Abstract
Studies of first (L1) and second (L2) language representation in the brain have not identified the timing and locations of neural regions involved in L1 and L2 processing. Magnetoencephalography offers high spatial and temporal resolution and can be employed to disentangle subtle timing and neural control differences between L1 and L2 use. We tested bilingual adults in the MEG as they completed a picture verb generation task in L1 and L2. We found the expected progression of activation from occipital to temporal to inferior frontal areas. We also observed the following differences. A sustained insula and early cingulate event-related desynchrony was observed only with L2; the fMRI literature suggests that the former reflects an activation, and the latter an inhibition, sub-process for language selection. L2 processes exhibited a lag and were bilateral compared to L1 processes. Finally, L1 and L2 activated adjacent language control in dorsolateral pre-frontal cortex.
Keywords: magnetoencephalography (MEG), bilingualism, event-related desynchrony (ERD), beamformer
1. Introduction
Second language representation in the brain has been a topic of great interest in neuroimaging research. Historically, some studies have reported distinct neural representations suggesting spatially separate networks for multiple languages; others have reported overlapping areas controlled by a common, integrated neural network for bilingual or multilingual use; the current working model assumes that bilingual language processing is not subsumed in spatially distinct areas, but different languages show functional distinctions in the brain (Abutalebi, 2008). A paper reporting the results of a meta-analysis of hemodynamic studies of bilingualism points to the huge variability in the literature, and suggests that this is primarily due to differences in experimental parameters; however, the author concludes that despite these limitations, there are differences in the activation patterns between L1 and L2 that likely are not due to coincidence although the factors of L2 onset, proficiency and exposure need to be controlled and consistent between subjects (Indefrey, 2006). These same issues have been raised in more recent reviews also (Kotz, 2009; Leonard, et al., 2010).
With regard to experimental parameters and paradigms, an important first distinction is the separation of language into its receptive and expressive components. A well-used model of language (Geschwind, 1970) localizes receptive language, or the processing of incoming language, to left posterior temporal brain (Wernicke’s) areas and expressive, or productive, language to left inferior frontal (Broca’s) areas. While simplistic, this division is helpful for comparing across paradigms. It may be that the neural representation of a second language dissociates into its receptive and expressive components depending on a number of different factors including age of acquisition, degree of second language exposure, level of comprehension (receptive language) and fluency (expressive language).
With regard to the use of different imaging modalities, the earliest investigations of bilingualism involved lesional and electrical stimulation studies and usually described expressive language deficits, i.e., aphasias. Event-related potential (ERP) studies, on the other hand, focused primarily on receptive language paradigms to avoid contamination by the muscle artifacts involved with language production. While ERPs have offered good temporal resolution to examine functional differences between L1 and L2 use, its spatial resolution is poorer and is probably unable to resolve questions of whether neural areas are truly distinct or simply adjacent or overlapping.
PET and fMRI (see van Heuven and Dijkstra, 2010 for a review) studies have used a combination of paradigms that activated both receptive and expressive language in the same task; however, one of the first PET studies showed left frontal lobe activation including inferior frontal gyrus (BA 47, 46, 45) and left pre-motor area (BA 8) for first (L1) and second (L2) language, regardless of task or language, suggesting that common brain areas are involved in within- and across- language searches (Klein, et al., 1995). One of the first fMRI studies (Kim, et al., 1997) reported that L2 was spatially separated from L1 if the subject learned their second language later in life, whereas, “early” bilinguals showed both languages in spatially common frontal cortical areas; however, subjects’ language proficiencies were not controlled and language comprehension was not directly tested, thus, interpretation of these results is difficult. Furthermore, late acquisition subjects showed more variability in neural areas activated (Bloch, et al., 2009). PET and fMRI have offered good spatial resolution but with poorer temporal resolution resulting in findings which represent only the strongest summed activations over time and may not capture the subtleties that are involved in L2 use. An event-related fMRI comparison of L1 and L2 picture naming showed bilateral anterior cingulate cortex, left inferior (BA 44, 47, 45), left middle (BA 10/46), and right dorsal frontal gyri (BA 9) and left pre-central gyrus (Abutalebi, et al., 2008).
Magnetoencephalography (MEG) data have high temporal and spatial resolution and this modality is thus a good candidate for examining the spatiotemporal dynamics of L1 and L2 representation (Salmelin, 2007). There is an extensive literature on MEG studies of bilingual receptive language processing. Schmidt and Roberts (2009) review these studies which involve the use of MEG to examine bilingual word processing, word listening, and sentence grammar violations primarily using mismatch negativity (MMF) and the M100. Comparing L1 and L2 reading, the right fusiform gyrus was found to be active at 273 ms for both languages, but the left superior temporal and supramarginal gyri were active at 616 ms for only first language use (Kamada, et al., 1998). Another reading study reported early left hemisphere gamma event-related synchrony (within 200 ms) for L1 and L2. This ERS was more pronounced in the right hemisphere only for L1 (Ihara and Kakigi, 2006). Although it has been found that event-related synchrony is most closely related to the hemodynamic response (Singh, et al., 2002), event-related desynchrony is more often seen in cognitive tasks (Niedermeyer and Lopes da Silva, 2005) and with language tasks (Fried, et al., 1981; Hirata, et al., 2004; Ihara, et al., 2003; Yamamoto, et al., 2006).
Expressive language tasks in the MEG are less common because the artefacts and trial-by-trial variability of speech production have been problematic for the small neuromagnetic signals (Hari, et al., 2010). Some groups have found creative approaches to these problems (e.g., Breier and Papanicolaou, 2008). The development of beamforming methods (Robinson and Vrba, 1999; Vrba and Robinson, 2001), a spatial filtering technique, has allowed us to directly compare oscillatory changes in power between active and baseline time windows on a single-trial basis (Herdman, et al., 2007; Ressel, et al., 2008). We recently reported a validation study of an MEG covert verb generation that identified left inferior frontal (Broca’s area) with high consistency when compared to fMRI (Pang, et al., 2011). These novel MEG expressive language tasks have not yet been applied to the question of bilingual language representation in the brain. In the current study, bilingual adults completed an MEG verb generation task in L1 and L2 and we compared the spatiotemporal profile of both languages using beamforming methods.
2. Results
2.1. Language Questionnaire Summary
Mean age of L2 acquisition was 5.1 years. All subjects confirmed that they had not received any speech language therapy or intervention in either L1 or L2. Table 1 (top panel) summarizes the subjects’ exposure to both L1 and L2 through their families, community and education. Table 1 (bottom panel) summarizes the subjects’ self ratings of fluency in L2. While all subjects were fluent in L2 by self-report; they clearly reported L1 dominance as evidenced by a balance towards L1 in both usage and ability.
Table 1.
Summary of subjects’ language exposure and fluency.
| (a) Language demographics: family, community and education exposure to L1 and L2.
| |||||
|---|---|---|---|---|---|
| Number of subjects (total n= 12) | |||||
|
| |||||
| Cantonese | English | French | Mandarin | Spanish | |
| L1 | 0 | 8 | 3 | 1 | 0 |
| L2 | 2 | 4 | 4 | 1 | 1 |
| Mother’s native language | 2 | 4 | 3 | 2 | 1 |
| Father’s native language | 2 | 1 | 6 | 2 | 1 |
| Language spoken: | L1 | L2 | Both |
|---|---|---|---|
| With parents | 9 | 3 | |
| With siblings | 12 | 0 | |
| With peers | 12 | 0 | |
| In community during childhood | 12 | 0 | |
| In primary school | 9 | 1 | 2 |
| In high school | 9 | 2 | 1 |
| In post-secondary school | 11 | 1 |
| (b) Self-reported rating of L2 fluency
| ||
|---|---|---|
| L2 fluency | Mean (on a 7-point scale)a | S.D. |
| Speaking | 5.4 | 1.7 |
| Understanding | 5.8 | 1.3 |
| Reading | 5.2 | 2.0 |
| Writing | 4.5 | 2.2 |
| Vocabulary | 5.0 | 1.3 |
| Balance of L1/L2 usageb | +1.0 | |
| Balance of L1/L2 abilityb | +2.1 | |
7-point Likert scale where 1 is basic fluency and 7 is perfectly fluent.
10-point scale where 0 is perfect balance between L1 and L2; +5 is L1 only; −5 is L2 only.
2.2. Occipital and Temporal activations prior to masking
Figure 1 (left panel) shows the strong desynchrony observed in left cuneus and right middle occipital gyrus areas for both L1 and L2. Time-frequency plots at each of these locations reveal similar patterns for L1 and L2: a rapid wide-band evoked response, probably the P100, followed by a sustained and broad (5-30 Hz) desynchrony. Figure 1 (right panel) shows the strong desynchrony observed in left middle and right superior temporal gyri for L1 and bilateral middle temporal gyri for L2. Time-frequency plots at these locations reveal sustained broadband desynchrony in the temporal regions.
Figure 1. Event-related desynchrony (ERD) in occipital (left panel) and temporal (right panel) regions for L1 and L2.
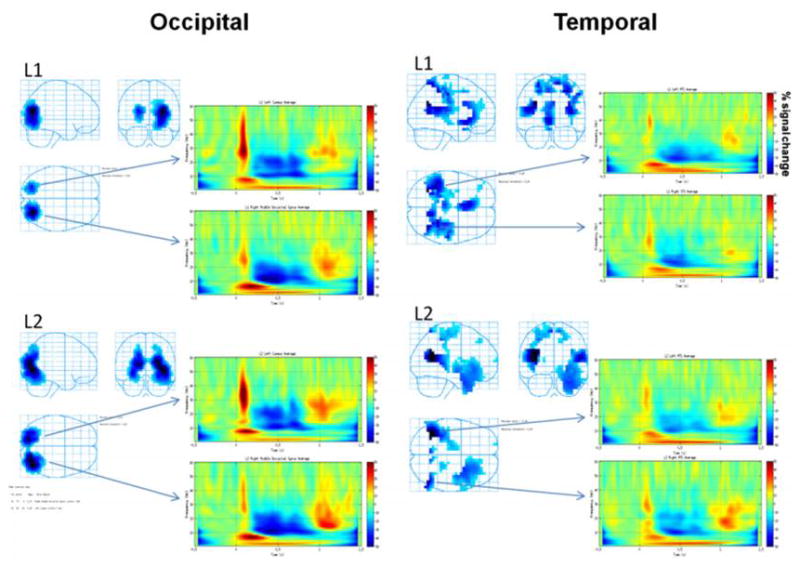
Occipital ERD (left panel): Glass brains show occipital locations for ERD with L1 and L2 use. Time-frequency plots at these locations show similar patterns of percent change in ERD for L1 and L2 at left cuneus and right middle occipital gyrus. Temporal ERD (right panel): Glass brains show temporal locations for ERD with L1 and L2 use. Time-frequency plots at these locations show similar patterns of percent change in ERD for L1 and L2 at middle and superior temporal gyri.
2.3. 5-15 Hz event-related desynchrony
Figure 2 shows thresholded ERD localizations in the lowest bandpass (5-15 Hz) for each of the time windows with L1 in the top row and L2 in the bottom row. For L1, left inferior frontal gyrus (BA 47) is active in the first window and remains active into the 250–400 msec window. As well, right hemisphere primary motor hand area (BA 4) is co-active with left hemisphere primary somatosensory (BA 3) area. Interestingly, L2 activation begins and is sustained in right insula (BA 13), with right inferior frontal gyrus (BA 47) coming on-line after 450 msec, followed by right hemisphere primary motor cortex (BA 4). This same sequence of BA 47 followed by BA 4 was seen in L1, but there is a lag with the L2 condition.
Figure 2. Thresholded event-related desynchrony localizations in the 5-15 Hz bandpass.
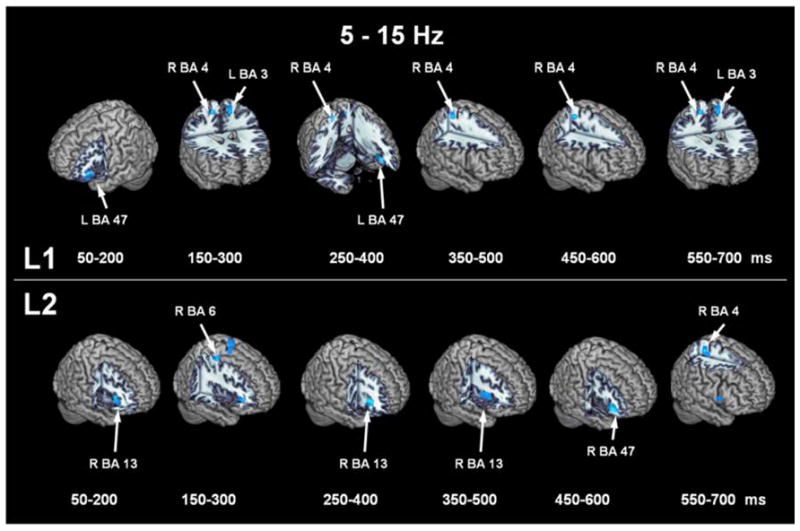
ERD locations for L1 (top row) and L2 (bottom) row for each time window in the 5-15 Hz bandpass.
To confirm the inferior frontal activations, a virtual sensor location was selected at the left inferior frontal location with maximal ERD on L1. TFRs were created at this location for both L1 and L2 and also for both languages at the homologous right hemisphere location. These are contained in Figure 3. Inspection of this figure shows that all four figures show desynchrony in approximately the 5-15 Hz bandpass and approximately between 250–550 msec. For L1, although the desynchrony is strongest in left IFG, it is clearly present in right IFG. For L2, the desynchrony is of approximately equivalent strength in both left and right hemispheres.
Figure 3. Time-frequency plot at inferior frontal locations.
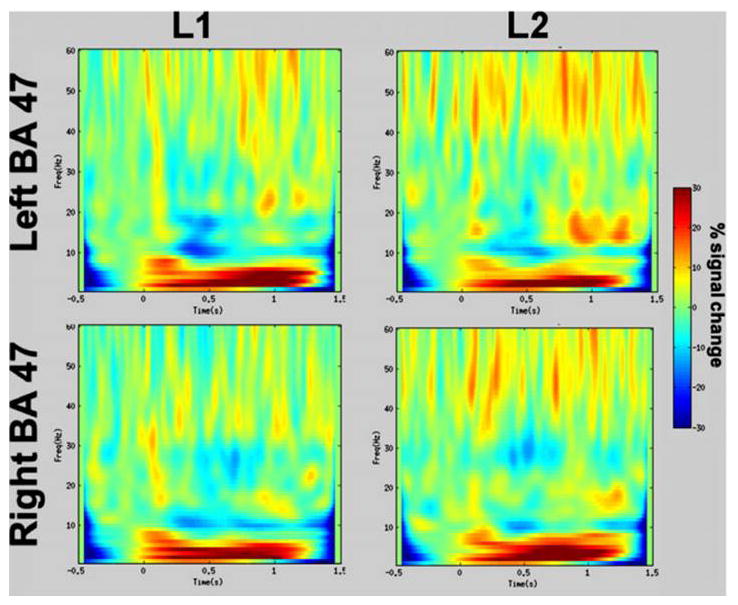
Time- frequency plots demonstrate percent change in ERD in left BA 47 and homologous right BA 47 for L1 and L2. The L1 ERD is more left lateralized while the L2 ERD is more bilateral.
2.4. 15-25 Hz (beta) and 25-50 Hz (gamma) event-related desynchrony
Figure 4 shows the thresholded ERD locations in the beta band (15-25 Hz) for each of the time windows with L1 on the top row and L2 on the bottom row. For L1, left premotor area (BA 6) shows a sustained activation starting at mouth motor areas and proceeding superiorly to hand motor areas. It should be noted that the left mouth premotor area is active in the same time window as the left inferior frontal gyrus in the lower frequency band. For L2, left premotor area (BA 6) shows a sustained activation starting in the 150–300 ms window, but this is preceded by activation in right anterior cingulate cortex (BA 32). Again, L2 follows a similar pattern of activations as L1, with the exception of the additional cingulate cortex response.
Figure 4. Thresholded event-related desynchrony localizations in the 15-25 Hz bandpass.
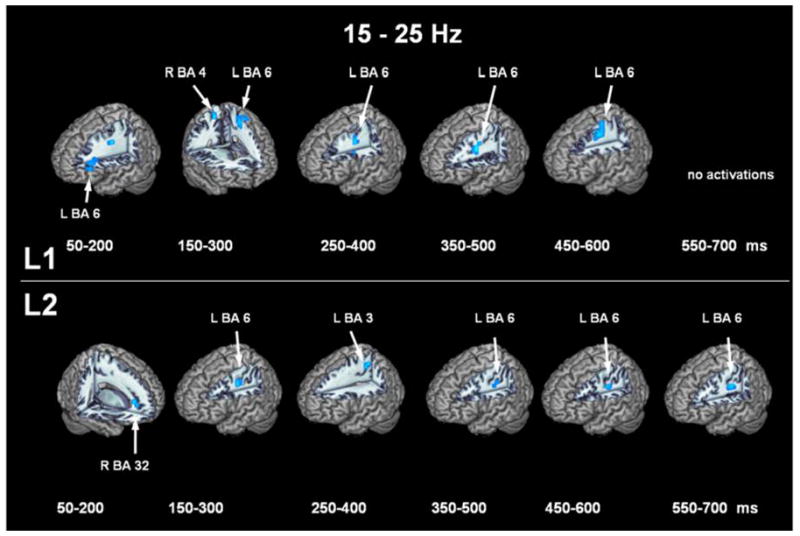
ERD locations for L1 (top row) and L2 (bottom) row for each time window in the 15-25 Hz bandpass.
Figure 5 shows the thresholded ERD locations for the gamma band (25-50 Hz) responses for each of the time windows with L1 and L2 on the top and bottom, respectively. For L1, a circuit is seen in left frontal cortex starting with left superior frontal gyrus (BA 9) to left insula (BA 13) and back to superior frontal gyrus in the last window. As well, right inferior gyrus (BA 47) becomes active in the 450–600 ms window. For L2, activations begin in bilateral frontopolar regions (BA 10 and 11) before switching into the motor network involving left supplementary motor (BA 6), primary motor (BA 3) and somatosensory (BA 4) areas.
Figure 5. Thresholded event-related desynchrony localizations in the 25-50 Hz bandpass.
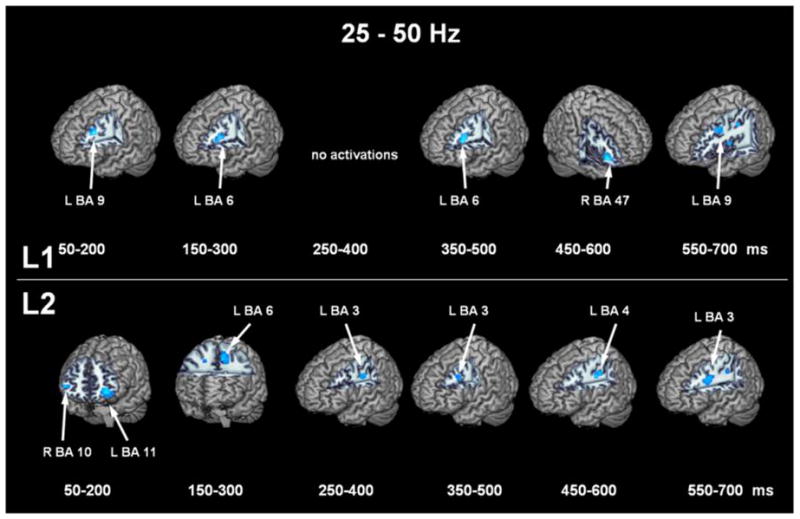
ERD locations for L1 (top row) and L2 (bottom) row for each time window in the 25-50 Hz bandpass.
3. Discussion
In this study, we used MEG to elucidate the spatiotemporal profile of healthy adult bilinguals as they completed a simple expressive language task in both their first and second languages. The MEG and ERP literature suggest that the generally accepted progression for language production, cued by visual stimuli, begins with object recognition and conceptualization processes at 0–175 ms involving occipital and ventrotemporal regions. This progresses to the left middle temporal gyrus at 175–250 ms where the word is selected from the mental lexicon; the phonological code is retrieved between 250–300 msec extending from left middle to left superior temporal gyri. At this point, preparation is made for oral output after 330 ms which engages Broca’s area in left inferior frontal gyrus and bilateral sensorimotor areas (Vihla, et al., 2006).
The literature suggests that this basic progression through the language network is followed regardless of the language being tested. Our choice to use a variety of first and second languages across subjects was built on the rationale that an ‘innate’ language system exists that is open to all languages, upon which all first languages are built. We also hypothesized that the basic mechanisms of second language control are consistent, and innate, across languages; thus, regardless of the first and second languages, the controllers are the same. The subtle differences between first and second language only emerge when one begins to probe the finer aspects of each language, such as its grammar, syntax, or roots (e.g., Romanic languages might be more similar to other Indo-European languages, and these families are quite different from Sino-Tibetan or American Indian languages). This latter investigation is beyond the scope of this paper. In this paper, as a first step, we were interested in elucidating the basic, innate mechanisms involved in L1 and L2 verb production.
The spatiotemporal progression reported in our findings is consistent with what has been suggested in the literature: occipital to ventrotemporal to inferior frontal/sensorimotor areas; however, we further identify areas that may be involved in the control of second language use. Our most striking finding is the sustained insula desynchrony with L2 language use, and the early and immediate involvement of anterior cingulate cortex, also with L2 use. An fMRI (Chee, et al., 2004) study, examining receptive language localization, reported left insula activation for the dominant language and left anterior cingulate activation for the less proficient language. While there is probably no direct relationship between the BOLD signal and ERD, as both are complex responses (Winterer, et al., 2007), Chee and colleagues (2004) were the first to identify insula and anterior cingulate as areas important in bilingualism.
In a verbal memory study, the insula was thought to be involved in subvocal rehearsal (Smith, et al., 1998), and another fMRI study which involved switching between L1 and L2 interpreted the insular activation as a priming effect that activated the appropriate language (Isel, et al., 2010). A recent MEG study using a semantic judgement task (Leonard, et al., 2011) found insular activation to be related to L2 proficiency and not L2 use. This most recent paper offers a plausible explanation of our findings of insula activation only in the L2 condition, as our subjects report that their balance between L1 and L2 proficiency is in favour of L1. The sustained insula desynchrony could reflect their lower proficiency in L2.
Our finding of anterior cingulate desynchrony is also of interest. Functional MRI studies by Chee and colleagues (2004) and Isel and colleagues (2010) report anterior cingulate activation which is interpreted as a subprocess inhibiting the inappropriate language. However, another model of bilingual control identifies the anterior cingulate cortex as being important in conflict monitoring and error detection (Abutalebi, 2008; Abutalebi and Green, 2007). Using this model, the results from the current study is consistent with the idea that the lower proficiency of L2 in our cohort would result in the need for greater error monitoring and thus, anterior cingulate response. A recent fMRI study (Guo et al., 2011) examined global versus local inhibition in bilingual picture naming whereby the dorsal anterior cingulate cortex was implicated in local inhibition (switching languages within a block) and the left frontal gyrus in global inhibition (switching between blocks). By this definition, our task was a global inhibition task, yet we found significant responses in anterior cingulate. We postulate that this may be due to differences in the study populations. We tested participants who acquired L2 relatively early in life whereas the participants in the study by Guo and colleagues (2011) acquired L2 later in life (after age 12 years). It may be that L1 and L2 processing is more integrated in individuals who acquired L2 early, and this is what requires greater monitoring and inhibition. This is an interesting finding that requires future empirical validation.
It has also been suggested in the literature that L2 processing requires greater right hemisphere involvement. An MEG study of semantic classification (Leonard, et al., 2010) reported greater right inferior precentral and pars opercularis activation to L2 than L1, and the authors concluded that L2 word processing required the additional recruitment of right hemisphere homologous areas; furthermore, peak activations of right hemisphere areas were delayed for L2 compared to L1. We saw this in our study where similar patterns of activations were repeated in the right hemisphere after a brief lag. This was particularly true of the inferior frontal (BA 47) ERD, which came on-line in the 50–200 ms window and again at 250–400 ms in L1, but was only seen later in right homologous BA 47 in the 450–600 ms window.
Classical thinking about the neural underpinnings of expressive language points solely to BA 44 and 45, that is, canonical Broca’s area. However, it is now known that BA 44/45 are functionally, and cytoarchitecturally, distinct (Horwitz, et al., 2003). BA 44 is more involved in the complex articulatory movements involved in speech, while BA 45 is involved in the narrative aspects of language. Extending past canonical Broca’s area, the pars orbitalis region of the inferior frontal gyrus, BA 47, has been identified as being involved in semantic processing with fMRI (Dapretto and Bookheimer 1999; Isel, et al., 2010; Parker Jones, et al., 2012), PET (Klein, et al., 1995), and MEG (Dhond, et al., 2001). Our finding of a BA 47 response, without BA 44/45 activation, is consistent with our use of a semantic task and the absence of any overt articulation or narration in our paradigm. In fact, new diffusion tensor imaging results highlight the importance of the inferior-occipito-frontal fasiculus (IOFF), a pathway that is not usually included in the canonical language network, in the comprehension and production of meaningful speech, and this fibre tract directly connects the middle temporal region to BA 47 (Turken and Dronkers, 2011) – regions that are identified in our study as important in both L1 and L2 language production.
The report by Turken and Dronkers (2011) of a direct fibre tract between middle temporal gyri and inferior frontal BA 47 regions offers a potential explanation for our finding of BA 47 ERD in the earliest time window. BA 47 is important for semantic processing and decisions, and this area may be active early to prime the system in preparation for the task to be performed. Other studies involving expressive language have found early activation in frontal areas (e.g., Herdman, et al., 2007) supporting a priming effect in frontal regions, and also highlighting the fact that a posterior-anterior model of language is inadequate and too simplistic.
In addition to the BA 47 region, we observed dorsolateral frontal involvement in the left hemisphere for L1 and bilaterally for L2. While the L1 area was identified as BA9, the L2 area was in bilateral BA 10/11. These regions are consistent with an event-related fMRI study (Abutalebi, et al., 2008) and models of bilingual language control which state that the dorsolateral frontal cortices are connected to the anterior cingulate, and are involved in language control, specifically response selection and response inhibition (Abutalebi, 2008; Abutalebi and Green, 2007). An fMRI connectivity study examining language recovery in a clinical population further demonstrated a strong connection between cingulate and dorsolateral prefrontal cortex and the connectivity studies suggest that these areas work together to inhibit interference from the non-target language. The cingulate serves to signal the prefrontal cortex of potential response conflicts and the prefrontal cortex seeks to avoid incorrect selection (Abutalebi, et al., 2009). Our finding of a very early cingulate response in the L2 condition supports this idea that the cingulate signals the possibility of response conflicts which is later responded to by the dorsolateral cortex. An intriguing interpretation for the left BA9 activity seen with L1, in contrast to the bilateral BA10/11 with L2, comes from the literature describing specific functional roles for these areas. Left BA9 has been implicated in many cognitive functions including monitoring and manipulation within working memory (Owen, 1997; Petrides, 1994), response selection (Rowe et al. 2000), and encoding and recognition for both working and long-term memory (Ranganath et al., 2003) – the involvement of this area is not surprising in our task. While right BA10 has been implicated in a number of cognitive processes including working memory (MacLeod et al., 1998), it seems be specifically engaged in situations where an integration of two or more separate cognitive operations are required to fulfill a higher behavioural goal (Ramnani and Owen, 2004). Further, BA 11 has been found to be involved in planning and verbal reasoning tasks (Colom et al., 2009). The involvement of bilateral BA10/11 in L2 verb generation suggests that L2 processing requires additional resources, in terms of process integration, planning and monitoring. This hypothesis requires further empirical confirmation.
In addition to finding neural regions involved in bilingual verb generation, our use of MEG allowed us to examine the role of different frequency bands and their involvement in both L1 and L2 use. Studies of motor cortex control (Salmelin and Sams, 2002) demonstrate that the human sensorimotor cortex displays 20 Hz oscillatory activity which is generated largely in the post-central primary somatosensory cortex in and around the hand area. Our study suggests that for L1, the desynchronization of BA 47 interrupts this oscillatory activity in primary somatosensory areas (BA 3/4) to prepare for the oral response. Further, this literature shows that the 20Hz activity originates in primary motor cortex and shows homuncular organization; this is consistent with our finding of modulation over mouth/face regions in BA 6 and BA 3. It was initially puzzling to observe activity in motor cortex with a covert response, but there are several potential explanations. Recent evidence shows that neurons in motor cortex are active with observation of a movement and with both imagined and covert movements (Cattaneo, et al., 2009; Kilner, et al., 2009; Lui, et al., 2008; Wadsworth, et al., 2011).
This study has several limitations that should be noted. One limitation is our use of subjects with different L1 and L2. Future studies should attempt to create homogeneous L1 and L2 groups so as to control for language subtleties, for example, phonologic or orthographic similarities). Another limitation is the inability of this study to disentangle the effects of noun versus verb processing. While verb generation was chosen in this study to follow on a long clinical tradition of using verb generation (Brown, et al., 2005; Gaillard, et al., 2003; Holland, et al., 2001; Wood, et al., 2004), this limits the interpretation of these results as it is well known that nouns and verbs are processed differently in the brain (Khader and Rösler, 2004; Liljestrom et al., 2008; Peran et al., 2009; Shapiro et al., 2006). A third limitation of our study is the use of an open-ended questionnaire that did not specifically query the subject as to their language choices in different environments, with different people, and for different functions. As well, we did not capture questionnaire data regarding the subject’s ability to switch languages within and between environments. Clearly, this study demonstrates that the brain data are sensitive enough to be correlated with specific behavioural questions so as to address particular hypotheses. This should be considered in future studies. A final limitation is our use of covert responding and thus our inability to obtain behavioural measures such as reaction time and accuracy. While this paradigm has been useful for us in the clinical setting for localizing expressive language, this is certainly a limitation in this study.
In summary, while our results clearly show consistencies with the literature, there are discrepancies with the extant fMRI literature. The relationship between the fMRI bold response and the MEG neurophysiological response are still poorly understood (Liljeströnm, et al., 2009). Both methods have differing preferences and sensitivities; the fMRI is incapable of capturing temporally brief or sparse events (Singh, et al., 2002), while the MEG may miss long-lasting non-time- and phase-locked events and may not encompass the entire extent of the neural network involved in the complexities of language production. This emphasizes the need for multi-modal recordings, cross-modal comparisons, and further studies of regional brain or network connectivity differences between L1 and L2 use.
In conclusion, our findings shed light on the discrepancies seen in the neuroimaging literature regarding first and second language use. We demonstrate that L2 processes are slower, due to the involvement of early control processes that activate appropriate, and inhibit inappropriate, language selection. We also demonstrate that L2 processing requires more bilateral resources compared to the primarily left-lateralized L1 processes. Finally, we demonstrate that L2 processes are subsumed in areas that are adjacent and non-overlapping with areas controlling to L1 processing.
4. Methods and Materials
4.1. Subjects
Twelve right-handed young adults (4 male, 8 female; mean age = 23.5±3.9 yrs) participated in the study. Subjects were screened and found to be free from academic, speech-language, neurological, neuropsychological, or psychiatric issues. Subjects had normal or corrected-to-normal vision and normal hearing, by self report. Subjects were also required to be free of magnetic appliances and compliant with MEG/MRI imaging requirements. Prior to entering the MEG, subjects completed a detailed language history, use and proficiency questionnaire to document age of L2 acquisition and self-reported proficiency in each language. All subjects gave informed consent and this study was approved by the Hospital for Sick Children Research Ethics Board.
4.2. Stimuli and task
Subjects completed a picture verb generation task (Kadis, et al., 2008). A verb generation task was selected as fMRI (Liljestrom et al., 2008; Peran et al., 2009; Shapiro et al., 2006) and ERP (Khader and Rösler, 2004) studies have shown that verbs activate inferior frontal areas more robustly than nouns. For this task, subjects were shown a picture of a common object and asked to think rapidly of a verb that corresponded to the object. Pictures were high resolution, colour drawings of common objects. Each condition consisted of 92 pictures which appeared sequentially, in random order, on the centre of the screen for 500 ms followed immediately by a colour-frequency scrambled picture, as baseline, for an inter-stimulus interval of 2 sec. Baseline and active pictures were matched for size, brightness and contrast. All pictures subtended 5 degrees of visual angle and were back-projected via mirrors to a screen located 65 cm from the subject’s nose. Stimuli were controlled using Presentation (Neurobehavioral Systems Inc., Albany CA). Subjects completed the verb generation task in either their first or second language with language of testing counter-balanced across subjects.
To minimize head movement and muscle artefact, this was a covert task which we have demonstrated to have high reliability compared with fMRI for identifying left inferior frontal areas associated with language production (Pang, et al., 2011). Due to the covert nature of the task response, and to ensure subject attention during the task, we inserted vigilance trials which consisted of a picture of a hand pressing a computer mouse. These appeared with a frequency of approximately 13% and subjects were trained to make an overt button press with their either right or left hand (counter-balanced across subjects) when they saw this stimulus. This allowed the MEG operator to track missed responses to button presses and query the subject for task compliance. Each condition was 4 minutes and there were no problems with compliance or subject inattention. After exiting the MEG shielded room, subjects sat at a computer in the lab and completed the same task but responded overtly so as to confirm adequate performance.
4.3. MEG Data and Structural MRI acquisition
Subjects were tested in a whole-head 151 channel MEG (with axial gradiometers and third order noise reduction) (CTF Omega, MISL, Coquitlam BC) located in the Neuromagnetic Lab at the Hospital for Sick Children. Prior to entering the magnetically shielded room, three fiducial markers were placed on the subject’s nasion, left and right pre-auricular points, to allow co-registration with anatomical MRI. Subjects were tested supine on the MEG bed in a darkened room. The subject’s head was localized before and after each test condition to check for head movements while in the MEG dewar. Motion was comparable between conditions and none of our subjects failed the movement limit of 5 mm; our criterion being that conditions with greater than 5 mm movement would be re-run. MEG data were acquired continuously with a digitization rate of 625 Hz and 0-100 Hz filter settings and third-order gradient noise reduction. After MEG testing, the three fiducial markers were replaced with radiological markers and a 3-dimensional T1-weighted SPGR anatomical MRI was acquired (1.5T, GE Medical Systems, Waukesha WI). The 3D volumes were transferred to BrainSuite2 (Dogdas, et al., 2005; Shattuck and Leahy, 2002; Shattuck, et al., 2001) and the tissue automatically segmented to establish inner skull morphology. A mask of each subject’s inner skull was used to develop multiple sphere models for beamforming analyses.
4.4. Data processing and Statistical testing
Continuous MEG data were epoched from 500 ms pre-stimulus to 1000 ms post-stimulus, resulting in 1.5 s trials with stimulus onset at time zero. Vigilance trials, which have hand movements, were discarded from further processing. Data were filtered with three bandpasses (5-15, 15-25, and 25-50 Hz), selected as per previous language studies using this approach (Kadis, et al., 2008; Mohamed, et al., 2008; Ressel, et al, 2008), and analysed with differential beamforming (see Robinson and Vrba, 1999; Sekihara, et al., 2001; Van Veen, et al., 1997; Vrba and Robinson, 2001). Time windows were set at 150 ms widths with baseline defined as −200 to −50 ms pre-stimulus. Sliding, overlapping active windows were used and defined as: 50–200, 150–300, 250–400, 350–500, 450–600, and 550–700 ms. The region of interest was set to include the whole cerebral cortex with a 5 mm voxel resolution. A bootstrap statistical procedure was applied where observed data were randomly sampled with replacement on a voxel-wise basis to create 99 pseudo runs of 80 trials which served as null distributions for non-parametric inferential statistical comparisons with actual data and a threshold set, a priori, at an alpha level of p<0.01 (uncorrected). Only voxels passing this threshold were included in further analyses.
4.5. Data Visualization and Averaging
Multiple areas in the brain passed the bootstrap thresholding procedure, however, initial observations of desynchrony results showed strong signals from posterior and temporal regions that precluded visualization of other brain areas. To isolate the extra- occipital and temporal components, we restricted the analyses of the thresholded data to a probabilistic volume (Shattuck, et al., 2008) that excluded the occipital and temporal lobes (developed by the International Consortium for Brain Mapping, made publicly available through the University of California’s Laboratory of Neuro Imaging at http://www.loni.ucla.edu). Individual subject data were spatially normalized using SPM2 routines (Friston, 2003), then the occipital and temporal areas masked so that the other loci of desynchrony could be visualized. The masked images were grand averaged across subjects. Talaraich coordinates were converted to Brodmann areas (Lancaster, et al., 1997; 2000).
Acknowledgments
The authors would like to thank Linda Zhang for help with questionnaire development and data acquisition. This work was supported by a Canadian Institutes for Health Research (CIHR) operating grant to the first author (MOP-89961).
References
- Abutalebi J. Neural aspects of second language representation and language control. Acta Psychol. 2008;128:466–478. doi: 10.1016/j.actpsy.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Annoni JM, Zimine I, Pegna AJ, Seghier ML, Lee-Jahnke H, Lazeyras F, Cappa SF, Khateb A. Language control and lexical competition in bilinguals: an event-related fMRI study. Cereb Cortex. 2008;18:1496–1505. doi: 10.1093/cercor/bhm182. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Della Rosa PA, Tettamanti M, Green DW, Cappa SF. Bilingual aphasia and language control: a follow-up fMRI and intrinsic connectivity study. Brain Lang. 2009;109:141–156. doi: 10.1016/j.bandl.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Green D. Bilingual language production: the neurocognition of language representation and control. J Neuroling. 2007;20:242–275. [Google Scholar]
- Bloch C, Kaiser A, Kuenzli E, Zappatore D, Haller S, Franceschini R, Luedi G, Radue EW, Nitsch C. The age of second language acquisition determines the variability in activation elicited by narration in three languages in Broca’s and Wernicke’s area. Neuropsychologia. 2009;47:625–633. doi: 10.1016/j.neuropsychologia.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Breier JI, Papanicolaou AC. Spatiotemporal patterns of brain activation during an action naming task using magnetoencephalography. J Clin Neurophysiol. 2008;25:7–12. doi: 10.1097/WNP.0b013e318163ccd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15(3):275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Caruana F, Jezzini A, Rizzolatti G. Representation of goal and movements without overt motor behaviour in the human motor cortex: a transcranial magnetic stimulation study. J Neurosci. 2009;29:11134–11138. doi: 10.1523/JNEUROSCI.2605-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Soon CS, Lee HL, Pallier C. Left insula activation: A marker for language attainment in bilinguals. Proc Natl Acad Sci USA. 2004;101(42):15265–15270. doi: 10.1073/pnas.0403703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom R, Haier RJ, Head K, Alvarez-Linera J, Angeles Quiroga M, Shih PC, Jung RE. Gray matter correlates of fluid, crystallized, and spatial intelligence: Testing the P-FIT model. Intelligence. 2009;37:124–135. [Google Scholar]
- Dapretto M, Bookheimer SY. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24:427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Dhond RP, Buckner RL, Dale AM, Marinkovic K, Halgren E. Spatiotemporal maps of brain activity underlying word generation and their modification during repetition priming. J Neurosci. 2001;21:3564–3571. doi: 10.1523/JNEUROSCI.21-10-03564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogdas B, Shattuck DW, Leahy RM. Segmentation of skull and scalp in 3-D human MRI using mathematical morphology. Human Brain Mapp. 2005;26(4):273–285. doi: 10.1002/hbm.20159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried I, Ojemann GA, Fetz EE. Language-related potentials specific to human language cortex. Science. 1981;212(4492):353–356. doi: 10.1126/science.7209537. [DOI] [PubMed] [Google Scholar]
- Friston K. Introduction: Experimental design and statistical parameter mapping. In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Price CM, Zeki S, Ashburner JT, Penny WD, editors. Human brain function. 2. Academic Press; San Diego: 2003. [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, Grandin CB. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Human Brain Mapp. 2003;18(3):176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. The organization of language and the brain. Science. 1970;170:940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- Guo T, Liu H, Misra M, Kroll JF. Local and global inhibition in bilingual word production: fMRI evidence from Chinese-English bilinguals. Neuroimage. 2011;56:2300–2309. doi: 10.1016/j.neuroimage.2011.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Parkkonen L, Nangini C. The brain in time: insights from neuromagnetic recordings. Annals of the New York Academy of Sciences. 2010;1191:89–109. doi: 10.1111/j.1749-6632.2010.05438.x. [DOI] [PubMed] [Google Scholar]
- Herdman AT, Pang EW, Ressel V, Gaetz W, Cheyne D. Task-related modulation of early evoked responses during language production: An event-related synthetic aperture magnetometry study. Cereb Cortex. 2007;17:2536–2543. doi: 10.1093/cercor/bhl159. [DOI] [PubMed] [Google Scholar]
- Hirata M, Kato A, Taniguchi M, Saitoh Y, Ninomiya H, Ihara A, Kishima H, Oshino S, Baba T, Yorifuji S, Yoshimine T. Determination of language dominance with synthetic aperture magnetometry: comparison with the Wada test. Neuroimage. 2004;23:46–53. doi: 10.1016/j.neuroimage.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14(4):837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Amunts K, Bhattacharyya R, Patkin D, Jeffries K, Zilles K, Braun AR. Activation of Broca’s area during the production of spoken and signed language: a combined cytoarchitectonic mapping and PET analysis. Neuropsychologia. 2003;41:1868–1876. doi: 10.1016/s0028-3932(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Ihara A, Hirata M, Sakihara K, Izumi H, Takahashi Y, Kono K, Imaoka H, Osaki Y, Kato A, Yoshimine T, Yorifuji S. Gamma-band desynchronization in language areas reflects syntactic process of words. Neurosci Lett. 2003;339:135–138. doi: 10.1016/s0304-3940(03)00005-3. [DOI] [PubMed] [Google Scholar]
- Ihara A, Kakigi R. Oscillatory activity in the occipitotemporal area related to the visual perception of a first and second language and pseudoletter. Neuroimage. 2006;29:789–796. doi: 10.1016/j.neuroimage.2005.08.036. [DOI] [PubMed] [Google Scholar]
- Indefrey P. A meta-analysis of hemodynamic studies on first and second language processing: which suggested differences can we trust and what do they mean? Lang Learning. 2006;56(suppl 1):279–304. [Google Scholar]
- Isel F, Baumgaertner A, Thrän J, Meisel JM, Büchel C. Neural circuitry of the bilingual mental lexicon: effect of age of second language acquisition. Brain Cogn. 2010;72:169–180. doi: 10.1016/j.bandc.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Kadis DS, Smith ML, Mills T, Pang EW. MEG localization of expressive language cortex in healthy children: Application to paediatric clinical populations. Downs Syndr Quart. 2008;10(2):5–12. [Google Scholar]
- Kamada K, Kober H, Saguer M, Möller M, Kaltenhauser M, Vieth J. Responses to silent Kanji reading of the native Japanese and German in task subtraction magnetoencephalography. Cogn Brain Res. 1998;7:89–98. doi: 10.1016/s0926-6410(98)00016-0. [DOI] [PubMed] [Google Scholar]
- Khader P, Rösler F. EEG power and coherence analysis of visually presented nouns and verbs reveals left frontal processing differences. Neurosci Lett. 2004;354:111–114. doi: 10.1016/j.neulet.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Marchant JL, Frith CD. Relationship between activity in human primary motor cortex during action observation and the mirror neuron system. PLoS ONE. 2009;4(3):e4925. doi: 10.1371/journal.pone.0004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KHS, Relkin NR, Lee KM, Hirsch J. Distinct cortical areas associated with native and second languages. Nature. 1997;388(10 July):171–174. doi: 10.1038/40623. [DOI] [PubMed] [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC. The neural substrates underlying word generation: A bilingual functional-imaging study. Proc Natl Acad Sci USA. 1995;92:2899–2903. doi: 10.1073/pnas.92.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz SA. A critical review of ERP and fMRI evidenceon L2 syntactic processing. Brain Lang. 2009;109:68–74. doi: 10.1016/j.bandl.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labelling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard MK, Brown TT, Travis KE, Gharapetian L, Hagler DJ, Jr, Dale AM, Elman JL, Halgren E. Spatiotemporal dynamics of bilingual word processing. Neuroimage. 2010;49:3286–3294. doi: 10.1016/j.neuroimage.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard MK, Torres C, Travis KE, Brown TT, Halger DJ, Jr, Dale AM, Elman JL, Halgren E. Language proficiency modulates the recruitment of non-classical language areas in bilinguals. PLoS One. 2011;6(3):e18240. doi: 10.1371/journal.pone.0018240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeström M, Hultén A, Parkkonen L, Salmelin R. Comparing MEG and fMRI views to naming actions and objects. Human Brain Mapp. 2009;30:1845–1856. doi: 10.1002/hbm.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeström M, Tarkiainen A, Parviainen T, Kujala J, Numminen J, Hiltunen J, Laine M, Salmelin R. Perceiving and naming actions and objects. Neuroimage. 2008;41(3):1132–1141. doi: 10.1016/j.neuroimage.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Lui F, Buccino G, Duzzi D, Benuzzi F, Crisi G, Baraldi P, Nichelli P, Porro CA, Rizzolatti G. Neural substrates for observing and imagining non-object-directed actions. Soc Neurosci. 2008;3(3–4):261–275. doi: 10.1080/17470910701458551. [DOI] [PubMed] [Google Scholar]
- MacLeod AK, Buckner RL, Miezin FM, Petersen SE, Raichle ME. Right anterior prefrontal cortex activation during semantic monitoring and working memory. Neuroimage. 1998;7:41–48. doi: 10.1006/nimg.1997.0308. [DOI] [PubMed] [Google Scholar]
- Mohamed IS, Cheyne D, Gaetz WC, Otsubo H, Logan WJ, Snead OC, III, Pang EW. Spatiotemporal patterns of oscillatory brain activity during auditory word recognition in children: A synthetic aperture magnetometry study. Int J Psychophysiol. 2008;68:141–148. doi: 10.1016/j.ijpsycho.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E, Lopes da Silva FH. Electroencephalography: basic principles, clinical applications and related fields. Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- Owen AM. The functional organization of working memory processes within human lateral frontal cortex: the contribution of functional neuroimaging. Eur J Neurosci. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Pang EW, Wang F, Malone M, Kadis DS, Donner EJ. Localization of Brocàs area using verb generation tasks in the MEG: Validation against fMRI. Neurosci Lett. 2011;490:215–219. doi: 10.1016/j.neulet.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker Jones O, Green DW, Grogan A, Pliatsikas C, Filippopolitis K, Ali N, Lee HL, Ramsden S, Gazarian K, Prejawa S, Seghier mI, Price CJ. Where, when and why brain activation differs for bilinguals and monolinguals during picture naming and reading aloud. Cereb Cortex. 2012;22(4):892–902. doi: 10.1093/cercor/bhr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péran P, Cardebat D, Cherubini A, Piras F, Luccichenti G, Peppe A, Caltagirone C, Rascol O, Démonet JF, Sabatini U. Object naming and action-verb generation in Parkinson’s disease: a fMRI study. Cortex. 2009;45(8):960–971. doi: 10.1016/j.cortex.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and behaviour. Curr Opin Neurobiol. 1994;4:207–211. doi: 10.1016/0959-4388(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D’Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychol. 2003;41:378–389. doi: 10.1016/s0028-3932(02)00169-0. [DOI] [PubMed] [Google Scholar]
- Ressel V, Wilke M, Lidzba K, Lutzenberger W, Krägeloh-Mann I. Increases in language lateralization in normal children as observed using magnetoencephalography. Brain Lang. 2008;106(3):167–176. doi: 10.1016/j.bandl.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Robinson SE, Vrba J. Recent Advances in Biomagnetism. Tohoku University Press; Sendai: 1999. Functional neuroimaging by synthetic aperture magnetometry (SAM) [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Salmelin R. Clinical neurophysiology of language: The MEG approach. Clinical Neurophysiology. 2007;118:237–254. doi: 10.1016/j.clinph.2006.07.316. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Sams M. Motor cortex involvement during verbal and non-verbal lip and tongue movements. Human Brain Mapp. 2002;16:81–91. doi: 10.1002/hbm.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt GW, Roberts TPL. Second language research using magnetoencephalography: a review. Second Lang Res. 2009;1:135–166. [Google Scholar]
- Seikihara K, Nagarajan SS, Poeppel D, Marantz A, Miyahita Y. Reconstructing spatio-temporal activities of neural sources using an MEG vector beamformer technique. IEEE Trans Biomed Eng. 2001;48(7):760–771. doi: 10.1109/10.930901. [DOI] [PubMed] [Google Scholar]
- Shapiro KA, Moo LR, Caramazza A. Cortical signatures of noun and verb production. Proc Natl Acad Sci USA. 2006;103(5):1644–1649. doi: 10.1073/pnas.0504142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck DW, Leahy RM. BrainSuite: An automated cortical surface identification tool. Medical Image Analysis. 2002;6(2):129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- Shattuck EW, Mirza M, Adisetivo V, Hojakashani C, Salamon G, Narr KL, Poldrack RA, Bilder RM, Toga AW. Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage. 2008;39(3):1064–1080. doi: 10.1016/j.neuroimage.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13(5):856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Singh KD, Barnes GR, Hillebrand A, Forde EME, Williams AL. Task-related changes in cortical synchronization are spatially coincident with the hemodynamic response. NeuroImage. 2002;16:103–114. doi: 10.1006/nimg.2001.1050. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe RA. Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Sys Neurosci. 2011;5:1–20. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heuven WJB, Dijkstra T. Language comprehension in the bilingual brain: fMRI and ERP support for psycholinguistic models. Brain Res Rev. 2010;64:104–122. doi: 10.1016/j.brainresrev.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Vihla M, Laine M, Salmelin R. Cortical dynamics of visual/semantic vs. phonological analysis in picture confrontation. Neuroimage. 2006;33:732–738. doi: 10.1016/j.neuroimage.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng. 1997;44(9):867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods (Duluth) 2001;25(2):249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- Winterer G, Carver FW, Musso F, Mattay V, Weinberger DR, Cuppola R. Complex relationship between BOLD signal and synchronization/desynchronization of human brain MEG oscillations. Human Brain Mapp. 2007;28:805–816. doi: 10.1002/hbm.20322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AG, Harvey AS, Wellard RM, Abbott DF, Anderson V, Kean M, Saling MM, Jackson GD. Language cortex activation in normal children. Neurology. 2004;63(6):1035–1044. doi: 10.1212/01.wnl.0000140707.61952.ca. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Ukai S, Shinosaki K, Ishii R, Kawaguchi S, Ogawa A, Mizuno-Matsumoto Y, Fujita N, Yoshimine T, Takeda M. Spatially filtered magnetoencephalographic analysis of cortical oscillatroy changes in basic brain rhythms during the Japanese ‘Shiritori’ Word Generation Task. Neuropsychobiology. 2006;53:215–222. doi: 10.1159/000094835. [DOI] [PubMed] [Google Scholar]


