissues even prior to tumor development.
Combined inhibition of MEK and Akt (both downstream of KRAS), resulted in an enhanced antitumor therapeutic efficacy greater than either single treatment (40). This results from induction of apoptosis and proliferation inhibition in tumor cells by these inhibitors (40). This is not surprising, as in tumors harboring mutant KRAS, MEK inhibition enhances Akt phosphorylation, thus enhancing cell proliferation (41). This is thought to be due to the interaction between the MEK-ERK and the PI3K-Akt pathway (42, 43). In fact, mutations in both these pathways are common in many cancers (44). Therefore, targeting both pathways results in an enhanced antitumor efficacy (40, 45). This has significant clinical implications, especially due to the role of the PI3K-Akt pathway in modulating the immune response in the tumor microenvironment. We previously reported that Akt inhibition results in a selective inhibition of Tregs, which translates into significant antitumor therapeutic efficacy (46). Our group and others have also shown that Akt inhibition enhances the effector arm of the immune response by enhancing the memory CD8 population and diminishing terminal differentiation of cytotoxic CD8 T cells. This translates into an enhanced antitumor immune response (47, 48).
Taken together, our findings demonstrate that oncogenic KRAS in cancer cells negatively regulates T cell immunity by inducing Tregs. We identified here a tumor cell–extrinsic role for oncogenic signaling pathways within tumor cells that ultimately promoted immunologic tolerance in the microenvironment through the expansion of the suppressive compartment.
We believe that our findings have important implications for therapeutic interventions in patients with mutated KRAS tumors. Indeed, oncogenic mutations of KRAS have emerged as a common mechanism of resistance against epidermal growth factor receptor (EGFR)-directed tumor therapy (49, 50). As tumor-mediated immune suppression represents a major obstacle to the stimulation of antitumor T-cell responses necessary for clinical effects, targeting IL10 and TGFβ1 might thus represent an attractive strategy to augment efficacy of antitumor immune therapies. Inhibition of MEK-ERK-AP-1 signaling pathway may also provide a route to blocking tumor immune evasion, as well as tumor proliferation and survival. These findings also suggest the utility of combining the inhibition of the MEK-ERK-AP-1 pathway with other immune modulators to further enhance the antitumor immune response.
In conclusion, here, we describe a cell-extrinsic mechanism through which mutated KRAS inhibits antitumor immune responses and augments its own cell-intrinsic oncogenic potential. This has significant clinical implications for immune modulation of tumors with KRAS mutations.
Supplementary Material
Acknowledgments
We thank Dr. D. Lowy, Dr. O. Nitzan and Dr J. Janik for critical comments and discussions.
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Financial Support: This work was supported by an intra-mural grant from NCI/NIH received by S.N. Khleif
Footnotes
Conflict of Interest: The authors declare that there are no conflicts of interest in the authorship or publication of this manuscript
REFERENCES
- 1.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 2.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Rostan G, Zhao H, Camp RL, Pollan M, Herrero A, Pardo J, et al. ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol. 2003;21:3226–3235. doi: 10.1200/JCO.2003.10.130. [DOI] [PubMed] [Google Scholar]
- 4.Castagnola P, Giaretti W. Mutant KRAS, chromosomal instability and prognosis in colorectal cancer. Biochim Biophys Acta. 2005;1756:115–125. doi: 10.1016/j.bbcan.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Abrams SI, Khleif SN, Bergmann-Leitner ES, Kantor JA, Chung Y, Hamilton JM, et al. Generation of stable CD4+ and CD8+ T cell lines from patients immunized with ras oncogene-derived peptides reflecting codon 12 mutations. Cell Immunol. 1997;182:137–151. doi: 10.1006/cimm.1997.1224. [DOI] [PubMed] [Google Scholar]
- 6.Khleif SN, Abrams SI, Hamilton JM, Bergmann-Leitner E, Chen A, Bastian A, et al. A phase I vaccine trial with peptides reflecting ras oncogene mutations of solid tumors. J Immunother. 1999;22:155–165. doi: 10.1097/00002371-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 8.Gjerdrum LM, Woetmann A, Odum N, Burton CM, Rossen K, Skovgaard GL, et al. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia. 2007;21:2512–2518. doi: 10.1038/sj.leu.2404913. [DOI] [PubMed] [Google Scholar]
- 9.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14:5166–5172. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 10.Seo N, Hayakawa S, Takigawa M, Tokura Y. Interleukin-10 expressed at early tumour sites induces subsequent generation of CD4(+) T-regulatory cells and systemic collapse of antitumour immunity. Immunology. 2001;103:449–457. doi: 10.1046/j.1365-2567.2001.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. 2002;129:263–276. doi: 10.1159/000067596. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 13.Granville CA, Memmott RM, Balogh A, Mariotti J, Kawabata S, Han W, et al. A central role for Foxp3+ regulatory T cells in K-Ras-driven lung tumorigenesis. PLoS One. 2009;4:e5061. doi: 10.1371/journal.pone.0005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion and characteristics of human T regulatory type 1 cells in co-cultures simulating tumor microenvironment. Cancer Immunol Immunother. 2007;56:1429–1442. doi: 10.1007/s00262-007-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertoncello I, McQualter J. Isolation and clonal assay of adult lung epithelial stem/progenitor cells. Curr Protoc Stem Cell Biol. 2011;Chapter 2(Unit 2G):1. doi: 10.1002/9780470151808.sc02g01s16. [DOI] [PubMed] [Google Scholar]
- 16.Groux H. Type 1 T-regulatory cells: their role in the control of immune responses. Transplantation. 2003;75:8S–12S. doi: 10.1097/01.TP.0000067944.90241.BD. [DOI] [PubMed] [Google Scholar]
- 17.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 19.Wang ZY, Sato H, Kusam S, Sehra S, Toney LM, Dent AL. Regulation of IL-10 gene expression in Th2 cells by Jun proteins. J Immunol. 2005;174:2098–2105. doi: 10.4049/jimmunol.174.4.2098. [DOI] [PubMed] [Google Scholar]
- 20.Jones EA, Flavell RA. Distal enhancer elements transcribe intergenic RNA in the IL-10 family gene cluster. J Immunol. 2005;175:7437–7446. doi: 10.4049/jimmunol.175.11.7437. [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Angel P, Lafyatis R, Hattori K, Kim KY, Sporn MB, et al. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol. 1990;10:1492–1497. doi: 10.1128/mcb.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang TS, Lee SC, Lin JK. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1991;88:5292–5296. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown PH, Chen TK, Birrer MJ. Mechanism of action of a dominant-negative mutant of c-Jun. Oncogene. 1994;9:791–799. [PubMed] [Google Scholar]
- 24.Westra WH, Baas IO, Hruban RH, Askin FB, Wilson K, Offerhaus GJ, et al. K-ras oncogene activation in atypical alveolar hyperplasias of the human lung. Cancer Res. 1996;56:2224–2228. [PubMed] [Google Scholar]
- 25.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wislez M, Spencer ML, Izzo JG, Juroske DM, Balhara K, Cody DD, et al. Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-ras. Cancer Res. 2005;65:3226–3235. doi: 10.1158/0008-5472.CAN-04-4420. [DOI] [PubMed] [Google Scholar]
- 27.Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 28.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 29.Matsuo Y, Campbell PM, Brekken RA, Sung B, Ouellette MM, Fleming JB, et al. K-Ras promotes angiogenesis mediated by immortalized human pancreatic epithelial cells through mitogen-activated protein kinase signaling pathways. Mol Cancer Res. 2009;7:799–808. doi: 10.1158/1541-7786.MCR-08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang XQ, Li H, Van Putten V, Winn RA, Heasley LE, Nemenoff RA. Oncogenic K-Ras regulates proliferation and cell junctions in lung epithelial cells through induction of cyclooxygenase-2 and activation of metalloproteinase-9. Mol Biol Cell. 2009;20:791–800. doi: 10.1091/mbc.E08-07-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulsen JE, Bjorheim J, Roe J, Eide TJ, Alexander J, Gaudernack G. Effect of vaccination with mutant KRAS peptides on rat colon carcinogenesis induced by azoxymethane. Anticancer Res. 2002;22:171–175. [PubMed] [Google Scholar]
- 32.Carbone DP, Ciernik IF, Kelley MJ, Smith MC, Nadaf S, Kavanaugh D, et al. Immunization with mutant p53- and K-ras-derived peptides in cancer patients: immune response and clinical outcome. J Clin Oncol. 2005;23:5099–5107. doi: 10.1200/JCO.2005.03.158. [DOI] [PubMed] [Google Scholar]
- 33.Smakman N, Veenendaal LM, van Diest P, Bos R, Offringa R, Borel Rinkes IH, et al. Dual effect of Kras(D12) knockdown on tumorigenesis: increased immune-mediated tumor clearance and abrogation of tumor malignancy. Oncogene. 2005;24:8338–8342. doi: 10.1038/sj.onc.1208995. [DOI] [PubMed] [Google Scholar]
- 34.Bradley SD, Chen Z, Melendez B, Talukder A, Khalili JS, Rodriguez-Cruz TG, et al. BRAF(V600E) co-opts a conserved MHC class I internalization pathway to diminish antigen presentation and CD8+ T-cell recognition of melanoma. Cancer Immunol Res. 2015 doi: 10.1158/2326-6066.CIR-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ott PA, Henry T, Baranda SJ, Frleta D, Manches O, Bogunovic D, et al. Inhibition of both BRAF and MEK in BRAF(V600E) mutant melanoma restores compromised dendritic cell (DC) function while having differential direct effects on DC properties. Cancer Immunol Immunother. 2013;62:811–822. doi: 10.1007/s00262-012-1389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalili JS, Liu S, Rodriguez-Cruz TG, Whittington M, Wardell S, Liu C, et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res. 2012;18:5329–5340. doi: 10.1158/1078-0432.CCR-12-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hossain DM, Panda AK, Chakrabarty S, Bhattacharjee P, Kajal K, Mohanty S, et al. MEK inhibition prevents tumor-shed TGFbeta-induced T-regulatory cell augmentation in tumor milieu. Immunology. 2014 doi: 10.1111/imm.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolcher AW, Khan K, Ong M, Banerji U, Papadimitrakopoulou V, Gandara DR, et al. Anti-tumour activity in RAS-driven tumours by blocking AKT and MEK. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holt SV, Logie A, Davies BR, Alferez D, Runswick S, Fenton S, et al. Enhanced apoptosis and tumor growth suppression elicited by combination of MEK (selumetinib) and mTOR kinase inhibitors (AZD8055) Cancer Res. 2012;72:1804–1813. doi: 10.1158/0008-5472.CAN-11-1780. [DOI] [PubMed] [Google Scholar]
- 42.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 43.She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18:39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janku F, Lee JJ, Tsimberidou AM, Hong DS, Naing A, Falchook GS, et al. PIK3CA mutations frequently coexist with RAS and BRAF mutations in patients with advanced cancers. PLoS One. 2011;6:e22769. doi: 10.1371/journal.pone.0022769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hofmann I, Weiss A, Elain G, Schwaederle M, Sterker D, Romanet V, et al. K-RAS mutant pancreatic tumors show higher sensitivity to MEK than to PI3K inhibition in vivo. PLoS One. 2012;7:e44146. doi: 10.1371/journal.pone.0044146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abu-Eid R, Samara RN, Ozbun L, Abdalla MY, Berzofsky JA, Friedman KM, et al. Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer Immunol Res. 2014;2:1080–1089. doi: 10.1158/2326-6066.CIR-14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abu Eid R, Friedman K, Mkrtichyan M, Walens A, King W, Janik J, et al. Akt 1 and 2 Inhibition Diminishes Terminal Differentiation and Enhances Central Memory CD8 T Cell Proliferation and Survival. OncoImmunology. 2015 doi: 10.1080/2162402X.2015.1005448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil R, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-14-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519–527. doi: 10.1038/nrclinonc.2009.111. [DOI] [PubMed] [Google Scholar]
- 50.Linardou H, Dahabreh IJ, Bafaloukos D, Kosmidis P, Murray S. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat Rev Clin Oncol. 2009;6:352–366. doi: 10.1038/nrclinonc.2009.62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.