Abstract
Allotetrahydrodeoxycorticosterone (THDOC) belongs to a class of pregnane neurosteroidal compounds that enhance brain inhibition by interacting directly with GABAA signaling, mainly through an increase in tonic inhibitory current. Here, we addressed the role of THDOC in the modulation of interictal- and ictal-like activity and associated high-frequency oscillations (HFOs, 80–500 Hz; ripples: 80–200 Hz, fast ripples: 250–500 Hz) recorded in vitro in the rat piriform cortex, a highly excitable brain structure that is implicated in seizure generation and maintenance. We found that THDOC: (i) increased the duration of interictal discharges in the anterior piriform cortex while decreasing ictal discharge duration in both anterior and posterior piriform cortices; (ii) reduced the occurrence of HFOs associated to both interictal and ictal discharges; and (iii) prolonged the duration of 4-aminopyridine-induced, glutamatergic independent synchronous field potentials that are known to mainly result from the activation of GABAA receptors. Our results indicate that THDOC can modulate epileptiform synchronization in the piriform cortex presumably by potentiating GABAA receptor-mediated signaling. This evidence supports the view that neurosteroids regulate neuronal excitability and thus control the occurrence of seizures.
Keywords: 4-aminopyridine, THDOC, epileptiform synchronization, high-frequency oscillations, ictogenesis
INTRODUCTION
The piriform cortex is a highly excitable region that extends over the ventrolateral forebrain in rodents (Galvan et al., 1982; Luskin and Price, 1983; Loscher and Ebert, 1996). This three-layered cortical area projects to several limbic structures including the amygdala, lateral entorhinal cortex, and subiculum (Luskin and Price, 1983; Haberly, 2001). With regard to connectivity, the piriform cortex can be divided into the anterior and posterior subregions (Haberly, 1998). Along these subregions, GABAergic inputs are asymmetrically arranged, with the strength of interneuron-to-pyramidal cell contact increasing along the rostro-caudal axis or from the anterior subregion toward the posterior (Luna and Pettit, 2010). Therefore, the anterior piriform cortex is presumably more excitable when compared to the posterior piriform.
Neuronal excitability can be modulated by neurosteroids, a class of compounds that act on ion channels and membrane receptors (Mellon and Griffin, 2002; Akk et al., 2009). By modulating the efficacy of GABAA receptor function, allotetrahydrodeoxycorticosterone (THDOC) enhances brain inhibition and acts as a broad spectrum anticonvulsant, protecting against seizures induced by pilocarpine, kindling, and GABAA receptor antagonists in animal models of epilepsy in vivo (Rupprecht et al., 1996; Stell et al., 2003; Reddy, 2004, 2011). Furthermore, in vitro studies performed in rat hippocampal slices have shown that neurosteroids produce a concentration-dependent suppression of the epileptiform activity induced by 4-aminopyridine (4AP) and picrotoxin (Salazar et al., 2003). Finally, the induction of neurosteroid synthesis in rats following pilocarpine treatment can delay epileptogenesis in vivo (Biagini et al., 2009).
High-frequency oscillations (HFOs, ripples: 80–200 Hz, fast ripples: 250–500 Hz) are recorded in the EEG of epileptic patients and in animal models of temporal lobe epilepsy (Engel and da Silva, 2012; Jefferys et al., 2012). HFOs occur in limbic structures such as the hippocampus and entorhinal cortex as well as in the neocortex, they are thought to reflect the activity of dysfunctional neural networks, and they are used to localize seizure onset zones (Bragin et al., 2004; Jacobs et al., 2009, 2010; Ibarz et al., 2010; Jiruska et al., 2010; Wu et al., 2010; Lévesque et al., 2011, 2012). Using sagittal and coronal brain slices maintained in vitro, Panuccio et al. (2012) have recently reported that HFOs coincide with the onset of ictal discharges induced by 4AP in the piriform cortex. Here, we further investigated the role of the piriform cortex in generating 4AP-induced epileptiform activity in vitro by addressing the impact of the neurosteroid THDOC on the epileptiform activity and associated HFOs in both anterior and posterior subregions. To improve access to both anterior and posterior aspects of the piriform cortex, which may differentially express epileptiform activity, we used horizontal rat brain slices that are known to better maintain rostro-caudal association fiber connections (Demir et al., 2001).
EXPERIMENTAL PROCEDURES
In vitro preparation
Horizontal brain slices with a thickness of 450 μm were obtained from male Sprague–Dawley rats weighing 250–275 g (Charles River Laboratories, Saint Constant, Quebec, Canada). Animals were anesthetized with isoflurane and decapitated. Their brains were then quickly removed and chilled for 3 min in ice-cold artificial cerebrospinal fluid (ACSF) with the following composition (mM): 124 NaCl, 2 KCl, 2 CaCl2, 2 MgSO4, 1.25 KH2PO4, 26 NaHCO3, 10 D-glucose. The ACSF was continuously bubbled with O2/CO2 (95/5%) gas mixture to maintain pH at ~7.4. Slices were prepared with a Vibratome (VT1000S; Leica, Concord, Ontario, Canada) and directly transferred to an interface chamber where they laid between warm (31–33 °C) ACSF (pH ~7.4, ~305 mOsm/kg) and humidified gas (O2/CO2, 95/5%). Slices were allowed to recover for at least 1 h before initiating the continual bath application of 4AP (50 μM) at a flow rate of ~2 ml/min. All pharmaceuticals were bath applied and obtained from Sigma–Aldrich Canada, Ltd. (Oakville, Ontario, Canada) or from Tocris Bioscience (Ellisville, MO, USA). All procedures were carried out in compliance with the guidelines of the Canadian Council on Animal Care and the McGill Animal Care Committee to minimize the suffering and number of animals.
Field potential recordings
Field potential recordings were obtained with ACSF-filled glass pipettes (1B150F-4; World Precision Instruments, Sarasota, Florida, USA; tip diameter <10 μm, resistance 5–10 MΩ) that were pulled with a Sutter P-97 puller (Sutter, Novato, CA, USA). Electrodes were placed in the anterior and posterior piriform cortices at a distance of approximately 1.5 mm. Signals were fed to an AI 401 amplifier and a CyberAmp 380 (Molecular Devices, Silicon Valley CA, USA), then digitized using the Digidata 1322A (Molecular Devices). Signals acquired with the CyberAmp 380 were sampled at 5 kHz and cut-off at 1 kHz.
Detection and analysis of high-frequency oscillatory events
To study HFOs, time-periods containing ictal and interictal discharges recorded from the piriform cortex were extracted and analyzed offline using Matlab (The Mathworks, Natick, MA, USA). A multiparametric algorithm using routines based on standardized functions (Signal Processing Toolbox) was used. Recordings were first band-pass filtered in the 80–200 and 250–500 Hz frequency ranges using a finite impulse response (FIR) filter. Zero phase digital filtering was used to avoid phase distortion. Each recording was then normalized using a 10-s artefact-free period. To be considered as an HFO candidate, oscillatory events in the 80–200 Hz and in the 250–500 Hz frequency range had to show four consecutive peaks at three standard deviations (SDs) above the mean as determined by the reference period. (Salami et al., 2012) The time lag between consecutive peaks varied from 5 to 12.5 ms for ripples and from 2 to 4 ms for fast ripples (Salami et al., 2012). HFOs were kept for analysis only if the oscillatory event was visible in either the 80–200 or 250–500 Hz range. Overlapping events, which may be caused by the filtering of sharp spikes (Bénar et al., 2010), were excluded from the analysis. HFO rates were obtained for each ictal discharge and interictal discharge in the anterior and posterior subregions of the piriform cortex.
Statistical analysis
Offline analysis of the duration and interval of occurrence of ictal and interictal discharges was performed using the software CLAMPFIT 8.2 (Molecular Devices). Specifically, in both cases, we measured the duration as the time span between the first deflection of the discharge from baseline to its return to baseline. Interval was measured as the time occurring between the onsets of consecutive discharges. Amplitude was measured from peak to peak. Time lag was measured as the difference in time onset between field potential recorded in the anterior and posterior subregion. Arbitrarily, discharges leading from the anterior piriform cortex were assigned a negative value and discharges originating in the posterior subregion were assigned positive value. When no difference was detected, a value of 0 was assigned. All visual analyses were conducted blind to the subregion of the piriform cortex being analyzed and the concentration of THDOC present in the ACSF.
Distributions were tested for skewness and kurtosis. Data were analyzed with two-way analysis of variances (ANOVAs) followed by the Tukey’s post hoc test, as appropriate. To account for the variability in ictal discharge duration between slices, Z-values were assigned to each discharge within a given experiment, allowing us to test the group effect of THDOC. HFOs occurrence was analyzed using the non-parametric Mann–Whitney rank sum test followed by the Bonferroni correction for multiple comparisons since values were not normally distributed.
When analyzing the dynamics of HFO occurrence, in order to account for differences in duration, ictal discharges were first transformed into a time scale from 1 (start of the ictal event) to 100 (end of the ictal event). The ictal period was then divided in three equal parts and rates of ripples and fast ripples in each subregion of the piriform cortex (anterior and posterior) were compared using non-parametric Wilcoxon signed-rank tests followed by Bonferroni–Holm corrections for multiple comparisons. This allowed us to evaluate if ripples or fast ripples predominated at specific moments of the ictal event in each piriform cortex subregion. Throughout the text, n indicates the number of slices studied, unless otherwise specified. Results were considered significantly different if p<0.05 and are expressed as mean±standard error of the mean (SEM).
RESULTS
Interictal and ictal discharges induced by 4AP
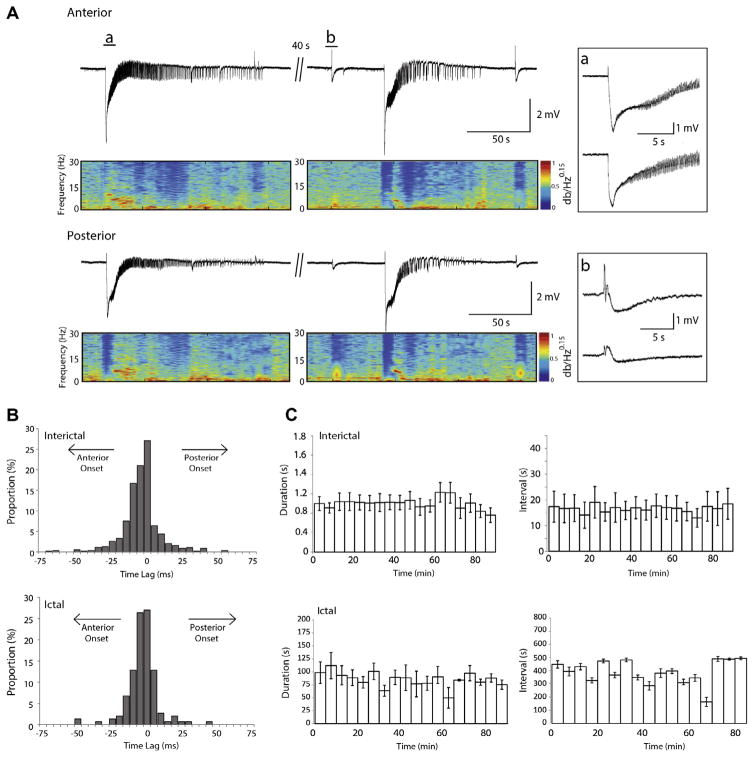
Fig. 1A shows examples of interictal and ictal activity induced by 4AP. Interictal discharges occurred synchronously in the anterior and posterior piriform cortex (Fig 1Ab) at an average interval of 9.6±3.5 s, and lasted on average 297.3±7.2 ms in the anterior piriform cortex and 322.6±8.3 ms in the posterior piriform cortex (n=19, 1171 events). These values were not significantly different. Moreover, interictal events were likely to initiate equally from the anterior and posterior subregions of the piriform cortex as shown by the time lag histograms (Fig. 1B).
Fig. 1.
Interictal and ictal discharges in the piriform cortex. (A) Epileptiform discharges recorded from the anterior and posterior piriform cortices of an intact brain slice following the application of 4AP. The spectrograms show the initial negative shift followed by oscillations in the 10–20 Hz range characterizing the ictal event. The ictal onset and an interictal discharge are expanded in insets a and b, respectively. (B) Time delay histograms between the anterior and posterior regions calculated for interictal and ictal discharges. These are pooled data from 19 slices. Note the jittering in the region of onset, as no event initiated in one region in particular. Events in the anterior piriform cortex are used as the reference (time 0). (C) Epileptiform activity does not change over time during 4AP application (n=5 slices). Upper panels show the duration and interval of occurrence of interictal events over time, respectively while lower panels show the duration and interval of occurrence of ictal events; data were averaged in epoques lasting 5 min. Time 0 represents the time of appearance of spontaneous epileptiform activity.
Ictal discharges occurred at an average rate of 20±2.4 events per hour (n=19, 152 events), with all discharges in the anterior piriform cortex co-occurring with a discharge in the posterior piriform cortex (Fig. 1A). Ictal discharges were characterized by an initial negative shift followed by the appearance of oscillations at approximately 15 Hz (spectrograms in Fig. 1A). The average duration of ictal discharges in the anterior and posterior piriform cortex was 66.8±6.0 and 69.6±6.4 s, respectively. These values were determined to be not significantly different. Ictal events were equally likely to initiate from either the anterior or the posterior piriform cortex as indicated by the time lag histograms (Fig. 1B).
In five additional experiments we plotted the duration and interval of occurrence of both interictal events and ictal discharges under control conditions up to 90 min after the appearance of spontaneous epileptiform activity. Since there was no significant difference in the duration and interval of occurrence of epileptiform events in the anterior and posterior piriform cortices, data were grouped for analysis. As illustrated in Fig. 1C, there were no significant changes in duration and interval of occurrence of interictal discharges, or any changes in these parameters with respect to ictal discharges.
Neurosteroids modulate 4AP-induced interictal activity
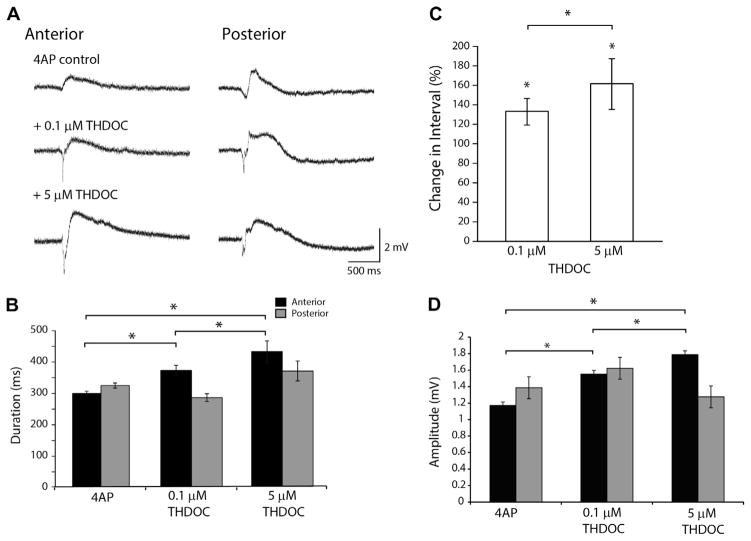
Fig. 2A shows examples of interictal discharges in the anterior and posterior piriform cortex under control conditions and during the application of 0.1 μM THDOC (n=7) and 5 μM of THDOC (n=6). Following the application of 0.1 μM THDOC, the duration of interictal events in the anterior piriform cortex increased significantly (p<0.05) compared to those seen under control conditions only (Fig. 2B). With 5 μM of THDOC (n=6), the duration of interictal discharges further increased compared to the 0.1 μM condition (p<0.05) (Fig. 2B). In contrast, interictal discharge duration in the posterior piriform cortex exhibited no change (Fig. 2B). However, in both anterior and posterior subregions, 0.1 μM THDOC significantly increased the interval between interictal discharges (p<0.05) from 4AP control, and 5 μM THDOC further increased the interval between discharges (p<0.05) (Fig. 2C). Moreover, compared to the 4AP control condition, 0.1 and 5 μM THDOC increased interictal amplitude in the anterior piriform cortex but did not induce a corresponding change in the posterior subregion (Fig. 2D). Following a 1.5-h wash out period, there was no full recovery to control conditions, possibly due to the hydrophobic nature of THDOC. Consistent with the 4AP control condition, interictal discharges during 4AP and THDOC were likely to originate from either the anterior or posterior piriform cortices.
Fig. 2.
Modulation of Interictal discharges by THDOC. (A) Representative examples of interictal events in the 4AP control, 4AP+0.1 μM THDOC and the 4AP +5 μM THDOC condition. (B) Bar graph showing the average duration of 4AP-induced slow events. THDOC induced a significant increase in duration of these slow events in the anterior region. (C) Bar graph showing the change in interval of interictal events normalized to the 4AP control condition (100%). THDOC induced a significant increase compared to 4AP. (D) Change in amplitude of interictal events, note that THDOC induced a significant increase of amplitude compared to 4AP. (*p<0.05; 4AP pooled data from n=13 slices, 0.1 μM THDOC n=7 slices, 5 μM THDOC n=7 slices).
Neurosteroidal modulation of 4AP-induced ictal activity
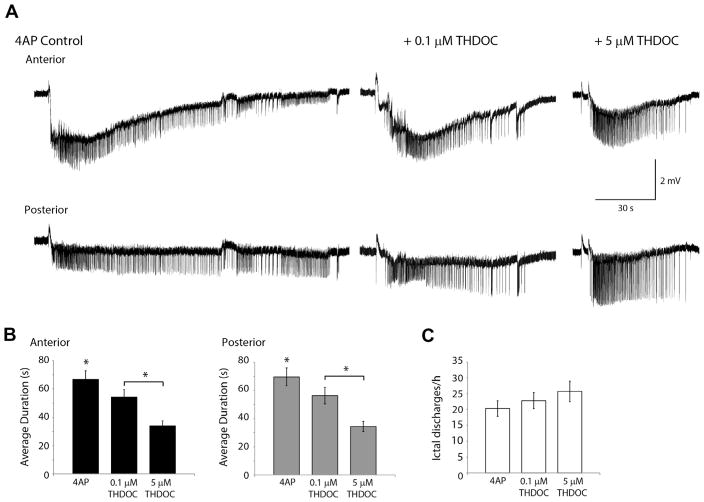
Fig. 3A shows ictal discharges recorded during the application of 4AP as well as following the addition of 0.1 and 5 μM THDOC. Application of 0.1 μM THDOC induced a decrease in ictal discharge duration compared to the control condition in recordings simultaneously performed in anterior piriform cortex (p<0.05) and posterior piriform cortex (p<0.05) (Fig. 3B). 5 μM THDOC also significantly reduced the duration of ictal events in both anterior (p<0.05) and posterior piriform cortex (p<0.05) (Fig. 3A, B). Moreover, 5 μM of THDOC led to a larger decrease in duration of ictal discharges compared to 0.1 μM condition (p<0.05) (Fig. 3B). Neither the 0.1 μM nor the 5 μM THDOC concentration affected the interval of ictal events (Fig. 3C) although a trend toward an increase in interval length was observed. In two of six experiments ictal events were completely abolished in 40 min following the application of 5 μM THDOC with only interictal events remaining. Finally, as reported for interictal discharges, THDOC application (both 0.1 and 5 μM) did not affect the “initiation jittering” observed by simultaneously recording ictal discharges from the anterior and posterior piriform subregions.
Fig. 3.
Effect of THDOC on Ictal-like events. (A) Representative examples of ictal discharges in the 4AP control condition, 4AP+0.1 μM THDOC and the 4AP +5 μM THDOC condition. (B) Bar graph showing the average duration of ictal discharges following the application of 4AP and THDOC. THDOC induced a significant decrease in ictal discharge duration compared to 4AP in both the anterior and posterior regions of the piriform cortex. (C) Number of ictal discharges per hour following in the 4AP control condition, 4AP+0.1 μM THDOC and 4AP+0.5 μM. The frequency of ictal discharges did not change significantly following the application of THDOC. (*p<0.05 4AP pooled data n=13, 0.1 μM THDOC n=7, 5 μM THDOC n=7).
Effects of THDOC on HFOs
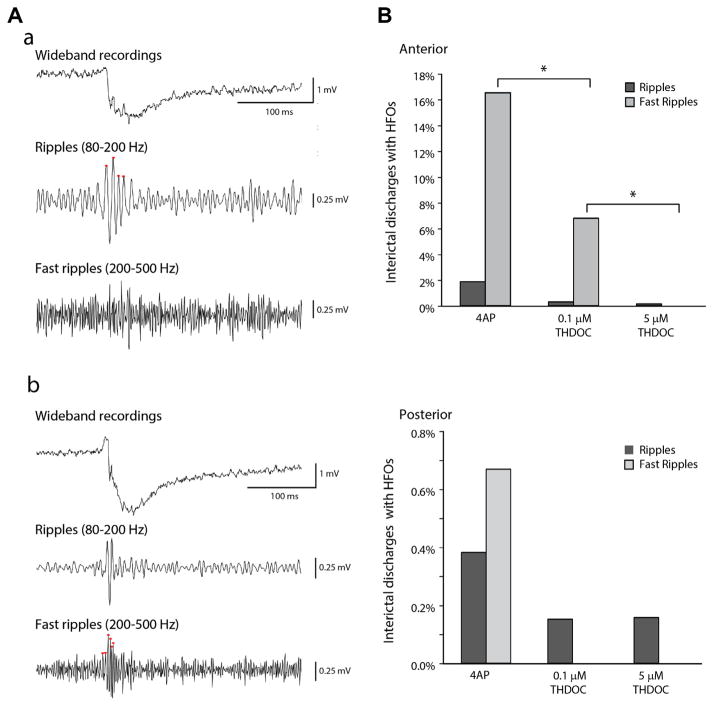
Fig. 4A shows examples of a ripple (Fig. 4Aa) and a fast ripple (Fig. 4Ab) occurring in the anterior piriform cortex in coincidence with interictal events induced by 4AP. Under this condition, only 1.9% (23/1235) of interictal spikes recorded in the anterior subregion co-occurred with ripples, whereas 16.5% of them (204/1235) co-occurred with fast ripples (n=19 slices) (Fig. 4Ba). Thus, fast ripples occurred more frequently than ripples (p<0.05). In the posterior subregion, HFO occurrence was markedly lower than the anterior subregion with only 1% of interictal discharges co-occurring with either ripples or fast ripples (11 of 1047 interictal events, n=19) (Fig. 4Bb). In both anterior and posterior subregions, HFOs occurring outside of interictal discharges were negligible.
Fig. 4.
HFOs during interictal discharges. (A) Example of an interictal discharge co-occurring with a ripple (a) or with a fast ripple (b) in the anterior piriform cortex under 4AP bath application. Note in b that the fast ripple is visible on the descending phase of the spike. (B) Bar graph showing the proportion of interictal events, in the anterior and posterior piriform cortex, co-occurring with HFOs in the 4AP control (anterior: n=19, events=1235; posterior: n=19, events=1047), 4AP+0.1 μM THDOC (anterior: n=7, events=665; posterior: n=7, events=659) and the 4AP+5 μM THDOC (anterior: n=6, events=633; posterior: n=6, events=636) condition. The application of THDOC induced a significant decrease in the proportion of interictal spikes co-occurring with HFOs, mainly in the fast ripple frequency range and in the anterior region. (*p<0.05) Note that the proportion of interictal spikes co-occurring with HFOs in the posterior region of the piriform cortex is almost negligible.
Application of 0.1 μM THDOC induced a significant decrease in the occurrence of fast ripples associated with interictal spikes in the anterior piriform cortex (44/665 events, n=7 slices) (p<0.01) while ripple occurrence did not change significantly (3/665 events, n=7 slices) (Fig. 4Ba). Following application of 5 μM THDOC, fast ripples were virtually abolished (633 events, n=7 slices) (p<0.05) and the occurrence of ripples remained low (2/633 events, n=7 slices) (Fig. 4Ba). In the posterior piriform cortex HFO rates remained low during the application 0.1 or 5 μM THDOC (Fig. 4Bb). Thus, THDOC can significantly reduce the proportion of fast ripples associated to interictal discharges in the anterior subregion of the piriform cortex.
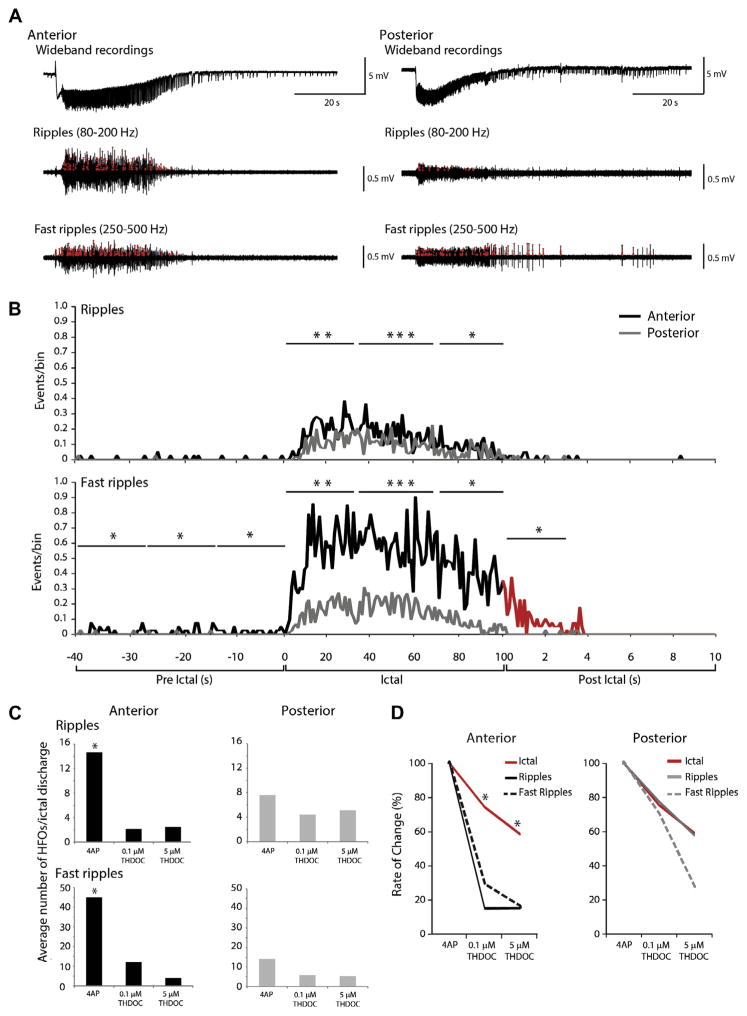
Next, we analyzed the incidence of ripples and fast ripples during ictal discharges recorded from the anterior and posterior piriform cortices during 4AP application (Fig. 5A). Throughout the duration of ictal discharges, fast ripples occurred at higher rates compared to ripples in both anterior and posterior subregions. Fast ripple occurrence in the pre-ictal period was higher in the anterior than the posterior piriform cortex. During the ictal period, ripple and fast ripple occurrence was higher in the anterior piriform cortex. In the post-ictal period, fast ripple rates remained higher compared to those in the posterior region (Fig. 5B).
Fig. 5.
HFOs during ictal discharges. (A) Representative 4AP-induced ictal discharge from the anterior and posterior piriform cortex, with filtered traces showing HFOs (red circles) in the ripple and fast ripple frequency ranges. (B) Rates of ripples and fast ripples over time during 4AP induced ictal discharges in the anterior and posterior regions (data pooled from n=19 slices). Note that ripples and fast ripple rates are significantly higher in the anterior compared to the posterior region. During the pre- and post-ictal periods, rates of fast ripples are also significantly higher in the anterior region of the piriform cortex compared to the posterior region. (C) Bar graphs showing the average number of HFOs per ictal discharge following the application of 4AP (n=13), 0.1 μM (n=7 slices) and 5 μM THDOC (n=6 slices). Application of THDOC induced a significant decrease in the occurrence of HFOs in the anterior region, but not in the posterior region. (D) Line graph showing the change in ictal duration compared to the change in HFO rate following the application of either 0.1 or 5 μM THDOC. All events are normalized to the 4AP control condition. (*p<0.05, **p<0.01, ***p<0.001). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We also established the proportion of ictal discharges containing ripples and fast ripples in the anterior and posterior piriform cortices under control conditions and following application of 0.1 and 5 μM THDOC. In the anterior piriform cortex, ripples and fast ripples were detected in 47.7% (71/149) and in 48.3% (72/149), respectively, of the ictal discharges occurring in the 4AP control condition (149 events, n=19) (Fig. 5C). In both subregions, treatment with 0.1 μM THDOC (n=7) led to a higher proportion of ictal discharges containing ripples (70%) while those containing fast ripples remained constant (50%). During the application of 5 μM THDOC (n=6), although the total proportion of ictal discharges containing ripples and fast ripples decreased, there was still a higher number of discharges that contained ripples (30%) than fast ripples (10%). A similar pattern was observed in the posterior region. Thus, THDOC increased the probability for an ictal discharge to contain HFOs in the ripple frequency range (if they contained HFOs at all) compared to the fast ripple frequency range, despite fast ripples occurring at a greater rate.
We then considered only those ictal discharges where HFOs were detected in order to determine the change in occurrence of ripples and fast ripples induced by THDOC. In the anterior piriform cortex, there was an average of 14.6±2.2 ripples and 45.1±7.6 fast ripples per discharge under control conditions; application of 0.1 or 5 μM THDOC led to a significant decrease of HFO occurrence (p<0.01 in both cases) (Fig. 5C). In the posterior piriform cortex, an average of 7.6±1.13 ripples and 14.1±2.2 fast ripples were detected per ictal event under 4AP; addition of 0.1 μM THDOC or 5 μM THDOC did not affect significantly HFO occurrence as the counts were low to begin with (Fig. 5C).
To rule out the possibility that the reduced occurrence of ripples and fast ripples during THDOC application was due to the decrease in ictal duration, we normalized the duration of ictal discharges, ripple occurrence, and fast ripple occurrence and compared their respective degree of change by assigning z-scores to values in each group. In the anterior piriform cortex, both 0.1 and 5 μM THDOC induced a decrease in ripple and fast ripple occurrence that was greater than the decrease in ictal duration (p<0.05) (Fig. 5D). However, in the posterior piriform cortex, ripple and fast ripple occurrence changed at a similar degree when compared to ictal discharge duration (Fig. 5D).
Pharmacologically isolated synchronous events
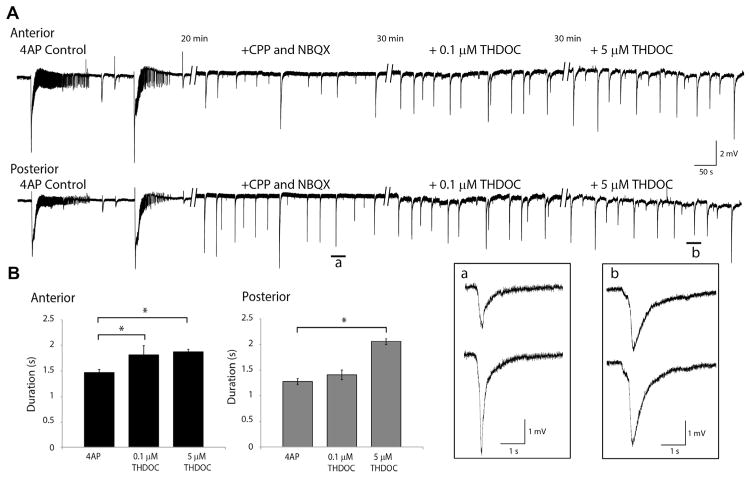
Finally, we pharmacologically blocked glutamatergic transmission with CPP and NBQX to analyze the effects of THDOC on the 4AP-induced synchronous, presumably GABAergic, events that are recorded under these conditions. As shown in Fig. 6A, CPP and NBQX (10 μM each) abolished the spontaneous epileptiform discharges, revealing the recurrence of slow field events with a duration of 1.4±0.1 s in the anterior piriform cortex (331 events, n=4) and 1.2±0.1 s in the posterior piriform cortex (288 events, n=4); these values, however, were not significantly different from each other. In both the anterior and posterior piriform cortices, the average interval between these slow field events was 24.0±1.2 s (n=4, 368 events). HFOs were virtually abolished by the application of CPP and NBQX.
Fig. 6.
Effects of THDOC on slow events (A). Recording of 4AP control activity, followed by the application of CPP and NBQX, 0.1 μM THDOC and then 5 μM THDOC in the anterior and posterior piriform cortex. Insets a and b show an example of a slow event on an expanded time scale. (B) Bar graphs showing the change in duration of these slow spikes in the 4AP+0.1 μM (n=4 slices) and 4AP+5 μM (n=4 slices) THDOC. Duration is expressed as the change from the 4AP control condition. (*p<0.05).
In the anterior piriform cortex, adding 0.1 or 5 μM THDOC to medium containing 4AP, CPP, and NBQX increased the duration of the isolated slow events to 1.7±0.1 or 1.8±0.05 s, respectively (p<0.05) (Fig. 6B). These values were however not significantly different from each other. In the posterior piriform cortex, application of 0.1 μM THDOC the duration of the field events averaged 1.4±0.1 s (not significant from control) and 2.1±0.1 s following application of 5 μM THDOC (significant from control p<0.05; not significant from 5 μM in anterior piriform) (Fig. 6B). THDOC application failed to induce a change in interval and amplitude of these slow field events.
DISCUSSION
The main findings of our study can be summarized as follows. First, the neurosteroid THDOC increases the duration of 4AP-induced interictal discharges in the anterior subregion of the piriform cortex. Second, THDOC reduces the duration of ictal discharges in both subregions of the piriform cortex. Third, THDOC decreases the occurrence of HFOs associated to interictal and ictal discharges in the anterior piriform cortex. Fourth, THDOC induces an increase in the duration of pharmacologically isolated synchronous GABAergic events.
THDOC modulation of 4AP-induced interictal and ictal activity
We have found that THDOC increases the duration of interictal discharges in the anterior piriform cortex. This could be due to the fact that THDOC modulates GABAA receptor signaling, which is known to participate in the generation of interictal events (Avoli and de Curtis, 2011). The different responsiveness to THDOC in the anterior and posterior piriform cortices could be due to the weaker interneuron-to-pyramidal cell connections in the anterior compared to the posterior piriform cortex (Luna and Pettit, 2010) and the different patterns of connectivity that characterize these two regions. It is known that the anterior piriform cortex receives sensory information directly from the olfactory tract (Hagiwara et al., 2012), whereas the posterior piriform cortex receives projections from the entorhinal, perihinal, amygdala, prefrontal and anterior piriform regions (Haberly, 2001). The anterior piriform cortex may be thus more sensitive to neurosteroids compared to the posterior piriform cortex due to differential levels of inhibition and different preservation of local networks following slicing (Suzuki and Bekkers, 2011).
Ictal discharge duration was also significantly reduced following THDOC application and in one third of experiments, ictal discharges were even completely abolished. Since THDOC potentiates GABAA receptor activity, with a greater preference to act on tonic currents (Reddy, 2010; Ferando and Mody, 2012), an increased level of “inhibitory tone” provided by THDOC during 4AP-induced hypersynchronous activity could have reduced the capacity for the maintenance of ictal discharges. These changes are not likely to reflect a rundown of the in vitro preparation since THDOC was applied 20 min from the start of the recording and data were analyzed 30 min from the start of the recording. In our baseline analysis, we found there was no significant decrease in interval of occurrence or duration of either interictal or ictal events for a period of up to 90 min.
The effects of THDOC in the 4AP model are congruent with studies investigating the anticonvulsant nature of neurosteroids in in vivo models of epilepsy and in human studies; however, these results must be duplicated in other models of epileptiform hyperexcitabilty such as low Mg2+ or high K+. The replication of these studies will provide a more thorough understanding of the role of neurosteroids in epilepsy. In rodent models of epilepsy, neurosteroidal compounds are effective in protecting against GABAA receptor antagonist-induced seizures, pilocarpine-induced limbic seizures and kindled seizures (Salazar et al., 2003; Reddy, 2010). In epileptic patients, finasteride, which inhibits neurosteroid synthesis, was recently shown to enhance seizure susceptibility (Pugnaghi et al., 2013). Moreover, the synthetic analog of anti-convulsant neurosteroids, ganaxolone, significantly reduced seizure frequency in patients with medically refractory partial epilepsy (Laxer et al., 2000).
Interictal and ictal HFOs can be modulated by neurosteroids
We have also discovered that THDOC decreases HFO occurrence (ripples and fast ripples) associated with both 4AP-induced interictal and ictal discharges. The occurrence of HFOs associated with interictal discharges was modulated with THDOC to a greater degree than the rate of interictal discharges, supporting the hypothesis that interictal spikes and HFOs may reflect different underlying neural mechanisms (Zijlmans et al., 2009; Demont-Guignard et al., 2012; Wendling et al., 2012). Regarding ictal discharges and associated HFOs, we found that discharges are more likely to contain ripples than fast ripples when THDOC is applied. This suggests that ripples might be more related to interneuron firing and their corresponding IPSPs recorded on principal cells compared to fast ripples (Buzsáki et al., 1992; Ylinen et al., 1995). However, when considering only those ictal events during which HFOs were detected, THDOC induced a decrease in HFO occurrence without influencing ictal discharge frequency. Moreover, HFO rates decreased independently of a decrease in ictal discharge duration following THDOC. This evidence suggests that neurosteroids can affect interneuronal synchronization underlying the occurrence of ripples (Buzsáki et al., 1992; Ylinen et al., 1995) as well as the pyramidal cell synchrony that is presumed to contribute to fast ripple generation (Dzhala and Staley, 2004; Bragin et al., 2011; Engel et al., 2009). This effect may be related to the ability for THDOC to potentiate tonic and phasic GABAA receptor-mediated currents (Wohlfarth et al., 2002). By doing so, the shunting of excitatory inputs would reduce cell firing and synchrony (Farrant and Nusser, 2005) and thus the capacity of neuronal networks to maintain epileptiform activity over time.
Neurosteroidal modulation of synchronous GABAergic events
Slow GABA receptor-dependent potentials were isolated in 4AP-treated slices with the blockade of glutamatergic signaling using NMDA and non-NMDA receptor antagonists. These slow discharges result from synchronous interneuronal network firing, leading to the postsynaptic activation of principal cells (Michelson and Wong, 1994; Avoli et al., 1996; Lamsa and Kaila, 1997; Avoli and de Curtis, 2011). Similar results were described in sagittal and coronal slices of the piriform cortex (Panuccio et al., 2012), insular cortex, perihinal cortex, hippocampal networks, and entorhinal cortex (Avoli and de Curtis, 2011).
In addition, we found that THDOC prolonged the duration of these synchronous events. Our findings substantiate previous reports on the importance of interneuronal networks in driving neuronal populations toward hypersynchronous states in the temporal lobe (Avoli and de Curtis, 2011) and additionally show that neurosteroids can be important modulators of interictal activity.
CONCLUSIONS
Neurosteroids act as a class of broad spectrum anticonvulsants, which can be implicated in catamenial epilepsy, stress-induced changes in seizure susceptibility, alcohol withdrawal epilepsy, and temporal lobe epilepsy when their endogenous levels are reduced (Reddy, 2010). Our findings demonstrate that THDOC can modulate interictal and ictal events as well as HFOs in the piriform cortex, although these effects are differentially expressed in the anterior and posterior subregions. To the best of our knowledge, this is the first study showing an effect of neurosteroids on HFOs and it supports the hypothesis that these compounds may modulate the overall excitability of the central nervous system thus playing relevant roles in both ictogenesis and epileptogenesis. This aspect is of paramount importance for providing new avenues of treatment for epilepsy, since ripples and fast ripples are thought to reflect the underlying pathological network activity that contributes to the generation and maintenance of ictal activity. However, the relationship between HFOs and epileptogenesis is complex and it remains to be established whether a drug that alters HFOs can be used as an effective treatment for controlling seizure occurrence.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (CIHR grants 8109 and 74609). We thank Dr. G. Panuccio and Ms. P. Salami for helping with recording procedures and data analysis as well as Dr. M. de Curtis for critical reviewing of an early draft of this paper.
Abbreviations
- 4AP
4-aminopyridine
- ACSF
artificial cerebrospinal fluid
- HFOs
high-frequency oscillations
- THDOC
allotetrahydrodeoxycorticosterone
References
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. The influence of the membrane on neurosteroid actions at GABAA receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S59–S66. doi: 10.1016/j.psyneuen.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, Barbarosie M, Lücke A, Nagao T, Lopantsev V, Köhling R. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci. 1996;16:3912–3924. doi: 10.1523/JNEUROSCI.16-12-03912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol. 2011;95:104–132. doi: 10.1016/j.pneurobio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin Neurophysiol. 2010;121:301–310. doi: 10.1016/j.clinph.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Biagini G, Longo D, Baldelli E, Zoli M, Rogawski MA, Bertazzoni G, et al. Neurosteroids and epileptogenesis in the pilocarpine model: evidence for a relationship between P450scc induction and length of the latent period. Epilepsia. 2009;50:53–58. doi: 10.1111/j.1528-1167.2008.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J. High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Benassi SK, Kheiri F, Engel J., Jr Further evidence that pathologic high-frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia. 2011;52:45–52. doi: 10.1111/j.1528-1167.2010.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Demir R, Haberly LB, Jackson MB. Epileptiform discharges with in-vivo-like features in slices of rat piriform cortex with longitudinal association fibers. J Neurophysiol. 2001;86:2445–2460. doi: 10.1152/jn.2001.86.5.2445. [DOI] [PubMed] [Google Scholar]
- Demont-Guignard S, Benquet P, Gerber U, Biraben A, Martin B, Wendling F. Distinct hyperexcitability mechanisms underlie fast ripples and epileptic spikes. Ann Neurol. 2012;71:342–352. doi: 10.1002/ana.22610. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Mechanisms of fast ripples in the hippocampus. J Neurosci. 2004;24:8896–8906. doi: 10.1523/JNEUROSCI.3112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, da Silva FL. High-frequency oscillations – where we are and where we need to go. Prog Neurobiol. 2012;98:316–318. doi: 10.1016/j.pneurobio.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Ferando I, Mody I. GABAA receptor modulation by neurosteroids in models of temporal lobe epilepsies. Epilepsia. 2012;53:89–101. doi: 10.1111/epi.12038. [DOI] [PubMed] [Google Scholar]
- Galvan M, Grafe P, Bruggencate G. Convulsant actions of 4-aminopyridine on the guinea-pig olfactory cortex slice. Brain Res. 1982;241:75–86. doi: 10.1016/0006-8993(82)91230-6. [DOI] [PubMed] [Google Scholar]
- Haberly LB. The synaptic organization of the brain. New York, NY, US: Oxford University Press; 1998. Olfactory cortex; pp. 377–416. [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Pal SK, Sato TF, Wienisch M, Murthy VN. Optophysiological analysis of associational circuits in the olfactory cortex. Front Neural Circuits. 2012:6. doi: 10.3389/fncir.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarz JM, Foffani G, Cid E, Inostroza M, Menendez de la Prida L. Emergent dynamics of fast ripples in the epileptic hippocampus. J Neurosci. 2010;30:16249–16261. doi: 10.1523/JNEUROSCI.3357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Châtillon CÉ, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CÉ, Hall J, Olivier A, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JGR, Menendez de la Prida L, Wendling F, Bragin A, Avoli M, Timofeev I, et al. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012;98:250–264. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiruska P, Csicsvari J, Powell AD, Fox JE, Chang WC, Vreugdenhil M, et al. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci. 2010;30:5690–5701. doi: 10.1523/JNEUROSCI.0535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa K, Kaila K. Ionic mechanisms of spontaneous GABAergic events in rat hippocampal slices exposed to 4-aminopyridine. J Neurophysiol. 1997;78:2582–2591. doi: 10.1152/jn.1997.78.5.2582. [DOI] [PubMed] [Google Scholar]
- Laxer K, Blum D, Abou-Khalil BW, Morrell MJ, Lee DA, Data JL, et al. Assessment of ganaxolone’s anticonvulsant activity using a randomized, double-blind, presurgical trial design. Ganaxolone presurgical study group. Epilepsia. 2000;41:1187–1194. doi: 10.1111/j.1528-1157.2000.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Bortel A, Gotman J, Avoli M. High-frequency (80–500 Hz) oscillations and epileptogenesis in temporal lobe epilepsy. Neurobiol Dis. 2011;42:231–241. doi: 10.1016/j.nbd.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Salami P, Gotman J, Avoli M. Two seizure-onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J Neurosci. 2012;32:13264–13272. doi: 10.1523/JNEUROSCI.5086-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Ebert U. The role of the piriform cortex in kindling. Prog Neurobiol. 1996;50:427–481. doi: 10.1016/s0301-0082(96)00036-6. [DOI] [PubMed] [Google Scholar]
- Luna VM, Pettit DL. Asymmetric rostro-caudal inhibition in the primary olfactory cortex. Nat Neurosci. 2010;13:533–535. doi: 10.1038/nn.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Michelson HB, Wong RK. Synchronization of inhibitory neurones in the guinea-pig hippocampus in vitro. J Physiol. 1994;477:35–45. doi: 10.1113/jphysiol.1994.sp020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panuccio G, Sanchez G, Lévesque M, Salami P, de Curtis M, Avoli M. On the ictogenic properties of the piriform cortex in vitro. Epilepsia. 2012;53:459–468. doi: 10.1111/j.1528-1167.2012.03408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugnaghi M, Monti G, Biagini G, Meletti S. Temporal lobe epilepsy exacerbation during pharmacological inhibition of endogenous neurosteroid synthesis. Case Rep. 2013 doi: 10.1136/bcr-2012-008204. http://dx.doi.org/10.1136/bcr-2012-008204. [DOI] [PMC free article] [PubMed]
- Reddy DS. Pharmacology of endogenous neuroactive steroids. Crit Rev Neurobiol. 2004;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Neurosteroids: endogenous role in the human brian and therapeutic potentials. Prog Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front Neuroendocr Sci. 2011;2:38. doi: 10.3389/fendo.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Hauser CAE, Trapp T, Holsboer F. Neurosteroids: molecular mechanisms of action and psychopharmacological significance. J Steroid Biochem Mol Biol. 1996;56:163–168. doi: 10.1016/0960-0760(95)00233-2. [DOI] [PubMed] [Google Scholar]
- Salami P, Lévesque M, Gotman J, Avoli M. A comparison between automated detection methods of high-frequency oscillations (80–500 Hz) during seizures. J Neurosci Methods. 2012;211:265–271. doi: 10.1016/j.jneumeth.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar P, Tapia R, Rogawski MA. Effects of neurosteroids on epileptiform activity induced by picrotoxin and 4-aminopyridine in the rat hippocampal slice. Epilepsy Res. 2003;55:71–82. doi: 10.1016/s0920-1211(03)00112-8. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farran M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Two layers of synaptic processing by principal neurons in piriform cortex. J Neurosci. 2011;31:2156–2166. doi: 10.1523/JNEUROSCI.5430-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling F, Bartolomei F, Mina F, Huneau C, Benquet P. Interictal spikes, fast ripples and seizures in partial epilepsies – combining multi-level computational models with experimental data. Eur J Neurosci. 2012;36:2164–2177. doi: 10.1111/j.1460-9568.2012.08039.x. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010;75:1686–1694. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]