Abstract
A large proportion of children with autism spectrum disorder (ASD) have speech and/or language difficulties. While a number of structural and functional neuroimaging methods have been used to explore the brain differences in ASD with regards to speech and language comprehension and production, the neurobiology of basic speech function in ASD has not been examined. Magnetoencephalography (MEG) is a neuroimaging modality with high spatial and temporal resolution that can be applied to the examination of brain dynamics underlying speech as it can capture the fast responses fundamental to this function. We acquired MEG from 21 children with high‐functioning autism (mean age: 11.43 years) and 21 age‐ and sex‐matched controls as they performed a simple oromotor task, a phoneme production task and a phonemic sequencing task. Results showed significant differences in activation magnitude and peak latencies in primary motor cortex (Brodmann Area 4), motor planning areas (BA 6), temporal sequencing and sensorimotor integration areas (BA 22/13) and executive control areas (BA 9). Our findings of significant functional brain differences between these two groups on these simple oromotor and phonemic tasks suggest that these deficits may be foundational and could underlie the language deficits seen in ASD. Autism Res 2016, 9: 249–261. © 2015 International Society for Autism Research, Wiley Periodicals, Inc.
Keywords: magnetoencephalography, oromotor control, phoneme production, phonemic sequencing, autism spectrum disorder
Introduction
Autism spectrum disorder (ASD) manifests with heterogeneous phenotypes characterized by repetitive and restricted behaviors as well as deficits in social communication [DSM‐V; American Psychiatric Association, 2013]. Within a group of affected individuals, a large number will have a range of language impairments including delayed or atypical language acquisition, regression of language skills, little or no language, or intact language with difficulties in appropriate usage especially within social contexts [Stefanatos & Baron, 2011]. However, even in individuals with ASD and “normal” language, a significant number will still have subtle articulation and speech problems [Shriberg, Paul, McSweeny, Klin, & Cohen, 2001; Kjelgaard & Tager‐Flusberg, 2001; Cleland, Gibbon, Peppe, O'Hare, & Rutherford, 2010; for reviews see Boucher, 2012; Kujala, Lepistö, & Näätänen, 2013].
With speech, children with ASD typically have a variety of difficulties. Data from the United States indicate that over 60% of children with ASD use, or have used, speech‐language services [Pringle, Colpe, Blumberg, Avila, & Kogan, 2012]. There is increasing evidence of an interplay between speech‐language facility, social interactions, and cognitive ability. It is known that limited practice with oral motor and articulatory control may constrain speech development early in life and restrict the acquisition of phonetic inventories. As well, it is known that young children with lower cognitive aptitudes show poorer and less proficient speech development [Nip, Green, & Marx, 2009]. Both of these factors place children with ASD at a disadvantage. Children with ASD often might not be interested, or have fewer opportunities, for social interaction; thus, they may not be motivated to practice vocal productions. They also can have cognitive delays which hinder the acquisition of vocabulary and thus the development of speech motor skills [Stefanatos & Baron, 2011; Boucher, 2012]. Despite these well‐established deficits and their behavioral interactions, the neurobiology of speech and language dysfunction within ASD is complex and not well understood.
Neuroimaging investigations have used both structural and functional imaging to explore brain differences in ASD [for reviews see Anagnostou & Taylor, 2011; Stigler, McDonald, Anand, Saykin, & McDougle, 2011; Lenroot & Yeung, 2013]. There have been sufficient data from meta‐analytic approaches which identified increased total volumes, enlarged cerebellum and caudate, and decreased volumes in midbrain areas, the cerebellar vermis, and portions of the corpus callosum [Stanfield et al., 2008]. Another meta‐analysis of studies using voxel‐based morphometry (VBM) found smaller gray matter volumes in the amygdalae, hippocampi, and medial parietal areas in the individuals with autism [Via, Radua, Cardoner, Happe, & Mataix‐Cols, 2011]. Functional differences have also been identified and a meta‐analysis of fMRI data showed decreased frontal activations with executive function tasks, decreased activation in the superior temporal gyri with language and auditory tasks, and increased activation in the superior temporal gyri with social processing [Philip et al., 2012].
Neuroimaging investigations into the nature of communication deficits in ASD have focused primarily on brain regions associated with language perception and comprehension [for reviews, see Dichter, 2012; Pina‐Camacho et al., 2012; Mody & Belliveau, 2013]. The predominant finding is of abnormalities in the temporal lobe in the region of Wernicke's area [e.g., Herbert et al., 2002, 2005; Rojas, Bawn, Benkers, Reite, & Rogers, 2002] and a meta‐analysis of VBM studies found white matter differences in arcuate and uncinate fascicule; fibre tracts known to be involved in language function [Radua et al., 2011]. Speech production has been less frequently studied with neuroimaging due to the difficulty of scanning while participants are speaking. A promising avenue to explore the functional deficits related to language processing in ASD is the use of magnetoencephalography (MEG). This neuroimaging modality has high temporal and good spatial resolution, is able to resolve task‐related brain activations on a millisecond time scale, and has great potential for application to the study of language [Salmelin, 2007].
MEG has been used extensively to examine speech perception in children with ASD and has identified distinct auditory processing delays that mediate language and communication impairments [for a review see Roberts et al., 2008]. The study of speech production has been a challenge in the MEG as the movements of the jaw muscles create enormous artefacts and only recently has this obstacle been overcome. There is now evidence that MEG can be applied to examine the brain dynamics of both speech [Saarinen, Laaksonen, Parviainen, & Salmelin, 2006; Memarian et al., 2012] and language production [Herdman, Pang, Ressel, Gaetz, & Cheyne, 2007; Breier & Papanicolaou, 2008; Liljestrom, Hulten, Parkkonen, & Salmelin, 2009; Pang, Wang, Malone, Kadis, & Donner, 2011], even in young children who stutter [Sowman, Crain, Harrison, & Johnson, 2014]. The study of speech production in ASD, however, has not been examined with MEG. Further, the application of beamforming source analyses has demonstrated success in separating mouth movement artefacts from brain activations [Cheyne, Bostan, Gaetz, & Pang, 2007; Memarian et al., 2012].
In the current study, we used MEG to examine differences in brain activations during a series of increasingly complex oromotor tasks underlying speech production in a group of children with high‐functioning ASD and age‐ and sex‐matched controls. We hypothesized that the high temporal and good spatial resolution of MEG could identify subtle abnormalities in oromotor control related to speech production in children with ASD.
Materials and Methods
Participants
Twenty‐one children (17 males; mean—11.43 ± 3.19 years; median = 11.49 years; range: 5.99–17.64 years) with ASD and 21 sex‐and age‐matched typically developing children (17 males; mean = 11.46 ± 3.14 years; median = 11.5 years; range: 5.98–16.8 years) participated in the study. The children were evenly distributed across the age range. The typically developing children were recruited by advertisements in the community and were free of any neurological, psychiatric, language, speech, hearing, vision, motoric, auditory, or academic problems. Children who had previously seen a developmental paediatrician, speech and language pathologist, psychologist or psychiatrist were not included, and all control children completed a brief screening test to rule out any oromotor dysfunction. All typically developing children were in age‐appropriate grades in school and were selected to match the ASD group by age and sex. All participants or their parents gave written informed consent, and this study received Institutional Ethics approval.
Twenty‐three children with ASD were identified through the Province of Ontario Neurodevelopmental Disorder Network (POND) database from Holland Bloorview Kids Rehabilitation Hospital in Toronto. The diagnosis of ASD was based on clinical observation and meeting of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition [DSM‐IV‐TR; American Psychiatric Association, 2004] criteria. The diagnosis of ASD was supported by either the Autism Diagnostic Observational Schedule [ADOS; Lord, Rutter, DiLavore, & Risi, 1999] or the Autism Diagnostic Observational Schedule‐2 (ADOS‐2) [Lord et al., 2012] and the Autism Diagnostic Interview—Revised (ADI‐R) [Le Couteur, Lord, & Rutter, 2003]. These diagnostic instruments were administered by research‐reliable research staff. Children with high‐functioning ASD were selected if they appeared able to comply with the demands of a neuroimaging study and were then invited to participate. As well, all children were asked about comorbid conditions and screened for symptomology using the Child Behavior Checklist [CBCL; Achenbach and Rescorla, 2001]. In particular, we screened for ADHD symptoms. Using a brief screening tool based on the Verbal motor production assessment for children [VMPAC; Hayden and Square, 1999], apraxia of speech could not be ruled out in two of the children; thus, the final number included in the study was 21 for the ASD group.
Speech and Language Measures
To characterize the groups, all children completed standardized measures of speech and language abilities. Typically developing children completed the Expressive Language Test [Williams, 2007], the Peabody Picture Vocabulary Test [Dunn & Dunn, 2007], and the Wechsler Non‐Verbal [Wechsler and Naglieri, 2006] to measure the constructs of expressive vocabulary and word retrieval, receptive vocabulary and non‐verbal (or performance) IQ, respectively. The children with ASD were assessed on similar constructs with the Oral and Written Language Scales, second edition for oral expression (OWLS II‐OE) and listening comprehension [OWLS II‐LC; Carrow‐Woolfolk, 2011] and the Wechsler Abbreviated Scale of Intelligence, second edition, nonverbal domain [WASI‐II; Wechsler, 2011]. Means and standards for the tests for each group are reported in Table 1.
Table 1.
Participant Characteristics
| Typically developing (mean ± SD) | ASD (mean ± SD) | P‐value | |
|---|---|---|---|
| N | 21 | 21 | |
| Males: Females | 17:4 | 17:4 | |
| Age (years) | 11.5 ± 3.1 | 11.4 ± 3.2 | P = 0.79 |
| Age range | 5.98–16.8 | 5.99–17.64 | |
| Oral expressiona | 113 ± 13.7 | 88.6 ± 24.4 | N/A |
| Listening comprehensionb | 117 ± 11.2 | 92.6 ± 27.9 | N/A |
| Non‐verbal IQc | 111 ± 9.0 | 96.4 ± 18.1 | N/A |
TD tested with EVT; ASD tested with OWLSII‐OE.
TD tested with PPVT; ASD tested with OWLSII‐LC.
TD tested with WNV; ASD tested with WASI.
MEG Data Acquisition and Co‐registration with Structural MRI
MEG data were acquired in a whole‐head 151 channel MEG (CTF Omega, MISL, Coquitlam, Canada) located in the Neuromagnetic Lab at the Hospital for Sick Children. Prior to examination, fiducial markers were placed on each subject's nasion and both preauricular points; these were replaced with MRI contrast markers after MEG data collection to allow co‐registration of MEG data with structural MRI. Subjects were tested supine on the MEG bed. Data were acquired continuously at a 4000 Hz to allow audio recording of the subject's voice output in conjunction with the MEG recording. Data were acquired with a third order spatial gradient and low pass filtered at 200 Hz.
After the MEG recording, a T1‐weighted MR image (3D SAG MPRAGE: GRAPPA = 2, TR/TE/TI/FA = 2300/2.96/900/9, FOV/Res = 192 × 240 × 256, 1.0mm isotropic voxels) was obtained on a 3T MR scanner (Magnetom Tim Trio, Siemens AG, Erlangen, Germany) with a 12‐channel head coil. MEG images were superimposed onto the co‐registered MRI for each individual subject.
MEG Tasks
Participants completed three tasks of increasing oromotor complexity in the MEG. One hundred fourteen trials of each task were completed for each condition in one block. Children fixated on a cue stimulus consisting of a small white circle with a small cross inside and were instructed to perform the oromotor task whenever the circle changed color to a light green. The choice to use a color change cue was intentional to minimize the visual onset/offset response and the visual evoked response, as these are usually several times larger than the cognitive responses of interest in this study. The interval between trials varied between 3500 and 3900 ms.
The three tasks consisted of increasingly difficult oromotor tasks with the same initial bilabial movement. The first task was a pure oromotor action which we refer to as “mouth open.” Children were instructed to open their jaw to mid‐aperture and then close it completely. The research assistant watched the first few movements to ensure a straight vertical motion downwards and upward. No sound productions or vocalizations were required in this task. In the second task, children were instructed to speak the phoneme/pa/. This phoneme required the same vertical open‐close motion as the “mouth open” condition with the addition of a phonemic vocalization. In the third task, children were instructed to speak the phonemic sequence/pa//da//ka/. Children were trained to produce this as one utterance, and again, the first phoneme involved the same vertical open–close mouth motion, while the remainder of the production was a more complex utterance that required the coordination and activation of the respiratory, vocal, and oral muscular systems, as well as the cognitive functions of oromotor planning and sequencing. The MEG data acquisition required a total of approximately 40 min, although somewhat longer for the younger children as more frequent breaks were required.
MEG Data Analysis
The continuously recorded MEG data were downsampled to 1000 Hz, filtered between 1 and 50 Hz and epoched from 500 ms before the stimulus cue to 1500 ms post‐cue (−500 to +1500 ms epoch lengths). An inner‐skull surface headmodel [FMRIB software library (FSL); Smith et al., 2004] was computed from each subject's anatomical MRI image and co‐registered to the MEG data. Beamformer source localization, a spatial filtering algorithm used to identify peak locations of brain activation, was applied using BrainWave (cheynelab. utoronto.ca) on the whole head data with 4 × 4 × 4 mm voxel resolution and 10 ms time windows across the whole epoch. Prominent activations were seen in the typically developing group in eight Brodmann areas in the left hemisphere. To explore subtle differences in the activation and timing of these processes in ASD, the time courses at these locations were studied. To achieve this, the coordinates for these eight activations, along with their homologous right hemisphere locations, were identified from beamforming and their Talairach [Talairach & Tournoux, 1988; Lancaster et al., 2000] coordinates were noted (See Table 2). These coordinates were then used as the locations where the time courses of activation (also called a “virtual sensor”) were reconstructed, using the absolute values of the beamformer output. This reconstruction resulted in waveforms at each location for each subject and condition. As a first pass, the data from each virtual sensor were averaged across subjects and within conditions to identify the locations with prominent peaks in the waveform that could be submitted to peak‐picking. Averaged sensors that did not show a clear peak in the waveform were not submitted to peak‐picking. Following recommended procedures for peak‐picking [Picton et al., 2000], if the averaged virtual sensor showed magnitude differences, then peak‐picking for each individual was performed at the latency of the peak in the averaged virtual sensor. If the averaged virtual sensor showed latency differences, a window around the peak in the average was specified and this window was applied to each individual tracing. The window was determined by inspection of the grand averaged waveform and was selected to encompass the onset and offset of the waveform of interest. The latency with the highest magnitude within the window was noted. Differences were submitted to separate paired t‐tests (as the ASD and control children were matched by age and sex) for each region of interest. Corrections for multiple comparisons were completed using the Benjamini and Hochberg correction factor which is calculated as:
where n is the number of comparisons and i is the rank of the P‐value in ascending order [Benjamini & Hochberg, 1995]. As each condition was analysed separately, the number of comparisons for magnitude were 14, 10, and 19 for mouth open,/pa/and/pa//ta//ka/, respectively, and for latency, the number of comparisons was 12.
Table 2.
Talairach Co‐ordinates for “virtual sensor” Locations for the Three Conditions
| MO | /pa/ | /pa//da//da/ | |||||
|---|---|---|---|---|---|---|---|
| Brodmann Area | Anatomical label | Left | Right | Left | Right | Left | Right |
| BA 47 | IFG | −36 15 −18 | 44 15 −7 | −40 15 −7 | 32 26 −25 | −44 23 3 | 36 19 −18 |
| BA 9 | MFG | −44 13 29 | 44 13 36 | −24 25 32 | 24 25 32 | −44 13 29 | 44 13 36 |
| BA 13 | Insula | −40 −15 8 | 44 −11 15 | −40 5 18 | 36 −42 21 | −40 −26 23 | 44 −20 −9 |
| BA 6 | PCG | −40 −10 30 | 48 −6 26 | −51 −3 26 | 36 −6 33 | −44 −3 26 | 48 −6 33 |
| BA 4 | PCG | −44 −14 38 | 40 −17 41 | −32 −17 41 | 36 −13 49 | −40 −14 38 | 32 −17 45 |
| BA 22 | STG | −32 −49 25 | 36 −49 25 | −40 −53 21 | 40 −53 21 | −40 −53 21 | 36 −49 25 |
| BA 40 | IPL | −32 −41 39 | 44 −30 31 | −28 −36 50 | 40 −41 28 | −48 −45 35 | 40 −33 35 |
| BA 18 | Cuneus | −24 −69 15 | 16 −80 22 | −16 −73 18 | 20 −81 15 | −24 −69 15 | 4 −77 8 |
IFG, in ferior frontal gyrus; MFG, middle frontal gyrus; PCG, pre‐central gyrus; STG, superior temporal gyrus; IPL, inferior parietal lobule
Results
All significant results, with both uncorrected and corrected P‐values, for magnitude differences are summarized Table 3 and for latency in Table 4. These are described by condition in the following sections.
Table 3.
Mean Magnitudes for Locations with Significant Differences (P < 0.05, Corrected) between the Typically Developing and ASD Groups in the Three Conditions
| Magnitude (mean ± SEM) | ||||
|---|---|---|---|---|
| Location (BA) | Typically developing | ASD | puncorr | pcorr |
| Mouth open | ||||
| R PCG (BA4) | −0.21 ± 1.46 | 0.46 ± 1.34 | 0.0042 | 0.029 |
| R MFG (BA9) | −0.31 ± 1.00 | 0.56 ± 1.01 | 0.0028 | 0.039 |
| L Cuneus (BA18) | 0.75 ± 2.69 | 1.92 ± 2.12 | 0.014 | 0.048 |
| /pa/ | ||||
| No significant differences | ||||
| /pa//da//ka/ | ||||
| R PCG (BA4) | 0.08 ± 0.78 | −0.58 ± 0.99 | 0.0025 | 0.047 |
| L Insula (BA13) | 0.26 ± 0.75 | 0.90 ± 0.76 | 0.0090 | 0.043 |
| R STG (BA22) | −0.33 ± 0.90 | −1.16 ± 1.19 | 0.0066 | 0.042 |
| L Cuneus (BA18) | 0.53 ± 2.63 | 1.67 ± 1.99 | 0.0033 | 0.032 |
PCG, pre‐central gyrus; MFG, middle frontal gyrus; STG, superior temporal gyrus.
Table 4.
Mean Latencies for Locations with Significant Differences (P < 0.05, Corrected) between the Typically Developing and ASD Groups in the Three Conditions
| Magnitude (mean ± SEM) | ||||
|---|---|---|---|---|
| Location (BA) | Typically developing | ASD | puncorr | pcorr |
| Mouth open | ||||
| R PCG (BA6) | 328 ± 13.1 | 366 ± 29.9 | 0.0000003 | 0.0000009 |
| L IPL (BA40) | 154 ± 12.6 | 180 ± 21.8 | 0.00003 | 0.00006 |
| /pa/ | ||||
| L IFG (BA47) | 219 ± 23.4 | 256 ± 17.4 | 0.000009 | 0.00002 |
| L MFG (BA9) | 231 ± 15.5 | 217 ± 19.2 | 0.011 | 0.015 |
| L IPL (BA40) | 174 ± 21.8 | 214 ± 36.6 | 0.00023 | 0.00039 |
| L Cuneus (BA18) | 193 ± 25.2 | 224 ± 30.8 | 0.0017 | 0.0025 |
| /pa//da//ka/ | ||||
| R IFG (BA47) | 158 ± 12.1 | 118 ± 15.8 | 0.000004 | 0.00002 |
| L Insula (BA13) | 242 ± 20.0 | 323 ± 27.2 | 0.000003 | 0.00003 |
Mouth Open Condition
Figure 1 shows the averaged virtual sensors for the eight left hemisphere and eight right hemisphere regions. Visual inspection shows that for some of the sensor locations (e.g., L IFG BA47), there is no difference in the morphology of the grand‐averaged response between groups. For other locations, peak‐picking and statistical testing were applied. After correction for multiple comparisons, only three sensors showed significant differences in magnitude (circled) and two in latency (boxed). For latency, the right motor area (R PCG BA6) and left Wernicke's Area (L IPL BA40) showed delays of approximately 30 ms in the ASD compared to control groups. Table 3 lists the mean magnitudes and Table 4 lists the latencies for both groups.
Figure 1.
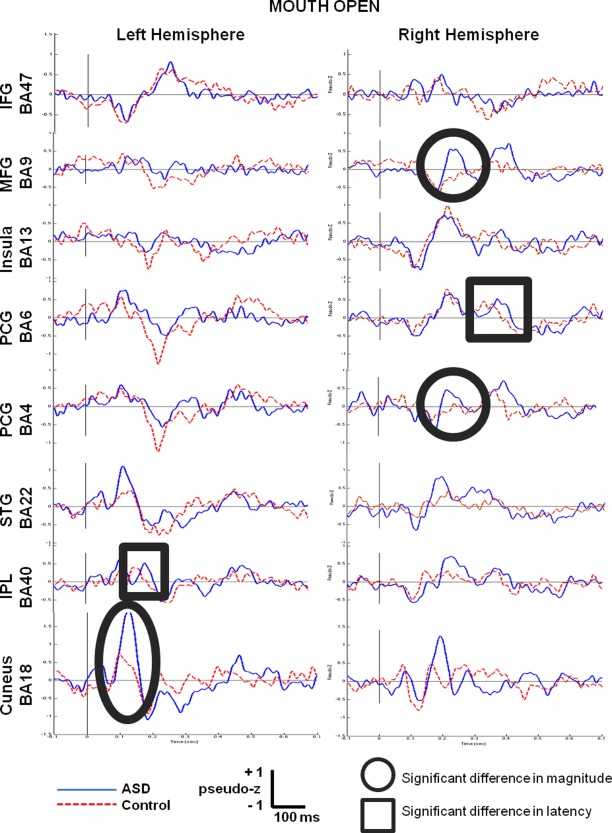
Grand‐averaged time courses of activation (virtual sensors) reconstructed from 8 regions of interest in the left hemisphere and their homologous right hemisphere locations for the mouth open condition. Responses from the children with ASD are shown by the blue solid line and the controls by the red dotted line. Significant between group differences in magnitude are circled and significant differences in latency are boxed.
/pa/Condition
For the/pa/condition, there were no significant differences in magnitude between groups; however, four left hemisphere sensors showed significant differences in latency (see Fig. 2). Delays of greater than 30 ms were seen in frontal and temporal language areas (L IFG BA47, L IPL BA40) and visual cortex (L cuneus BA18) for the ASD compared to control groups. Interestingly, in executive control areas (L MFG BA9), the group with ASD showed a peak that was approximately 15 ms earlier than the controls. These group differences are listed in Table 4.
Figure 2.
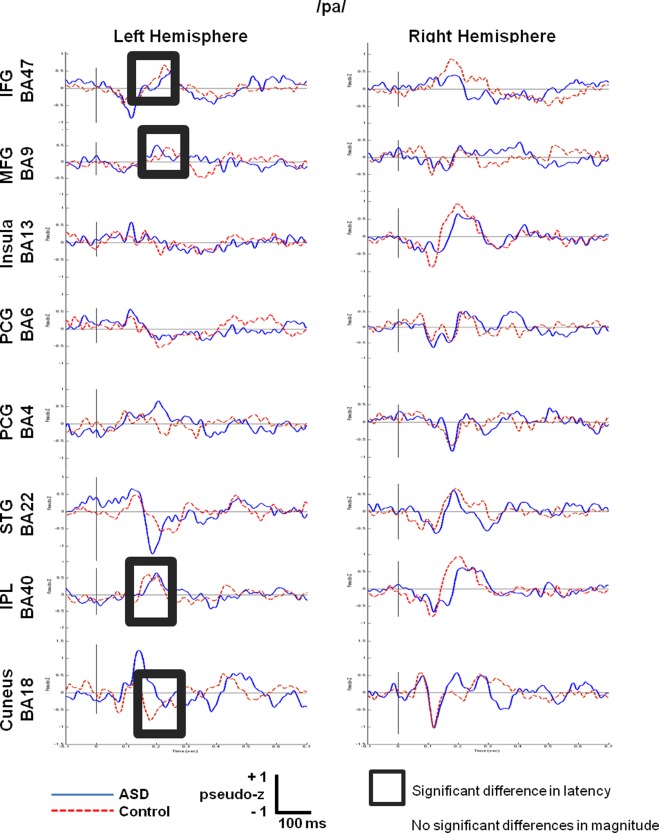
Grand‐averaged time courses of activation (‘virtual sensors’) reconstructed from 8 regions of interest in the left hemisphere and their homologous right hemisphere locations for the/pa/condition.
/pa//da//ka/Condition
Figure 3 shows the grand averaged virtual sensors for the 16 regions of interest. Peak picking identified four sensors with significant differences in magnitude (circled) and two with significant differences in latency (boxed). Compared to the control subjects, activation in the group with ASD peaked earlier by 40 ms in homologous Broca's Area (R IFG BA47) but was delayed by 80 ms in left insula (BA13). Means of these significant differences are contained in Table 4.
Figure 3.
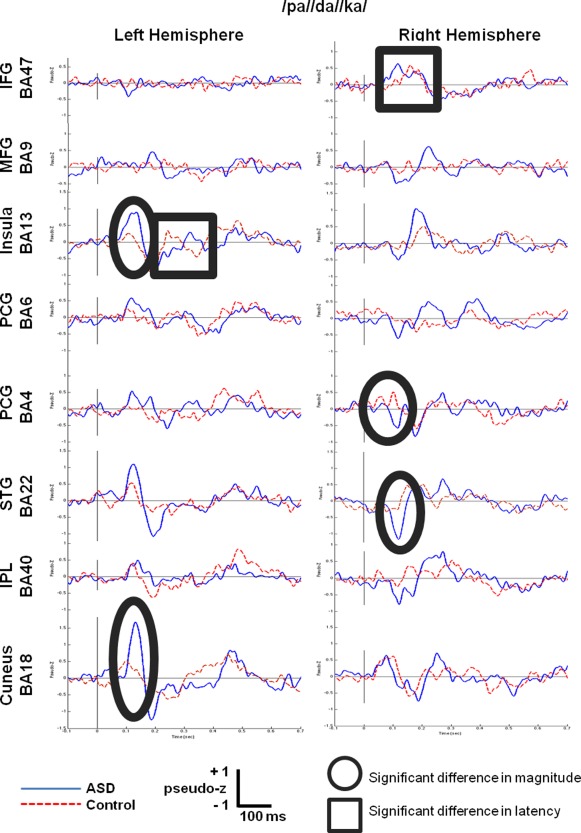
Grand‐averaged time courses of activation (‘virtual sensors’) reconstructed from 8 regions of interest in the left hemisphere and their homologous right hemisphere locations for the/pa//da//ka/condition.
Discussion
We identified amplitude and latency differences between typically developing children and children with ASD on a set of simple tasks involving oromotor control and phoneme production, using a classic peak‐picking approach on virtual sensors reconstructed from regions of interest identified from beamformer source localization. While studies of language have suggested that children with ASD would likely have difficulties with language tasks, this is the first neuroimaging study to demonstrate timing delays related to ASD at the more basic speech production level.
To summarize the most salient findings, for the oromotor task, we found increased magnitude and delayed latency for ASD in motor control areas (BA4, BA6), as well as increased magnitude in an executive control area (BA9). With the phoneme task, latency delays were seen for the ASD group in both frontal (BA47) and temporal language (BA40) processing areas. Finally, for the oromotor sequencing task, in the ASD group, both an increased magnitude and a delayed latency were seen in a sensory integration area (insula). Typically, when increased magnitudes are seen, this is interpreted as a process requiring more synchronized brain activity, or possibly more effort to complete, while increased latencies are interpreted as a demonstration that the task was more difficult and required more time to complete. Below, we interpret the specific findings for each of the three tasks.
Difficulties with Oromotor Control and Oromotor Sequencing in ASD
In the mouth open and/pa//da//ka/conditions, differences were seen in the right hemisphere primary motor control area (R PCG/BA4) only for magnitude. It is known that primary motor areas located in pre‐central gyrus (PCG/BA4) are organized in a homuncular fashion and activate with motor tasks [Penfield & Roberts, 1959]. Typically, this activation is unilateral and contralateral to the limb that is moved; however, mouth and jaw movements are the exception, and typically result in activation of bilateral motor cortices [Salmelin & Sams, 2002; Saarinen et al., 2006]. Interestingly, our finding of a unilateral right hemisphere over‐activation of oromotor control is consistent with an fMRI resting state study which found enhanced engagement of the right hemisphere for components of the sensorimotor network in ASD [Cardinale, Shih, Fishman, Ford, & Muller, 2013]. Further, our findings of delayed and larger activations in the ASD group suggest that children with ASD are slower in activating, and require a greater neural response, to generate the required motor output. As well, in the mouth open condition, the children with ASD showed significantly increased latency for the activation in the right premotor cortex, or motor planning area, (R PCG/BA6), offering additional confirmation that they were slower in activating the appropriate brain regions required to complete the task.
For the/pa//da//ka/condition, differences were also observed in the left insula (BA13) and right superior temporal gyrus (R STG/BA22), with the ASD group showing delayed and larger activations in these regions, respectively. The insula is known to subserve a number of functions, one of which is sensorimotor integration [Kurth, Zilles, Fox, Laird, & Eickhoff, 2010]. The STG has been implicated in sequencing as well as melodic and prosodic processing [e.g., Buchsbaum, Hickok, & Humphries, 2001; Meyer, Alter, Friederici, Lohmann, von Cramon, 2002; Patterson, Uppenkamp, Johnsrude, & Griffiths, 2002]. Our findings of larger and slower activations in these two regions support observations that children with ASD have difficulty with sensorimotor integration and oromotor sequencing.
Even with the simple oromotor tasks used in our study, significant differences were seen in the children with ASD. This suggests that their oromotor difficulties may be foundational to some of the speech deficits seen in ASD and, with more complex sequences or more difficult tasks, these difficulties become more pronounced. Of note, in the/pa/condition, no differences were observed in the primary motor control and supplementary motor control areas between control and ASD children. This may be due to the over‐learned nature of this phoneme. It is one of the first phonemes to be learned in the English language and has been extensively rehearsed and practised by both groups of children. This is consistent with literature showing that in most cases of high‐functioning ASD, productive phonology, or articulation, is normal [Kjelgaard & Tager‐Flusberg, 2001]. Our inclusion of the/pa//da//ka/utterance is helpful in demonstrating that while children with ASD might perform well at simple tasks, they may begin to experience greater difficulties once the tasks become more complex, such as in this case where sequencing was required.
Difficulties with Executive Control in ASD
The above suggestion that children with ASD may perform adequately on simple tasks, but will fail on more complex tasks, is supported by our finding of differences between the ASD and control groups at the middle frontal gyrus (BA9). Brodmann Area 9 is part of the dorsolateral prefrontal cortex and is known to be involved in sustaining attention, working memory and also movement preparation [Pochon et al., 2001]. We see larger and earlier activation of this area in the ASD group for both the mouth open and/pa/tasks. This suggests that the ASD group rely more heavily on this region as they recruit it sooner and to a greater degree than the controls. An fMRI study found that adolescents with ASD showed a disruption of modulatory control and connectivity between Broca's area and dorsolateral prefrontal cortex when performing a verb generation task [Verly et al., 2014]. It is not clear how MEG activations relate to fMRI connectivity due to the very different time‐scales and distinct signal origins; however, both studies point to abnormalities in ASD in the prefrontal regions.
Functional Deficits in Canonical Language Areas in ASD
For both the mouth open and/pa/tasks, children with ASD showed significant delays in activation in the left inferior parietal lobule (L IPL BA 40) or Wernicke's Area. Typically this area is activated with receptive language processing, but it also plays an important role in language‐related sensory feedback [Jardri et al., 2007]. The delayed activation in this area suggests that children with ASD experience a lag in their sensory feedback of the speech production information, and this may be a contributing factor to their language deficits. The hypothesis that abnormalities in this region contribute to both language and ASD symptomology is supported by an MRI study which found increased gray matter volumes in the parietal lobe that correlated with increased delay of age of onset of words [Zoccante et al., 2010] and a functional connectivity MRI study which reported a lack of left hemisphere connectivity between Wernicke's area and the posterior cingulate cortex which correlated with autism severity [Nielsen et al., 2014].
For both of the phonemic stimuli,/pa/and/pa//da//ka/, significant differences were observed in inferior frontal areas (IFG BA47). In the left hemisphere, this difference was seen as a delayed activation for ASD with the/pa/production, while for/pa//da//ka/, the children with ASD showed the opposite effect; an earlier activation in the right hemisphere. Typically, with word production and language production tasks, right hemisphere activation is observed in younger children and in clinical groups that struggle with the task. Involvement of the homologous right hemisphere language areas suggests that the recruitment of additional neural resources is required to complete the task. This is consistent with a pragmatic language task in fMRI which yielded comparable behavioral results between TD and ASD, while ASD activated homologous BA47 to complete the task—this was interpreted to indicate the compensatory nature of the right BA47 [Tesink et al., 2009]. Similar results have been seen in fMRI naming tasks where adolescents with ASD showed less left‐lateralized responses with word production [Knaus, Silver, Lindgren, Hadjikhani, & Tager‐Flusberg, 2008] and a diffusion tensor imaging study which showed that abnormalities in left hemisphere frontotemporal language connections correlated with severity of clinical language impairment [Nagae et al., 2012].
Furthermore, there are a number of studies reporting over‐engagement of frontal and temporal brain areas in the right hemisphere when processing language in ASD. It may be that the left hemisphere abnormalities seen in our study, with oromotor control and basic speech tasks, result in an inability for the left hemisphere to carry out its language tasks, and thus the right hemisphere is recruited to supplement the processing. The outcome is the asymmetrical right hemisphere over‐activation frequently seen in ASD with language tasks [e.g., Boddaert et al., 2004; Just, Cherkassky, Keller, & Minshew, 2004; Gendry Meresse et al., 2005; Harris et al., 2006; Gaffrey et al., 2007; Wang, Lee, Sigman, & Dapretto, 2006; Kleinhans, Muller, Cohen, & Courchesne, 2008; Mason, Williams, Kana, Minshew, & Just, 2008; Groen et al., 2009; Knaus et al., 2010; Eyler, Pierce, & Courchesne, 2012]. This over‐involvement of right hemisphere seen in functional neuroimaging is supported by structural neuroimaging studies. For example, using DTI, children with ASD showed less left lateralization in fractional anisotrophy measures in uncinate, arcuate and cingulum [Lo et al., 2011], as well as in radial diffusivity of the arcuate fasciculus. Further, greater rightward asymmetry of connections to Broca's area was associated with higher language scores in ASD; whereas in the typically developing group, greater leftward lateralization was associated with higher language scores [Joseph et al., 2014].
Abnormal Activation of Primary Visual Areas in ASD
Across all three conditions, the left cuneus (BA18) response was significantly larger or later in ASD than in controls. Functional MRI studies have already demonstrated significantly greater activation in extrastriate visual cortex (BA18 and 19) with visual language processing in ASD. This additional activation is interpreted as increased visualization of target items; however, behaviorally, the individuals with ASD rely heavily on perceptual components and visual imagery but their performance remains poorer [Gaffrey et al., 2007; Shen et al., 2012]. In our study, we specifically chose a very simple stimulus (small cross in a circle) with a change in color as the cue, as we had hoped to not evoke extensive visual perceptive processing and thus minimize the visual response differences between groups. This was not the case. Clearly even with a simple visual stimulus, the hyperactivity of the extrastriatal visual region is found in children with ASD.
Limitations and Future Directions
While this is the first report using MEG to characterize the spatiotemporal dysfunctions that underlie oromotor processing and speech production, there are a number of limitations to this study including its small sample size, the use of different language and IQ measures between groups, its focus on high‐functioning autism, and its emphasis on speech but not language processing. Future studies should aim to recruit a larger sample of children across the spectrum of language abilities. Further, these participants should be extensively phenotyped with various oromotor, speech and language measures, and the participants should undergo a battery of tasks in the MEG that encompass both simple and higher‐order, complex language processes. This would elucidate the association between brain processes and specific language/speech functions. This would allow the isolation of specific brain dysfunctions that are tied to specific behavioral dysfunctions. With this knowledge of the neurobiology of atypical language function, it would be possible to begin to develop interventions and tailor therapies that target the specific dysfunction in question.
Summary and Conclusions
This is the first study to show significant differences in the early temporal dynamics underlying simple oromotor tasks involving nonspeech, speech, and phoneme sequencing that demonstrate brain abnormalities in children with high‐functioning ASD compared to age‐ and sex‐matched controls. These differences were located in primary motor control areas (BA4), motor control areas (BA6), dorsolateral prefrontal cortex (BA9), motor integration (BA13) and sequencing areas (BA22), as well as classical language production (BA47) and language processing (BA40) areas. To our surprise, even the simple mouth open condition and simple phoneme production condition evoked significant between‐group differences. Often the latency delays were of 30 ms or more in the ASD group. Certainly, these delays at the basic speech production level would contribute to downstream impact at the language production level. Our finding of brain abnormalities using basic speech and oromotor tasks in children with high‐functioning ASD points to the fundamental nature of these oromotor deficits, although future studies are required to determine whether these results extend to the rest of the spectrum. As well, these findings highlight the value of using MEG, as it was the high temporal resolution of MEG that allowed us to identify these important delays in the ASD group.
Acknowledgments
This study was completed by Tatiana Valica in partial fulfillment of the requirements for the degree of Master of Science from the University of Toronto. The authors would like to thank Anna Oh and Marc Lalancette for help with MEG data collection, Paul Ferrari and Sarah Vinette for task development, Susan Day Fragiadakis and Naomi Sklar for ASD subject recruitment, and the clinical team at Holland Bloorview Kids Rehabilitation Hospital for completing the clinical assessments. Thanks to Dr. Elizabeth Johnson for comments on the work, and Talya Wolff for consultation on the speech language aspects of the project. This work was partially funded by a Canadian Institutes for Health Research Operating Grant to Dr. Elizabeth Pang (MOP‐89961). The recruitment and ASD assessments were made possible by funding from the Province of Ontario Neurodevelopmental Disorders Network of the Ontario Brain Institute to Dr. Evdokia Anagnostou.
References
- Achenbach, T.M. & Rescorla, L.A. (2001). Manual for the ASEBA school‐age forms & profiles. Burlington, VT: University of Vermont, Research Centre for Children, Youth, & Families. [Google Scholar]
- American Psychiatric Association . (2004). Diagnostic and statistical manual of mental disorders (DSM IV‐TR). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Anagnostou, E. & Taylor, M.J. (2011). Review of neuroimaging in autism spectrum disorders: What have we learned and where we go from here. Molecular Autism, 2(1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y. & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Boucher, J. (2012). Research review: Structural language in autistic spectrum disorder—characteristics and causes. Journal of Child Psychology and Psychiatry 53, 219–233. [DOI] [PubMed] [Google Scholar]
- Boddaert, N. , Chabane, N. , Belin, P. , Bourgeois, M. , Royer, V. , Barthelemy, C. , et al. (2004). Perception of complex sounds in autism: Abnormal auditory cortical processing in children. American Journal of Psychiatry, 161, 2117–20. [DOI] [PubMed] [Google Scholar]
- Breier, J.I. & Papanicolaou, A.C. (2008). Spatiotemporal patterns of brain activation during an action naming task using magnetoencephalography. Journal of Clinical Neurophysiology, 25, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum, B.R. , Hickok, G. , & Humphries, C. (2001). Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cognitive Science, 25, 663–678. [Google Scholar]
- Cardinale, R.C. , Shih, P. , Fishman, I. , Ford, L.M. , & Muller, R.‐A. (2013). Pervasive rightwards asymmetry shifts of functional networks in autism spectrum disorder. JAMA Psychiatry, 70(9), 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow‐Woolfolk, E. (2011). Oral and written language scales (2nd ed.). (OWLS‐II). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Cheyne, D. , Bostan, A.C. , Gaetz, W. , & Pang, E.W. (2007). Event‐related beamforming: A robust method for presurgical functional mapping using MEG. Clinical Neurophysiology, 118, 1691–1704. [DOI] [PubMed] [Google Scholar]
- Cleland, J. , Gibbon, F. , Peppe, S. , O'Hare, A. , & Rutherford, M. (2010). Phonetic and phonological errors in children with high functioning autism and Asperger syndrome. International Journal of Speech and Language Pathology, 12, 69–76. [DOI] [PubMed] [Google Scholar]
- Dichter, G.S. (2012). Functional magnetic resonance imaging of autism spectrum disorders. Dialogues in Clinical Neuroscience, 14, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, L.M. & Dunn, D.M. (2007). Peabody picture vocabulary test (4th ed.). Minneapolis, MN: Pearson Assessments. [Google Scholar]
- Eyler, L.T. , Pierce, K. , & Courchesne, E. (2012). A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain, 135(3), 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey, M.S. , Kleinhans, N.M. , Haist, F. , Akshoomoff, N. , Campbell, A. , Courchesne, E. , et al. (2007). Atypical [corrected] participation of visual cortex during word processing in autism: An fMRI study of semantic decision. Neuropsychologia, 45, 1672–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendry Meresse, I. , Zilbovicius, M. , Boddaert, N. , Robel, L. , Philippe, A. , Sfaello, I. , et al. (2005). Autism severity and temporal lobe functional abnormalities. Annals of Neurology, 58, 466–469. [DOI] [PubMed] [Google Scholar]
- Groen, W.B. , van Orsouw, L. , Huurne, N. , Swinkels, S. , van der Gaag, R.J. , Buitelaar, J.K. , et al. (2009). Intact spectral but abnormal temporal processing of auditory stimuli in autism. Journal of Autism Developmental Disorders, 39, 742–750. [DOI] [PubMed] [Google Scholar]
- Harris, G.J. , Chabris, C.F. , Clark, J. , Urban, T. , Aharon, I. , Steele, S. , et al. (2006). Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain and Cognition, 61, 54–68. [DOI] [PubMed] [Google Scholar]
- Hayden, D.A. & Square, P.A. (1999). The verbal motor production assessment for children. San Antonio, Texas: Psychological Corporation. [VMPAC; ] [Google Scholar]
- Herbert, M.R. , Harris, G.J. , Adrien, K.T. , Ziegler, D.A. , Makris, N. , Kennedy, D.N. , et al. (2002). Abnormal asymmetry in language association cortex in autism. Annals of Neurology, 52(5), 588–596. [DOI] [PubMed] [Google Scholar]
- Herbert, M.R. , Ziegler, D.A. , Deutsch, C.K. , O'Brien, L.M. , Kennedy, D.N. , Filipek, P.A. , et al. (2005). Brain asymmetries in autism and developmental language disorder: A nested whole‐brain analysis. Brain, 128(Pt 1), 213–226. [DOI] [PubMed] [Google Scholar]
- Herdman, A.T. , Pang, E.W. , Ressel, V. , Gaetz, W. , & Cheyne, D. (2007). Task‐related modulation of early cortical responses during language production: An event‐related synthetic aperture magnetometry study. Cerebral Cortex, 17, 2536–2543. [DOI] [PubMed] [Google Scholar]
- Jardri, R. , Pins, D. , Bubrovsky, M. , Despretz, P. , Pruvo, J‐P. , Steinling, M. , & Thomas, P. (2007). Self awaremess and speech processing: An fMRI study. Neuroimage, 35, 1656–1653. [DOI] [PubMed] [Google Scholar]
- Joseph, R.M. , Fricker, Z. , Fenoglio, A. , Lindgren, K.A. , Knaus, T.A. , & Tager‐Flusberg, H. (2014). Structural asymmetries of language‐related gray and white matter and their relationship to language function in young children with ASD. Brain Imaging and Behaviour, 8, 60–72. [DOI] [PubMed] [Google Scholar]
- Just, M.A. , Cherkassky, V.L. , Keller, T.A. , & Minshew, N.J. (2004). Cortical activation and synchronization during sentence comprehension in high‐functioning autism: Evidence of underconnectivity. Brain, 127, 1811–1821. [DOI] [PubMed] [Google Scholar]
- Kjelgaard, M.M. & Tager‐Flusberg, H. (2001). An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes, 16, 287–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans, N.M. , Muller, R.A. , Cohen, D.N. , & Courchesne, E. (2008). Atypical functional lateralization of language in autism spectrum disorders. Brain Research, 1221, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus, T.A. , Silver, A.M. , Lindgren, K.A. , Hadjikhani, N. , & Tager‐Flusberg, H. (2008). fMRI activation during a language task in adolescents with ASD. JINS, 14, 967–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus, T.A. , Silver, A.M. , Kennedy, M. , Lindgren, K.A. , Dominick, K.C. , Siegel, J. , et al. (2010). Language laterality in autism spectrum disorder and typical controls: A functional, volumetric, and diffusion tensor MRI study. Brain and Language, 112(2), 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala, T. , Lepistö, T. , & Näätänen, R. (2013). The neural basis of aberrant speech and audition in autism spectrum disorders. Neuroscience and Biobehavioral Reviews, 37, 697–704. [DOI] [PubMed] [Google Scholar]
- Kurth, F. , Zilles, K. , Fox, P.T. , Laird, A.R. , & Eickhoff, S.B. (2010). A link between the systems: Functional differentiation and integration within the human insula revealed by meta‐analysis. Brain Structure & Function, 214(5/6), 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, J.L. , Woldorff, M.G. , Parsons, L.M. , Liotti, M. , Freitas, C.S. , Rainey, L. , et al. (2000). Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping, 10, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur, A. , Lord, C. , & Rutter, M. (2003). The Autism Diagnostic Interview–Revised (ADI‐R). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Lenroot, R.K. & Yeung, P.K. (2013). Heterogeneity within autism spectrum disorders: What have we learned from neuroimaging studies? Frontiers Human Neuroscience, 7, 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljestrom, M. , Hulten, A. , Parkkonen, L. , & Salmelin, R. (2009). Comparing MEG and fMRI views to naming actions and objects. Human Brain Mapping, 30, 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y‐C., Soong, W‐T. , Gau, S.S‐F. , Wu, Y‐Y. , Lai, M‐C. , Yeh, F‐C. , et al. (2011). The loss of asymmetry and reduced interhemispheric connectivity in adolescents with autism: A study using diffusion spectrum imaging tractography. Psychiatry Research: Neuroimaging, 192(1), 60–66. [DOI] [PubMed] [Google Scholar]
- Lord, C. , Rutter, M. , DiLavore, P.C. , & Risi, S. (1999). Autism diagnostic observation schedule: Manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Lord, C. , Rutter, M. , DiLavore, P.C. , Risi, S. , Gotham, K. , & Bishop, S.L. (2012). Autism diagnostic observation schedule (2nd ed.) (ADOS‐2): Manual. Los Angeles: Western Psychological Services. [Google Scholar]
- Mason, R.A. , Williams, D.L. , Kana, R.K. , Minshew, N. , & Just, M.A. (2008). Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia, 46, 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memarian, N. , Ferrari, P. , Macdonald, M.J. , Cheyne, D. , De Nil, L.F. , & Pang, E.W. (2012). Cortical activity during speech and non‐speech oromotor tasks: A magnetoencephalography (MEG) study. Neuroscience Letters, 527, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, M. , Alter, K. , Friederici, A.D. , Lohmann, G. , & von Cramon, D.Y. (2002). fMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Human Brain Mapping, 17(2), 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody, M. & Belliveau, J.W. (2013). Speech and language impairments in autism: Insights from behavior and neuroimaging. North American Journal of Medical Sciences, 5(3), 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae, L.M. , Zarnow, D.M. , Blaskey, L. , Dell, J. , Khan, S.Y. , Qasmieh, S. , et al., (2012). Elevated mean diffusivity in the left hemisphere superior longitudinal fasciculus in autism spectrum disorders increases with more profound language impairment. AJNR, 33, 1720–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, J.A. , Zielinski, B.A. , Fletcher, P.T. , Alexander, A.L. , Lange, N. , Bigler, E.D. , et al. (2014). Abnormal lateralization of functional connectivity between language and default mode regions in autism. Molecular Autism, 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nip, I.S.B. , Green, J.R. , & Marx, D.B. (2009). Early speech motor development: Cognitive and linguistic considerations. Journal of Communication Disorders, 42(4), 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang, E.W. , Wang, F. , Malone, M. , Kadis, D.S. , & Donner, E.J. (2011). Localization of Broca's area using verb generation tasks in the MEG: Validation against fMRI. Neuroscience Letters, 490, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, R.D. , Uppenkamp, S. , Johnsrude, I.S. , & Griffiths, T.D. (2002). The processing of temporal pitch and melody in auditory cortex. Neuron, 36(4), 767–776. [DOI] [PubMed] [Google Scholar]
- Penfield, W. & Roberts, L. (1959). Speech and brain mechanisms. Princeton, NJ: Princeton University Press. [Google Scholar]
- Philip, R.C. , Dauvermann, M.R. , Whalley, H.C. , Baynham, K. , Lawrie, S.M. , & Stanfield, A.C. (2012). A systematic review and meta‐analysis of the fMRI investigation of autism spectrum disorders. Neuroscience and Biobehavoiral Reviews, 36(2), 901–942. [DOI] [PubMed] [Google Scholar]
- Picton, T.W. , Bentin, S. , Berg, P. , Donchin, E. , Hillyard, S.A. , Johnson, R. Jr. , et al. (2000). Guidelines for using human event‐related potentials to study cognition: Recording standards and publication criteria. Psychophysiology, 37, 127–152. [PubMed] [Google Scholar]
- Pina‐Camacho, L. , Villero, S. , Fraguas, D. , Boada, L. , Janssen, J. , Navas‐Sanchez, et al. (2012). Autism spectrum disorder: Does neuroimaging support the DSM‐5 proposal for a symptom dyad? A systematic review of functional magnetic resonance imaging and diffusion tensor imaging studies. Journal of Autism and Developmental Disorders, 42, 1326–1341. [DOI] [PubMed] [Google Scholar]
- Pochon, J‐B. , Levy, R. , Poline, J‐B. , Crozier, S. , Lehericy, S. , Pillon, B. , et al. (2001). The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: An fMRI study. Cerebral Cortex, 11(3), 260–266. [DOI] [PubMed] [Google Scholar]
- Pringle, B. , Colpe, L.J. , Blumberg, S.J. , Avila, R.M. , & Kogan, M.D. (2012). Diagnostic history and treatment of school‐aged children with autism spectrum disorder and special health care needs. NCHS Data Brief, 97, 1–8. [PubMed] [Google Scholar]
- Radua, J. , Via, E. , Catani, M. , & Mataix‐Cols D. (2011). Voxel‐based meta‐analysis of regional white‐matter volume differences in autism spectrum disorder versus healthy controls. Psychological Medicine, 41(7), 1539–1550. [DOI] [PubMed] [Google Scholar]
- Roberts, T.P.L. , Schmidt, G.L. , Egeth, M. , Blaskey, L. , Rey, M.M. , Edgar, J.C. , et al. (2008). Electrophysiological signatures: Magnetoencephalographic studies of the neural correlates of language impairment in autism spectrum disorders. International Journal of Psychophysiology, 68(2), 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas, D.C. , Bawn, S.D. , Benkers, T.L. , Reite, M.L. , & Rogers, S.J. (2002). Smaller left hemisphere planum temporale in adults with autistic disorder. Neuroscience Letters, 328(3), 237–240. [DOI] [PubMed] [Google Scholar]
- Saarinen, T. , Laaksonen, H. , Parviainen, T. , & Salmelin, R. (2006). Motor cortex dynamics in visuomotor production of speech and non‐speech mouth movements. Cerebral Cortex, 16, 212–222. [DOI] [PubMed] [Google Scholar]
- Salmelin, R. (2007). Clinical neurophysiology of language: The MEG approach. Clinical Neurophysiology, 118, 237–254. [DOI] [PubMed] [Google Scholar]
- Salmelin, R. & Sams, M. (2002). Motor cortex involvement during verbal versus nonverbal lip and tongue movements. Human Brain Mapping, 16, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, M.D. , Shih, P. , Ottl, B. , Keehn, B.M. , Leyden, K.M. , Gaffrey, M.S. , & Muller, R.‐A. (2012). Atypical lexicosemantic function of extrastriate cortex in autism spectrum disorder: Evidence from functional and effective connectivity. Neuroimage, 62, 1780–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg, L. , Paul, R. , McSweeny, J. , Klin, A. , & Cohen, D. (2001). Speech and prosody characteristics of adolescents and adults with high‐functioning autism and Asperger syndrome. Journal of Speech, Language, and Hearing Research, 44, 1097–1115. [DOI] [PubMed] [Google Scholar]
- Sowman, P.F. , Crain, S. , Harrison, E. , & Johnson, B.W. (2014). Lateralization of brain activation in fluent and non‐fluent preschool children: A magnetoencephalographic study of picture naming. Frontiers in Human Neuroscience, 8, 3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. , Jenkinson, M. , Woolrich, M. , Beckmann, C. , Behrens, T. , Johansen‐Berg, H. , et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(S1), 208–219. [DOI] [PubMed] [Google Scholar]
- Stanfield, A.C. , McIntosh, A.M. , Spencer, M.D. , Philip, R. , Gaur, S. , & Lawrie, S.M. (2008). Towards a neuroanatomy of autism: A systematic review and meta‐analysis of structural magnetic resonance imaging studies. European Journal of Psychiatry, 23(4), 289–299. [DOI] [PubMed] [Google Scholar]
- Stefanatos, G.A. , Baron, I.S. (2011). The ontogenesis of language impairment in autism: A neuropsychological perspective. Neuropsychological Review, 21, 252–270. [DOI] [PubMed] [Google Scholar]
- Stigler, K.A. , McDonald, B.C. , Anand, A. , Saykin, A.J. , & McDougle, C.J. (2011). Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Research, 1380, 146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach, J. & Tournoux, P. (1988). Co‐planar stereotactic atlas of the human brain. Stuttgart: Beorg Thieme Verlag. [Google Scholar]
- Tesink, C.M. , Buitelaar, J.K. , Petersson, K.M. , van der Gaag, R.J. , Kan, C.C. , Tendolkar, I. , et al. (2009). Neural correlates of pragmatic language comprehension in autism spectrum disorders. Brain, 132, 1941–1952. [DOI] [PubMed] [Google Scholar]
- Via, E. , Radua, J. , Cardoner, N. , Happe, F. , & Mataix‐Cols, D. (2011). Meta‐analysis of gray matter abnormalities in autism spectrum disorder: Should Asperger disorder be subsumed under a broader umbrella of autistic spectrum disorder. Archives General Psychiatry, 68, 409–418. [DOI] [PubMed] [Google Scholar]
- Verly, M. , Verhoeven, J. , Zink, I. , Mantini, D. , Peeters, R. , Deprez, S. , et al. (2014). Altered functional connectivity of the language network in ASD: Role of classical language areas and cerebellum. Neuroimage: Clinical, 4, 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, A.T. , Lee, S.S. , Sigman, M. , & Dapretto, M. (2006). Neural basis of irony comprehension in children with autism: The role of prosody and context. Brain, 129, 932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (2011). Wechsler Abbreviated Scale of Intelligence (2nd ed.) Manual (WASI‐II). Bloomington, MN: Pearson. [Google Scholar]
- Wechsler, D. & Naglieri, J.A. (2006). Wechsler Nonverbal Scale of Ability (WNV). San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Williams, K.T. (2007). Expressive vocabulary test, second edition: Manual. Minneapolis, MN: Pearson Assessments. [Google Scholar]
- Zoccante, L. , Viviani, A. , Ferro, A. , Cerini, R. , Cerruti, S. , Rambaldelli, G. , et al. (2010). Increased left parietal volumes related to delayed language development in autism: A structural MRI study. Functional Neurology, 25(4), 217–221. [PubMed] [Google Scholar]


