SUMMARY
The H3K9me3 repressive histone conformation of p53 target promoters is abrogated in response to p53 activation by MDM2-mediated SUV39H1 degradation. Here, we present evidence that the USP7 deubiquitinase protects SUV39H1 from MDM2-mediated ubiquitination in the absence of p53 stimulus. USP7 occupies p53 target promoters in unstressed conditions, which is abrogated with p53 activation associated with loss of the H3K9me3 mark on these same promoters. Mechanistically, USP7 forms a trimeric complex with MDM2 and SUV39H1, independent of DNA, and modulates MDM2-dependent SUV39H1 ubiquitination. Further, we show that this protective function of USP7 on SUV39H1 is independent of p53. Finally, USP7 blocking cooperates with p53 in inducing apoptosis by enhancing p53 promoter occupancy and dependent transactivation of target genes. These results uncover a new layer of the p53 transcriptional program mediated by USP7, which restrains relaxation of local chromatin conformation at p53 target promoters.
Keywords: USP7, SUV39H1, H3K9me3, p53, MDM2
Graphical Abstract
eTOC Blurb
Mungamuri et al. show that in the absence of a p53 stimulus, USP7 plays a role in maintaining the H3K9me3 repressive mark on p53 targets by protecting SUV39H1 from MDM2-mediated degradation and enforcing heterochromatinization of these promoters.
INTRODUCTION
TP53 is a well-established tumor suppressor and cellular gatekeeper of genome stability (Bieging et al., 2014; Khoo et al., 2014). In response to homeostatic stresses, p53 is stabilized and recruits core transcriptional machinery proteins to its target promoters enabling transactivation of these genes with cellular outcomes including cell cycle arrest and apoptosis (Kruiswijk et al., 2015; Mandinova and Lee, 2011; Vousden and Prives, 2009; Zilfou and Lowe, 2009).
Chromatin conformation plays a major role in p53-dependent transcription (Allen et al., 2014; Beckerman and Prives, 2010; Botcheva, 2014; Su et al., 2015). p53 interacts with several co-factors, which have intrinsic histone-modifying activities (Liu et al., 1999; Vaziri et al., 2001), and with histone deacetylase complexes that act specifically to remodel chromatin (Brooks and Gu, 2011; Dai and Gu, 2010). We and others have shown that p53 target promoters are enriched with the H3K9me3 (histone H3 lysine9 trimethylation) mark and that p53 activation abrogates this repressive chromatin conformation through MDM2-mediated degradation of SUV39H1 (KMT1A), a major methyltransferase responsible for writing this mark (Bosch-Presegue et al., 2011; Choi et al., 2012; Mungamuri et al., 2012; Zheng et al., 2014). Further, lysine 87 of SUV39H1 has been identified as the primary site of MDM2-mediated ubiquitination (Bosch-Presegue et al., 2011).
In the absence of genotoxic stress, p53 is known to occupy target promoters such as p21, Gadd45α and PUMA, but remains transcriptionally inactive (Allen et al., 2014; Espinosa et al., 2003; Jackson and Pereira-Smith, 2006; Kaeser and Iggo, 2002). Specifically, MDM2 has been proposed to repress p53 activity when co-occupied on these promoters, with p53 stress stimuli relieving such repression (Kruse and Gu, 2009; Minsky and Oren, 2004). There is also evidence that degradation of SUV39H1, also present on these same promoters, is required for p53-dependent transcription (Choi et al., 2012; Mungamuri et al., 2012). However, the mechanisms responsible for protecting SUV39H1 from MDM2-mediated degradation and preserving the repressive H3K9me3 mark in the absence of stress-induced p53 stabilization remain to be elucidated.
USP7 (Ubiquitin Specific Peptidase 7) is known to have a dual role in the p53-MDM2 pathway, as it deubiquitinates both p53 and MDM2. USP7 overexpression counteracts MDM2-mediated p53 ubiquitination, which stabilizes p53, leading to apoptosis (Li et al., 2004; Li et al., 2002). Disruption of the USP7 gene is also lethal to p53 wt cells, as loss of USP7 expression enhances the auto-ubiquitination of MDM2 leading to its degradation, resulting in p53 stabilization and apoptosis (Cummins et al., 2004; Cummins and Vogelstein, 2004). The present studies define a new role of USP7 in the regulation of MDM2-mediated SUV39H1 degradation and maintenance of the H3K9me3 repressive chromatin conformation on p53 target promoters in unstressed cells.
RESULTS
USP7 enhances SUV39H1 stability, independent of p53
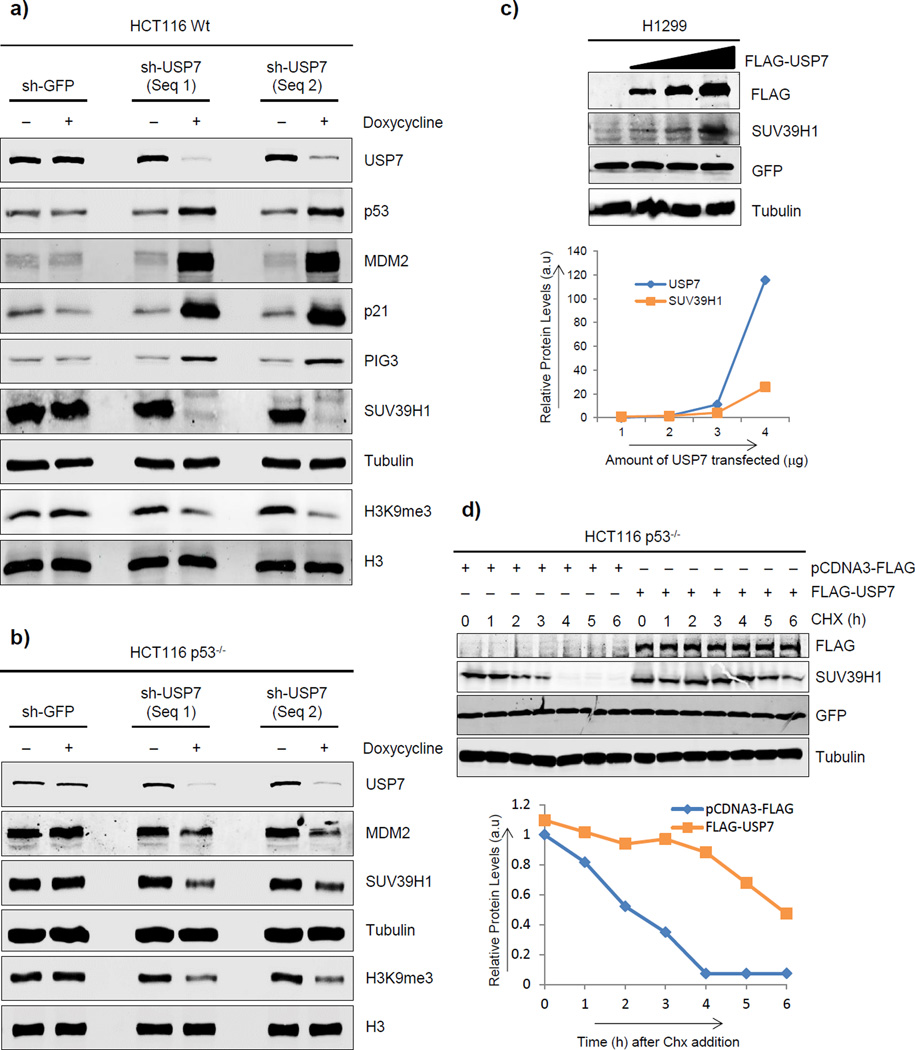
To investigate whether USP7 modulates SUV39H1 expression levels, we generated HCT116 p53 wt cells stably expressing doxycycline-inducible USP7 shRNA. Addition of doxycycline to the culture medium reduced USP7 expression as measured at both mRNA (Supplementary Fig. 1a) and protein (Fig. 1a) levels, and as expected, USP7 silencing stabilized p53, resulting in induction of its target genes (Fig. 1a; Supplementary Fig. 1a). We showed previously that p53 regulates SUV39H1 at the mRNA level through p21 and at the protein level through MDM2 (Mungamuri et al., 2012). Consistent with these data, we observed a decrease in SUV39H1 mRNA and protein levels under these conditions (Fig. 1a; Supplementary Fig. 1a), which also correlated with loss of the H3K9me3 histone mark (Fig. 1a). As a control, we generated similar stables in isogenic HCT116 p53−/− cells and analyzed the effect of USP7 silencing on SUV39H1 levels. USP7 knockdown in these cells had no effect on either the induction of p21 or down regulation of SUV39H1 mRNA levels (Supplementary Fig. 1b), but resulted in reduction both of SUV39H1 protein expression and H3K9me3 mark levels (Fig. 1b). Further, downregulation of SUV39H1 protein expression in response to USP7 silencing was inhibited when cells were treated with the proteasomal inhibitor, MG-132 (Supplementary Fig. 1c). All of these findings indicated that USP7 loss destabilizes the SUV39H1 protein independent of p53.
Figure 1. USP7 enhances SUV39H1 protein stability, independent of p53.
a, b) Western blot analysis of HCT116 p53 wt (a) and HCT116 p53−/− (b) cells stably transduced with doxycyclineinducible shGFP or shUSP7 (two sequences) and cultured in the presence of doxycycline for 48h. c) Western blot analysis of H1299 cells transiently overexpressing 1, 2 or 4µg of FLAG-USP7 for 24h. The amounts of proteins expressed (arbitrary units) are shown as a line diagram. d) Western blot analysis of HCT116 p53−/− cells transiently overexpressing FLAG-USP7 and treated with cycloheximide for indicated time points. The amounts of proteins expressed (arbitrary units) are shown as a line diagram. See also Figures S1 and S2.
To further investigate the role of USP7 in regulating SUV39H1 protein stability, we exogenously overexpressed USP7 in p53 null H1299 cells and observed a USP7 dose-dependent increase in SUV39H1 protein levels (Fig. 1c). These results further substantiated that USP7 modulates steady-state SUV39H1 levels independent of p53. Finally, we transiently overexpressed USP7 in HCT116 p53−/− cells, treated with cycloheximide to block new protein synthesis and observed a significant increase in SUV39H1 half-life (Fig. 1d), confirming that USP7 positively regulates SUV39H1 protein stability.
USP7 protects SUV39H1 from MDM2-mediated degradation
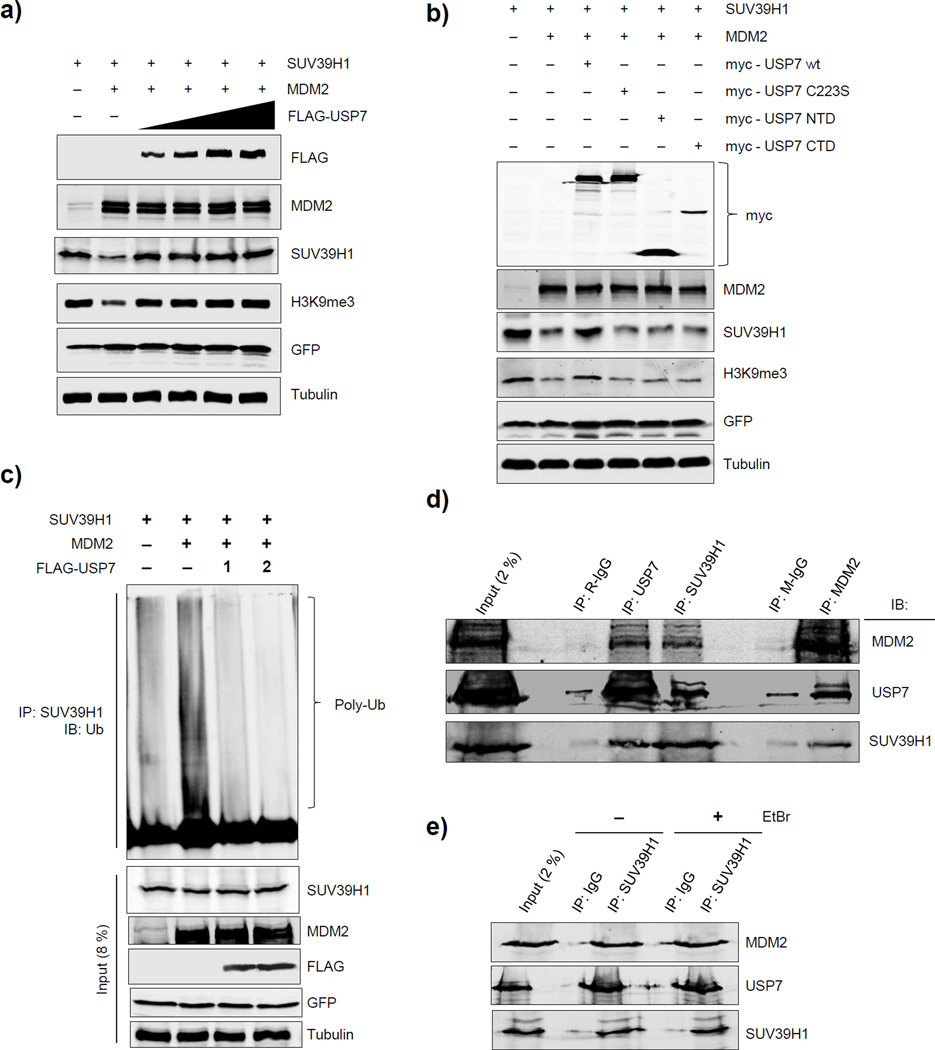
MDM2 ubiquitinates SUV39H1 and targets it for proteasomal degradation (Bosch-Presegue et al., 2011; Mungamuri et al., 2012). Thus, we tested whether USP7 protects SUV39H1 from MDM2-mediated degradation. Transient transfection of H1299 or HCT116 p53−/− cells revealed that exogenous MDM2 expression led to downregulation of exogenous SUV39H1 protein levels (Fig. 2a; Supplementary Fig. 2a), while USP7 co-transfection rescued SUV39H1 expression in a dose-dependent manner (Fig. 2a). Exogenous MDM2 expression in HCT116 p53−/− cells downregulated levels of exogenously expressed SUV39H1 wt but not an MDM2-resistant SUV39H1-K87A mutant, confirming previous findings (Bosch-Presegue et al., 2011) that lysine 87 is the site of ubiquitination by MDM2 (Supplementary Fig. 2a). Finally, doxycycline-induced shUSP7 expression in HCT116 p53−/− cells resulted in downregulation of exogenously expressed SUV39H1 wt but not SUV39H1-K87A protein levels (Supplementary Fig. 2a). These results support the conclusion that endogenous USP7 protects SUV39H1 from endogenous MDM2-mediated degradation.
Figure 2. USP7 interacts with SUV39H1 through MDM2 and protects SUV39H1 from MDM2-mediated degradation.
a) Western blot analysis of H1299 cells transfected with SUV39H1, MDM2 and 1, 2, 3 or 4µg of FLAG-USP7 for 24h. b) Western blot analysis of H1299 cells transfected with SUV39H1, MDM2 and either myc-tagged USP7 wt, C223S, NTD or CTD expression constructs for 24h. c) Western blot analysis of SUV39H1 immunoprecipitated samples. H1299 cells were transfected with the indicated plasmids and 24h later SUV39H1 protein was immunoprecipitated and loaded on a gradient SDS-PAGE gel, and probed with an anti-ubiquitin antibody. 8% of the sample used for IP was also loaded and showed as the input. d) Western blot analysis of immunoprecipitates from HCT116 p53 wt cell lysates. 2% of the sample used for IP was also loaded and showed as the input. e) Western blot analysis of immunoprecipitates from HCT116 p53 wt cell lysates. The cell lysates for treated with EtBr to denature the DNA, before performing immunoprecipitation. 2% of the sample used for IP was also loaded and showed as the input. See also Figures S3.
USP7 activity can also be inhibited at sub-micro molar concentrations by HBX41108, a cyano-indenopyrazine derivative (Colland et al., 2009). In MDM2 non-silenced p53 null H1299 cells, USP7 inhibition using HBX41108 resulted in reduced levels of SUV39H1 wt but not of SUV39H1 K87A (site of MDM2 ubiquitination) (Supplementary Fig. 2b). When the same cells were pre-silenced for MDM2, HBX41108 treatment did not result in a decrease in either SUV39H1 wt or SUV39H1 K87A protein levels (Supplementary Fig. 2b). These results indicate that MDM2 is the E3 ligase for SUV39H1 (ubiquitinating at K87 residue) and that USP7 acts to stabilize SUV39H1 only in the presence of basal levels of MDM2. Furthermore, a USP7 catalytically inactive mutant (C223S), as well as the wild type protein’s N-terminal domain (NTD) or C-terminal domain (CTD) were unable to protect SUV39H1 from MDM2-mediated degradation, substantiating that functional, full length USP7 is required for this protection (Fig. 2b). In previous studies, we observed that MDM2-mediated SUV39H1 loss correlated with global reduction of the H3K9me3 mark (Mungamuri et al., 2012). In good agreement, USP7-mediated protection of SUV39H1 from MDM2-mediated degradation rescued H3K9me3 levels in the same cells, under the same conditions (Fig. 2a, b; Supplementary Fig. 2a).
Overexpressed SUV39H1 displayed constitutive ubiquitination, which was enhanced by exogenous MDM2 expression (Fig. 2c), whereas exogenous USP7 expression decreased this ubiquitination in a dose-dependent manner (Fig. 2c). Collectively, these findings argue strongly that USP7 protects SUV39H1 from MDM2-mediated degradation.
USP7 forms a trimeric protein complex with SUV39H1 and MDM2
USP7 has been shown to protect both MDM2 from auto-ubiquitination and p53 from MDM2-mediated ubiquitination via physical interactions (Cummins et al., 2004; Li et al., 2002; Sheng et al., 2006). Thus, we tested the nature of the interactions, if any, between USP7, SUV39H1 and MDM2. Immunoprecipitation followed by immunoblot analysis of endogenous proteins using USP7, SUV39H1 or MDM2 antibody in HCT116 wt cells resulted in pull down of the other two endogenous proteins, implying the presence of a tri-molecular protein complex in unstressed cells (Fig. 2d). Further, this tri-molecular complex was equally detectable in cell lysates treated with ethidium bromide (Fig. 2e). Whether or not the chromatin landscape, specifically the p53 RE (response element) is required for formation of this tri-molecular complex, these findings suggest that its stability is independent of DNA.
To further understand the role of USP7 catalytic activity and protein domains required for its interaction with SUV39H1, we used different FLAG-tagged USP7 constructs. Co-expression of FLAG-tagged USP7 wt and SUV39H1, followed by FLAG-antibody pull-down and immunoblot analysis established that USP7 forms an easily detectable protein complex with SUV39H1 (Supplementary Fig. 3a). Furthermore, a FLAG-tagged USP7-C223S mutant was also able to pull-down SUV39H1 to a similar extent as USP7 wt (Supplementary Fig. 3b). These results indicated that USP7-SUV39H1 complex formation was independent of USP7 catalytic activity. Next, we asked which domains of USP7 mediate this interaction by co-immunoprecipitation experiment using constructs expressing different USP7 domains. While the USP7-NTD was able to pull down SUV39H1 to a similar extent as full-length USP7, the USP7-CTD showed no detectable interaction (Supplementary Fig. 3b), indicating that the N-terminal domain of USP7 is essential for protein complex formation with SUV39H1.
Previous studies have shown that MDM2 physically interacts with USP7 (Sheng et al., 2006), and also forms a complex with SUV39H1 (Fahraeus and Olivares-Illana, 2014). We observed a trimolecular protein complex between endogenous USP7, SUV39H1 and MDM2 (Fig. 2d, e). To assess whether USP7-SUV39H1 complex formation was dependent on MDM2, we performed USP7 immunoprecipitation in the absence and presence of MDM2 silencing (Supplementary Fig. 3c). USP7 was unable to form a protein complex with SUV39H1 in MDM2 pre-silenced cells (Supplementary Fig. 3d). Moreover, MDM2 silencing in p53−/− cells did not increase SUV39H1 steady-state levels, arguing that USP7 stabilization of SUV39H1 occurred only in the presence of MDM2 (Supplementary Fig. 3e). Further, overexpression of USP7 enhanced the endogenous study-state levels of SUV39H1 only in control cells, but not in the cells in which MDM2 was pre-silenced (Supplementary Fig. 3f). Collectively, these results indicate that shared physical interactions of USP7 and SUV39H1 with MDM2 are required for either for USP7 to deubiquitinate SUV39H1 or for MDM2 to ubiquitinate SUV39H1; both resulting in enhanced SUV39H1 stability. Further, our data that silencing MDM2 in p53 null cells, does not further stabilize SUV39H1, argues that silencing MDM2 not only abrogated the degradation of SUV39H1, but also inhibited SUV39H1 interaction with USP7, and thus as a net effect has no change in SUV39H1 protein levels.
USP7 occupies p53 target promoters and maintains the H3K9me3 mark
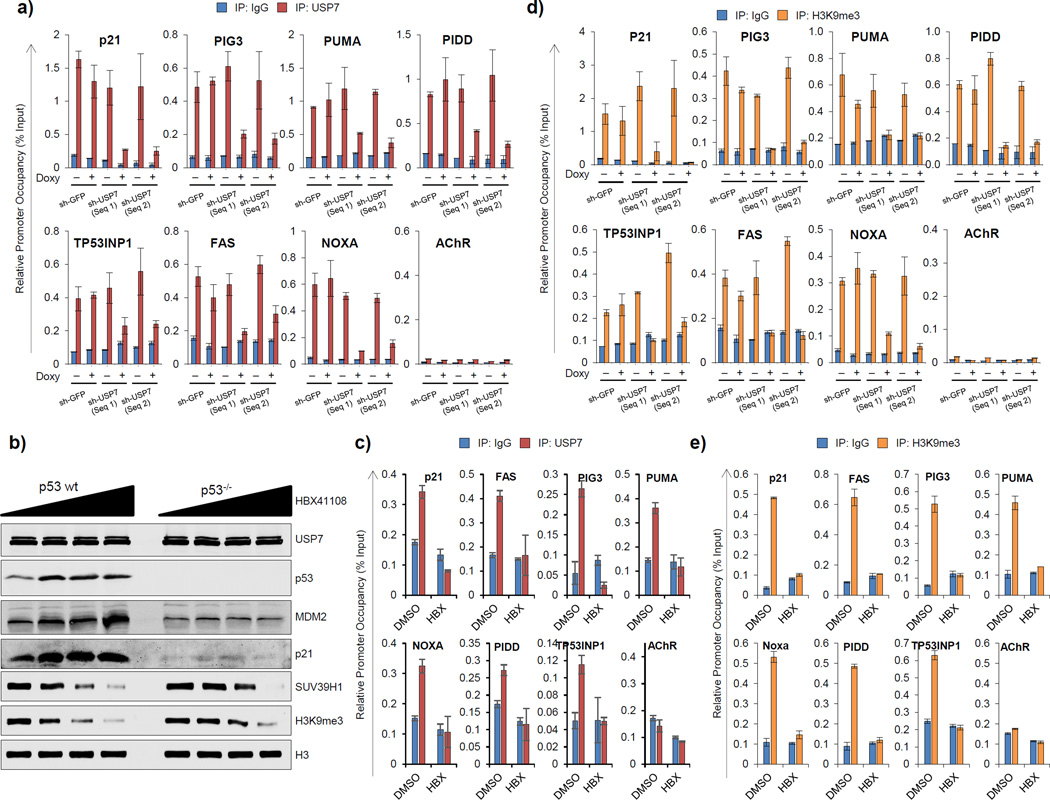
One possible mechanism for retention of SUV39H1/ H3K9me3 mark on p53 target promoters in unstressed p53 wt cells could be that USP7 is also recruited at these sites and protects SUV39H1 from MDM2-mediated degradation. In fact, chromatin immunoprecipitation (ChIP) analysis indicated that USP7 was enriched on p53 target promoters in HCT116 (Supplementary Fig. 4a) and B5/589 cells (Supplementary Fig. 4b), both harboring wt p53.
In response to nutlin3a-treatment, we observed increased p53 occupancy on its target promoters (Supplementary Fig. 4c), associated with a decrease in USP7 occupancy on these same p53 target promoters (Supplementary Fig. 4a, b). To further validate this data, we have performed a time course analysis (0, 1, 2, 3 and 4hrs) of nutlin3 treatment of p53 wt cells to investigate how p53 enrichment influences the presence of USP7 and the H3K9me3 mark on p53 target promoters. The results establish that as p53 promoter occupancy increases with time of nutlin3 treatment, there is a decrease in USP7 occupancy and H3K9me3 enrichment on these same promoters (Supplementary Fig. 5b, c, d). Moreover these results establish that the loss of USP7 occupancy and H3K9me3 enrichment on p21 and PUMA promoters precede detectable increases in p53 promoter occupancy. All of these findings are consistent with a model in which basal recruitment of MDM2 by p53 leads to basal recruitment of USP7-SUV39H1 and a basal level of H3K9me3. Under these same conditions, we observed no detectable changes in total cellular USP7 levels, as measured at both mRNA (Supplementary Fig. 6a, b) and protein (Supplementary Fig. 6c, 5a) levels.
We showed previously that the H3K9me3 repressive histone mark is enriched on p53 target promoters (Mungamuri et al., 2012; Mungamuri et al., 2014). USP7 knockdown by shRNA reduced total cellular USP7 levels (Fig. 1a, b) as well as USP7 promoter occupancy in p53 wt cells (Fig. 3a). Pharmacological inhibition of USP7 did not reduce the total cellular pool of USP7 either in p53 wt or in p53 null cells, as measured at both mRNA (Supplementary Fig. 6d) and protein (Fig. 3b) levels. In HCT116 p53 wt cells, such inhibition stabilized p53 protein (Fig. 3b), associated with enhanced p53 promoter occupancy (data not shown) and transactivation of p53 target genes (Fig. 3b; Supplementary Fig. 6d), as well as reduced USP7 occupancy on these same p53 target promoters (Fig. 3c). In contrast, in p53 null cells, HBX41108 treatment did not induce p53 pro-apoptotic target gene expression (Supplementary Fig. 6d). These results, collectively argue that p53 stabilization/promoter occupancy is required for removal of USP7 from p53 target promoters.
Figure 3. USP7 occupies p53 target promoters and maintains the H3K9me3 mark on these promoters.
a) ChIP analysis showing USP7 occupancy on p53 target promoters in HCT116 wt cells stably transduced with inducible shGFP or shUSP7 (two sequences) and cultured in the presence of doxycycline for 48h. b) Western blot analysis of HCT116 p53 wt and p53−/− cells treated with 0, 2.5, 5 or 10µM of HBX41108 for 24h. c) ChIP analysis showing H3K9me3 enrichment on p53 target promoters in HCT116 p53 wt cells stably transduced with inducible shGFP or shUSP7 (two sequences) and cultured in the presence of doxycycline for 48h. d, e) ChIP analysis showing USP7 occupancy (d) and H3K9me3 enrichment (e) in HCT116 p53 wt cells treated with 10µM of HBX41108 for 24h. See also Figures S4, S5 and S6.
Both shRNA-mediated USP7 silencing and pharmacological USP7 inhibition in HCT116 p53 wt cells led to global reduction of H3K9me3 levels (Fig. 1a, 3b) as well as abrogation of H3K9me3 mark enrichment on each of the p53 target promoters analyzed (Fig. 3d, e). Further, the loss of USP7 occupancy on p53 target promoters correlated with the abrogation of the H3K9me3 mark on these same promoters in a time-dependent manner (Supplementary Fig. 5d). These results argue that functional USP7 is required to maintain SUV39H1-dependent H3K9me3 enrichment on p53 target promoters.
Reduced USP7 expression/activity enhances chemotherapy-induced p53-dependent apoptosis
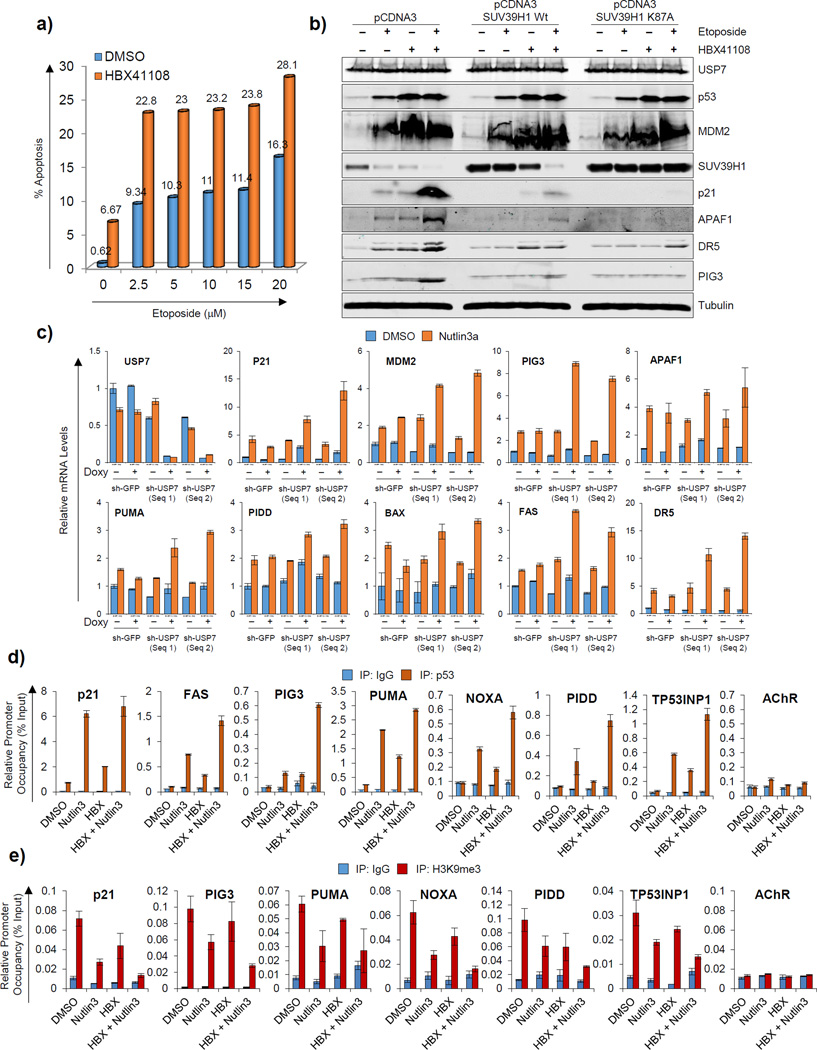
Next, we sought to determine the functional significance of USP7-mediated stabilization of SUV39H1 on p53-dependent cell fate decisions. Etoposide, a topoisomerase II inhibitor, induced apoptosis in HCT116 p53 wt cells in a dose-dependent manner, as analyzed by propidium iodide staining (Fig. 4a; Supplementary Fig. 7a). The same cells showed significantly increased apoptosis when either USP7 was pharmacologically inhibited by pretreatment with HBX41108 (Fig. 4a; Supplementary Fig. 7a) or when USP7 expression was inhibited using shRNA (Supplementary Fig. 8). The increases in apoptosis observed at different levels of etoposide in combination with HBX41108 and with shUSP7 were greater in each case than the sum of either HBX41108 / shUSP7 or etoposide treatment alone. Further, this enhanced apoptosis correlated well with increased induction of p53 target genes as measured at both protein (Fig. 4b) and mRNA levels (Fig. 4c; Supplementary Fig. 7b). HBX41108 also cooperated with nutlin3a-stablized p53 to transactivate its target genes (data not shown), which was associated with enhanced p53 promoter occupancy (Fig. 4d). Further, we also analyzed the H3K9me3 enrichment on p53 target promoters in HCT116 p53 wt cells under these conditions. The results show that while etoposide or HBX41108 treatment alone decreased H3K9me3 enrichment on p53 target promoters, their combined treatment further down regulated H3K9me3 enrichment on these same promoters (Fig. 4e). Thus loss of USP7 function favors increased p53 promoter occupancy and dependent transcription of its target genes through abrogation of the H3K9me3 mark.
Figure 4. Pharmacological inhibition of USP7 or USP7 pre-silencing enhances chemotherapy-induced apoptosis in a P53-dependent manner.
a) Propidium iodide (PI) staining in HCT116 p53 wt cells pre-treated with either DMSO or 2.5µM of HBX41108 for 24h followed by treatment with increasing doses of etoposide for 48h. The percentage of cells showing less than a 2N content of DNA (apoptosis) in each condition is shown in the table (see Supplementary Fig. 7a for actual FACS graphs). b) Western blot analysis of HCT116 p53 wt cells stably expressing SUV39H1 wt or SUV39H1-K87A are pre-treated with either DMSO or 2.5µM of HBX41108 for 24h followed by treatment with 5µM of etoposide for another 24h. c) Real-time quantitative PCR analysis of HCT116 p53 wt cells stably transduced with inducible shGFP or shUSP7 (two sequences) and cultured in the presence of doxycycline for 24h (instead of 48h; in order to minimize the extent of p53 activation of its target genes and optimize the ability to detect cooperation) followed by treating cells with 10µM of nutlin3a for another 16h. d, e) ChIP analysis showing p53 occupancy (d) and H3K9me3 enrichment (e) on p53 target promoters in HCT116 p53 wt cells pre-treated with either DMSO or 2.5µM of HBX41108 for 24h followed by treatment with 10µM of nutlin3a for another 16h. See also Figures S7, S8 and S9.
Finally, we confirmed the enhanced p53 occupancy/transactivation in response to USP7 inhibition was indeed due to abrogation of the H3K9me3 mark on its target promoters, using HCT116 p53 wt cells stably overexpressing either SUV39H1 wt or SUV39H1-K87A. In contrast to vector stables, expression of p53 targets was inhibited in response to etoposide or HBX41108-mediated stabilization of p53 in cells stably overexpressing SUV39H1 wt (Fig. 4b). In SUV39H1 wt overexpressing cells, etoposide and HBX41108, either as single agents or in combination induced p53 targets less efficiently compared to vector stables, and is associated with higher residual levels of SUV39H1 (Fig. 4b). In SUV39H1-K87A overexpressing cells, neither etoposide nor HBX41108 alone or in combination was able to induce p53 target genes (Fig. 4b). In good agreement, we did not observe degradation of SUV39H1-K87A under any of these conditions (Fig. 4b), despite comparable levels of p53 stabilization and MDM2 induction across all of the cells analyzed (Fig. 4b). All of these findings argue that decreased USP7 expression/activity leads to abrogation of the H3K9me3 repressive histone mark on p53 target promoters, enhancing p53-promoter occupancy, transactivation of its target genes and the proapoptotic response.
SUV39H1 is known to influence global gene expression (Kondo et al., 2008; Peters et al., 2001), and we reported previously that p53 regulates the expression of growth hormone receptor (GHR), myostatin (GDF8), scinderin (SCIN) and ets homologous factor (EHF) through downregulation of SUV39H1, even though these gene promoters lack p53 binding sites (Mungamuri et al., 2012). Thus, we analyzed the expression of these same genes in response to USP7 and MDM2 silencing. While USP7 silencing induced the expression of GHR, GDF8, SCIN and EHF (Supplementary Fig. 9a), silencing MDM2 had no effect on the induction of these genes (Supplementary Fig. 9b). All of these results argue that USP7 regulates SUV39H1 steady-state protein levels and that down regulation of USP7 mimics down regulation of SUV39H1, leading to abrogation of the H3K9me3 mark and transactivation of target promoters. Further studies will be necessary to determine the global effects on gene expression mediated by USP7 and specifically how many of these changes can be attributed to its modulation of SUV39H1/H3K9me3 levels.
DISCUSSION
A number of studies have indicated that in unstressed wt p53 harboring cells, p53 occupies target promoters, yet remains transcriptionally inactive (Espinosa et al., 2003; Jackson and Pereira-Smith, 2006; Kaeser and Iggo, 2002). An anti-repression mechanism has been postulated in which MDM2 co-occupies these promoters and represses p53-dependent transcription (Kruse and Gu, 2009; Minsky and Oren, 2004). Cellular stresses, which stabilize p53, result in MDM2 promoter clearance, thus relieving MDM2 repression and allowing p53 to function. The presence of the H3K9me3 histone repressive mark represents another major obstacle to p53 transactivation in unstressed cells. We recently established that the repressive chromatin conformation associated with this mark inhibits p53-dependent transcription of its target genes and that p53 overcomes such repression through MDM2-mediated degradation of SUV39H1, the writer of this mark (Mungamuri et al., 2012).
The MDM2 E3 ubiquitin ligase is both a p53 transcriptional target and in a negative feedback loop, targets p53 for proteasomal degradation (Beckerman and Prives, 2010). The USP7 deubiquitinase is known to modulate the p53 pathway both by protecting p53 from MDM2-mediated degradation (Cummins et al., 2004) and MDM2 from auto-ubiquitination (Li et al., 2004). Evidence that USP7 is present in the chromatin fraction (Maertens et al., 2010) and that MDM2 also degrades SUV39H1, led us to investigate a possible role of USP7 in protecting SUV39H1 from MDM2-mediated degradation. Our present studies establish that USP7 is enriched on p53 target promoters and modulates SUV39H1/H3K9me3 levels in a MDM2-dependent manner. Moreover, either USP7 knockdown or a USP7 small molecule inhibitor led to a global decrease in SUV39H1 protein and H3K9me3 levels as well as abrogation of the H3K9me3 mark on p53 pro-apoptotic target promoters. Thus, our findings support the concept that USP7 through its interactions with SUV39H1 and MDM2 plays an important role in enforcing MDM2-mediated repression of p53-dependent transcription of its target genes in unstressed cells.
Previous reports have indicated that MDM2 binds USP7 through interactions mediated by MDM2 aa147–159 (Sheng et al., 2006), while aa200–262 are involved in MDM2 binding to SUV39H1 (Fahraeus and Olivares-Illana, 2014). Our present studies demonstrate that USP7, SUV39H1 and MDM2 exist in a trimeric complex and that the USP7-MDM2-SUV39H1 complex keeps MDM2 inactive under basal conditions. These findings are consistent with a model in which USP7 regulates SUV39H1 protein levels indirectly by modulating MDM2 E3 ligase activity. Alternatively, MDM2 binding of both USP7 and SUV39H1 may place these molecules in close proximity such that low affinity interaction not detectable in the absence of MDM2 may allow USP7 to directly deubiquitinate SUV39H1. By either mechanism, our results establish that USP7 modulates the repressive H3K9me3 mark by protecting SUV39H1 from MDM2-mediated degradation and blocking aberrant p53 transactivation of target genes in unstressed p53 wt cells. Whether this protection occurs on p53 target promoters alone and/or elsewhere within the cell as well as the physiological significance of these interactions in a p53 null context remain to be elucidated.
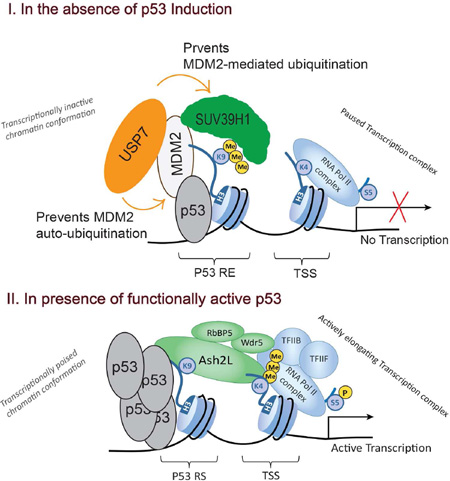
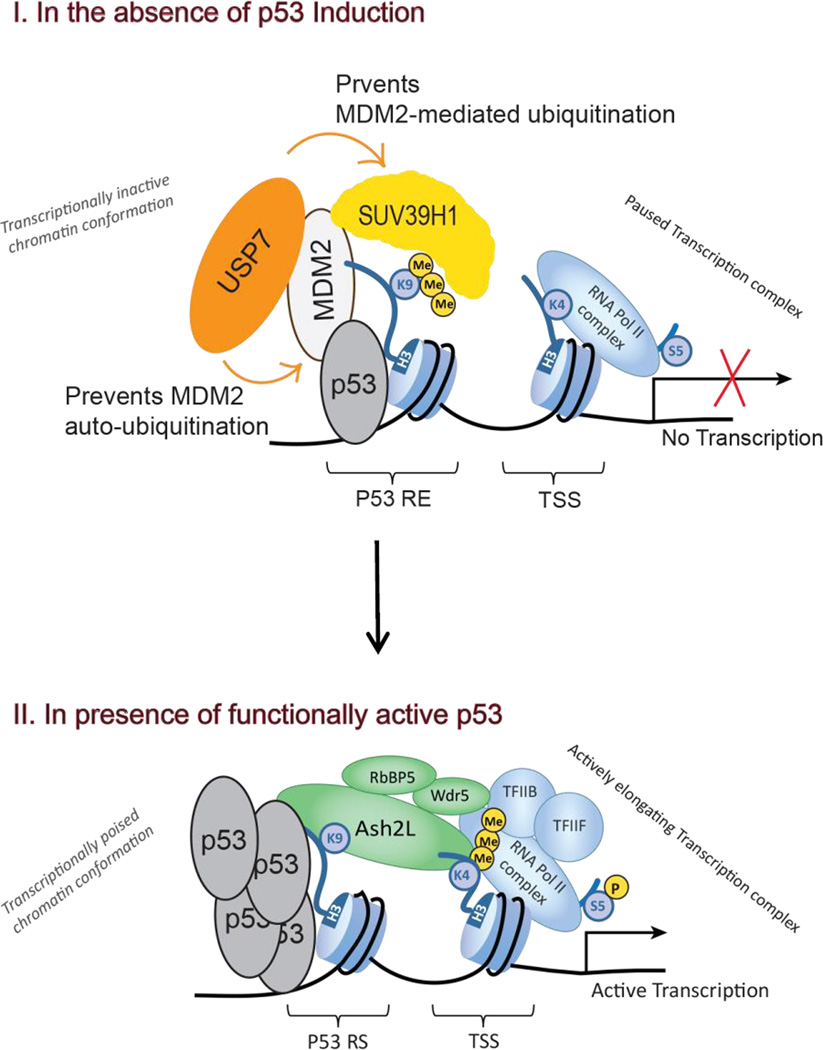
Our present findings as well as data available from previous reports indicate that 'basal' levels of mostly transcriptionally inactive p53 enable the recruitment of the USP7-MDM2-SUV39H1 complex to p53 REs via p53-MDM2 interaction, placing the H3K9me3 repressive mark and maintaining low basal transcription activity of p53 target genes (Fig. 5). Upon p53 activation by stress stimuli or by nutlin3, p53-MDM2 interaction is disrupted, leading to increased p53 accumulation and chromatin binding, leading to productive transcription. MDM2 accumulation, as a result of p53-dependent transcription, blocks SUV39H1-mediated H3K9me3 enrichment and its repressive effect by triggering SUV39H1 degradation. Thus, p53-dependent MDM2 accumulation serves two purposes: 1) a negative feedback loop via p53 degradation once the stressful stimulus is relieved, and 2) a fast forward loop whereas p53 transactivation is enhanced via degradation of the SUV39H1.
Figure 5. Schematic diagram illustrating the role of USP7 in maintaining H3K9me3 mark on p53 target promoters.
I) In the absence of p53 induction, 'basal' levels of mostly transcriptionally inactive p53 enable the recruitment of the MDM2-USP7-SUV39H1 complex to p53 REs via the p53-MDM2 interaction, placing the repressive mark H3K9me3 and keeping basal transcription activity of the target genes low. USP7 prevents both MDM2 auto-ubiquitination and MDM2-mediated SUV39H1 degradation. II) With p53 induction, USP7 promoter occupancy reduces, MDM2 accumulates in the cell, both leading to degradation of SUV39H1 and loss of H3K9me3 mark on p53 target promoters favoring enhanced p53 promoter occupancy, subsequently leading to H3K4me3 enrichment at TSS (also see Mungamuri et al., 2012 and Mungamuri et al., 2014).
Several studies have shown that USP7 directly affects chromatin structure, by deubiquitinating and stabilizing histone H2A (Lecona et al., 2015), and histone acetyl transferase TIP60 (Gao et al., 2013), both of which enhance p53 function (Dar et al., 2013; Shema et al., 2008). Our present findings demonstrate that USP7 also has a major impact on p53 pro-apoptotic signaling by altering H3K9me3 enrichment on p53 target promoters, uncovering a new layer of p53 regulation at the chromatin level. We showed that inhibition of USP7 function through shRNA-mediated knock-down or by pharmacological inhibition cooperated with chemotherapy in inducing apoptosis in a p53-dependent manner by favoring p53 promoter occupancy and transactivation of its target genes. Such cooperation may be clinically beneficial in allowing the use of lower levels of chemotherapy to achieve a better therapeutic index (Cheon and Baek, 2006; Nicholson and Suresh Kumar, 2011).
EXPERIMENTAL PROCEDURES
Cell lines Plasmids, and treatments
HCT116 p53 wt, HCT116 p53−/−, B5/589 (p53 wt), H1299 (p53 null) cells (Mungamuri et al., 2012; Mungamuri et al., 2014) cancer cells were used. Specific gene silencing was achieved by using either pTripZ (Open Biosystems) or Tet-pLKO-puro (Addgene # 21915) vectors. Human SUV39H1 cDNA is previously described (Mungamuri et al., 2012). MDM2 and FLAG-USP7 cDNA (Cummins et al., 2004; Zhou et al., 2001) were obtained from Addgene (#16233 and #16655). Myc-USP7 wt and other deletion constructs are described previously (Sarkari et al., 2010). Please see the supplementary extended procedures for the list of chemicals and their concentrations used in this study.
Real-Time Quantitative PCR and Western Blotting
RNA extraction and quantitative real-time PCR was performed as described previously (Mungamuri et al., 2012). Western blotting and Flow cytometry were done as described previously (Mungamuri et al., 2012). Please see the supplementary information for the list of antibodies used in the study. All blots were developed using the Odyssey fluorescence image scanner. Please see Table S1 for the sequences of primers used.
Immunoprecipitation assay
For Immunoprecipitation, cells were lysed in IP lysis buffer and pre cleared with Protein A beads before immunoprecipitation. Protein lysates were incubated with antibody overnight, and the antibody complex was precipitated using Protein A beads. To analyze the role of DNA in protein interactions the immunoprecipitate was treated with ethedium bromide.
ChIP assay
ChIP experiments were performed as described previously (Mungamuri et al., 2014). The target sequences were detected by quantitative real-time PCR analysis of eluted DNA. The relative promoter occupancy over the input percentage is shown as a bar diagram. Acetylcholine receptor (AChR) is used as a negative control. Please see Table S1 for the sequences of primers used.
Generation of stable cell lines
HCT116 p53 wt, HCT116 p53−/− and H1299 cells were infected with either Tet-pLKO-shUSP7 or pTripZ: shMDM2 lentivirus and selected for puromycin (2µg/ml) resistance. Resistant clones were pooled to generate doxycycline-inducible shRNA cell lines.
For further details, please refer to the Extended Experimental Procedures.
Supplementary Material
Highlights.
-
➢
USP7 enhances SUV39H1 stability.
-
➢
USP7 forms a trimeric protein complex with SUV39H1 and MDM2.
-
➢
USP7 occupies p53 target promoters and maintains the H3K9me3 mark.
-
➢
Reduced USP7 activity enhances chemotherapy-induced p53-dependent apoptosis.
Acknowledgments
We thank Dr. L Frappier, Canada for providing USP7 deletion constructs. SKM acknowledges the Leo and Julia Forchheimer Foundation for fellowship support. This research was supported by grants from the National Cancer Institute (P01CA080058) and Breast Cancer Research Foundation. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the US National Institutes of Health.
Abbreviations
- USP7
Ubiquitin-specific-processing protease 7
- p53
Tumor suppressor protein tp53
- SUV39H1
suppressor of variegation 3–9 homolog 1 (Drosophila)
- MDM2
MDM2 protooncogene
- E3
ubiquitin ligase
- H3K9me3
Histone H3 lysine 9 trimethylation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
AUTHOR CONTRIBUTIONS
S.K.M. planned the project and conducted all the experiments. RFQ and SY provided the technical help. S.A.A. supervised the study. WG and JJM provided advice. S.K.M. and S.A.A. wrote the manuscript.
Contributor Information
Sathish Kumar Mungamuri, Email: Sathish.Mungamuri@mssm.edu.
Rui F. Qiao, Email: rui.qiao@mssm.edu.
Shen Yao, Email: shen.yao@mssm.edu.
James J. Manfredi, Email: wg8@cumc.columbia.edu.
Wei Gu, Email: james.manfredi@mssm.edu.
REFERENCES
- 1.Allen MA, Andrysik Z, Dengler VL, Mellert HS, Guarnieri A, Freeman JA, Sullivan KD, Galbraith MD, Luo X, Kraus WL, et al. Global analysis of p53-regulated transcription identifies its direct targets and unexpected regulatory mechanisms. eLife. 2014;3:e02200. doi: 10.7554/eLife.02200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckerman R, Prives C. Transcriptional regulation by p53. Cold Spring Harbor perspectives in biology. 2010;2:a000935. doi: 10.1101/cshperspect.a000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nature reviews. Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch-Presegue L, Raurell-Vila H, Marazuela-Duque A, Kane-Goldsmith N, Valle A, Oliver J, Serrano L, Vaquero A. Stabilization of Suv39H1 by SirT1 is part of oxidative stress response and ensures genome protection. Molecular cell. 2011;42:210–223. doi: 10.1016/j.molcel.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 5.Botcheva K. p53 binding to human genome: crowd control navigation in chromatin context. Frontiers in genetics. 2014;5:447. doi: 10.3389/fgene.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks CL, Gu W. The impact of acetylation and deacetylation on the p53 pathway. Protein Cell. 2011;2:456–462. doi: 10.1007/s13238-011-1063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheon KW, Baek KH. HAUSP as a therapeutic target for hematopoietic tumors (review) International journal of oncology. 2006;28:1209–1215. [PubMed] [Google Scholar]
- 8.Choi JD, Park MA, Lee JS. Suppression and recovery of BRCA1-mediated transcription by HP1gamma via modulation of promoter occupancy. Nucleic acids research. 2012;40:11321–11338. doi: 10.1093/nar/gks947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colland F, Formstecher E, Jacq X, Reverdy C, Planquette C, Conrath S, Trouplin V, Bianchi J, Aushev VN, Camonis J, et al. Small-molecule inhibitor of USP7/HAUSP ubiquitin protease stabilizes and activates p53 in cells. Molecular cancer therapeutics. 2009;8:2286–2295. doi: 10.1158/1535-7163.MCT-09-0097. [DOI] [PubMed] [Google Scholar]
- 10.Cummins JM, Rago C, Kohli M, Kinzler KW, Lengauer C, Vogelstein B. Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature. 2004;428 doi: 10.1038/nature02501. 1 p following 486. [DOI] [PubMed] [Google Scholar]
- 11.Cummins JM, Vogelstein B. HAUSP is required for p53 destabilization. Cell cycle. 2004;3:689–692. [PubMed] [Google Scholar]
- 12.Dai C, Gu W. p53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med. 2010;16:528–536. doi: 10.1016/j.molmed.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dar A, Shibata E, Dutta A. Deubiquitination of Tip60 by USP7 determines the activity of the p53-dependent apoptotic pathway. Molecular and cellular biology. 2013;33:3309–3320. doi: 10.1128/MCB.00358-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinosa JM, Verdun RE, Emerson BM. p53 functions through stress-and promoter-specific recruitment of transcription initiation components before and after DNA damage. Molecular cell. 2003;12:1015–1027. doi: 10.1016/s1097-2765(03)00359-9. [DOI] [PubMed] [Google Scholar]
- 15.Fahraeus R, Olivares-Illana V. MDM2's social network. Oncogene. 2014;33:4365–4376. doi: 10.1038/onc.2013.410. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Koppen A, Rakhshandehroo M, Tasdelen I, van de Graaf SF, van Loosdregt J, van Beekum O, Hamers N, van Leenen D, Berkers CR, et al. Early adipogenesis is regulated through USP7-mediated deubiquitination of the histone acetyltransferase TIP60. Nature communications. 2013;4:2656. doi: 10.1038/ncomms3656. [DOI] [PubMed] [Google Scholar]
- 17.Jackson JG, Pereira-Smith OM. p53 is preferentially recruited to the promoters of growth arrest genes p21 and GADD45 during replicative senescence of normal human fibroblasts. Cancer research. 2006;66:8356–8360. doi: 10.1158/0008-5472.CAN-06-1752. [DOI] [PubMed] [Google Scholar]
- 18.Kaeser MD, Iggo RD. Chromatin immunoprecipitation analysis fails to support the latency model for regulation of p53 DNA binding activity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:95–100. doi: 10.1073/pnas.012283399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoo KH, Verma CS, Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. Nature reviews. Drug discovery. 2014;13:217–236. doi: 10.1038/nrd4236. [DOI] [PubMed] [Google Scholar]
- 20.Kondo Y, Shen L, Ahmed S, Boumber Y, Sekido Y, Haddad BR, Issa JP. Downregulation of histone H3 lysine 9 methyltransferase G9a induces centrosome disruption and chromosome instability in cancer cells. PloS one. 2008;3:e2037. doi: 10.1371/journal.pone.0002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nature reviews. Molecular cell biology. 2015;16:393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 22.Kruse JP, Gu W. Modes of p53 Regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lecona E, Narendra V, Reinberg D. Usp7 Cooperates with Scml2 to Regulate the Activity of Prc1. Molecular and cellular biology. 2015 doi: 10.1128/MCB.01197-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Brooks CL, Kon N, Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Molecular cell. 2004;13:879–886. doi: 10.1016/s1097-2765(04)00157-1. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416:648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Scolnick DM, Trievel RC, Zhang HB, Marmorstein R, Halazonetis TD, Berger SL. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Molecular and cellular biology. 1999;19:1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maertens GN, El Messaoudi-Aubert S, Elderkin S, Hiom K, Peters G. Ubiquitin-specific proteases 7 and 11 modulate Polycomb regulation of the INK4a tumour suppressor. EMBO J. 2010;29:2553–2565. doi: 10.1038/emboj.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandinova A, Lee SW. The p53 pathway as a target in cancer therapeutics: obstacles and promise. Science translational medicine. 2011;3:64rv61. doi: 10.1126/scitranslmed.3001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minsky N, Oren M. The RING domain of Mdm2 mediates histone ubiquitylation and transcriptional repression. Molecular cell. 2004;16:631–639. doi: 10.1016/j.molcel.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Mungamuri SK, Benson EK, Wang S, Gu W, Lee SW, Aaronson SA. p53-mediated heterochromatin reorganization regulates its cell fate decisions. Nature structural & molecular biology. 2012;19:478–484. S471. doi: 10.1038/nsmb.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mungamuri SK, Wang S, Manfredi JJ, Gu W, Aaronson SA. Ash2L enables P53-dependent apoptosis by favoring stable transcription pre-initiation complex formation on its pro-apoptotic target promoters. Oncogene. 2014;0 doi: 10.1038/onc.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholson B, Suresh Kumar KG. The multifaceted roles of USP7: new therapeutic opportunities. Cell biochemistry and biophysics. 2011;60:61–68. doi: 10.1007/s12013-011-9185-5. [DOI] [PubMed] [Google Scholar]
- 33.Peters AH, O'Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 34.Sarkari F, Sheng Y, Frappier L. USP7/HAUSP promotes the sequence-specific DNA binding activity of p53. PloS one. 2010;5:e13040. doi: 10.1371/journal.pone.0013040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shema E, Tirosh I, Aylon Y, Huang J, Ye C, Moskovits N, Raver-Shapira N, Minsky N, Pirngruber J, Tarcic G, et al. The histone H2B-specific ubiquitin ligase RNF20/hBRE1 acts as a putative tumor suppressor through selective regulation of gene expression. Genes Dev. 2008;22:2664–2676. doi: 10.1101/gad.1703008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheng Y, Saridakis V, Sarkari F, Duan S, Wu T, Arrowsmith CH, Frappier L. Molecular recognition of p53 and MDM2 by USP7/HAUSP. Nature structural & molecular biology. 2006;13:285–291. doi: 10.1038/nsmb1067. [DOI] [PubMed] [Google Scholar]
- 37.Su D, Wang X, Campbell MR, Song L, Safi A, Crawford GE, Bell DA. Interactions of Chromatin Context, Binding Site Sequence Content, and Sequence Evolution in Stress-Induced p53 Occupancy and Transactivation. PLoS genetics. 2015;11:e1004885. doi: 10.1371/journal.pgen.1004885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 39.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 40.Zheng H, Chen L, Pledger WJ, Fang J, Chen J. p53 promotes repair of heterochromatin DNA by regulating JMJD2b and SUV39H1 expression. Oncogene. 2014;33:734–744. doi: 10.1038/onc.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nature cell biology. 2001;3:973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 42.Zilfou JT, Lowe SW. Tumor suppressive functions of p53. Cold Spring Harbor perspectives in biology. 2009;1:a001883. doi: 10.1101/cshperspect.a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.