Abstract
Exposure to cholinergic agonists is a widely used paradigm to induce epileptogenesis in vivo and synchronous activity in brain slices maintained in vitro. However, the mechanisms underlying these effects remain unclear. Here, we used field potential recordings from the lateral entorhinal cortex in horizontal rat brain slices to explore whether two different K+ currents regulated by muscarinic receptor activation, the inward rectifier (KIR) and the M-type (KM) currents, have a role in carbachol (CCh)-induced field activity, a prototypical model of cholinergic-dependent epileptiform synchronization. To establish whether KIR or KM blockade could replicate CCh effects, we exposed slices to blockers of these currents in the absence of CCh. KIR channel blockade with micromolar Ba2+ concentrations induced interictal-like events with duration and frequency that were lower than those observed with CCh; by contrast, the KM blocker linopirdine was ineffective. Pre-treatment with Ba2+ or linopirdine increased the duration of epileptiform discharges induced by subsequent application of CCh. Baclofen, a GABAB receptor agonist that activates KIR, abolished CCh-induced field oscillations, an effect that was abrogated by the GABAB receptor antagonist CGP 55845, and prevented by Ba2+. Finally, when applied after CCh, the KM activators flupirtine and retigabine shifted leftward the cumulative distribution of CCh-induced event duration; this effect was opposite to what seen during linopirdine application under similar experimental conditions. Overall, our findings suggest that KIR rather than KM plays a major regulatory role in controlling CCh-induced epileptiform synchronization.
Keywords: Inward rectifiers, M-Current, Entorhinal cortex, Temporal lobe epilepsy, Muscarinic receptors, GABAB receptors, Retigabine
1. Introduction
Cholinergic agonists like carbachol (CCh) or pilocarpine, by activating M1 muscarinic receptors (Cruickshank et al., 1994; Bymaster et al., 2003), induce seizure activity when administered in vivo (Turski et al.,1983), and epileptiform discharges in brain slices (Dickson and Alonso, 1997). It is still unclear which of the different transductional events activated upon M1 receptor stimulation is responsible for the appearance of this synchronous activity. A wealth of experimental evidence points to the activation of non-specific cation currents as the major factor involved even though the identity of these currents remains poorly defined. D’Antuono et al. (2001) provided evidence for a major role of the so called ICAN current (Colino and Halliwell,1993), a non-specific cationic current activated by [Ca2+]i increases, which induces the appearance of depolarizing plateau potentials in limbic areas involved in temporal lobe epilepsy (TLE) (see Gloor, 1997) including the subiculum (D’Antuono et al., 2001), the entorhinal cortex (EC) (Klink and Alonso, 1997), and the hippocampal CA1 subfield (Fraser and MacVicar, 1996). By contrast, Egorov et al. (2003) provided evidence for a CCh-activated Ca2+-independent non-specific cation channel.
In addition to non-specific cationic channels, CCh interferes with a number of additional targets, which may play a relevant role in CCh-induced epileptiform discharges. In particular, M1 receptors activate phospholipase C (PLC), which, in turn, leads to a depletion of phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid crucially involved in the control of a wide variety of ionic currents (Suh and Hille, 2005). Among PIP2-modulated currents are inward rectifiers (KIR) (Huang et al., 1998; Sohn et al., 2007; Carr and Surmeier, 2007) and the M-current (KM), which may therefore regulate the genesis or maintenance of CCh-induced epileptiform discharges. KIR-M1receptor interaction may be particularly relevant to TLE, since inward rectifier K+ channels are highly expressed in the hippocampus and in the EC (Karschin et al., 1996), where they participate in TLE pathogenesis (Young et al., 2009). Depending upon their subunit composition, KIR may be open at resting conditions (constitutively active KIR) and control resting membrane potential, or require the stimulation of G-protein-coupled receptors to become active (Nichols and Lopatin, 1997). The latter, also referred to as GIRK channels, largely mediate the responses to inhibitory neurotransmitters (Mark and Herlitze, 2000).
KM, as well, is highly expressed in the hippocampus (Cooper et al., 2001), where it controls neuronal excitability by regulating action potential frequency adaptation (Yue and Yaari, 2004), after-depolarization/after-hyperpolarization amplitude and duration (Yue and Yaari, 2004) and, in some instances, resting membrane potential (Shah et al., 2008). Moreover, KM modulates intrinsic firing properties and subthreshold membrane oscillations in EC cells (Yoshida and Alonso, 2007), and the epileptiform activity induced by perfusion with low Mg2+-containing medium in the hippocampus (Qiu et al., 2007).
Despite these evidences, the possible involvement of KIR and KM in epileptiform synchronization consequent to cholinergic activation remains unexplored. Therefore, we employed field potential recordings from the EC, a limbic area having a major role in TLE (Du et al., 1995; de Guzman et al., 2008) and in generating the epileptiform activity induced by muscarinic agonists in brain slices (Nagao et al., 1996), along with pharmacological manipulations aimed at modulating KIR and KM, to investigate the roles of these two K+ currents in CCh-elicited synchronous activity. Our data suggest that KIR exerts a major regulation of CCh-induced epileptiform synchronization, whereas KM appears to play a complementary modulatory role.
2. Methods
Brain slices were obtained from adult male Sprague–Dawley rats (Charles River, St. Constant, QC, Canada), 2–3 months of age (250–400 g). Animal housing and experimental procedures were performed according to the recommendations of the Canadian Council on Animal Care following a research protocol approved by the McGill Animal Care Committee. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Combined hippocampus-EC slices were obtained as described by de Guzman et al. (2008). Slices were transferred to a custom-made interface recording chamber and let equilibrate for 1–1.5 h while continuously superfused (1 ml/min) with artificial cerebrospinal fluid (ACSF) at ~32 °C, equilibrated at pH = 7.4 with gas mixture (95% O2, 5% CO2), and containing in mM: 124 NaCl, 2 KCl, 1.25 KH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3 and 10 D-glucose. Extracellular field potentials were recorded using ACSF-filled borosilicate electrodes (5–10 MΩ) (World Precision Instruments, Sarasota, FL, USA) that were placed under visual control in the deep layers of the lateral EC. Field potentials were recorded with preamplifier probes connected to a Cyberamp 380 amplifier (Molecular Devices, Sunnyvale, CA) driven by the pClamp 8.2 software (Molecular Devices). Data were digitized at 5 kHz with a Digidata 1322A A/D converter (Molecular Devices) and stored on the hard drive for offline analysis performed using the Clampfit 9 software (Molecular Devices).
Unless otherwise stated, data are expressed as mean ± SEM. Statistical analysis was performed using the Sigma Plot 10 software with Sigma Stat 3.5 module. Normal distribution was assessed prior to statistical comparison, which was performed with one way or repeated measure ANOVA as appropriate, followed by Newman–Keuls post-hoc test. Statistical analysis of non-normally distributed data was performed with the Kruskall–Wallis or Friedman analysis, as appropriate, followed by the Dunn post-hoc test. Two-group comparisons were performed using the Student’s t test for paired data (normally distributed datasets) or the Mann–Whitney Rank Sum Test (non-normally distributed datasets). Significance was set at p < 0.05.
The effect of different compounds on the duration of CCh- or Ba2+-induced events was quantified by applying the Kolmogorov–Smirnov test to compare the cumulative distributions of these events (bin = 250 ms) before and after application of the test drug. Significance was set at p < 0.05. The concentration-dependence curve of baclofen-induced decrease of the frequency of CCh-induced events was obtained by sequentially exposing each slice to 1, 3, 10, 30 and 100 μM baclofen, and by fitting the data to the four parameter logistic equation
where min and max were the minimal and maximal normalized values of the frequency of CCh-evoked events, respectively, y was the average frequency of CCh-induced events normalized to the values obtained in the absence of baclofen, x was the baclofen concentration and n the Hill slope.
All chemicals were obtained from Sigma–Aldrich (Oakville, Ontario, Canada) with the exception of flupirtine and CGP 55485 (Tocris, Bristol, UK). Retigabine was obtained from Valeant Pharmaceuticals (Aliso Viejo, CA, USA). CGP 55845, flupirtine and retigabine were dissolved in DMSO, and linopirdine was dissolved in ethanol. To rule-out any possible interference of the solvents on field activity, control experiments were performed by adding the appropriate vehicle to the ACSF.
3. Results
3.1. CCh-induced epileptiform discharges in the EC
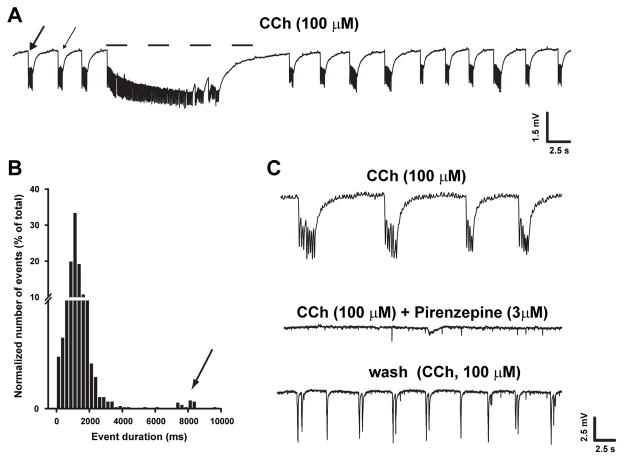
Bath-application of CCh (100 μM) readily (10–30 min) induced the generation of synchronized oscillatory field potentials in the lateral EC in all slices (n = 32; Fig. 1A). Two types of epileptiform activity could be distinguished according to their duration (cf. de Guzman et al., 2004): (i) events shorter than 4 s (Fig. 1A, arrows), thereafter defined as ‘interictal-like’ and (ii) events longer than 4 s, termed as ‘ictal-like’ (Fig. 1A, dashed line). As indicated by the frequency distribution in Fig. 1B, interictal-like discharges represented the majority (n = 3090/3116 events, 99%) of CCh-induced events, and had a mean duration of 1.2 ± 0.4 s, whereas ictal-like discharges had a mean duration of 10.1 ± 1.2 s. Given the minor contribution of ictal-like discharges on average CCh-induced excitability, subsequent analysis was performed on all events pooled together, regardless of their classification as ictal-like or interictal-like discharges. Using this approach, the mean frequency of CCh-induced events was 0.33±0.02 Hz whereas their mean duration was 1.4±0.08 s. CCh-induced events were abolished by bath-application of 3 μM pirenzepine (n = 6; Fig. 1C), confirming that activation of muscarinic receptors is primarily involved in CCh excitatory actions within the EC.
Fig. 1.
CCh-induced synchronized field oscillations in EC. A: Field potential recording obtained from EC deep layers during application of CCh. Note the occurrence of frequent, brief interictal-like events (arrows) and of a prolonged ictal-like discharge (dotted line). B: Bin distribution of the duration of CCh-induced events. Note that two components can be identified in the distribution plot, the second of which (indicated by the arrow) represents ictal-like events. C: Suppression of CCh-induced oscillations by pirenzepine indicates that the effect of CCh is primarily mediated by its interaction with muscarinic receptors.
3.2. KIR and KM regulate CCh-induced oscillations
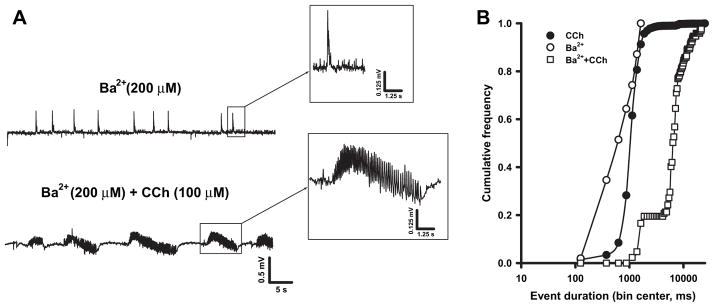
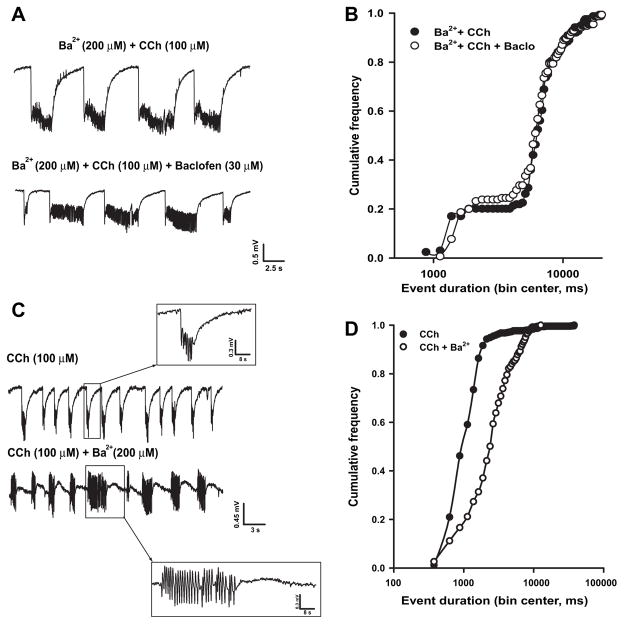
Since muscarinic stimulation is known to decrease the activity of KIR and KM (Carr and Surmeier, 2007; Delmas and Brown, 2005; Sohn et al., 2007), we tested the hypothesis that epileptiform synchronous events similar to those triggered by CCh could be induced upon blockade of either of these two potassium currents. To this aim, we used micromolar concentrations of Ba2+ that should selectively block KIR without affecting KM (Kubo et al.,1993; Cloues and Marrion,1996; Schoots et al., 1996), and linopirdine, a selective KM blocker (Miceli et al., 2008). Bath-application of Ba2+ (200 μM; n = 5) caused the appearance of field discharges (Fig. 2A, top) with a frequency (0.06 ± 0.01 Hz) and duration (0.8 ± 0.2 s) significantly lower than those triggered by CCh (frequency = 0.33 ± 0.02 Hz and duration = 1.4 ± 0.08 s; n = 32, p < 0.01). As shown in Fig. 2B, the cumulative distribution curve of Ba2+-induced events was shifted leftward when compared to that obtained for CCh-induced events (p < 0.01).
Fig. 2.
Ba2+ induces the appearance of interictal-like events and influences CCh-induced oscillations. A: Field discharges recorded in the same slice exposed to Ba2+ (top) and Ba2+ + CCh (bottom). The insets show expanded traces of the boxed events. B: Cumulative frequency distribution of the events duration induced by Ba2+ (open circles, n = 5 slices), CCh (filled circles, n = 32) and Ba2+ + CCh (open squares, n = 5).
Subsequent application of CCh to Ba2+-perfused slices (Fig. 2A, bottom) caused the appearance of field discharges that were longer in duration (7.3 ± 1.5 s, p < 0.001) and occurred less frequently (0.09 ± 0.01 Hz, p < 0.001) than those observed in the presence of CCh alone. These field events were also significantly longer (p < 0.001) and less frequent (p < 0.001) than those seen with Ba2+ alone. As shown in Fig. 2B, the cumulative distribution of the duration of CCh-induced events recorded in the presence of Ba2+ was shifted rightward compared to that describing the events occurring during application of either Ba2+ or CCh alone (p < 0.001). Noticeably, in our system, as in electrically stimulated CA1 region (Gabriel et al.,1998), field transients recorded in the presence of Ba2+ often showed a prominent positive-going component (Fig. 2A).
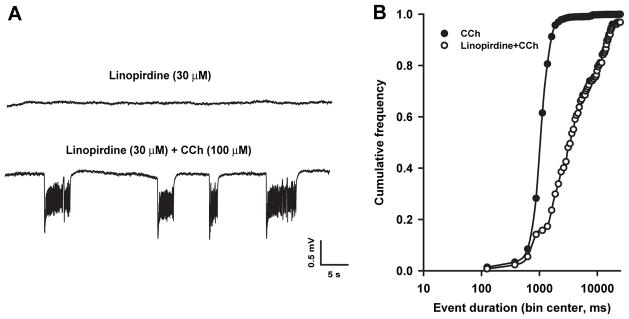
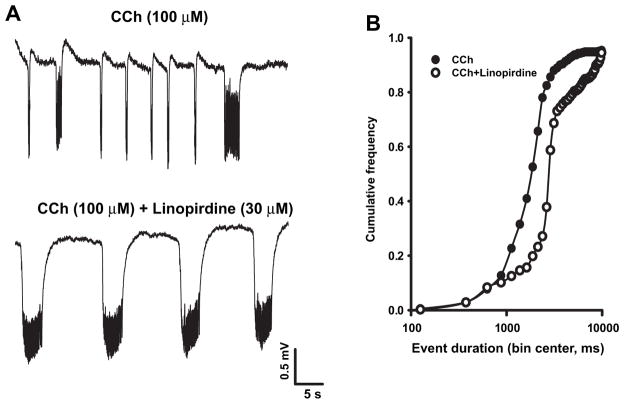
To verify whether CCh-induced field activity could be disclosed by KM blockade, we used the selective KM blocker linopirdine (Miceli et al., 2008) at a dose (30 μM) that is known to induce maximal KM blockade in hippocampal neurons (Schnee and Brown, 1998). Linopirdine by itself did not elicit synchronous activity in the EC (Fig. 3A, upper trace; n = 6); however, further application of CCh (100 μM) induced synchronized field oscillations (Fig. 3A, lower trace) that were significantly slower in frequency (0.09 ± 0.02 Hz, p < 0.001) and longer in duration (12.1 ± 3.0 s; p < 0.01) than those seen during CCh alone. Fig. 3B shows the cumulative distributions of event duration during CCh application alone and following linopirdine treatment. The latter is significantly (p < 0.01) rightward-shifted (Fig. 3B).
Fig. 3.
Linopirdine by itself does not induce field discharges but influences CCh-induced oscillations. A: Field activity recorded during application of linopirdine and after further application of CCh. B: Cumulative frequency of the duration of the events induced by CCh (filled circles, n = 32) and linopirdine + CCh (open circles, n = 6).
3.3. KIR but not KM activation abolishes CCh-induced synchronous activity
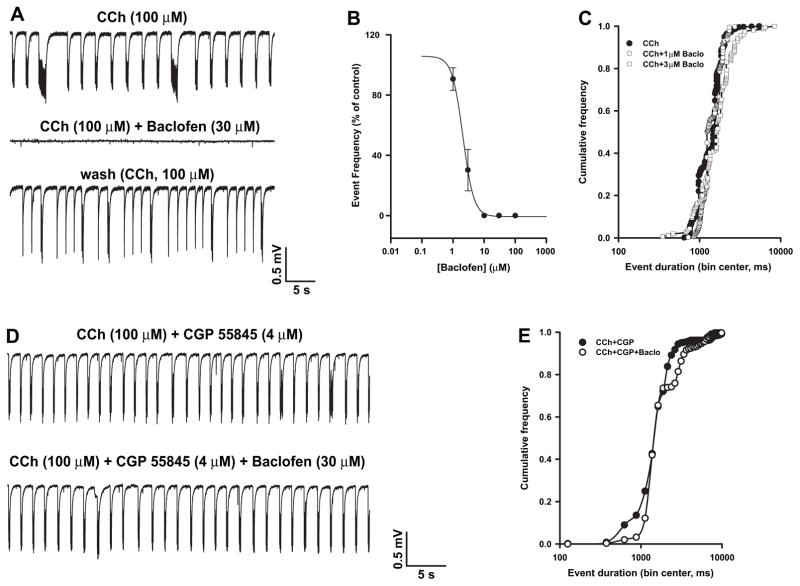
The observation that the duration of CCh-induced events in the EC was significantly increased by pre-treatment with either KIR or KM blockers suggests that both K+ currents play a role in regulating CCh-induced synchronous activity. To further validate this conclusion, we explored the changes induced by drugs that enhance KIR or KM on already established CCh-induced field events. To enhance KIR, we used the GABAB receptor agonist baclofen, which is known to promote the activation of neuronal KIR by a G-protein βγ-mediated stabilization of the channel interaction with PIP2 (Mark and Herlitze, 2000). When applied 30–60 min after the start of CCh perfusion, baclofen (1–100 μM, n = 6) caused a concentration-dependent decrease in CCh-induced event frequency (Fig. 4A and B) without modifying their duration (Fig. 4C); 30 μM baclofen was sufficient to completely suppress field oscillations triggered by CCh.
Fig. 4.
Baclofen suppresses CCh-induced field discharges via GABAB receptors. A: Baclofen reversibly suppresses CCh-induced events in the EC. B: Concentration-dependence curve of baclofen-induced decrease of the frequency of CCh-evoked events; baclofen IC50 was 2.24 μM and the Hill slope = 1. C: Cumulative frequency curves of the duration of the events recorded in the presence of CCh (filled circles, n = 32) and after addition of baclofen, 1 μM (open circles, n = 6) or 3 μM (open squares, n = 6). D: The GABAB receptor blocker CGP 55845 prevents baclofen-induced suppression of the field oscillations triggered by CCh. E: Cumulative frequency curves of the duration of the events recorded in the presence of CCh + CGP 55845 (filled circles, n = 6) or CCh + CGP 55845 + baclofen (open circles, n = 6).
The effects induced by baclofen were not observed when slices were pre-treated with the GABAB receptor antagonist CGP 55845 (4 μM; n = 6) (Fig. 4D and E). It is worth noticing that CGP 55845 by itself did not modify CCh-induced synchronous events (frequency of occurrence during CCh and CCh + CGP 55845: 0.37 ± 0.05 and 0.28 ± 0.05 Hz, respectively; duration during CCh and CCh + CGP 55845: 1.3 ± 0.2 and 1.7 ± 0.2 ms, respectively; n = 6, p > 0.05 for both parameters).
Mechanisms different from the activation of KIR channel activity, including presynaptic cAMP accumulation and the inhibition of voltage-gated Ca2+ channel (VGCC) activity, could be involved in the disappearance of CCh-induced events in the presence of baclofen (Bowery and Enna, 2000). Therefore, in order to understand whether baclofen-induced suppression of field activity evoked by CCh actually involved KIR, we tested whether KIR blockade with 200 μMBa2+ could prevent baclofen effects. CCh-induced field oscillations recorded in Ba2+-pretreated slices (duration: 7.3 ± 1.5 s; frequency: 0.09 ± 0.01 Hz), were not modified by 30 μM baclofen (duration: 8.2±1.9 s; frequency: 0.09±0.02 Hz; n=6; p> 0.05) (Fig. 5A and B), therefore suggesting that the effect of this GABAB receptor agonist on CCh-induced discharges largely involved the activation of KIR channels.
Fig. 5.
Baclofen suppression of CCh-induced field activity depends on KIR activation. A: Field discharges recorded in the EC during application of Ba2+ + CCh and after addition of baclofen. B: Cumulative frequency curves of the duration of the discharges recorded during perfusion with Ba2+ + CCh (filled circles, n = 6) and after baclofen addition (open circles, n = 6). C: Established CCh-induced events change in polarity and become more robust after subsequent application of Ba2+ (cf. Figs. 1A and 2A). D: Cumulative frequency curves of the duration of field discharges recorded in Ba2+ + CCh (filled circles, n = 6) and after baclofen addition (open circles, n = 6).
To further explore the role of KIR in CCh-induced epileptiform synchronization, we evaluated whether blockade of these channels by Ba2+ could modify the frequency or duration on established CCh-induced field discharges. As shown in Fig. 5C, Ba2+ (200 μM) increased in the duration of CCh-induced events (CCh: 1.8 ± 0.6 s; +Ba2+: 3.6±0.7 s; p<0.05, paired t-test), thus significantly shifting rightward the cumulative distribution of event duration (p < 0.05, Kolmogoronov–Smirnov test). Conversely, event frequency was significantly reduced (0.27 ± 0.04 vs 0.17 ± 0.03; p < 0.01, paired t-test).
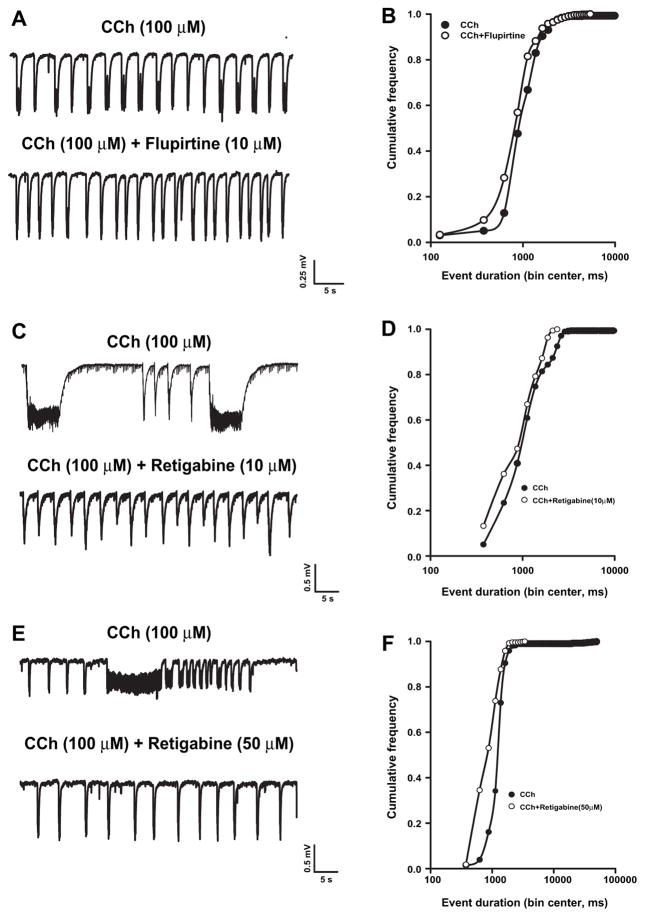
Similarly, the role of KM in modulating established CCh-induced field oscillations was investigated by exploring the effect of the KM activators flupirtine and retigabine in slices that had been already perfused with CCh for 30–60 min. Both these compounds enhance the M-current by shifting its voltage dependence of activation toward more negative potentials but they differ in their efficacy, retigabine being more effective than flupirtine (Miceli et al., 2008). Flupirtine (10 μM, cf. Martire et al., 2004; Wladyka and Kunze, 2006) did not abolish CCh-induced oscillations (Fig. 6A), but caused a significant leftward shift in the cumulative distribution of their duration (Fig. 6B) (n = 6, p < 0.05, Kolmogorov–Smirnov test). The mean duration (1.35 ± 0.2 s) and frequency (0.39 ± 0.05 Hz) of the oscillations recorded in the presence of CCh+flupirtine were not significantly different when compared with CCh alone (respectively, 1.16 ± 0.2 s and 0.40 ± 0.04 Hz; n = 6). The effect of retigabine (10 μM) was tested in 8 different slices, three of which showed ictal-like discharges. Retigabine (10 μM) caused the disappearance of ictal-like but not of interictal-like discharges (Fig. 6C) and shifted leftward the cumulative distribution of CCh-induced event duration (Fig. 6D). As observed with flupirtine, the mean event duration was not significantly modified by retigabine (1.7 ± 0.35 vs 1.17 ± 0.51 s, n = 8, p > 0.05 at paired t-test). Conversely, event frequency was significantly increased in the presence of this drug (CCh: 0.31 ± 0.07 Hz; CCh + Retigabine: 0.43 ± 0.08 Hz; p < 0.05, paired t-test) presumably because of the suppression of ictal-like events. We also tested the higher retigabine concentration of 50 μM because at this concentration retigabine is effective in suppressing not only the ictal-like but also the interictal-like events elicited by 4-aminopyridine (Armand et al., 1999). Interestingly, even at this concentration retigabine, although suppressing ictal-like events, failed to modify CCh-induced interictal activity (Fig. 6E). In the presence of retigabine (50 μM) a significant leftward shift of the cumulative distribution of CCh-induced event duration was observed (Fig. 6F). The mean duration (1.8 ± 0.29 vs 1.1 ± 0.1 s) and the mean frequency (0.28 ± 0.05 vs 0.43 ± 0.09 Hz) of CCh-induced events were not modified by 50 μM retigabine although a non-significant (p = 0.054) trend toward higher event frequency was observed.
Fig. 6.
Effects of flupirtine and retigabine on CCh-evoked event duration. Representative traces (left) and cumulative frequency curves of event duration (right) of field recordings obtained in slices exposed to CCh before and after the addition of (A and B) flupirtine (10 μM, n = 6), and (C and D) retigabine 10 μM (n = 8) and (E and F) retigabine 50 μM (n = 6). Note the disappearance of ictal-like discharges in slices exposed to retigabine.
In CCh-treated brain slices the M-current blocker linopirdine modified the pattern of synchronous discharges in a way opposite to that observed in the presence of the KM activators flupirtine and retigabine. Indeed, linopirdine (30 μM) caused a significant (p < 0.05) rightward shift of the cumulative distribution of event duration (Fig. 7), although, as observed with flupirtine, the mean frequency and duration of CCh-induced events were not significantly modified (frequency: 0.13 ± 0.04 Hz vs 0.18 ± 0.04 Hz (p > 0.05) and duration: 5.3 ± 1.3 and 4.7 ± 2.0 s (p > 0.05) respectively in the presence and in the absence of the drug).
Fig. 7.
Effect of linopirdine on CCh-evoked event duration. Representative traces (A) and cumulative event duration frequency curves (B) of field recordings obtained in slices exposed to CCh before and after the addition of linopirdine (30 μM) (n = 6).
4. Discussion
The present study investigated whether KIR or KM, two K+ currents that are influenced by muscarinic receptor activation, have a role in generating and/or maintaining CCh-induced synchronous activity in the rat lateral EC in an in vitro brain slice preparation. By using field potential recordings, we found that CCh-induced epileptiform discharges are facilitated by KIR blockade and suppressed by KIR activation; in addition, they are tuned by KM but cannot be reproduced by the pharmacological blockade of either of these two currents. Therefore, our results indicate that, in concert with other mechanisms, KM acts as a secondary modulator of CCh-induced epileptiform synchronization, whereas KIR may play a major role.
4.1. Involvement of KIR in the generation and maintenance of CCh-induced epileptiform activity
The non-selective KIR antagonist Cs+ induces the appearance of epileptiform activity in the CA1 area of hippocampal slices, suggesting that KIR plays a crucial role in controlling resting excitability in the hippocampus (Zhang et al., 1998; Andreasen et al., 2007). Here, we have shown that concentrations of Ba2+ that selectively block KIR (Kubo et al., 1993; Schoots et al., 1996) without affecting KM (Cloues and Marrion, 1996) can trigger spontaneous interictal-like events in the EC. However, epileptiform discharges induced by Ba2+ were shorter in duration and occurred less frequently than those induced by CCh alone. Moreover, CCh could induce field oscillations even when KIR was already blocked by Ba2+ (cf., Klink and Alonso, 1997). Therefore, these results suggest that, similarly to what observed in the prefrontal cortex (Carr and Surmeier, 2007) and in the CA1 subfield (Sohn et al., 2007), muscarinic-induced suppression of KIR, while cooperating to the generation of CCh-induced field oscillations, is not the primary mechanism responsible for this process. In line with our findings, other mechanisms, such as the activation of ICAN (Klink and Alonso, 1997), have been proposed.
A crucial modulatory role for muscarinic-induced KIR suppression during CCh-induced oscillation is also suggested by the observation that CCh-induced synchronous events are facilitated when KIR is blocked by pre-treatment with Ba2+, and are abolished after KIR activation by baclofen, a procedure that promotes KIR activation by stabilizing the channel-PIPs interaction (Mark and Herlitze, 2000). GABAB receptor activation, in addition to increasing KIR activity, can also suppress cAMP generation and inhibit VGCC activity (Bowery and Enna, 2000). However, it is unlikely that these transductional mechanisms played a significant role in the suppression of CCh-induced synchronous activity caused by baclofen, since KIR blockade by Ba2+ fully prevented the effect of this GABAB receptor agonist. Noticeably, in our slice preparation, the lack of effect of the GABAB receptor antagonist CGP 55845 by itself argues against a ‘tonic’ GABAergic activation of GIRK, although other neurotransmitters, such as adenosine or opioids (Mark and Herlitze, 2000) might be involved. Further experiments are needed to clarify this point and better define which class of KIR is the main regulator of CCh, given that low concentrations of Ba2+ can block both constitutively active KIR and GIRK (Kubo et al., 1993; Schoots et al., 1996).
Since KIR channels (Sontheimer, 1994), and M1 (Shelton and McCarthy, 2000) and GABAB (Charles et al., 2003) receptors are expressed not only by neurons but also by astrocytes, the effect that we observed could have been determined by an action on neurons, glia or both. Based on the results of our pharmacological experiments, however, we cannot distinguish among these different possibilities, since Ba2+ and baclofen could interfere with both of these cell populations. However, the fact that Ba2+ modified the shape of CCh-induced field discharges by inducing the appearance of a positive-going component could be explained by a Ba2+-induced inhibition of the glial K+ sink. Similar mechanisms have been put forward to explain the findings of Gabriel et al. (1998), who showed that an inversion of the negative field potentials evoked in the CA1 subfield by alvear electrical stimulation occurs when the slices are exposed to Ba2+.
4.2. Involvement of KM in the modulation of CCh-induced synchronous activity
Here we showed that the KM blocker linopirdine failed to trigger spontaneous activity in the EC, but significantly increased the duration of CCh-induced events. These results suggest that although KM does not provide a major contribution to resting excitability in the EC, it participates in sustaining neuronal hyperexcitability during CCh exposure. Interestingly, previous studies have shown that the contribution of KM to resting membrane potential vary among different neuronal populations; in fact while the blockade of KM increased resting excitability in rat visceral sensory neurons (Wladyka and Kunze, 2006) it failed to do so in peripheral unmyelinated C-type nerve fibers (Lang et al., 2008) and in central myelinated axons (Rivera-Arconada and Lopez-Garcia, 2005). Our evidence that the contribution of KM in the control of membrane excitability becomes relevant when the slices are exposed to CCh is consistent with the data reported by Yoshida and Alonso (2007) who showed that KM blockers markedly modify the intrinsic firing properties of EC neurons when they are depolarized. CCh does induce, indeed, membrane depolarization in EC neurons, probably by activating non-selective cationic currents (see the Introduction) and this depolarization could be responsible for KM activation merely because of the voltage dependence of this current. KM participation in CCh-induced epileptiform activity is further suggested by the fact that two KM openers, retigabine and flupirtine, significantly reduced the duration of CCh-induced interictal-like events. Since none of the slices in the flupirtine group generated ictal-like discharges we were unable to evaluate whether flupirtine could modify this type of field activity as well. However, retigabine completely suppressed these long-lasting events whenever present. The fact that retigabine failed to fully suppress all types of CCh-induced events is in apparent contrast with the ability of this drug to abolish both ictal- and interictal-like events induced in vitro by 4-aminopyridine (Armand et al., 1999), or by perfusion with low Mg2+- (Qiu et al., 2007) or low Ca2+/high K+- (Piccinin et al., 2006) containing medium. It should be, however, mentioned that distinct levels of KM activity could be recruited by different epileptogenic stimuli; in particular, in the present model of ictogenesis triggered by muscarinic stimulation, KM contribution would be less pronounced since CCh induces a profound depletion in plasma membrane PIP2, thus leading to a substantial suppression of KM. Indeed, due to the low affinity for this phospholipid (significantly smaller than that of KIR) of KV7 channels, which carry the KM current (Huang et al., 1998; Zhang et al., 2003), KM is extremely sensitive to PIP2 depletion. Therefore, our findings are compatible with the hypothesis that KM does not contribute significantly to resting excitability in the EC and that, despite being activated during CCh-induced depolarization, it only provides a limited contribution in modulating CCh-evoked interictal-like activity, possibly due to a simultaneous depletion of PIP2. It is, however, important to emphasize that despite its limited activity on interictal-like discharges, KM pharmacological modulation with retigabine was effective in suppressing ictal-like activity. This suggests that the M-current plays a role in ictal-like discharge generation. Since ictal-like discharges are the “in vitro” counterpart of the seizures occurring in vivo in animal models and patients, our findings are fully consistent with the anticonvulsant actions shown by retigabine.
5. Conclusive remarks
We have shown in the present study that two different K+ currents, both sensitive to muscarinic activation, exert distinct modulatory effects on CCh-induced epileptiform activity. Specifically, while KM appears to play only a limited role, KIR seems to be a major regulator of CCh-induced events. Further studies are needed to investigate whether these currents might be useful pharmacological targets to counteract muscarinic-dependent mechanisms possibly involved in TLE (Friedman et al., 2007).
Acknowledgments
This study was supported by the following grants: Canadian Institutes of Health Research (Grant MOP-8109) and the Savoy Foundation to, M.A., E-RARE and Telethon GGPO 7125 to M.T., PRIN 2007 to M.C.; GP received fellowships from Epilepsy Canada and the Savoy Foundation.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- CCh
carbachol
- EC
entorhinal cortex
- KIR
inward rectifier K+ current
- KM
M-current
- TLE
temporal lobe epilepsy
References
- Andreasen M, Skov J, Nedergaard S. Inwardly rectifying K+ (Kir) channels antagonize ictal-like epileptiform activity in area CA1 of the rat hippocampus. Hippocampus. 2007;17:1037–1048. doi: 10.1002/hipo.20335. [DOI] [PubMed] [Google Scholar]
- Armand V, Rundfeldt C, Heinemann U. Effects of retigabine (D-23129) on different patterns of epileptiform activity induced by 4-aminopyridine in rat entorhinal cortex hippocampal slices. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:33–39. doi: 10.1007/pl00005320. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Enna SJ. Gamma-aminobutyric acid(B) receptors: first of the functional metabotropic heterodimers. J Pharmacol Exp Ther. 2000;292:2–7. [PubMed] [Google Scholar]
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci. 2003;17:1403–1410. doi: 10.1046/j.1460-9568.2003.02588.x. [DOI] [PubMed] [Google Scholar]
- Carr DB, Surmeier DJ. M1 muscarinic receptor modulation of Kir2 channels enhances temporal summation of excitatory synaptic potentials in prefrontal cortex pyramidal neurons. J Neurophysiol. 2007;97:3432–3438. doi: 10.1152/jn.00828.2006. [DOI] [PubMed] [Google Scholar]
- Charles KJ, Deuchars J, Davies CH, Pangalos MN. GABA B receptor subunit expression in glia. Mol Cell Neurosci. 2003;24:214–223. doi: 10.1016/s1044-7431(03)00162-3. [DOI] [PubMed] [Google Scholar]
- Cloues R, Marrion NV. Conduction properties of the M-channel in rat sympathetic neurons. Biophys J. 1996;70:806–812. doi: 10.1016/S0006-3495(96)79620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colino A, Halliwell JV. Carbachol potentiates Q current and activates a calcium-dependent non-specific conductance in rat hippocampus in vitro. Eur J Neurosci. 1993;5:1198–1209. doi: 10.1111/j.1460-9568.1993.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci. 2001;21:9529–9540. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank JW, Brudzynski SM, McLachlan RS. Involvement of M1 muscarinic receptors in the initiation of cholinergically induced epileptic seizures in the rat brain. Brain Res. 1994;643:125–129. doi: 10.1016/0006-8993(94)90017-5. [DOI] [PubMed] [Google Scholar]
- D’Antuono M, Kawasaki H, Palmieri C, Avoli M. Network and intrinsic contributions to carbachol-induced oscillations in the rat subiculum. J Neurophysiol. 2001;86:1164–1178. doi: 10.1152/jn.2001.86.3.1164. [DOI] [PubMed] [Google Scholar]
- de Guzman P, D’Antuono M, Avoli M. Initiation of electrographic seizures by neuronal networks in entorhinal and perirhinal cortices in vitro. Neuroscience. 2004;123:875–886. doi: 10.1016/j.neuroscience.2003.11.013. [DOI] [PubMed] [Google Scholar]
- de Guzman P, Inaba Y, Baldelli E, de Curtis M, Biagini G, Avoli M. Network hyperexcitability within the deep layers of the pilocarpine-treated rat entorhinal cortex. J Physiol. 2008;586:1867–1883. doi: 10.1113/jphysiol.2007.146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Dickson CT, Alonso A. Muscarinic induction of synchronous population activity in the entorhinal cortex. J Neurosci. 1997;17:6729–6744. doi: 10.1523/JNEUROSCI.17-17-06729.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Eid T, Lothman EW, Kohler C, Schwarcz R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci. 1995;15:6301–6313. doi: 10.1523/JNEUROSCI.15-10-06301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov AV, Angelova PR, Heinemann U, Müller W. Ca2+-independent muscarinic excitation of rat medial entorhinal cortex layer V neurons. Eur J Neurosci. 2003;18:3343–3351. doi: 10.1111/j.1460-9568.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- Fraser DD, MacVicar BA. Cholinergic-dependent plateau potential in hippocampal CA1 pyramidal neurons. J Neurosci. 1996;16:4113–4128. doi: 10.1523/JNEUROSCI.16-13-04113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Behrens CJ, Heinemann U. Cholinergic dysfunction in temporal lobe epilepsy. Epilepsia. 2007;48(Suppl 5):126–130. doi: 10.1111/j.1528-1167.2007.01300.x. [DOI] [PubMed] [Google Scholar]
- Gabriel S, Eilers A, Kivi A, Kovacs R, Schulze K, Lehmann TN, Heinemann U. Effects of barium on stimulus induced changes in extracellular potassium concentration in area CA1 of hippocampal slices from normal and pilocarpine-treated epileptic rats. Neurosci Lett. 1998;242:9–12. doi: 10.1016/s0304-3940(98)00012-3. [DOI] [PubMed] [Google Scholar]
- Gloor P. The Temporal Lobe and Limbic System. Oxford University Press; Oxford: 1997. [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbeta-gamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Karschin C, Dissmann E, Stühmer W, Karschin A. IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, Alonso A. Ionic mechanisms of muscarinic depolarization in entorhinal cortex layer II neurons. J Neurophysiol. 1997;77:1829–1843. doi: 10.1152/jn.1997.77.4.1829. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Lang PM, Fleckenstein J, Passmore GM, Brown DA, Grafe P. Retigabine reduces the excitability of unmyelinated peripheral human axons. Neuropharmacology. 2008;54:1271–1278. doi: 10.1016/j.neuropharm.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Mark MD, Herlitze S. G-protein mediated gating of inward-rectifier K+ channels. Eur J Biochem. 2000;267:5830–5836. doi: 10.1046/j.1432-1327.2000.01670.x. [DOI] [PubMed] [Google Scholar]
- Martire M, Castaldo P, D’Amico M, Preziosi P, Annunziato L, Taglialatela M. M channels containing KCNQ2 subunits modulate norepinephrine, aspartate, and GABA release from hippocampal nerve terminals. J Neurosci. 2004;24:592–597. doi: 10.1523/JNEUROSCI.3143-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli F, Soldovieri MV, Martire M, Taglialatela M. Molecular pharmacology and therapeutic potential of neuronal Kv7-modulating drugs. Curr Opin Pharmacol. 2008;8:65–74. doi: 10.1016/j.coph.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Nagao T, Alonso A, Avoli M. Epileptiform activity induced by pilocarpine in the rat hippocampal-entorhinal slice preparation. Neuroscience. 1996;72:399–408. doi: 10.1016/0306-4522(95)00534-x. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- Piccinin S, Randall AD, Brown JT. KCNQ/Kv7 channel regulation of hippocampal gamma-frequency firing in the absence of synaptic transmission. J Neurophysiol. 2006;95:3105–3112. doi: 10.1152/jn.01083.2005. [DOI] [PubMed] [Google Scholar]
- Qiu C, Johnson BN, Tallent MK. K+ KM regulates the transition to seizures in immature and adult hippocampus. Epilepsia. 2007;48:2047–2058. doi: 10.1111/j.1528-1167.2007.01193.x. [DOI] [PubMed] [Google Scholar]
- Rivera-Arconada I, Lopez-Garcia JA. Effects of KM modulators on the excitability of immature rat spinal sensory and motor neurones. Eur J Neurosci. 2005;22:3091–3098. doi: 10.1111/j.1460-9568.2005.04507.x. [DOI] [PubMed] [Google Scholar]
- Schnee ME, Brown BS. Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons. J Pharmacol Exp Ther. 1998;286:709–717. [PubMed] [Google Scholar]
- Schoots O, Yue KT, MacDonald JF, Hampson DR, Nobrega JN, Dixon LM, Van Tol HH. Cloning of a G protein-activated inwardly rectifying potassium channel from human cerebellum. Brain Res Mol Brain Res. 1996;39:23–30. doi: 10.1016/0169-328x(95)00349-w. [DOI] [PubMed] [Google Scholar]
- Shah MM, Migliore M, Valencia I, Cooper EC, Brown DA. Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2008;105:7869–7874. doi: 10.1073/pnas.0802805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton MK, McCarthy KD. Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ. J Neurochem. 2000;74:555–563. doi: 10.1046/j.1471-4159.2000.740555.x. [DOI] [PubMed] [Google Scholar]
- Sohn JW, Lee D, Cho H, Lim W, Shin HS, Lee SK, Ho WK. Receptor-specific inhibition of GABAB-activated K+ currents by muscarinic and metabotropic glutamate receptors inimmature rat hippocampus. J Physiol. 2007;580:411–422. doi: 10.1113/jphysiol.2006.125914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H. Voltage-dependent ion channels in glial cells. Glia. 1994;11:156–172. doi: 10.1002/glia.440110210. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Wladyka CL, Kunze DL. KCNQ/KMs contribute to the resting membrane potential in rat visceral sensory neurons. J Physiol. 2006;575:175–189. doi: 10.1113/jphysiol.2006.113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Alonso A. Cell-type specific modulation of intrinsic firing properties and subthreshold membrane oscillations by the M(Kv7)-current in neurons of the entorhinal cortex. J Neurophysiol. 2007;98:2779–2794. doi: 10.1152/jn.00033.2007. [DOI] [PubMed] [Google Scholar]
- Young CC, Stegen M, Bernard R, Müller M, Bischofberger J, Veh RW, Haas CA, Wolfart J. Upregulation of inward rectifier K+ (Kir2) channels in dentate gyrus granule cells in temporal lobe epilepsy. J Physiol. 2009;587:4213–4233. doi: 10.1113/jphysiol.2009.170746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Yaari Y. KCNQ/M channels control spike after depolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24:4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Perez Velazquez JL, Tian GF, Wu CP, Skinner FK, Carlen PL, Zhang L. Slow oscillations (≤1 Hz) mediated by GABAergic interneuronal networks in rat hippocampus. J Neurosci. 1998;18:9256–9268. doi: 10.1523/JNEUROSCI.18-22-09256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Craciun LC, Mirshahi T, Rohács T, Lopes CM, Jin T, Logothetis DE. PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]