Abstract
Purpose
Sigma receptor 1 (Sigma1R), a nonopioid putative molecular chaperone, has neuroprotective properties in retina. This study sought to determine whether delaying administration of (+)-pentazocine, a high-affinity Sigma1R ligand after onset of diabetes in Ins2Akita/+ diabetic mice would afford retinal neuroprotection and to determine consequences on retinal phenotype in Ins2Akita/+ diabetic mice in the absence of Sigma1R.
Methods
Ins2Akita/+ diabetic and WT mice received intraperitoneal injections of (+)-pentazocine beginning 4 or 8 weeks after onset of diabetes; eyes were harvested at 25 weeks. Retinal histologic sections were analyzed to determine thicknesses of retinal layers, number of ganglion cells, and evidence of gliosis (increased glial fibrillary acidic protein levels). Ins2Akita/+/Sig1R−/−mice were generated and subjected to in vivo assessment of retinal architecture (optical coherence tomography [OCT]) and retinal vasculature using fluorescein angiography (FA) at 12 and 16 weeks compared with age-matched Ins2Akita/+ mice. Eyes were then harvested for retinal morphometric assessment and gliosis assessment.
Results
Wild-type mice had 13 ± 0.06 cells/100 μm retinal length; cell bodies in Ins2Akita/+ mice injected 4 and 8 weeks after onset of diabetes with (+)-pentazocine retained significantly more ganglion cells compared with Ins2Akita/+ mice (9 ± 0.04) and demonstrated significant attenuation of gliosis. Ins2Akita/+/Sig1R−/−mouse retinas, analyzed to determine whether the Ins2Akita/+ phenotype was accelerated when lacking Sigma1R, revealed increased nerve fiber layer thickness (OCT), evidence of vitreal opacities, and vessel beading (FA) compared with Ins2Akita/+ mice. Morphometric analysis revealed significantly fewer ganglion cells in Ins2Akita/+/Sig1R−/−mice compared with Ins2Akita/+ mice.
Conclusions
Sigma1R may be a novel retinal stress modulator, and targeting it even after disease onset may afford retinal neuroprotection.
Keywords: retina, sigma 1 receptor (σ1R), ganglion cells, GFAP, (+)-pentazocine, OCT, fluorescein angiography, FA, mouse
Diabetic retinopathy is a leading cause of sight-threatening disabilities worldwide.1 Evidence compiled from clinical and basic research investigations supports the view that diabetic retinopathy constitutes a change in the retinal neurovascular unit, which specifically refers to neurons, glia, and vasculature.2 The vascular pathology in diabetic retinopathy involves occlusion of retinal vessels and leakiness that can result in macular edema, frequently associated with severe vision loss.3 Retinal neurons and glial cells are also altered in diabetic retinopathy as microvascular lesions develop, especially loss of neurons in the ganglion cell layer (GCL) and the inner nuclear layer (INL).4 A mouse model that has proven useful for studies of the retinal neuronal phenotype associated with diabetes is the Ins2Akita/+ mouse.5 This mouse has a point mutation in the Ins2 gene causing a conformational change in the protein, which accumulates in the β cells of the pancreas, inducing cell death.6 The homozygous mice are rarely viable, but the heterozygous mice develop hyperglycemia, hypoinsulinemia, polydipsia, and polyuria at approximately 4 weeks. Subclinical changes occur in the retina of Ins2Akita/+, consistent with the prodromal phase of diabetic retinopathy. Most notably, there is at least 20% reduction in the number of cell bodies in the retinal GCL of these diabetic mice as they age.5 Although this model has increased retinal vascular permeability and a modest increase in acellular capillaries after approximately 8 months, the classical vascular pathology as assessed by scanning laser ophthalmoscopy (SLO), fluorescein angiography (FA), and optical coherence tomography (OCT) is not prominent in this mouse model.7
Several years ago, our laboratory used the Ins2Akita/+ mouse to investigate whether targeting a unique protein, sigma 1 receptor (Sigma1R), would attenuate ganglion cell loss.8 We found that sustained administration of a high-affinity Sigma1R ligand, (+)-pentazocine [(+)-PTZ] conferred significant retinal neuroprotection, reduced evidence of oxidative stress, and preserved retinal architecture. In particular, ganglion cell death was markedly reduced in (+)-PTZ–treated Ins2Akita/+mice, suggesting that SigmaR1 ligands are promising therapeutic agents for intervention in neurodegenerative diseases of the retina, including diabetic retinopathy.
Sigma1R is 223-amino-acid nonopioid protein that shares no homology with any other mammalian protein. It is a unique integral membrane protein.9 Aside from N,N-dimethyltryptamine,10 no natural ligands for Sigma1R have been identified. Sigma1R is a putative molecular chaperone11 that regulates Ca2+ mobilization from endoplasmic reticulum (ER) stores12 and contributes to the stability of inositol 1,4,5-triphosphate type 3 receptor channels to ensure proper Ca2+ transport between the ER and the mitochondrial-associated membrane.13 These cellular survival properties have prompted speculation that ligands for Sigma1R could be promising for treatment of neurodegenerative diseases,14 including retinal diseases. Sigma1R is present in several ocular tissues including retina,15–20 cornea, ciliary body, and lens.15,20 A number of in vitro and in vivo studies demonstrate the powerful neuroprotective effects of Sigma1R ligands, most notably in protection against retinal ganglion cell death.8,21–24 Ligands for Sigma1R modulate ER stress in retinal ganglion cells25 and ER stress and osmolar stress in retinal Müller glia cells26,27; they also regulate intracellular Ca2+ calcium levels in retinal ganglion cells.28 Studies from Tchedre et al.29 provide evidence that Sigma1R interacts directly with voltage-gated Ca2+calcium channels in primary cultures of ganglion cells. Mice lacking Sigma1R (Sig1R−/− mice) have a late-onset retinal degeneration characterized by loss of ganglion cells.30 Under stress, such as optic nerve crush18 or streptozotocin-induced diabetes31 in Sig1R−/− mice, there is acceleration of the ganglion cell loss.
The finding that ligands for Sigma1R were neuroprotective in Ins2Akita/+ mice8 combined with the observation that streptozotocin-induced diabetes accelerated the phenotype in Sig1R−/− mice prompted the present investigation. First, we asked whether delaying injection of (+)-PTZ to Ins2Akita/+ mice beyond the onset of diabetes would confer neuroprotection. This is relevant clinically because it would be rare that treatment of humans would commence at diabetes onset. Our data suggest that (+)-PTZ has neuroprotective properties even when administered several weeks after the onset of diabetes. Second, we asked whether the retinal phenotypic changes observed in Ins2Akita/+ mice would be accelerated when Sigma1R was absent and whether there would be additional ocular manifestations including vasculopathy. Our data indicate that lack of Sigma1R in Ins2Akita/+ mice worsens the neurodegenerative phenotype and accentuates vasculopathy.
Materials and Methods
Animals
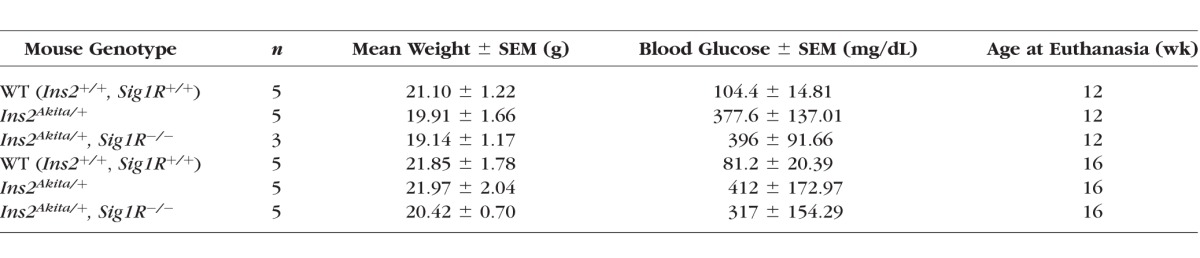
C57BL/6J Ins2Akita/+ mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA), Ins2Akita/+ female mice were bred to wild-type (WT) male mice in our animal facilities. Genotyping of Ins2Akita/+ mice was performed per the protocol recommended by the Jackson Laboratories. Retinas of male and female mice were examined; however, only males were used in the morphometric retinal analyses because disease progression in females is slower and less uniform.5 Diabetes onset was verified by measuring urine glucose using the Diastic Reagent Strips for urinalysis (Bayer, Mishawaka, IN, USA) and blood glucose using a Blood Glucose Monitoring System (Abbott Laboratories, Alameda, CA, USA). Fasting blood glucose levels >240 mg/dL were considered diabetic. Diabetic animals were not maintained on insulin. Age-matched, nondiabetic WT mice were used as controls. All mice received food and water ad libitum and were maintained on a 12-hour light-dark cycle. Body weight and blood glucose were measured at time of death, and data are provided in Tables 1 and 2.
Table 1.
Average Weights and Blood Glucose Levels of Mice Used in the Delayed (+)-PTZ Administration Study
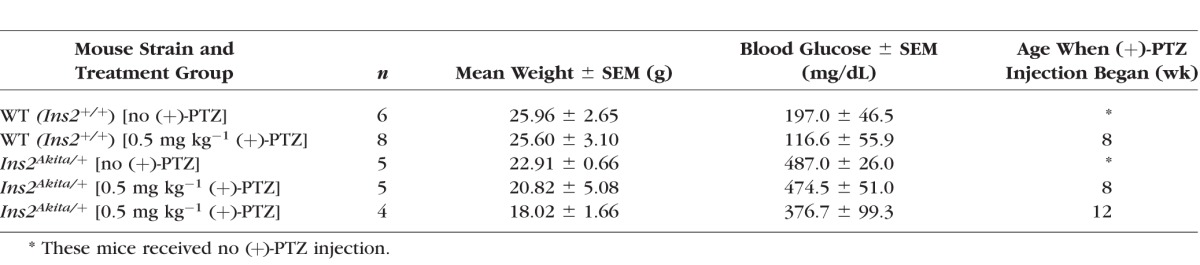
Table 2.
Average Weights and Blood Glucose Levels of Mice Used in the Analysis of Ins2Akita/+- Sig1R−/− Mice
Two studies were conducted. In the first study, we examined the effects of delaying (+)-PTZ injection (after onset of diabetes) on retinal neuronal death in diabetic retinopathy. Details of (+)-PTZ injections are provided below. In the second study, we determined whether the retinal neurodegeneration of Ins2Akita/+ mice would be accelerated if the mice lacked Sigma1R, and we asked whether the retinal phenotype would involve vasculopathy when Sigma1R was absent. C57BL/6-Ins2Akita/J mice were bred with Sig1R−/− mice (the generation of which has been previously described30,32) to produce Ins2Akita/+/Sig1R± mice, which were crossed with Sig1R−/− mice to generate Ins2Akita/+/Sig1R−/−mice. Genotyping was performed to confirm gene expression in all mice. In addition, we confirmed that the mice did not carry the rd8 (Crb1) mutation, which manifests as disrupted morphology including retinal rosettes.33 Mice were maintained for either 12 or 16 weeks, after which they were subjected to functional and morphologic analysis. Maintenance of animals adhered to the institutional guidelines for the humane treatment of animals following our Institutional Animal Care and Use Committee (IACUC)-approved protocol and to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Administration of (+)-PTZ After Onset of Diabetes
Ins2Akita/+ mice become diabetic at 3–4 weeks.5,6 Mice were administered (+)-PTZ either 4 or 8 weeks after onset of diabetes (thus, one group of Ins2Akita/+ mice was injected beginning at age 8 weeks and a second was injected beginning at age 12 weeks). Additional groups of mice included Ins2Akita/+ noninjected, WT (Ins2+/+) noninjected, and WT (Ins2+/+) injected beginning at 8 weeks (Table 1). (+)-Pentazocine (Cat. No. P127; Sigma-Aldrich Corp., St. Louis, MO, USA) was dissolved initially in dimethyl sulfoxide (DMSO) and diluted with 0.01 M PBS, and it was administered via intraperitoneal injection at a dosage 0.5 mg kg−1 two times per week for 22 weeks. Mice were euthanized at 25 weeks, as was the case in the previous study in which Ins2Akita/+ mice were administered (+)-PTZ at diabetes onset.8 Retinas were evaluated morphometrically (described below).
Spectral-Domain OCT Imaging, Fundoscopy, and FA
To evaluate retinal morphology in vivo, spectral-domain (SD)-OCT imaging was performed in Ins2Akita/+/Sig1R−/−mice. Mice were anesthetized using a rodent anesthesia cocktail (ketamine hydrochloride/xylazine hydrochloride solution [80:12 mm/mL]; Sigma-Aldrich Corp.). Pupils were dilated with 1% tropicamide (Bausch and Lomb, Tampa, FL, USA) followed by application of GenTeal Lubricant Eye Gel, 10 g (Alcon, Ft. Worth, TX, USA). Systane lubricant eye drops (Alcon) were applied to keep the cornea moist. The SD-OCT images were obtained using the Bioptigen Spectral-Domain Ophthalmic Imaging System (SD OIS; Bioptigen Envisu R2200; Bioptigen, Inc., Durham, NC, USA). Imaging included averaged single B-scan and volume intensity scans (VIPs) with images centered on the optic nerve head. Postimaging analysis included auto segmentation report analysis and manual assessment of all retinal layers using InVivoVueTM DIVER 2.4 software (Bioptigen, Inc.).
Fundus imaging was performed in Ins2Akita/+/Sig1R−/−mice using the Micron III camera (Phoenix Research Laboratories, Inc., Pleasanton, CA, USA) in mice that had been anesthetized as described for SD-OCT. Pupils were dilated with 1% tropicamide, and the cornea was kept moist. Following fundus imaging, fluorescein angiography was performed following a 20-μL 10% fluorescein lite (Apollo Ophthalmic, Newport Beach, CA, USA) intraperitoneal injection, which permitted visualization of retinal vasculature. The procedures for OCT and FA followed our previously reported methods.34,35
Tissue Processing and Morphometric Analysis
Mice were killed by CO2 asphyxiation followed by cervical dislocation. Eyes were removed immediately and were either oriented in Tissue-Tek OCT (Miles Laboratories, Elkhart, IN, USA) and were frozen slowly by immersion in liquid nitrogen (without fixation) or were fixed in 4% paraformaldehyde/2% glutaraldehyde in 0.1 M cacodylate buffer and embedded in JB-4 embedding compound (EMS, Hatfield, PA, USA) per our method.30 Sections were stained with hematoxylin and eosin (H&E) and used for systematic morphometric analysis using a Zeiss Axioplan2 fluorescent microscope equipped with HRM camera and the Axiovision 4.6.3 program (Carl Zeiss, Oberkochen, Germany). Measurements included the thickness of the (1) total retina, (2) inner plexiform layer (IPL), (3) INL, (4) outer plexiform layer (OPL), and (5) the photoreceptor inner/outer segments. The number of rows of photoreceptor cell nuclei in the outer nuclear layer (ONL) was counted. The number of cell bodies in the GCL was quantified by counting cells from the temporal ora serrata to the nasal ora serrata and expressing the data as number of cells per 100 μm length of retina. Sections analyzed were from the center of the eyeball; thus, the optic nerve was visible. Three measurements were made on each side (temporal and nasal) of the optic nerve at 200- to 300-μm intervals, resulting in a total of six measurements obtained per eye. Per mouse, at least two sections were analyzed for the left and right eye. In addition, eyes were examined for evidence of gross pathology.
Assessment of Gliosis and Cell Death
Glial fibrillary acidic protein (GFAP) is a 54-kDa intermediate filament protein that is a major constituent of retinal astrocytes; under retinal stress conditions, GFAP expression is up-regulated in retinal Müller glial cells in a process termed gliosis.36 Retinal cryosections were fixed with 4% paraformaldehyde, washed with PBS–Triton X-100, and incubated with Power Block, after which sections were incubated with rabbit anti-GFAP (1:500; Dako, Carpentaria, CA, USA) overnight at 4°C. Sections were washed and incubated Triton X-100, followed by incubation with secondary antibody Alexa Fluor 488–conjugated donkey–anti-rabbit IgG (1:1,000; Invitrogen, Carlsbad, CA, USA) for 1 hour at room temperature. Sections were washed three times with PBS-Triton X-100 and coverslipped with Fluoroshield with 4′,6-diamidino-2-phenylindole (DAPI). Sections were examined by epifluorescence using a Zeiss Axioplan-2 microscope, equipped with the axiovision program, an HRM camera, and filters to detect FITC and DAPI. Fluorescence intensity was analyzed using Metamorph Image Analysis software (Molecular Devices, Sunnyvale, CA, USA). Additional immunofluorescence experiments were performed to detect dying cells using the ApopTag Fluorescein In Situ Apoptosis Detection Kit (TUNEL assay; EMD Millipore, Billerica, MA, USA) per our published methods.30
Statistical Analysis
Analysis of variance was used to determine whether there were significant differences in morphologic measurements in mice administered (+)-PTZ at the two time intervals compared with noninjected animals. For OCT measurements and cell counting in GCL studies, 2-way ANOVA was used to determine whether there were significant differences between WT, Ins2Akita/+, and Ins2Akita/+/Sig1R−/−mouse groups at the two ages studied. Tukey's paired comparison test was the post hoc statistical test. Statistical analysis was conducted using the GraphPad Prism software (version 6; GraphPad Software, Inc., La Jolla, CA, USA). P < 0.05 was considered significant.
Results
Effects of Administration of (+)-PTZ After Diabetes Onset on Neuronal Death in Diabetic Retinopathy
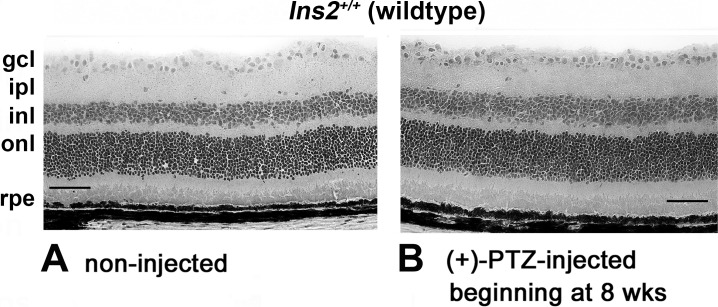
Earlier studies demonstrated that twice weekly administration of (+)-PTZ at the 0.5-mg kg−1 dosage reduced apoptotic neuron death characteristic of Ins2Akita/+mice and preserved retinal architecture, when treatment started at diabetes onset.8 We used this same (+)-PTZ dosage and treatment regimen in the present study, but initiated injections either 4 or 8 weeks after diabetes onset, which meant that injections began at age 8 or 12 weeks in Ins2Akita/+mice. Representative photomicrographs of retinas of WT mice receiving either no (+)-PTZ injection or receiving (+)-PTZ twice weekly beginning at age 8 weeks are shown (Fig. 1). There was no deleterious effect on retinal architecture when WT mice were administered the Sigma1R ligand (Fig. 1) and no differences in morphometric analysis of retinal layers (data not shown).
Figure 1.
Effects of (+)-PTZ on retinas of WT mice. Representative photomicrographs of H&E-stained retinas of control (C57BL/6) mice (WT) that received (A) no (+)-PTZ injection or (B) 0.5 mg kg−1 (+)-PTZ injection twice weekly beginning at 8 weeks. Retinal appearance was similar between noninjected and injected WT mice. gcl, ganglion cell layer; ipl, inner plexiform layer; inl, inner nuclear layer; onl, outer nuclear layer; rpe, retinal pigment epithelial layer.
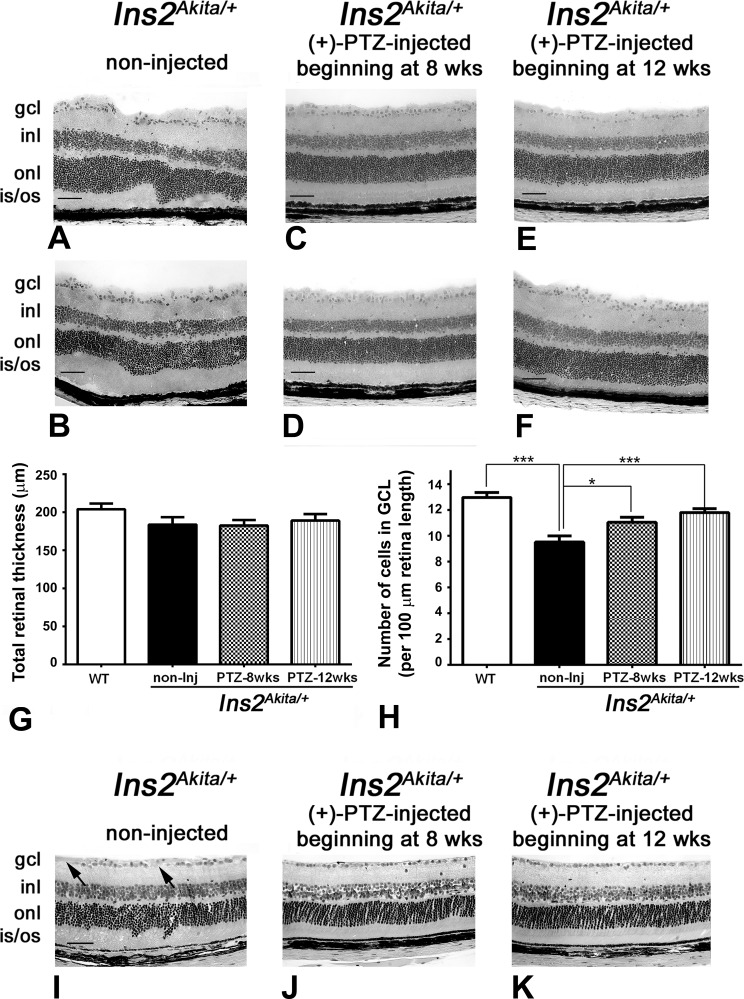
In the case of the Ins2Akita/+ mice that received no (+)-PTZ injections, the retinas showed mild disruption. Figures 2A and 2B show representative photomicrographs of retinas from two of the mice in the nontreated group. Other nontreated mice had similar retinal architecture. The alterations in the INL by 25 weeks in the Ins2Akita/+ mice have been reported.5 The nuclear layers did not have their characteristic uniform stratified appearance, but this was not attributable to presence of the rd8 mutation. Mice that had been injected intraperitoneally beginning at 4 or 8 weeks after onset of diabetes onset with 0.5 mg kg−1 (+)-PTZ twice weekly showed preservation of retinal architecture (Figs. 2C–2F). The panels shown are representative of two mice per group at each age studied. The nuclear layers were uniform in appearance. The retinas were subjected to morphometric analysis, and the total retinal thickness did not differ between the WT mice, Ins2Akita/+-noninjected mice, and Ins2Akita/+-injected mice beginning at 4 or 8 weeks (Fig. 2G). Nor did the thickness of the individual layers (IPL, INL, OPL, and ONL) differ significantly between groups (data not shown). However, when the numbers of cells in the GCL were quantified, there was a significant difference between the WT mice and the Ins2Akita/+-noninjected mice (Fig. 2H). Wild-type mice had approximately 13 cells per 100 μm length of retina, whereas the Ins2Akita/+-noninjected mice had approximately 9 cells for the same retinal length (Fig. 2H). For the mice treated with Sigma1R ligand at either 4 or 8 weeks after onset of diabetes, there were approximately 11 cells per 100 μm retinal length, which was significantly greater than the Ins2Akita/+-noninjected mice. Although the numbers of cells in the GCL of (+)-PTZ-treated Ins2Akita/+ mice were not equal to that of the WT mice, there was clear preservation of these cells compared with diabetic mice not treated with the Sigma1R ligand. We also noted that there was waveform irregularity of the ONL accompanied by shallow retinal detachment and irregularly elongated photoreceptor outer segments in the retinas of Ins2Akita/+-noninjected mice. To evaluate whether the waveform appearance and occasional retinal detachment might be a consequence of the cryosectioning method of preparing sections, we examined eyes that had been fixed prior to embedding and processed for JB-4 plastic embedding. In this case, the Ins2Akita/+-noninjected mice showed minimal detachment, although slight irregularities of the nuclear layers were still detectable (Fig. 2I). There continue to be regions within the GCL devoid of cells (Fig. 2I, arrows). The JB-4–embedded retinas of the (+)-PTZ–treated Ins2Akita/+ mice had a generally uniform appearance (Figs. 2J, 2K) similar to that observed in the cryosections of retinas of these mice (Figs. 2C–2F).
Figure 2.
Preservation of retinal structure and cells in the GCL in Ins2Akita/+ mice administered (+)-PTZ after diabetes onset. All images are from mice age 25 weeks. (A, B) Representative H&E-stained retinal cryosections of Ins2Akita/+ mice that received no (+)-PTZ for the duration of the study; the INL is slightly disrupted, and GCL density is decreased. Representative H&E-stained retinal cryosections of (C, D) Ins2Akita/+ mice that received 0.5 mg kg−1 (+)-PTZ beginning 4 weeks after onset of diabetes (at age 8 weeks) or (E, F) Ins2Akita/+ mice that received 0.5 mg kg−1 (+)-PTZ beginning 8 weeks after onset of diabetes (at age 12 weeks). Retinas of (+)-PTZ injected mice demonstrated marked preservation of retinal structure. Morphometric analysis revealed no significant change in total retinal thickness in any of the eyes evaluated (G), but a significant difference in the number of cell bodies in the GCL per 100 μm length of retina (H). Eyes were processed for JB-4 embedding and stained with H&E to permit visualization of retinal architecture of (I) Ins2Akita/+ mice that received no (+)-PTZ; (J) Ins2Akita/+ mice that received 0.5 mg kg−1 (+)-PTZ beginning 4 weeks after onset of diabetes (at age 8 weeks); or (K) Ins2Akita/+ mice that received 0.5 mg kg−1 (+)-PTZ beginning 8 weeks after onset of diabetes (at age 12 weeks). Arrows in I point to areas of cell dropout. gcl, ganglion cell layer; inl, inner nuclear layer; onl, outer nuclear layer; is/os, inner/outer segments. Magnification bar: 50 μm. Data are means ± SE. Significantly different at *P < 0.05 and ***P < 0.001.
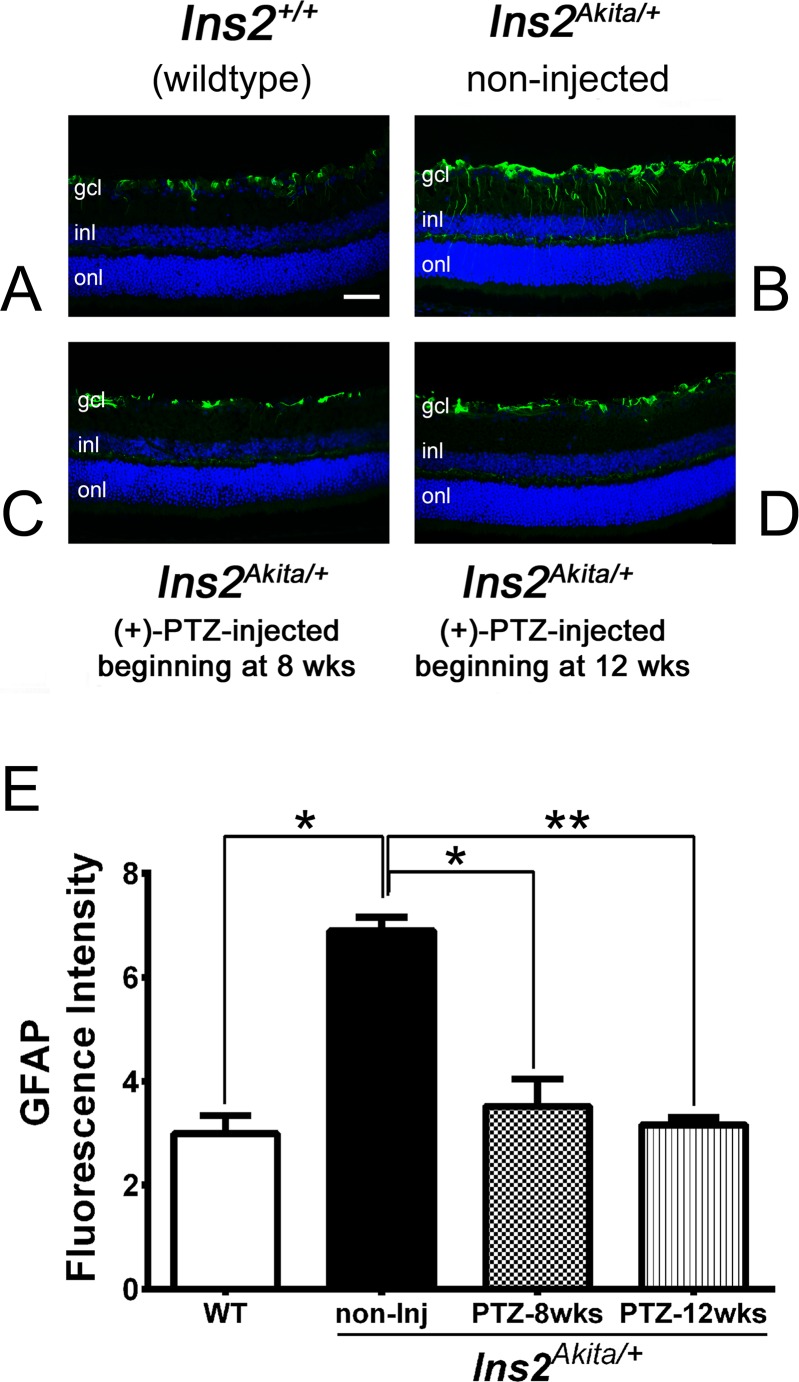
We examined the expression levels of GFAP, which is up-regulated during retinal gliosis.36 We observed labeling in the WT retina limited to astrocytes, which typically express this protein (Fig. 3A). In the Ins2Akita/+-noninjected mice, the level of GFAP increased, and the distribution of labeling was detected along the radial fibers of Müller glial cells (Fig. 3B). This is an indication of gliosis characteristic of this diabetic retinopathy model. In the Ins2Akita/+ (+)-PTZ mice, the level of GFAP was very low in the Müller cells. The observation of minimal detection of GFAP (except the normal detection in astrocytes) was true for mice treated beginning at 8 weeks (4 weeks after onset of diabetes; Fig. 3C) and in mice treated beginning at 12 weeks (8 weeks after onset of diabetes; Fig. 3D). The quantification of fluorescence intensity is shown in Figure 3E.
Figure 3.
Immunofluorescent detection of GFAP, a marker of gliosis in Ins2Akita/+ mice administered (+)-PTZ after diabetes onset. Representative photomicrographs of immunofluorescent detection of GFAP in retinal cryosections from (A) Ins2+/+ (WT), (B) Ins2Akita/+ [no (+)-PTZ treatment], (C) Ins2Akita/+ mice that received 0.5 mg kg−1 (+)-PTZ beginning 4 weeks after onset of diabetes (at age 8 weeks), and (D) Ins2Akita/+ mice that received 0.5 mg kg−1 (+)-PTZ beginning 8 weeks after onset of diabetes (at age 12 weeks). Immunofluorescence analysis used anti-GFAP followed by incubation with Alexa Fluor 488 (green-labeled secondary antibody), showing marked increase in GFAP in radially oriented fibers of Müller glial cells (radial labeling) in Ins2Akita/+ [no (+)-PTZ treatment], but minimal labeling in Müller cells of the WT or (+)-PTZ–treated mice. Astrocytic labeling with GFAP is typical and is observed in retinas of all mice. (E) Quantification of GFAP fluorescence intensity data obtained from metamorphic analysis (significant difference: *P < 0.05, **P < 0.01).
Regarding the monitoring of blood glucose in this study (Table 1), WT mice had blood glucose values below 240 mg/dL and were not hyperglycemic. The (+)-PTZ–treated Ins2Akita/+ mice remained hyperglycemic throughout the study; blood glucose levels were approximately 400 mg/dL (similar to untreated Ins2Akita/+ mice) and were significantly higher than WT mice (116–197 mg/dL), suggesting that hyperglycemia per se may not be sufficient to trigger neuronal loss in diabetes. Treatment with (+)-PTZ does not alter blood glucose levels.
SD-OCT Imaging, Fundoscopy, and FA in Ins2Akita/+/Sig1R−/− Mice
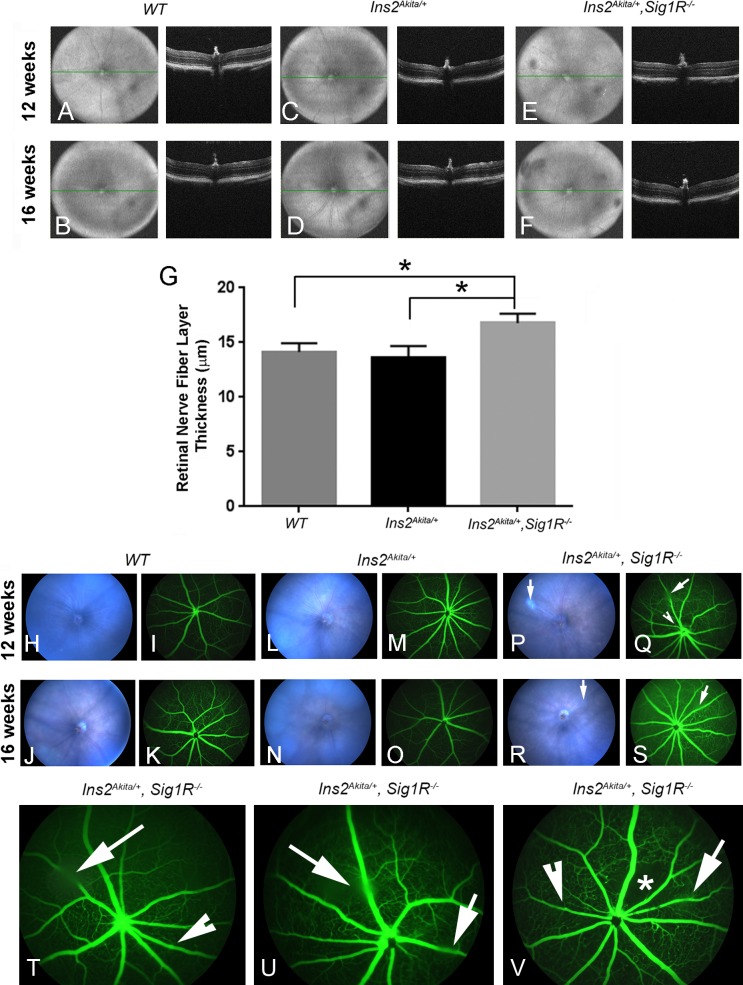
In addition to investigating the effects of delayed administration of (+)-PTZ on the retinal structure in the Ins2Akita/+diabetic mice described above, we examined the consequences on retinal phenotype when Sigma1R was absent by generating Ins2Akita/+/Sig1R−/−mice. We performed SD-OCT in WT (Ins2+/+), Ins2Akita/+ mice, and Ins2Akita/+/Sig1R−/−mice that were either 12 or 16 weeks of age. Volume image projections and the accompanying B-scans showed generally normal retinal architecture and no evidence of gross disruption in the retinal nuclear or plexiform layers among the three groups (Figs. 4A–4F). Postimage analysis of retinal layers used InVivoVue 2.4 DIVER software (Bioptigen, Inc., Durham, NC, USA) and confirmed that the thickness of most retinal layers was comparable among the three groups of mice, with the exception of the measurement of the nerve fiber layer (NFL). In this case, the NFL was thicker in retinas of Ins2Akita/+/Sig1R−/−mice compared with WT (Ins2+/+) or Ins2Akita/+ mice (Fig. 4G). Measurement of other retinal layers did not differ significantly among the three mouse groups (data not shown).
Figure 4.
Spectral-domain OCT imaging, fundoscopy, and FA in Ins2Akita/+ and Ins2Akita/+/Sig1R−/−mice. Wild-type (Ins2+/+), Ins2Akita/+, and Ins2Akita/+/Sig1R−/− mice were anesthetized and subjected to SD-OCT to obtain images of the retina in situ. Representative VIP for WT (Ins2+/+) mice: age 12 (A) and 16 weeks (B); Ins2Akita/+ mice: age 12 (C) and 16 weeks (D); and Ins2Akita/+/Sig1R−/−mice: age 12 (E) and 16 weeks (F). The panel to the right of each VIP is the B-scans of the same animal. Autosegmentation analysis was performed to determine whether there were significant differences in thickness of retinal layers among the three mouse groups. There was a significant increase in thickness of the nerve fiber layer (G) (*significantly different at P < 0.05). Mice were subjected to fundoscopy WT (Ins2+/+) at 12 (H) and 16 weeks (J); Ins2Akita/+ at 12 (L) and 16 weeks (N); Ins2Akita/+/Sig1R−/− at 12 (P) and 16 weeks (R). Fundus images were normal for WT and Ins2Akita/+, whereas vitreal opacities (denoted by long arrows) were detected in the Ins2Akita/+/Sig1R−/− mice (P, R). Mice were subjected to FA to observe vascular filling with a fluorescent dye. Wild-type (Ins2+/+) showed no alterations at either 12 (I) or 16 weeks (K). Ins2Akita/+ mice also had normal vascularization patterns at 12 (M) and 16 weeks (O), whereas Ins2Akita/+/Sig1R−/− mice had beading at 12 (Q) and at 16 weeks (S). The long arrows in Q and S point to areas of vitreal opacity; the indented arrowhead points to a vessel with beading (S). (T) Enlarged image of image shown in S. (U, V) FA images of two additional mice at 16 weeks showing vitreal opacity (long arrow) and occasional beading of vessels (indented arrowhead). The asterisk in V denotes an area of capillary dropout.
We then examined WT (Ins2+/+), Ins2Akita/+, and Ins2Akita/+/Sig1R−/− mice by retinal fundoscopy and FA at 12 and 16 weeks of age. The fundus was normal in appearance in WT (Figs. 4H, 4J) and Ins2Akita/+ mice (Figs. 4L, 4N). There was evidence of vitreal opacities in several Ins2Akita/+/Sig1R−/− mice (Figs. 4P, 4R, long arrows). To visualize retinal vessels, fluorescein dye was injected, and images were acquired 30 seconds after injection and every minute thereafter for 5 minutes. Images were arranged according to capture time, and data were compared among the three mouse groups at ages 12 and 16 weeks. Fluorescein angiography performed in WT mice showed normal vessel filling, no leakage, and uniform capillary network around blood vessels at both ages studied (Figs. 4I, 4K). Similarly, vessel filling in Ins2Akita/+ mice appeared normal, and leakage was not observed at either 12 (Fig. 4M) or 16 weeks (Fig. 4O). In Ins2Akita/+/Sig1R−/−mice, however, there was evidence of vessel beading at 12 weeks (Fig. 4Q, indented arrowhead) and at 16 weeks (Fig. 4S, indented arrowhead). A larger image of Figure 4S is provided (Fig. 4T). The long arrow in Figure 4T points to a region of vitreal opacity, not an area of leakiness. Higher-magnification FA images for two additional 16-week Ins2Akita/+/Sig1R−/−mice are shown (Figs. 4U, 4V). In Figure 4V, there appears to be an area of capillary dropout (denoted by an asterisk) in the retina of a 16-week Ins2Akita/+/Sig1R−/−mouse. It is noteworthy that all of the 16-week Ins2Akita/+/Sig1R−/−mice examined showed some evidence of beading and of vitreal opacities. The data suggest that in the absence of Sigma1R subtle retinal vascular abnormalities develop that are not normally observed by FA in the Ins2Akita/+ diabetic mouse retina.7
Retinal Morphometric Analysis in Ins2Akita/+/Sig1R−/− Mice
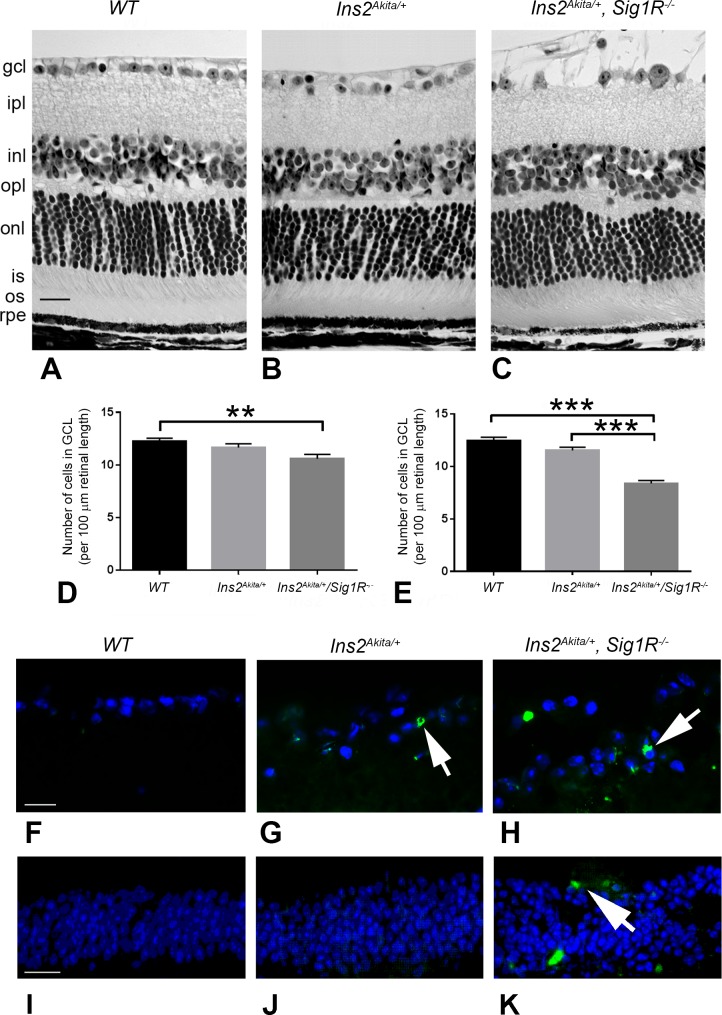
Eyes from WT (Ins2+/+), Ins2Akita/+, and Ins2Akita/+/Sig1R−/− mice were processed in JB-4 embedding compound for light microscopic evaluation and retinal morphometric analysis at 12 and 16 weeks. Although the retinal architecture becomes mildly disrupted by 25 weeks in the Ins2Akita/+ mice as reported previously5 and Figure 2 of this study, the retinal architecture of Ins2Akita/+ mice at the younger ages (12 and 16 weeks) analyzed in this study (Fig. 5B) was similar to that of WT (Ins2+/+) mice (Fig. 5A). A notable feature that distinguished the Ins2Akita/+/Sig1R−/− mice from the WT and Ins2Akita/+ mice was the fragmented appearance and disruption of the NFL in these mice compared with the WT (Ins2+/+) and Ins2Akita/+ mice (Fig. 5C). Although the disrupted appearance of the NFL may reflect increased sensitivity to the fixative used in processing the tissue, there appears to be involvement of NFL in the Ins2Akita/+/Sig1R−/− mice when examined in vivo by SD-OCT imaging (Fig. 4G). The NFL is comprised of the axons of the ganglion cells; thus, the decrease in the numbers of cells observed in the Ins2Akita/+/Sig1R−/− mice could account for disruption of their axons. The images in Figures 5A–5C are from 16-week mice.
Figure 5.
Retinal structure of WT (Ins2+/+), Ins2Akita/+, and Ins2Akita/+/Sig1R−/−mice. Eyes of (A) WT (Ins2+/+), (B) Ins2Akita/+, and (C) Ins2Akita/+/Sig1R−/−mice were processed for JB-4 embedding and stained with H&E to permit visualization of retinal architecture. Animals were either 12 or 16 weeks at the time eyes were harvested. Panels shown are from 16-week-old mice. In WT (Ins2+/+) mice, the retinas are well organized and layers are of normal thickness. The retinas of the Ins2Akita/+ and Ins2Akita/+/Sig1R−/−mice are similar in appearance, with the exception of the nerve fiber layer in the Ins2Akita/+/Sig1R−/−mice (arrow), which is markedly disrupted. Retinas were subjected to morphometric analysis; there was a significant decrease in the number of cells in the GCL in the Ins2Akita/+/Sig1R−/−mice at 12 (D) and 16 weeks (E). Retinal cryosections of the same mice were subjected to immunofluorescent detection of TUNEL-positive cells (green labeling), cell nuclei were blue (DAPI) in (F) WT (Ins2+/+), (G) Ins2Akita/+, and (H) Ins2Akita/+/Sig1R−/−mice. Images were obtained using a high-powered objective focused on the GCL. Additional images of TUNEL staining were obtained using a lower-powered objective focused on the INL for (I) WT (Ins2+/+), (J) Ins2Akita/+, and (K) Ins2Akita/+/Sig1R−/−mice. gcl, ganglion cell layer; ipl, inner plexiform layer; inl, inner nuclear layer; opl, outer plexiform layer; onl, outer nuclear layer; is, inner segment; os, outer segment; rpe, retinal pigment epithelium. Calibration bar: 50 μm. Statistical significance: **P < 0.01, ***P < 0.001.
In the mouse retina, the number of cell bodies in the GCL is typically 12–14 per 100 μm length of retina. This was observed in the WT (Ins2+/+) mouse retina at both 12 and 16 weeks (Figs. 5D, 5E). In Ins2Akita/+ mice, there was minimal difference at 12 and 16 weeks in the number of cells bodies in the GCL compared with WT mice (Figs. 5D, 5E). In Ins2Akita/+/Sig1R−/− mice, however, there was a significant decrease in the number of cells in the GCL at 12 (∼11 cells/100 μm retinal length) and 16 weeks (∼8 cells/100 μm retinal length). The data suggest that, in the absence of Sigma1R, the loss of ganglion cells observed at 25 weeks in the Ins2Akita/+ is markedly accelerated. We subjected retinal cryosections from the three mouse groups to immunofluorescence to detect dying cells using the ApopTag Fluorescein In Situ Apoptosis Detection Kit (TUNEL staining). We did not detect dying cells in GCL of WT (Ins2+/+) mice (Fig. 5F), indicating minimal cell death in this layer under normal conditions. We observed some TUNEL-positive cells in the GCL of the Ins2Akita/+ retina (Fig. 5G) and many more TUNEL-positive cells in the GCL of Ins2Akita/+/Sig1R−/− mice (Fig. 5H) at age 16 weeks. The findings confirm the reduced numbers of cells in the GCL determined by morphometric analysis. We also examined the INL for TUNEL-positive cells and observed very few TUNEL-positive cells in retinas of WT (Ins2+/+) (Fig. 5I) or Ins2Akita/+ mice (Fig. 5J), but a significant increase in TUNEL-positive cells in the INL of retinas of Ins2Akita/+/Sig1R−/− mice (Fig. 5K).
Assessment of Gliosis in Ins2Akita/+/Sig1R−/− Mice
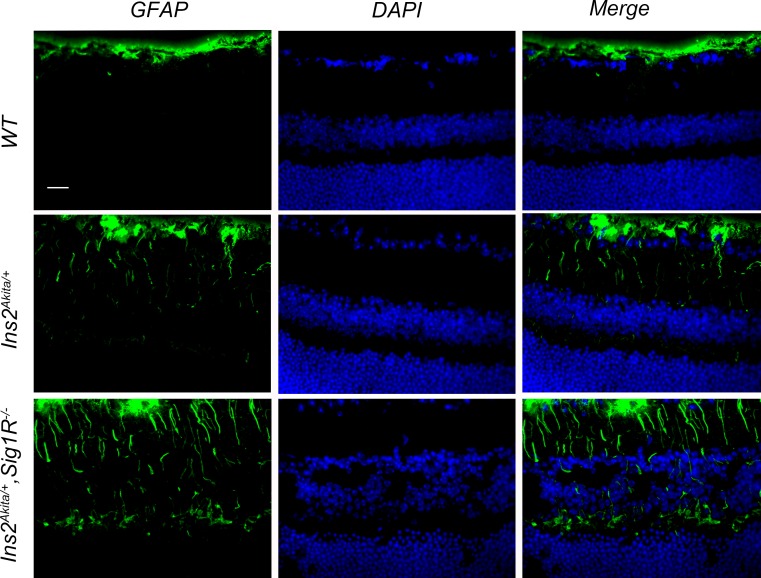
In the Ins2Akita/+ retina, increased expression of GFAP has been reported in Müller cells at 6 months7 (and Fig. 3 of this study). In the current study, we investigated the levels of GFAP in retinal cryosections of WT (Ins2+/+), Ins2Akita/+, and Ins2Akita/+/Sig1R−/− mice by immunofluorescence at 12 and 16 weeks. Photomicrographs shown in Figure 6 are from 16-week mice. Aside from GFAP labeling typically observed in retinal astrocytes, we detected minimal GFAP in retinas of WT (Ins2+/+) mice (Fig. 6A). There was a slight increase in GFAP levels in retinas of Ins2Akita/+ mice (Fig. 6B) and a substantial increase in GFAP detected in retinas of Ins2Akita/+/Sig1R−/− mice (Fig. 6C). The data suggest that, in younger Ins2Akita/+ mice lacking Sigma1R, there is increased retinal gliosis compared with age-matched diabetic mice that have Sigma1R.
Figure 6.
Immunofluorescent detection of GFAP, a marker of gliosis in WT (Ins2+/+), Ins2Akita/+, and Ins2Akita/+/Sig1R−/−mice at 16 weeks. Representative photomicrographs of immunofluorescent detection of GFAP in retinal cryosections from (A) WT (Ins2+/+), (B) Ins2Akita/+, and (C) Ins2Akita/+/Sig1R−/−mice at age 16 weeks. Immunofluorescence analysis used anti-GFAP followed by incubation with Alexa Fluor 488 (green-labeled secondary antibody), showing marked increase in GFAP in radially oriented fibers of Müller glial cells (radial labeling) in Ins2Akita/+/Sig1R−/−mice but much less GFAP labeling in Müller cells of the WT or Ins2Akita/+ mice at this age. Astrocytic labeling with GFAP is typical and is observed in retinas of all mice. gcl, ganglion cell layer; inl, inner nuclear layer; onl, outer nuclear layer.
Effects of Sigma1R on Blood Glucose Levels in Ins2Akita/+/Sig1R−/− Mice
The blood glucose levels measured in WT (Ins2+/+) were within normal limits, whereas levels were hyperglycemic in both Ins2Akita/+ and Ins2Akita/+/Sig1R−/− mice (Table 2). The absence of Sigma1R did not alter blood glucose levels in Ins2Akita/+ mice.
Discussion
This study had two objectives. One was to investigate whether delaying administration of a high-affinity Sigma1R ligand after onset of diabetes could afford neuroprotection in a model of diabetic retinopathy and the second was to evaluate whether absence of Sigma1R would accelerate neuron loss in this model. Diabetic retinopathy is one of the foremost retinopathies afflicting humans.1 Although diabetic retinopathy has long been recognized as a retinal vascular disease, significant neuronal loss is also a feature of this disease.37 We chose to use the diabetic Ins2Akita/+ mouse as an endogenous diabetic model with modest progressive loss of cells in the GCL.
In our study examining whether administration of (+)-PTZ after onset of diabetes would be neuroprotective, we investigated four mouse groups. We used WT mice, Ins2Akita/+ mice that received no injection, and two groups of Ins2Akita/+ mice that were injected beginning at 8 or 12 weeks (which was either 4 or 8 weeks after diabetes onset). Earlier studies had shown that by the time Ins2Akita/+ mice were 25 weeks, there was an approximate 20% loss of cells in the GCL.5,8 We used that same time point to investigate whether (+)-PTZ could attenuate cell loss and found that, in both the 8- and 12-week injection groups, there was attenuation of cell loss. That is, even though (+)-PTZ was not administered at the onset of diabetes, there still appeared to be significant neuroprotection when the retinas were examined at 25 weeks. The retinas of Ins2Akita/+ mice that were in the delayed injection groups retained significantly more cells in the GCL than noninjected Ins2Akita/+ mice. In addition, examination of retinal architecture showed that retinas of (+)-PTZ-injected mice were well organized. Most intervention studies (including our own study with the Ins2Akita/+ mouse several years ago8) start therapeutic intervention as early as possible, sometimes prior to onset of disease, to achieve the greatest likelihood of a beneficial outcome. A recent example of this involving a Sigma1R ligand was a report from Shimazawa et al.,38 in which the effects of cutamesine dihydrochloride (SA4503) were investigated in a light-induced mouse model of photoreceptor degeneration. In this case, the mice were pretreated with the Sigma1R ligand (intravitreal injection) 1 hour prior to exposure to intense light. The effects of cutamesine on the photoreceptor cell loss were impressive, but it is not known whether administering the ligand after the exposure to intense light would be protective. Similarly, Sun et al.39 induced ganglion cell death in rats using episcleral vein cauterization (EVC) and tested whether the Sigma1R ligand pregnenolone sulfate would lower EVC-induced increase in intraocular pressure (IOP). They observed normalization of IOP in this treatment regimen, but again it is not known whether the treatment could lower IOP if administered after the EVC insult. Our findings that administration of (+)-PTZ after onset of diabetes can afford some degree of neuroprotection is important because most human patients would not receive treatment at the onset of disease. Our findings that gliosis was attenuated as shown by decreased GFAP expression in the Ins2Akita/+ mouse retina, even though (+)-PTZ administration was delayed until after diabetes onset, is noteworthy given the recent report that activation of Sigma1R using PRE-084 inhibits osmotic swelling of retinal Müller glial cells and may contribute to the neuroprotective properties of Sigma1R ligands.26
(+)-Pentazocine is a potent and highly specific SigmaR1 ligand.40 It binds SigmaR1 with a very high affinity and is presumed to be an agonist for the receptor; however, because the actual signaling events associated with SigmaR1 activation have not been characterized, it is difficult to ascribe definitively an agonist/antagonist function to this ligand. There is convincing evidence that (+)-PTZ is specific for SigmaR1 because in experiments where the gene is absent, (+)-PTZ does not afford protection.25,28,41,42 The preservation of retinal structure and attenuated neuronal death do not appear to be a direct function of decreased hyperglycemia because (+)-PTZ–injected Ins2Akita/+ mice continue to have elevated blood glucose throughout the study. Retinal neuronal loss associated with diabetes may not involve hyperglycemia directly; rather, it may be due to complications secondary to hyperglycemia such as oxidative stress.43
The beneficial effects of (+)-PTZ administered at the onset of diabetes,8 as well as after onset of diabetes reported herein, suggest that activation of the Sigma1R may be a promising treatment strategy for retinal degenerative diseases. The precise physiologic role of Sigma1R in retina is not known; however, findings from many studies support the notion that Sigma1R is a molecular chaperone and modulates cellular stress. Sigma1R has been shown to modulate oxidative stress via Nrf2 signaling,42 to activate extracellular signal–regulated kinases1/2,44,45 to suppress inflammatory responses in microglial cells,45 to attenuate calcium influx in ganglion cells,28 to modulate voltage-gated calcium channels,29 to decrease retinal glial cytokine release and promote cytosolic-to-nuclear NF-κB translocation,42 and to modulate ER stress.25,27
The multiple protective functions of Sigma1R prompted the present study to investigate whether absence of Sigma1R would accelerate the retinal phenotype in Ins2Akita/+ mice. Sig1R−/− mice have a late-onset retinal degeneration characterized by optic nerve axonal degeneration at approximately 6 months and progressive loss of ganglion cells by 1 year; the retina is normal in appearance in the first few months of life.30 No retinal vasculopathy has been reported in Sig1R−/− mice. In the present study, we evaluated retinas of Ins2Akita/+/Sig1R−/− mice and compared them with Ins2Akita/+ mice at 12 and 16 weeks, time points earlier than the reported ganglion cell loss (25 weeks). We found that ganglion cell loss was minimal in Ins2Akita/+ mice at 12 and 16 weeks but was significantly worse in Ins2Akita/+/Sig1R−/− mice, particularly at 16 weeks. The data suggest that in the absence of Sigma1R, retinal neuronal loss is accelerated. The data were confirmed by detection of significantly more TUNEL-positive cells (indicative of apoptosis), in retinas of Ins2Akita/+/Sig1R−/− mice compared with WT or Ins2Akita/+ mice. In addition, the NFL examined in retinal sections was fragmented and disrupted in Ins2Akita/+/Sig1R−/− mice. This was not due simply to fixation artifacts because OCT analysis showed that, although the total retinal thickness and most of the individual retinal layers did not differ from WT, there was a significant increase in the NFL thickness in the Ins2Akita/+/Sig1R−/− mice.
In addition to OCT analysis, imaging of the retinal vessels of Ins2Akita/+/Sig1R−/− mice showed subtle vascular changes that are not typically observed in Ins2Akita/+ mice.7,46 Especially at 16 weeks, FA revealed vessel beading and occasional capillary dropout. The findings suggest that despite the minimal vascular involvement of retina in Ins2Akita/+ mice normally,7,46 lack of Sigma1R increases slightly the incidence of vascular alterations. In addition to neuronal and vascular features, the Ins2Akita/+/Sig1R−/− mice also demonstrate significant increase in GFAP compared with Ins2Akita/+ mice, suggesting that retinal gliosis, which has been reported in the Ins2Akita/+ mice, is more pronounced when Sigma1R is absent. The findings suggest a role for Sigma1R as a modulator of stress; in the absence of Sigma1R, there appears to be heightened sensitivity to stress.
Taken collectively, the data from the present study provide insights into the role of Sigma1R in retinal disease. The findings that (1) administration of a high affinity Sigma1R ligand after onset of diabetes affords a degree of retinal neuroprotection and (2) absence of the receptor in diabetic retinopathy worsens the phenotype suggest that Sigma1R may have an important role in preservation of retinal structure. Ligands for Sigma1R are in clinical trials for treatment of neurodegenerative diseases. It remains to be determined whether other types of retinal degeneration, for example, glaucoma, will respond positively to targeting of Sigma1R. In addition it will be important to explore targeting Sigma1R in treatment of other more severe retinopathies including photoreceptor cell degeneration.
There is an additional aspect of the present study that warrants discussion, which concerns the retinal phenotype of the Ins2Akita/+ diabetic mouse. Several years ago, our group published in Investigative Ophthalmology and Visual Science observations about the protective effects of (+)-PTZ8 in Ins2Akita/+ diabetic mice. In that study, we observed and reported a very severe retinal phenotype in Ins2Akita/+ mice affecting the INL and the GCL. Barber and others5 had initially reported the ganglion cell loss and decreased thickness of INL in this mouse, although they did not report a pronounced disruption as we had observed. We do not know what factors caused the Ins2Akita/+ mice to have such a disrupted retinal architecture in our colony at that time. For several years thereafter, we did not use the Ins2Akita/+ mice in our studies. When we reestablished our colony more recently, we again purchased the mice from the Jackson Laboratories (the source of the original mice), but the animals derived from establishing the new colony showed a much milder phenotype, which is reported here. The ganglion cell loss, reported initially by Barber and others5 and by our group,8 is still a characteristic feature of this mouse model, but the fulminant retinopathy we observed8 is not present. We cannot account for the apparent phenotypic drift that appears to have occurred between our initial studies and the current work. We do not know whether our original colony harbored the rd8 mutation; however, we do not think this was the case because the characteristic retinal rosette pattern was not observed. We do not know whether a pathogen in our laboratory animal facilities was responsible. At any rate, the Ins2Akita/+ mice phenotype observed and reported in the present work is very similar to that observed by other investigators,5 and the severe phenotype is no longer detectable in our animals.
Acknowledgments
The authors thank the administration and especially the Provost and the VP for Research of Augusta University for institutional support of the EM/histology Core Facility and the Imaging Core Facility. The authors acknowledge the support for equipment necessary to test visual function provided by the Office of the Dean, Medical College of Georgia. S. B. Smith, J. Wang, and X. Cui are members of the James and Jean Culver Vision Discovery Institute, and the authors thank the institute for facilitating interactions and fruitful discussions about this work.
Supported by National Institutes of Health Grant R01 EY014560 and the James and Jean Culver Vision Discovery Institute.
Disclosure: J. Wang, None; X. Cui, None; P. Roon, None; S.B. Smith, None
References
- 1. Klein R,, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci. 2013; 54:ORSF5 – ORSF13. [DOI] [PMC free article] [PubMed]
- 2. Antonetti DA,, Klein R,, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012; 366: 1227–1239. [DOI] [PubMed] [Google Scholar]
- 3. Gardner TW,, Larsen M,, Girach A,, Zhi X. Diabetic macular oedema and visual loss: relationship to location, severity and duration. Acta Ophthalmol (Copenh). 2009; 87: 709–713. [DOI] [PubMed] [Google Scholar]
- 4. Barber AJ,, Lieth E,, Khin SA,, Antonetti DA,, Buchanan AG,, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes: early onset and effect of insulin. J Clin Invest. 1998; 102: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barber AJ,, Antonetti DA,, Kern TS,, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005; 46: 2210–2218. [DOI] [PubMed] [Google Scholar]
- 6. Yoshioka M,, Kayo T,, Ikeda T,, Koizumi A. A novel locus Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997; 46: 887–894. [DOI] [PubMed] [Google Scholar]
- 7. McLenachan S,, Chen X,, McMenamin PG,, Rakoczy EP. Absence of clinical correlates of diabetic retinopathy in the Ins2Akita retina. Clin Experiment Ophthalmol. 2013; 41: 582–592. [DOI] [PubMed] [Google Scholar]
- 8. Smith SB,, Duplantier J,, Dun Y,, et al. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest Ophthalmol Vis Sci. 2008; 49: 4154–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brune S,, Pricl S,, Wunsch B. Structure of the sigma1 receptor and its ligand binding site. J Med Chem. 2013; 56: 9809–9819. [DOI] [PubMed] [Google Scholar]
- 10. Fontanilla D,, Johannessen M,, Hajipour AR,, Cozzi NV,, Jackson MB,, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009; 323: 934–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayashi T,, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007; 131: 596–610. [DOI] [PubMed] [Google Scholar]
- 12. Hayashi T,, Su TP. Intracellular dynamics of sigma-1 receptors sigma(1)binding sites in NG108-15 cells. J Pharmacol Exp Ther. 2003; 306: 726–733. [DOI] [PubMed] [Google Scholar]
- 13. Su TP,, Hayashi T,, Maurice T,, Buch S,, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010; 31: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nguyen L,, Lucke-Wold BP,, Mookerjee SA,, et al. Role of sigma-1 receptors in neurodegenerative diseases. J Pharmacol Sci. 2015; 127: 17–29. [DOI] [PubMed] [Google Scholar]
- 15. Ola MS,, Moore P,, El-Sherbeny A,, et al. Expression pattern of sigma receptor 1 mRNA and protein in mammalian retina. Brain Res Mol Brain Res. 2001; 95: 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mavlyutov TA,, Epstein M,, Guo LW. Subcellular localization of the sigma-1 receptor in retinal neurons - an electron microscopy study. Sci Rep. 2015; 5: 10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang G,, Mysona B,, Dun Y,, et al. Expression, subcellular localization, and regulation of sigma receptor in retinal Müller cells. Invest Ophthalmol Vis Sci. 2006; 47: 5576–5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mavlyutov TA,, Nickells RW,, Guo LW. Accelerated retinal ganglion cell death in mice deficient in the Sigma-1 receptor. Mol Vis. 2011; 17: 1034–1043. [PMC free article] [PubMed] [Google Scholar]
- 19. Liu LL,, Wang L,, Zhong YM,, Yang XL. Expression of sigma receptor 1 mRNA and protein in rat retina. Neuroscience. 2010; 167: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 20. Duncan G,, Wang L. Focus on molecules: the Sigma-1 receptor. Exp Eye Res. 2005; 81: 121–122. [DOI] [PubMed] [Google Scholar]
- 21. Tchedre KT, Yorio T. sigma-1 receptors protect RGC-5 cells from apoptosis by regulating intracellular calcium, Bax levels, and caspase-3 activation. Invest Ophthalmol Vis Sci. 2008; 49: 2577–2588. [DOI] [PubMed] [Google Scholar]
- 22. Martin PM,, Ola MS,, Agarwal N,, Ganapathy V,, Smith SB. The sigma receptor ligand (+)-pentazocine prevents apoptotic retinal ganglion cell death induced in vitro by homocysteine and glutamate. Brain Res Mol Brain Res. 2004; 123: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dun Y,, Thangaraju M,, Prasad P,, Ganapathy V,, Smith SB. Prevention of excitotoxicity in primary retinal ganglion cells by (+)-pentazocine a sigma receptor-1 specific ligand. Invest Ophthalmol Vis Sci. 2007; 48: 4785–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cantarella G,, Bucolo C,, Di Benedetto G,, et al. Protective effects of the sigma agonist Pre-084 in the rat retina. Br J Ophthalmol. 2007; 91: 1382–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ha Y,, Dun Y,, Thangaraju M,, et al. Sigma receptor 1 modulates endoplasmic reticulum stress in retinal neurons. Invest Ophthalmol Vis Sci. 2011; 52: 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vogler S,, Winters H,, Pannicke T,, Wiedemann P,, Reichenbach A,, Bringmann A. Sigma-1 receptor activation inhibits osmotic swelling of rat retinal glial (Müller) cells by transactivation of glutamatergic and purinergic receptors. Neurosci Lett. 2015; 610: 13–18. [DOI] [PubMed] [Google Scholar]
- 27. Ha Y,, Shanmugam AK,, Markand S,, Zorrilla E,, Ganapathy V,, Smith SB. Sigma receptor 1 modulates ER stress and Bcl2 in murine retina. Cell Tissue Res. 2014; 356: 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mueller BH,, II, Park Y,, Daudt DR III, et al. Sigma-1 receptor stimulation attenuates calcium influx through activated L-type voltage gated calcium channels in purified retinal ganglion cells. Exp Eye Res. 2013; 107: 21–31. [DOI] [PubMed] [Google Scholar]
- 29. Tchedre KT,, Huang RQ,, Dibas A,, Krishnamoorthy RR,, Dillon GH,, Yorio T. Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Invest Ophthalmol Vis Sci. 2008; 49: 4993–5002. [DOI] [PubMed] [Google Scholar]
- 30. Ha Y,, Saul A,, Tawfik A,, et al. Late-onset inner retinal dysfunction in mice lacking sigma receptor 1 (σR1). Invest Ophthalmol Vis Sci. 2011; 52: 7749–7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ha Y,, Saul A,, Tawfik A,, Zorrilla EP,, Ganapathy V,, Smith SB. Diabetes accelerates retinal ganglion cell dysfunction in mice lacking sigma receptor 1. Mol Vis. 2012; 18: 2860–2870. [PMC free article] [PubMed] [Google Scholar]
- 32. Sabino V,, Cottone P,, Parylak SL,, Steardo L,, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009; 198: 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van de Pavert A,, Meuleman J,, Malysheva A,, et al. A single amino acid substitution (Cys249Trp) in CRB1 causes retinal degeneration and deregulates expression of pituitary tumor transforming gene Pttg1. J. Neurosci. 2007; 27: 564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tawfik A,, Markand S,, Al-Shabrawey M,, et al. Alterations of retinal vasculature in cystathionine-β-synthase heterozygous mice: a model of mild to moderate hyperhomocysteinemia. Am J Pathol. 2014; 184: 2573–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Markand S,, Saul A,, Roon P,, et al. Retinal ganglion cell loss and mild vasculopathy in methylene tetrahydrofolate reductase (Mthfr)-deficient mice: a model of mild hyperhomocysteinemia. Invest Ophthalmol Vis Sci. 2015; 56: 2684–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bringmann A,, Wiedemann P. Müller glial cells in retinal disease. Ophthalmologica. 2012; 227: 1–19. [DOI] [PubMed] [Google Scholar]
- 37. Barber AJ,, Gardner TW,, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011; 52: 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shimazawa M,, Sugitani S,, Inoue Y,, Tsuruma K,, Hara H. Effect of a sigma-1 receptor agonist cutamesine dihydrochloride (SA4503), on photoreceptor cell death against light-induced damage. Exp Eye Res. 2015; 132: 64–72. [DOI] [PubMed] [Google Scholar]
- 39. Sun X,, Cheng F,, Meng B,, Yang B,, Song W,, Yuan H. Pregnenolone sulfate decreases intraocular pressure and changes expression of sigma receptor in a model of chronic ocular hypertension. Mol Biol Rep. 2012; 39: 6607–6614. [DOI] [PubMed] [Google Scholar]
- 40. de Costa BR,, Bowen WD,, Hellewell SB,, et al. Synthesis and evaluation of optically pure [3H]-(+)-pentazocine, a highly potent and selective radioligand for sigma receptors. FEBS Lett. 1989; 251: 53–58. [DOI] [PubMed] [Google Scholar]
- 41. Wang J,, Shanmugam A,, Markand S,, Zorrilla E,, Ganapathy V,, Smith SB. Sigma 1 receptor regulates the oxidative stress response in primary retinal Müller glial cells via NRF2 signaling and system xc(-) the Na(+)-independent glutamate-cystine exchanger. Free Radic Biol Med. 2015; 86: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shanmugam A,, Wang J,, Markand S,, et al. Sigma receptor 1 activation attenuates release of inflammatory cytokines MIP1γ, MIP2, MIP3α, and IL12 (p40/p70) by retinal Müller glial cells. J Neurochem. 2014; 132: 546–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kowluru RA,, Kowluru A,, Mishra M,, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2015; 48: 40–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mueller BH,, II, Park Y,, Ma HY,, et al. Sigma-1 receptor stimulation protects retinal ganglion cells from ischemia-like insult through the activation of extracellular-signal-regulated kinases 1/2. Exp Eye Res. 2014; 128: 156–169. [DOI] [PubMed] [Google Scholar]
- 45. Zhao J,, Ha Y,, Liou GI,, Gonsalvez GB,, Smith SB,, Bollinger KE. Sigma receptor ligand (+)-pentazocine, suppresses inflammatory responses of retinal microglia. Invest Ophthalmol Vis Sci. 2014; 55: 3375–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McLenachan S,, Magno AL,, Ramos D,, et al. Angiography reveals novel features of the retinal vasculature in healthy and diabetic mice. Exp Eye Res. 2015; 138: 6–21. [DOI] [PubMed] [Google Scholar]